Microglial Function in the Effects of Early-Life Stress on Brain and Behavioral Development
Abstract
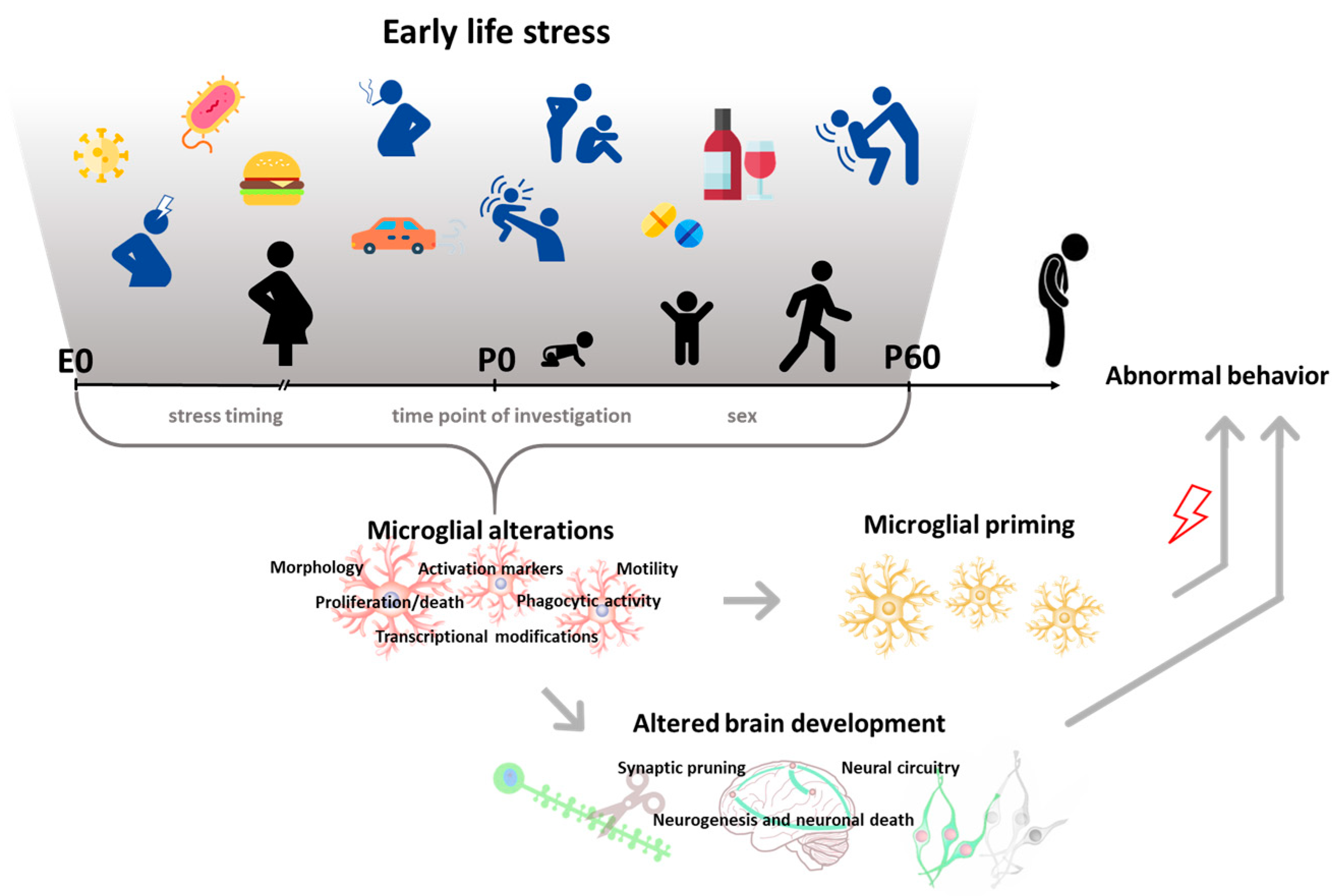
:1. Introduction
2. Discussion
2.1. Early-Life Stress and Microglia: Preclinical Studies
2.1.1. Prenatal Behavioral Stressors
2.1.2. Prenatal Environmental Agents and Infection
2.1.3. Effects of Prenatal-Stress-Induced Microglial Alterations on Brain and Behavior
2.1.4. Postnatal Behavioral Stressors
2.1.5. Postnatal Environmental Agents and Infection
2.1.6. Effects of Postnatal-Stress-Induced Microglial Alterations on Brain and Behavior
2.1.7. Early Preventative Interventions
2.2. Early-Life Stress and Microglia: Clinical Studies
2.3. Perspectives
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kessler, R.C.; McLaughlin, K.A.; Green, J.G.; Gruber, M.J.; Sampson, N.A.; Zaslavsky, A.M.; Aguilar-Gaxiola, S.; Alhamzawi, A.O.; Alonso, J.; Angermeyer, M. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br. J. Psychiatry 2010, 197, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Teicher, M.H.; Samson, J.A.; Anderson, C.M.; Ohashi, K. The effects of childhood maltreatment on brain structure, function, and connectivity. Nat. Rev. Neurosci. 2016, 17, 652–666. [Google Scholar] [CrossRef]
- Vaiserman, A.M.; Koliada, A.K. Early-life adversity and long-term neurobehavioral outcomes: Epigenome as a bridge? Hum. Genom. 2017, 11, 432–445. [Google Scholar] [CrossRef] [Green Version]
- Iacono, L.L.; Valzania, A.; Visco-Comandini, F.; Aricò, E.; Viscomi, M.T.; Castiello, L.; Oddi, D.; D’Amato, F.R.; Bisicchia, E.; Ermakova, O.; et al. Social threat exposure in juvenile mice promotes cocaine-seeking by altering blood clotting and brain vasculature. Addict. Biol. 2017, 22, 911–922. [Google Scholar] [CrossRef]
- Blaisdell, K.N.; Imhof, A.M.; Fisher, P.A. Early adversity, child neglect, and stress neurobiology: From observations of impact to empirical evaluations of mechanisms. Int. J. Dev. Neurosci. 2019, 78, 139–146. [Google Scholar] [CrossRef]
- Agorastos, A.; Pervanidou, P.; Chrousos, G.P.; Baker, D.G. Developmental Trajectories of Early Life Stress and Trauma: A Narrative Review on Neurobiological Aspects Beyond Stress System Dysregulation. Front. Psychiatry 2019, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Johnson, F.K.; Kaffman, A. Early life stress perturbs the function of microglia in the developing rodent brain: New insights and future challenges. Brain Behav. Immun. 2018, 69, 18–27. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Cardona, A.E. The myeloid cells of the central nervous system parenchyma. Nature 2010, 468, 253–262. [Google Scholar] [CrossRef]
- Schafer, D.P.; Stevens, B. Microglia Function in Central Nervous System Development and Plasticity. Cold Spring Harb. Perspect. Biol. 2015, 7, a020545. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Thion, M.S.; Ginhoux, F.; Garel, S. Microglia and early brain development: An intimate journey. Science 2018, 362, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Kopec, A.M.; Smith, C.J.; Ayre, N.R.; Sweat, S.C.; Bilbo, S.D. Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Cowan, M.; Petri, W.A. Microglia: Immune Regulators of Neurodevelopment. Front. Immunol. 2018, 9, 2576. [Google Scholar] [CrossRef] [Green Version]
