Bone Mineral Affinity of Polyphosphodiesters
Abstract
1. Introduction
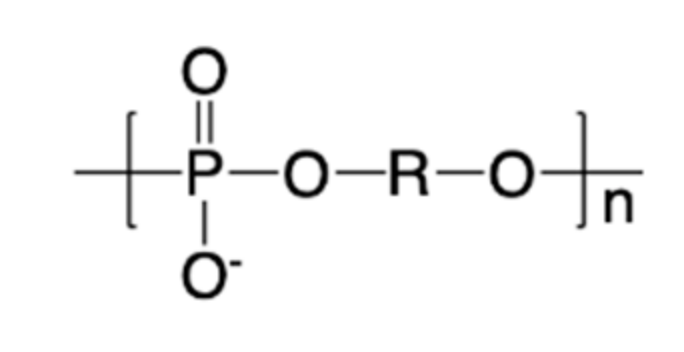
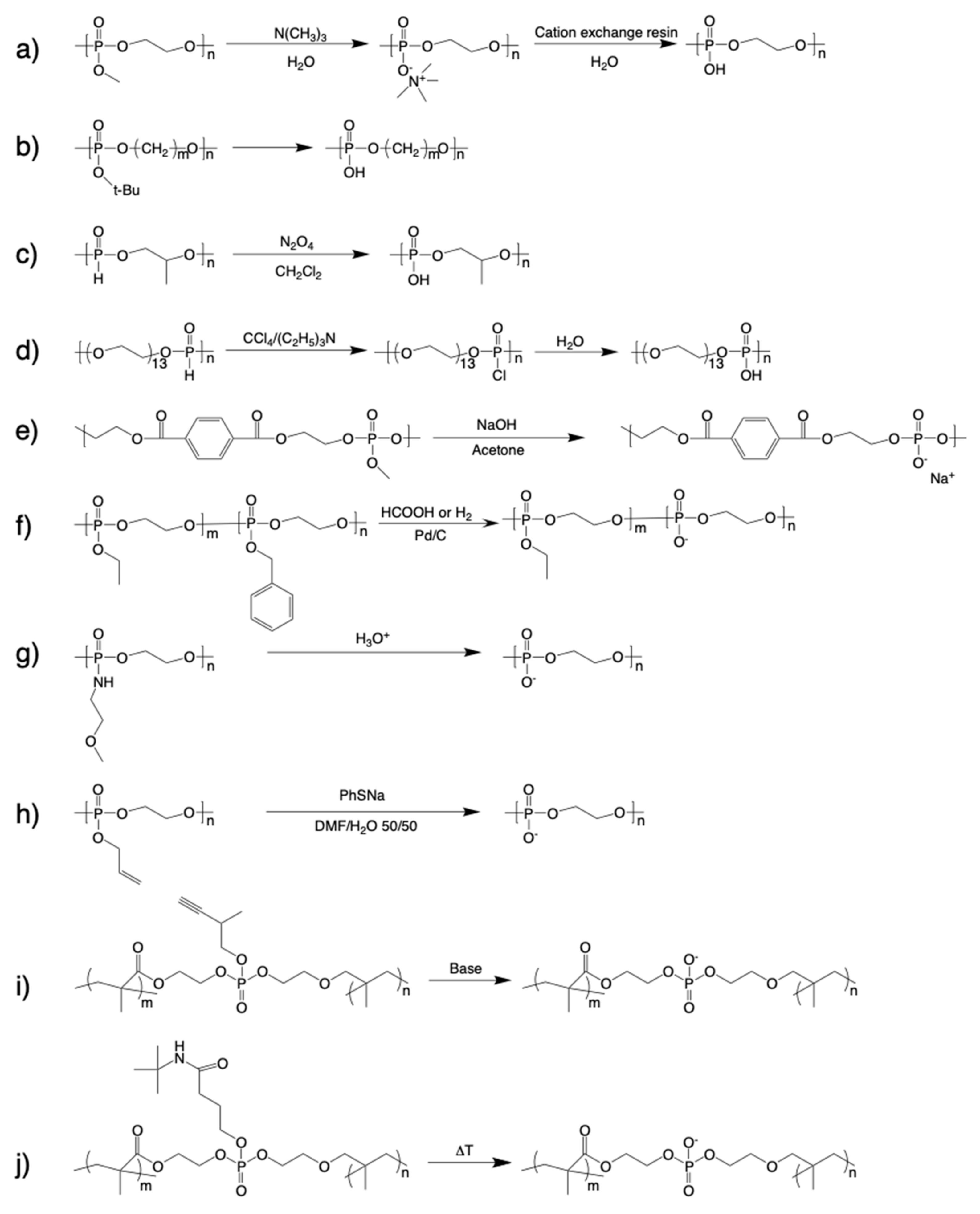
2. Synthesis of Polyphosphodiesters (PPDEs)
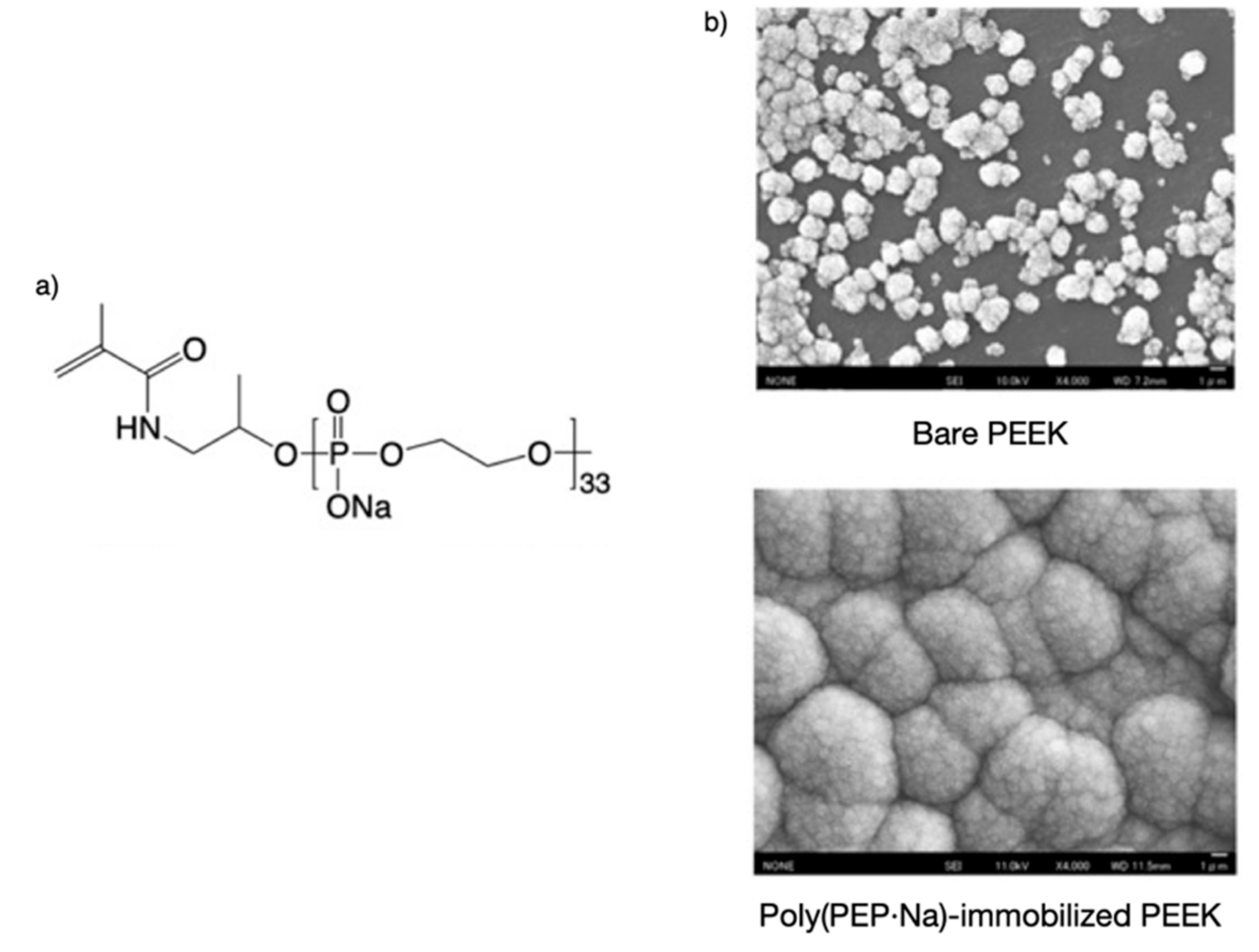
3. Mineral Affinity of Polyphosphodiesters (PPDEs)
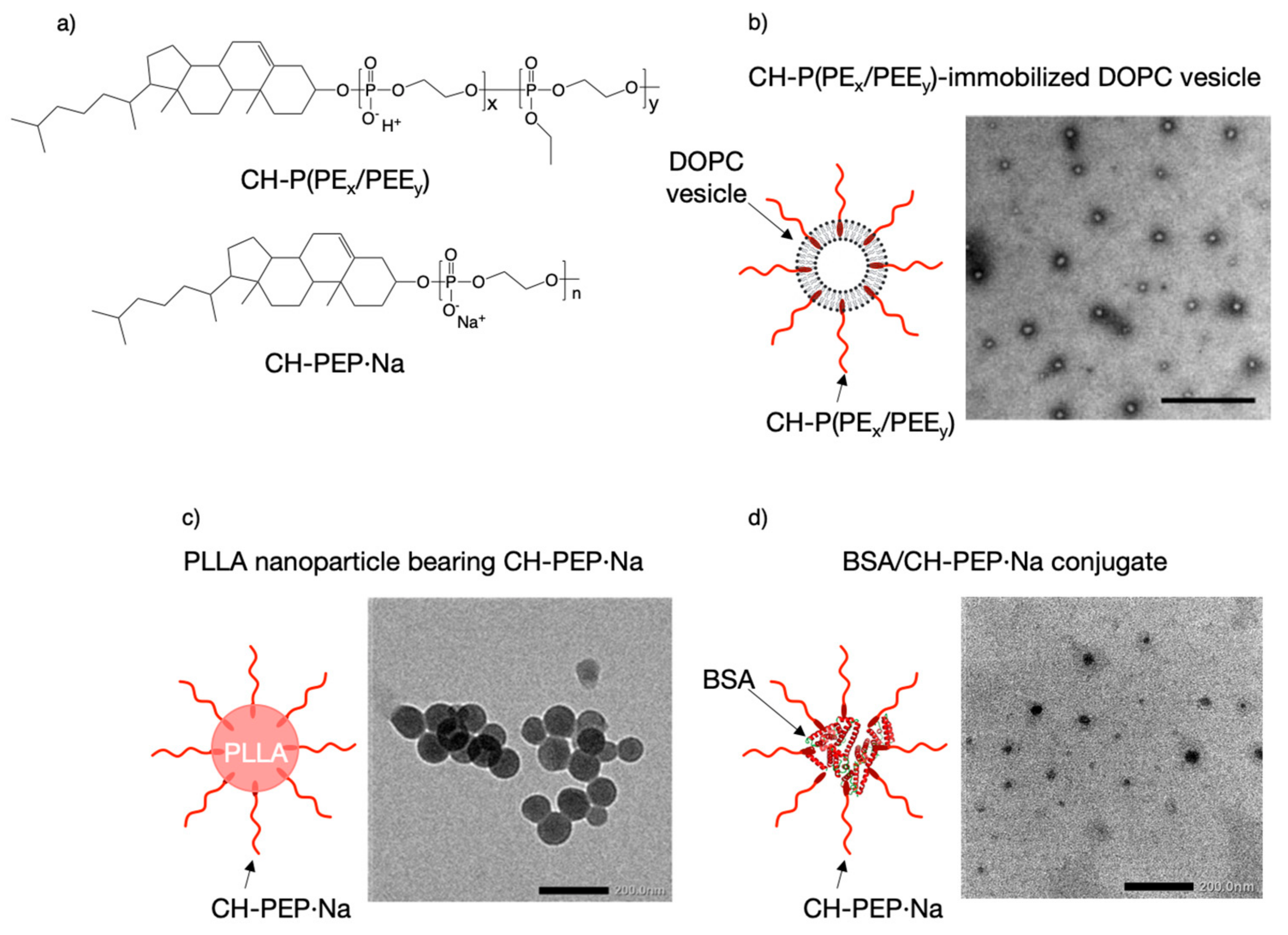
4. Polyphosphodiesters (PPDEs) in Mineral-Binding Nanoparticles
5. Cellular Interaction of Polyphosphodiesters (PPDEs)
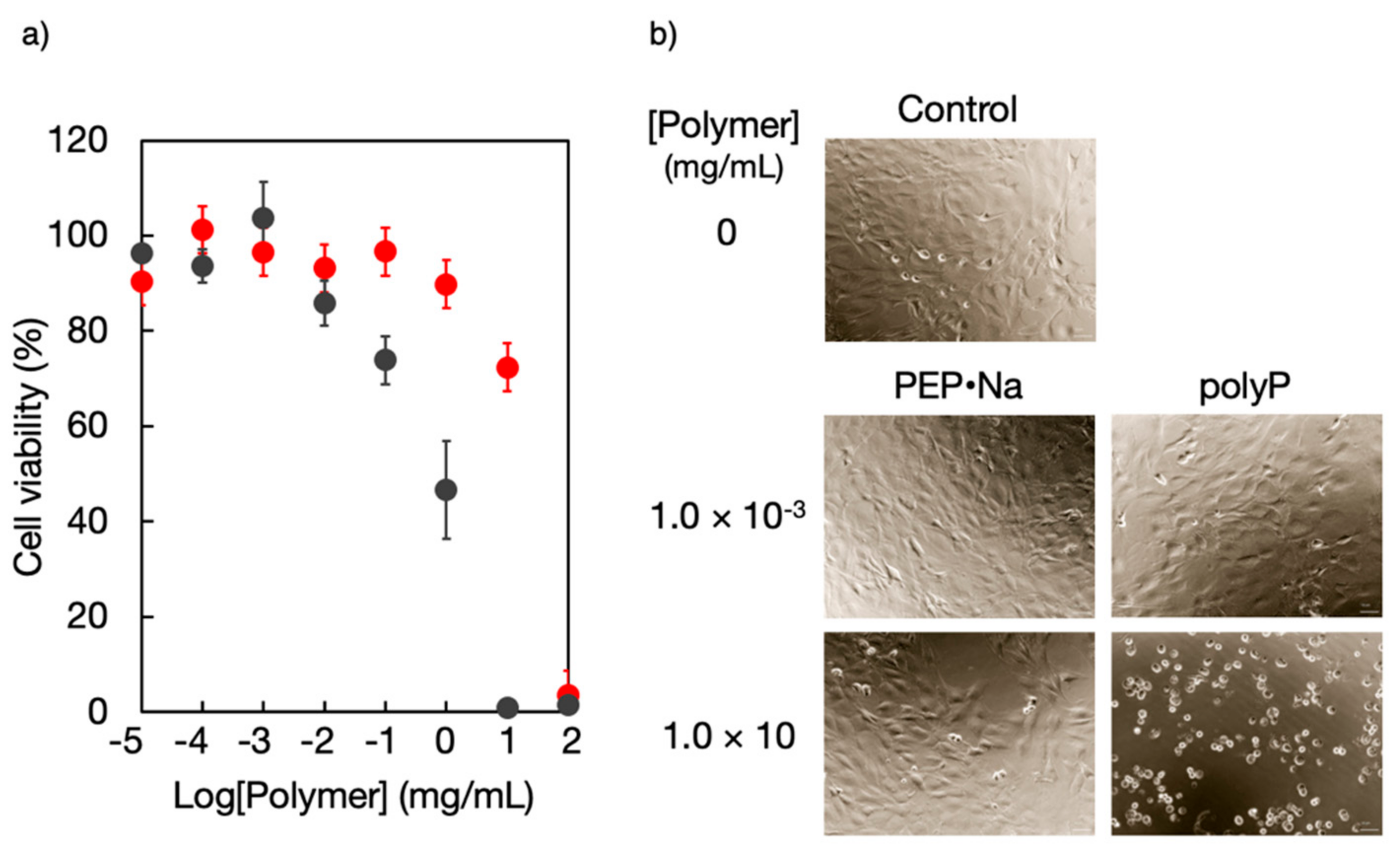
5.1. Cytocompatibility
5.2. Osteoblast Differentiation
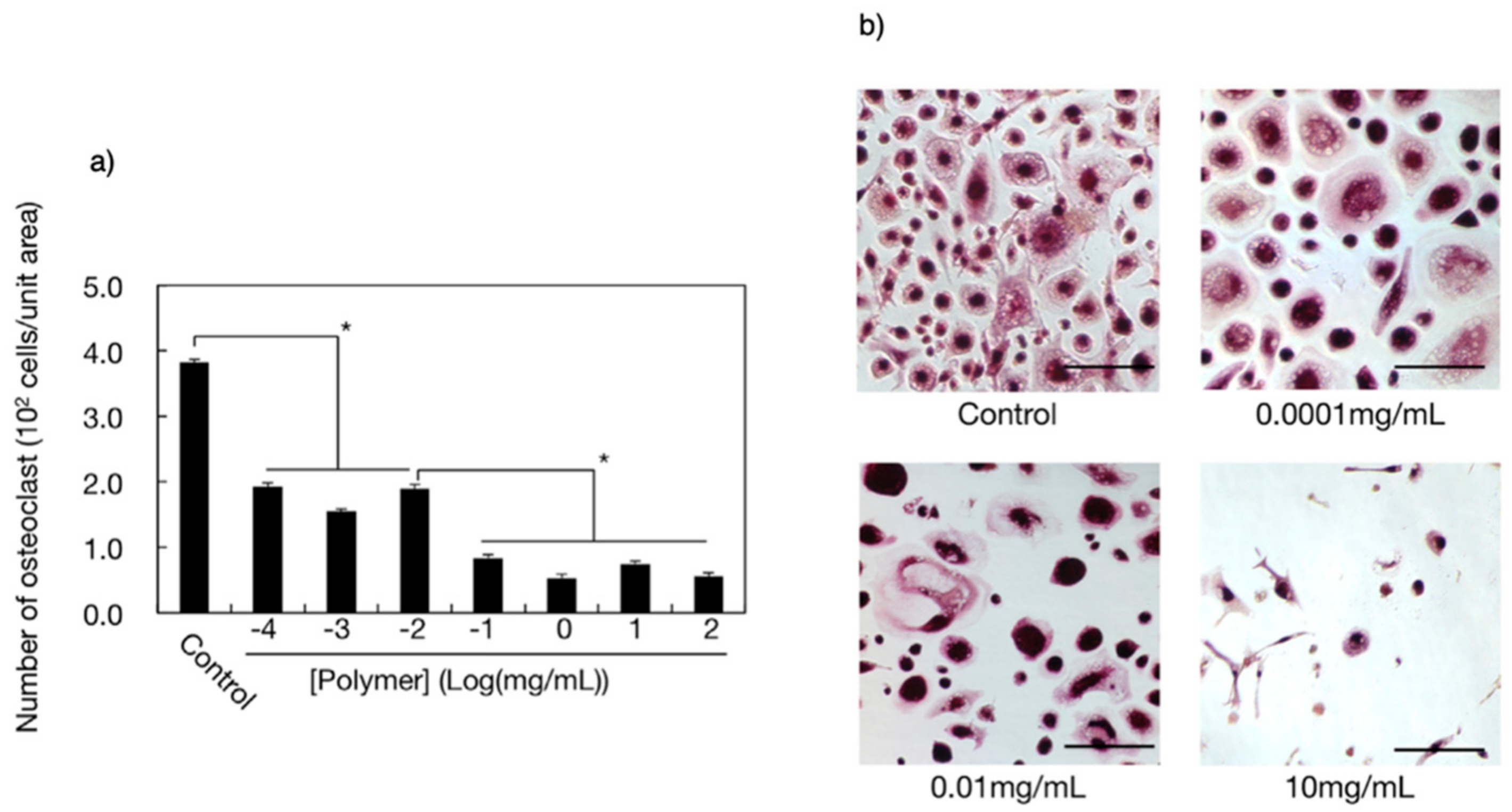
5.3. Osteoclastic Differentiation
6. In Vivo Bone Targeting
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular Firefighting—How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Yuan, Y.Y.; Du, J.Z.; Yang, X.Z.; Wang, J. Recent Progress in Polyphosphoesters: From Controlled Synthesis to Biomedical Applications. Macromol. Biosci. 2009, 9, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.N.; Tee, H.T.; Velencoso, M.M.; Wurm, F.R. Main-chain poly(phosphoester)s: History, Syntheses, Degradation, Bio- and Flame-Retardant Applications. Prog. Polym. Sci. 2017, 73, 61–122. [Google Scholar] [CrossRef]