- Wright-Jin, E.C.; Gutmann, D.H. Microglia as Dynamic Cellular Mediators of Brain Function. Trends Mol. Med. 2019, 25, 967–979. [Google Scholar] [CrossRef]
- Frank, M.G.; Fonken, L.K.; Watkins, L.R.; Maier, S.F. Microglia: Neuroimmune-sensors of stress. Semin. Cell Dev. Biol. 2019, 94, 176–185. [Google Scholar] [CrossRef]
- Wolf, S.A.; Boddeke, H.W.G.M.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Ferretti, M.T. Function and Dysfunction of Microglia during Brain Development: Consequences for Synapses and Neural Circuits. Front. Synaptic Neurosci. 2017, 9, 9. [Google Scholar] [CrossRef]
- Burke, N.N.; Fan, C.Y.; Trang, T. Microglia in health and pain: Impact of noxious early life events. Exp. Physiol. 2016, 101, 1003–1021. [Google Scholar] [CrossRef] [Green Version]
- Harry, G.J. Microglia During Development and Aging. Pharmacol. Ther. 2013, 139, 313–326. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Dissing-Olesen, L.; MacVicar, B.A.; Stevens, B. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 2015, 36, 605–613. [Google Scholar] [CrossRef]
- York, E.M.; Bernier, L.-P.; MacVicar, B.A. Microglial modulation of neuronal activity in the healthy brain. Dev. Neurobiol. 2018, 78, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, S.; Basilico, B.; Marrone, M.C.; Ragozzino, D. Microglia-neuron crosstalk: Signaling mechanism and control of synaptic transmission. Semin. Cell Dev. Biol. 2019, 94, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Xu, F.; Previti, M.L.; Davis, J.; Grande, A.M.; Robinson, J.K.; Van Nostrand, W.E. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J. Neurosci. 2007, 27, 3057–3063. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, K.; Mishima, K.; Nozako, M.; Hazekawa, M.; Mishima, S.; Fujioka, M.; Orito, K.; Egashira, N.; Iwasaki, K.; Fujiwara, M. Delayed treatment with minocycline ameliorates neurologic impairment through activated microglia expressing a high-mobility group box1-inhibiting mechanism. Stroke 2008, 39, 951–958. [Google Scholar] [CrossRef] [Green Version]
- Mishra, M.K.; Basu, A. Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J. Neurochem. 2008, 105, 1582–1595. [Google Scholar] [CrossRef]
- Chen, M.-K.; Guilarte, T.R. Translocator protein 18 kDa (TSPO): Molecular sensor of brain injury and repair. Pharmacol. Ther. 2008, 118, 1–17. [Google Scholar] [CrossRef]
- Rupprecht, R.; Papadopoulos, V.; Rammes, G.; Baghai, T.C.; Fan, J.; Akula, N.; Groyer, G.; Adams, D.; Schumacher, M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2010, 9, 971–988. [Google Scholar] [CrossRef]
- Gómez-González, B.; Escobar, A. Prenatal stress alters microglial development and distribution in postnatal rat brain. Acta Neuropathol. 2010, 119, 303–315. [Google Scholar] [CrossRef]
- Zhao, Q.; Peng, C.; Wu, X.; Chen, Y.; Wang, C.; You, Z. Maternal sleep deprivation inhibits hippocampal neurogenesis associated with inflammatory response in young offspring rats. Neurobiol. Dis. 2014, 68, 57–65. [Google Scholar] [CrossRef]