- Yilmaz, E.; Jerome, C. Polyphosphoesters: New Trends in Synthesis and Drug Delivery Applications. Macromol. Biosci. 2016, 16, 1745–1761. [Google Scholar] [CrossRef] [PubMed]
- Appukutti, N.; Serpell, C.J. High Definition Polyphosphoesters: Between Nucleic Acids and Plastics. Polym. Chem. 2018, 9, 2210–2226. [Google Scholar] [CrossRef]
- Troev, K.D. Polyphosphoesters, 1st ed.; Elsevier: London, UK, 2012; pp. 129–261. [Google Scholar]
- Penczek, S. Models of Biopolymers by Ring-Opening Polymerization of Cyclic Phosphorus Containing Compounds. In Phosphorus Chemistry Directed towards Biology; Stec, W.J., Ed.; Pergamon Press: Oxford, UK, 1980; pp. 133–143. [Google Scholar]
- Alexandrino, E.M.; Ritz, S.; Marsico, F.; Baier, G.; Mailänder, V.; Landfester, K.; Wurm, F.R. Paclitaxel-Loaded Polyphosphate Nanoparticles: A Potential Strategy for Bone Cancer Treatment. J. Mater. Chem. B 2014, 2, 1298–1306. [Google Scholar] [CrossRef]
- Wen, J.; Zhuo, R.-X. Enzyme-Catalyzed Ring-Opening Polymerization of Ethylene Isopropyl Phosphate. Macromol. Rapid Commun. 1998, 19, 641–642. [Google Scholar] [CrossRef]
- Steinbach, T.; Alexandrino, E.M.; Wurm, F.R. Unsaturated Poly(phosphoester)s via Ring-Opening Metathesis Polymerization. Polym. Chem. 2013, 4, 3800–3806. [Google Scholar] [CrossRef]
- Penczek, S. Models of Biopolymers by Ring-Opening Polymerization; CRC Press: Boca Raton, FL, USA, 1990; pp. 291–378. [Google Scholar]
- Penczek, S.; Biela, T.; Klosinski, P.; Lapienis, G. Polymerization of Phosphorus Containing Cyclic Monomers: Synthesis of Polymers Related to Biopolymers. Makromol. Chem. Macromol. Symp. 1986, 6, 123–153. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Yamaguchi, E. Synthesis of Well-Defined Thermoresponsive Polyphosphoester Macroinitiators Using Organocatalysts. Macromolecules 2010, 43, 2664–2666. [Google Scholar] [CrossRef]
- Clément, B.; Grignard, B.; Koole, L.; Jérôme, C.; Lecomte, P. Metal-Free Strategies for the Synthesis of Functional and Well-Defined Polyphosphoesters. Macromolecules 2012, 45, 4476–4486. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Katayama, K.; Yoshida, M.; Yamamoto, M.; Tabata, Y. Comparative Physicochemical Properties and Cytotoxicity of Polyphosphoester Ionomers with Bisphosphonates. J. Biomater. Sci. Polym. Ed. 2013, 24, 882–895. [Google Scholar] [CrossRef]