- Zhao, Q.; Xie, X.; Fan, Y.; Zhang, J.; Jiang, W.; Wu, X.; Yan, S.; Chen, Y.; Peng, C.; You, Z. Phenotypic dysregulation of microglial activation in young offspring rats with maternal sleep deprivation-induced cognitive impairment. Sci. Rep. 2015, 5, 9513. [Google Scholar] [CrossRef] [Green Version]
- Diz-Chaves, Y.; Astiz, M.; Bellini, M.J.; Garcia-Segura, L.M. Prenatal stress increases the expression of proinflammatory cytokines and exacerbates the inflammatory response to LPS in the hippocampal formation of adult male mice. Brain Behav. Immun. 2013, 28, 196–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolders, S.; Notter, T.; Smolders, S.M.T.; Rigo, J.-M.; Brône, B. Controversies and prospects about microglia in maternal immune activation models for neurodevelopmental disorders. Brain Behav. Immun. 2018, 73, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Mattei, D.; Djodari-Irani, A.; Hadar, R.; Pelz, A.; de Cossío, L.F.; Goetz, T.; Matyash, M.; Kettenmann, H.; Winter, C.; Wolf, S.A. Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav. Immun. 2014, 38, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Van den Eynde, K.; Missault, S.; Fransen, E.; Raeymaekers, L.; Willems, R.; Drinkenburg, W.; Timmermans, J.-P.; Kumar-Singh, S.; Dedeurwaerdere, S. Hypolocomotive behaviour associated with increased microglia in a prenatal immune activation model with relevance to schizophrenia. Behav. Brain Res. 2014, 258, 179–186. [Google Scholar] [CrossRef]
- Zhu, F.; Zheng, Y.; Liu, Y.; Zhang, X.; Zhao, J. Minocycline alleviates behavioral deficits and inhibits microglial activation in the offspring of pregnant mice after administration of polyriboinosinic–polyribocytidilic acid. Psychiatry Res. 2014, 219, 680–686. [Google Scholar] [CrossRef]
- Giovanoli, S.; Engler, H.; Engler, A.; Richetto, J.; Feldon, J.; Riva, M.A.; Schedlowski, M.; Meyer, U. Preventive effects of minocycline in a neurodevelopmental two-hit model with relevance to schizophrenia. Transl. Psychiatry 2016, 6, 772. [Google Scholar] [CrossRef] [Green Version]
- Fernández de Cossío, L.; Guzmán, A.; van der Veldt, S.; Luheshi, G.N. Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain Behav. Immun. 2017, 63, 88–98. [Google Scholar] [CrossRef]
- Hui, C.W.; St-Pierre, A.; El Hajj, H.; Remy, Y.; Hébert, S.S.; Luheshi, G.N.; Srivastava, L.K.; Tremblay, M.-È. Prenatal Immune Challenge in Mice Leads to Partly Sex-Dependent Behavioral, Microglial, and Molecular Abnormalities Associated with Schizophrenia. Front. Mol. Neurosci. 2018, 11, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Thion, M.S.; Mosser, C.-A.; Férézou, I.; Grisel, P.; Baptista, S.; Low, D.; Ginhoux, F.; Garel, S.; Audinat, E. Biphasic Impact of Prenatal Inflammation and Macrophage Depletion on the Wiring of Neocortical Inhibitory Circuits. Cell Rep. 2019, 28, 1119–1126. [Google Scholar] [CrossRef] [Green Version]