- Kalużyński, K.; Libiszowski, J.; Penczek, S. A New Class of Synthetic Polyelectrolytes. Acidic Polyesters of Phosphoric Acid (Poly(hydroxyalkylene phosphates)). Macromolecules 1976, 9, 365–367. [Google Scholar] [CrossRef]
- Yasuda, H.; Sumitani, M.; Nakamura, A. Novel Synthesis of Acidic Polyesters of Phosphoric Acid by Thermal Elimination of Isobutylene from Poly(alkylene tert-butyl phosphates). Macromolecules 1981, 14, 458–460. [Google Scholar] [CrossRef]
- Biela, T.; Penczek, S.; Słomkowski, S.; Vogl, O. Racemic and Optically Active Poly(4-methyl-2-oxo-2-hydro-1,3,2-dioxaphospholane). Synthesis and Oxidation to the Polyacids. Makromol. Chem. Rapid Commun. 1983, 4, 443. [Google Scholar] [CrossRef]
- Troev, K.; Tsatcheva, I.; Koseva, N.; Georgieva, R.; Gitsov, I. Immobilization of Aminothiols on Poly(oxyethylene H-phosphonate)s and Poly(oxyethylene phosphate)s—An Approach to Polymeric Protective Agents for Radiotherapy of Cancer. J. Polym. Sci. A Polym. Chem. 2007, 45, 1349–1363. [Google Scholar] [CrossRef]
- Wan, A.C.A.; Mao, H.Q.; Wang, S.; Phua, S.H.; Lee, G.P.; Pan, J.; Lu, S.; Wang, J.; Leong, K.W. Poly(phosphoester) Ionomers as Tissue-Engineering Scaffolds. J. Biomed. Mater. Res. B 2004, 70, 91–102. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Kawakita, T.; Yusa, S.-I. Thermoresponsive Polyphosphoesters Bearing Enzyme-Cleavable Side Chains. Chem. Lett. 2009, 38, 1054–1055. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Shen, Y.; Zhang, F.; Seetho, K.; Zou, J.; Taylor, J.-S.A.; Dove, A.P.; Wooley, K.L. A Simple and Efficient Synthesis of an Acid-Labile Polyphosphoramidate by Organobase-Catalyzed Ring-Opening Polymerization and Transformation to Polyphosphoester Ionomers by Acid Treatment. Macromolecules 2013, 46, 5141–5149. [Google Scholar] [CrossRef]
- Clément, B.; Molin, D.G.; Jérôme, C.; Lecomte, P. Synthesis of Polyphosphodiesters by Ring-Opening Polymerization of Cyclic Phosphates Bearing Allyl Phosphoester Protecting Groups. J. Polym. Sci. A Polym. Chem. 2015, 53, 2642–2648. [Google Scholar] [CrossRef]
- Dera, R.; Diliën, H.; Billen, B.; Gagliardi, M.; Rahimi, N.; Van Den Akker, N.M.S.; Molin, D.G.M.; Grandfils, C.; Adriaensens, P.; Guedens, W.; et al. Phosphodiester Hydrogels for Cell Scaffolding and Drug Release Applications. Macromol. Biosci. 2019, 19, 1900090. [Google Scholar] [CrossRef] [PubMed]