- Giovanoli, S.; Engler, H.; Engler, A.; Richetto, J.; Voget, M.; Willi, R.; Winter, C.; Riva, M.A.; Mortensen, P.B.; Feldon, J.; et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 2013, 339, 1095–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, M.P.; Zaghouani, H.; Niklas, V. Gut microbiota, the immune system, and diet influence the neonatal gut-brain axis. Pediatr. Res. 2015, 77, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Ke, X.; Liu, Q.; Fu, Q.; Majnik, A.; Lane, R. Adverse early life environment increases hippocampal microglia abundance in conjunction with decreased neural stem cells in juvenile mice. Int. J. Dev. Neurosci. 2016, 55, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Edlow, A.G.; Glass, R.M.; Smith, C.J.; Tran, P.K.; James, K.; Bilbo, S. Placental Macrophages: A Window Into Fetal Microglial Function in Maternal Obesity. Int. J. Dev. Neurosci. 2019, 77, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Mairesse, J.; Zinni, M.; Pansiot, J.; Hassan-Abdi, R.; Demene, C.; Colella, M.; Charriaut-Marlangue, C.; Rideau Batista Novais, A.; Tanter, M.; Maccari, S.; et al. Oxytocin receptor agonist reduces perinatal brain damage by targeting microglia. Glia 2019, 67, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Komada, M.; Hara, N.; Kawachi, S.; Kawachi, K.; Kagawa, N.; Nagao, T.; Ikeda, Y. Mechanisms underlying neuro-inflammation and neurodevelopmental toxicity in the mouse neocortex following prenatal exposure to ethanol. Sci. Rep. 2017, 7, 234–257. [Google Scholar] [CrossRef] [Green Version]
- Bolton, J.L.; Auten, R.L.; Bilbo, S.D. Prenatal air pollution exposure induces sexually dimorphic fetal programming of metabolic and neuroinflammatory outcomes in adult offspring. Brain Behav. Immun. 2014, 37, 30–44. [Google Scholar] [CrossRef]
- Bolton, J.L.; Marinero, S.; Hassanzadeh, T.; Natesan, D.; Le, D.; Belliveau, C.; Mason, S.N.; Auten, R.L.; Bilbo, S.D. Gestational Exposure to Air Pollution Alters Cortical Volume, Microglial Morphology, and Microglia-Neuron Interactions in a Sex-Specific Manner. Front. Synaptic Neurosci. 2017, 9, 672–695. [Google Scholar] [CrossRef] [Green Version]
- Matcovitch-Natan, O.; Winter, D.R.; Giladi, A.; Vargas Aguilar, S.; Spinrad, A.; Sarrazin, S.; Ben-Yehuda, H.; David, E.; Zelada González, F.; Perrin, P.; et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016, 353, 8670. [Google Scholar] [CrossRef]
- Mattei, D.; Ivanov, A.; Ferrai, C.; Jordan, P.; Guneykaya, D.; Buonfiglioli, A.; Schaafsma, W.; Przanowski, P.; Deuther-Conrad, W.; Brust, P.; et al. Maternal immune activation results in complex microglial transcriptome signature in the adult offspring that is reversed by minocycline treatment. Transl. Psychiatry 2017, 7, 1120. [Google Scholar] [CrossRef]
- Field, E.J. Observations on the development of microglia together with a note on the influence of cortisone. J. Anat. 1955, 89, 201–208. [Google Scholar] [PubMed]
- Ling, E.A.; Kaur, C.; Wong, W.C. Light and electron microscopic demonstration of non-specific esterase in amoeboid microglial cells in the corpus callosum in postnatal rats: A cytochemical link to monocytes. J. Anat. 1982, 135, 385–394. [Google Scholar] [PubMed]