- Dera, R.; Diliën, H.; Billen, B.; Adriaensens, P.; Guedens, W.; Cleij, T.J. An Efficient Thermal Elimination Pathway toward Phosphodiester Hydrogels via a Precursor Approach. Macromol. Chem. Phys. 2020. [Google Scholar] [CrossRef]
- Penczek, S.; Pretula, J.; Kaluzynski, K. Synthesis of a Triblock Copolymer: Poly(ethylene glycol)-Poly(alkylene phosphate)-Poly(ethylene glycol) as a Modifier of CaCO3 Crystallization. J. Polym. Sci. A Polym. Chem. 2005, 43, 650–657. [Google Scholar] [CrossRef]
- Penczek, S.; Pretula, J.; Kaluzynski, K. Poly(alkylene phosphates): From Synthetic Models of Biomacromolecules and Biomembranes toward Polymer—Inorganic Hybrids (mimicking biomineralization). Biomacromolecules 2005, 6, 547–551. [Google Scholar] [CrossRef]
- Zeynep, E.Y.; Antoine, D.; Brice, C.; Frank, B.; Christine, J. Double Hydrophilic Polyphosphoester Containing Copolymers as Efficient Templating Agents for Calcium Carbonate Microparticles. J. Mater. Chem. B 2015, 3, 7227–7236. [Google Scholar] [CrossRef]
- Kunomura, S.; Iwasaki, Y. Immobilization of Polyphosphoesters on Poly(ether ether ketone) (PEEK) for Facilitating Mineral Coating. J. Biomater. Sci. Polym. Ed. 2019, 30, 861–876. [Google Scholar] [CrossRef]