- Kaur, C.; Wu, C.H.; Wen, C.Y.; Ling, E.A. The Effects of Subcutaneous Injections of Glucocorticoids on Amoeboid Microglia in Postnatal Rats. Arch. Histol. Cytol. 1994, 57, 449–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.H.; Chien, H.F.; Chang, C.Y.; Chen, S.H.; Huang, Y.S. Response of amoeboid and differentiating ramified microglia to glucocorticoids in postnatal rats: A lectin histochemical and ultrastructural study. Neurosci. Res. 2001, 40, 235–244. [Google Scholar] [CrossRef]
- Yam, K.-Y.; Schipper, L.; Reemst, K.; Ruigrok, S.R.; Abbink, M.R.; Hoeijmakers, L.; Naninck, E.F.G.; Zarekiani, P.; Oosting, A.; Van der Beek, E.M.; et al. Increasing availability of ω-3 fatty acid in the early-life diet prevents the early-life stress-induced cognitive impairments without affecting metabolic alterations. FASEB J. 2019, 33, 5729–5740. [Google Scholar] [CrossRef]
- Hoeijmakers, L.; Ruigrok, S.R.; Amelianchik, A.; Ivan, D.; van Dam, A.-M.; Lucassen, P.J.; Korosi, A. Early-life stress lastingly alters the neuroinflammatory response to amyloid pathology in an Alzheimer’s disease mouse model. Brain Behav. Immun. 2017, 63, 160–175. [Google Scholar] [CrossRef]
- Roque, A.; Ochoa-Zarzosa, A.; Torner, L. Maternal separation activates microglial cells and induces an inflammatory response in the hippocampus of male rat pups, independently of hypothalamic and peripheral cytokine levels. Brain Behav. Immun. 2016, 55, 39–48. [Google Scholar] [CrossRef]
- Delpech, J.-C.; Wei, L.; Hao, J.; Yu, X.; Madore, C.; Butovsky, O.; Kaffman, A. Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain Behav. Immun. 2016, 57, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Saavedra, L.M.; Fenton Navarro, B.; Torner, L. Early Life Stress Activates Glial Cells in the Hippocampus but Attenuates Cytokine Secretion in Response to an Immune Challenge in Rat Pups. Neuroimmunomodulation 2017, 24, 242–255. [Google Scholar] [CrossRef]
- Baldy, C.; Fournier, S.; Boisjoly-Villeneuve, S.; Tremblay, M.-È.; Kinkead, R. The influence of sex and neonatal stress on medullary microglia in rat pups. Exp. Physiol. 2018, 103, 1192–1199. [Google Scholar] [CrossRef]
- Réus, G.Z.; Silva, R.H.; de Moura, A.B.; Presa, J.F.; Abelaira, H.M.; Abatti, M.; Vieira, A.; Pescador, B.; Michels, M.; Ignácio, Z.M.; et al. Early Maternal Deprivation Induces Microglial Activation, Alters Glial Fibrillary Acidic Protein Immunoreactivity and Indoleamine 2,3-Dioxygenase during the Development of Offspring Rats. Mol. Neurobiol. 2019, 56, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Chocyk, A.; Dudys, D.; Przyborowska, A.; Majcher, I.; Maćkowiak, M.; Wędzony, K. Maternal separation affects the number, proliferation and apoptosis of glia cells in the substantia nigra and ventral tegmental area of juvenile rats. Neuroscience 2011, 173, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Banqueri, M.; Méndez, M.; Gómez-Lázaro, E.; Arias, J.L. Early life stress by repeated maternal separation induces long-term neuroinflammatory response in glial cells of male rats. Stress 2019, 22, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Genty, J.; Tetsi Nomigni, M.; Anton, F.; Hanesch, U. Maternal separation stress leads to resilience against neuropathic pain in adulthood. Neurobiol. Stress 2018, 8, 21–32. [Google Scholar] [CrossRef]
- Takatsuru, Y.; Nabekura, J.; Ishikawa, T.; Kohsaka, S.; Koibuchi, N. Early-life stress increases the motility of microglia in adulthood. J. Physiol. Sci. 2015, 65, 187–194. [Google Scholar] [CrossRef]
- Gong, Y.; Tong, L.; Yang, R.; Hu, W.; Xu, X.; Wang, W.; Wang, P.; Lu, X.; Gao, M.; Wu, Y.; et al. Dynamic changes in hippocampal microglia contribute to depressive-like behavior induced by early social isolation. Neuropharmacology 2018, 135, 223–233. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Wang, J.; Ren, F.; Shao, F.; Ellenbroek, B.; Lin, W.; Wang, W. Transient upregulation of immune activity induced by adolescent social stress is involved in cognitive deficit in adult male mice and early intervention with minocycline. Behav. Brain Res. 2019, 374, 112–136. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Zhang, F.; Shao, F.; Ellenbroek, B.; Wang, J.; Wang, W. Deficiencies of microglia and TNFα in the mPFC-mediated cognitive inflexibility induced by social stress during adolescence. Brain Behav. Immun. 2019, 79, 256–266. [Google Scholar] [CrossRef]
- Lo Iacono, L.; Catale, C.; Martini, A.; Valzania, A.; Viscomi, M.T.; Chiurchiù, V.; Guatteo, E.; Bussone, S.; Perrone, F.; Di Sabato, P.; et al. From Traumatic Childhood to Cocaine Abuse: The Critical Function of the Immune System. Biol. Psychiatry 2018, 84, 905–916. [Google Scholar] [CrossRef] [Green Version]
- McCormick, C.M.; Thomas, C.M.; Sheridan, C.S.; Nixon, F.; Flynn, J.A.; Mathews, I.Z. Social instability stress in adolescent male rats alters hippocampal neurogenesis and produces deficits in spatial location memory in adulthood. Hippocampus 2012, 22, 1300–1312. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Wang, Q.; Zhang, D.; Zhao, Q.; Zhang, J.; Xie, L.; Liu, G.; You, Z. Minocycline inhibits microglial activation and alleviates depressive-like behaviors in male adolescent mice subjected to maternal separation. Psychoneuroendocrinology 2019, 107, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Yao, S.; Wang, R.; Fang, Z.; Zhong, K.; Nie, L.; Zhang, Q. PI3K/Akt/NF-κB signaling pathway regulates behaviors in adolescent female rats following with neonatal maternal deprivation and chronic mild stress. Behav. Brain Res. 2019, 362, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Thompson, V.; Gildawie, K.; Brenhouse, H.C. Adolescent food restriction in rats alters prefrontal cortex microglia in an experience-dependent manner. Stress 2018, 21, 162–168. [Google Scholar] [CrossRef]
- Wang, H.-T.; Huang, F.-L.; Hu, Z.-L.; Zhang, W.-J.; Qiao, X.-Q.; Huang, Y.-Q.; Dai, R.-P.; Li, F.; Li, C.Q. Early-Life Social Isolation-Induced Depressive-Like Behavior in Rats Results in Microglial Activation and Neuronal Histone Methylation that Are Mitigated by Minocycline. Neurotox. Res. 2017, 31, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.M.; Hutchinson, M.R.; Bilbo, S.D. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J. Neurosci. 2011, 31, 17835–17847. [Google Scholar] [CrossRef] [PubMed]