- Ikeuchi, R.; Iwasaki, Y. High Mineral Affinity of Polyphosphoester Ionomer-Phospholipid Vesicles. J. Biomed. Mater. Res. A 2013, 101, 318–325. [Google Scholar] [CrossRef]
- Hirano, Y.; Iwasaki, Y. Bone-Specific Poly(ethylene sodium phosphate)-Bearing Biodegradable Nanoparticles. Colloids Surf. B 2017, 153, 104–110. [Google Scholar] [CrossRef]
- Noree, S.; Iwasaki, Y. Thermally Assisted Generation of Protein-Poly(ethylene sodium phosphate) Conjugates with High Mineral Affinity. ACS Omega 2019, 4, 3398–3404. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Yokota, A.; Otaka, A.; Inoue, N.; Yamaguchi, A.; Yoshitomi, T.; Yoshimoto, K.; Neo, M. Bone-Targeting Poly(ethylene sodium phosphate). Biomater. Sci. 2018, 6, 91–95. [Google Scholar] [CrossRef]
- Yang, X.Z.; Sun, T.M.; Dou, S.; Wu, J.; Wang, Y.C.; Wang, J. Block Copolymer of Polyphosphoester and Poly(l-Lactic Acid) Modified Surface for Enhancing Osteoblast Adhesion, Proliferation, and Function. Biomacromolecules 2009, 10, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Nifant, I.; Bukharova, T.; Dyakonov, A.; Goldshtein, D.; Galitsyna, E.; Kosarev, M.; Shlyakhtin, A.; Gavrilov, D.; Ivchenko, P. Osteogenic Differentiation of Human Adipose Tissue-Derived MSCs by Non-Toxic Calcium Poly(ethylene phosphate)s. Int. J. Mol. Sci. 2019, 20, 6242. [Google Scholar]