- Tuan, L.-H.; Lee, L.-J. Microglia-mediated synaptic pruning is impaired in sleep-deprived adolescent mice. Neurobiol. Dis. 2019, 130, 104517. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Shah, S.A.; Badshah, H.; Kim, M.J.; Ali, T.; Yoon, G.H.; Kim, T.H.; Abid, N.B.; Rehman, S.U.; Khan, S.; et al. Neuroprotection by Vitamin C Against Ethanol-Induced Neuroinflammation Associated Neurodegeneration in the Developing Rat Brain. CNS Neurol. Disord. Drug Targets Former. Curr. Drug Targets-CNS Neurol. Disord. 2016, 15, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Chastain, L.G.; Franklin, T.; Gangisetty, O.; Cabrera, M.A.; Mukherjee, S.; Shrivastava, P.; Jabbar, S.; Sarkar, D.K. Early life alcohol exposure primes hypothalamic microglia to later-life hypersensitivity to immune stress: Possible epigenetic mechanism. Neuropsychopharmacology 2019, 44, 1579–1588. [Google Scholar] [CrossRef]
- Boschen, K.E.; Ruggiero, M.J.; Klintsova, A.Y. Neonatal binge alcohol exposure increases microglial activation in the developing rat hippocampus. Neuroscience 2016, 324, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Ji, Z.; Yuan, L.; Lu, X.; Ding, H.; Luo, J.; Ke, Z.-J. Binge Alcohol Exposure Causes Neurobehavioral Deficits and GSK3β Activation in the Hippocampus of Adolescent Rats. Sci. Rep. 2018, 8, 540–561. [Google Scholar] [CrossRef] [Green Version]
- Ward, R.J.; Colivicchi, M.A.; Allen, R.; Schol, F.; Lallemand, F.; de Witte, P.; Ballini, C.; Corte, L.D.; Dexter, D. Neuro-inflammation induced in the hippocampus of “binge drinking” rats may be mediated by elevated extracellular glutamate content. J. Neurochem. 2009, 111, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Topper, L.A.; Baculis, B.C.; Valenzuela, C.F. Exposure of neonatal rats to alcohol has differential effects on neuroinflammation and neuronal survival in the cerebellum and hippocampus. J. Neuroinflamm. 2015, 12, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClain, J.A.; Morris, S.A.; Deeny, M.A.; Marshall, S.A.; Hayes, D.M.; Kiser, Z.M.; Nixon, K. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav. Immun. 2011, 25, S120–S128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drew, P.D.; Johnson, J.W.; Douglas, J.C.; Phelan, K.D.; Kane, C.J.M. Pioglitazone Blocks Ethanol Induction of Microglial Activation and Immune Responses in the Hippocampus, Cerebellum, and Cerebral Cortex in a Mouse Model of Fetal Alcohol Spectrum Disorders. Alcohol. Clin. Exp. Res. 2015, 39, 445. [Google Scholar] [CrossRef] [Green Version]
- Kane, C.J.M.; Phelan, K.D.; Han, L.; Smith, R.R.; Xie, J.; Douglas, J.C.; Drew, P.D. Protection of neurons and microglia against ethanol in a mouse model of fetal alcohol spectrum disorders by peroxisome proliferator-activated receptor-γ agonists. Brain Behav. Immun. 2011, 25, S137–S145. [Google Scholar] [CrossRef] [Green Version]
- Ruggiero, M.J.; Boschen, K.E.; Roth, T.L.; Klintsova, A.Y. Sex Differences in Early Postnatal Microglial Colonization of the Developing Rat Hippocampus Following a Single-Day Alcohol Exposure. J. Neuroimmune Pharmacol. 2018, 13, 189–203. [Google Scholar] [CrossRef]