- Kootala, S.; Tokunaga, M.; Hilborn, J.; Iwasaki, Y. Anti-Resorptive Functions of Poly(ethylene sodium phosphate) on Human Osteoclasts. Macromol. Biosci. 2015, 15, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Yokota, A.; Otaka, A.; Neo, M. Bone-Targeting Polyphosphoesters and Their Interaction with Bone Cells. In Proceedings of the 256th National Meeting and Exposition of the American-Chemical-Society, Boston, MA, USA, 19–23 August 2018. [Google Scholar]
Sample Availability: Not available. |


© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, Y. Bone Mineral Affinity of Polyphosphodiesters. Molecules 2020, 25, 758. https://doi.org/10.3390/molecules25030758
Iwasaki Y. Bone Mineral Affinity of Polyphosphodiesters. Molecules. 2020; 25(3):758. https://doi.org/10.3390/molecules25030758
Chicago/Turabian StyleIwasaki, Yasuhiko. 2020. "Bone Mineral Affinity of Polyphosphodiesters" Molecules 25, no. 3: 758. https://doi.org/10.3390/molecules25030758
APA StyleIwasaki, Y. (2020). Bone Mineral Affinity of Polyphosphodiesters. Molecules, 25(3), 758. https://doi.org/10.3390/molecules25030758