- Teixeira, F.B.; Santana, L.N.S.; Bezerra, F.R.; De Carvalho, S.; Fontes-Júnior, E.A.; Prediger, R.D.; Crespo-López, M.E.; Maia, C.S.F.; Lima, R.R. Chronic ethanol exposure during adolescence in rats induces motor impairments and cerebral cortex damage associated with oxidative stress. PLoS ONE 2014, 9, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Ahlers, K.E.; Karaçay, B.; Fuller, L.; Bonthius, D.J.; Dailey, M.E. Transient activation of microglia following acute alcohol exposure in developing mouse neocortex is primarily driven by BAX-dependent neurodegeneration. Glia 2015, 63, 1694–1713. [Google Scholar] [CrossRef]
- Wong, E.L.; Lutz, N.M.; Hogan, V.A.; Lamantia, C.E.; McMurray, H.R.; Myers, J.R.; Ashton, J.M.; Majewska, A.K. Developmental alcohol exposure impairs synaptic plasticity without overtly altering microglial function in mouse visual cortex. Brain Behav. Immun. 2018, 67, 257–278. [Google Scholar] [CrossRef]
- Vetreno, R.P.; Crews, F.T. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and toll-like receptors in the adult prefrontal cortex. Neuroscience 2012, 226, 475–488. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, J.M.; Bilbo, S.D. Adolescent morphine exposure affects long-term microglial function and later-life relapse liability in a model of addiction. J. Neurosci. 2013, 33, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Cardis, R.; Cabungcal, J.-H.; Dwir, D.; Do, K.Q.; Steullet, P. A lack of GluN2A-containing NMDA receptors confers a vulnerability to redox dysregulation: Consequences on parvalbumin interneurons, and their perineuronal nets. Neurobiol. Dis. 2018, 109, 64–75. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Cheng, C.-W.; Wang, W.-H.; Chen, P.-S.; Tzeng, S.-F. Postnatal Stress Induced by Injection with Valproate Leads to Developing Emotional Disorders Along with Molecular and Cellular Changes in the Hippocampus and Amygdala. Mol. Neurobiol. 2016, 53, 6774–6785. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Santos, V.; Cardoso, F.L.; Leitão, R.A.; Fontes-Ribeiro, C.A.; Silva, A.P. Impact of developmental exposure to methylphenidate on rat brain’s immune privilege and behavior: Control versus ADHD model. Brain Behav. Immun. 2018, 68, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Streifel, K.M.; Sullivan, K.A.; Legare, M.E.; Tjalkens, R.B. Developmental exposure to manganese increases adult susceptibility to inflammatory activation of glia and neuronal protein nitration. Toxicol. Sci. 2009, 112, 405–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carias, E.; Hamilton, J.; Robison, L.S.; Delis, F.; Eiden, R.; Quattrin, T.; Hadjiargyrou, M.; Komatsu, D.; Thanos, P.K. Chronic oral methylphenidate treatment increases microglial activation in rats. J. Neural Transm. 2018, 125, 18671875. [Google Scholar] [CrossRef] [PubMed]
- Claypoole, L.D.; Zimmerberg, B.; Williamson, L.L. Neonatal lipopolysaccharide treatment alters hippocampal neuroinflammation, microglia morphology and anxiety-like behavior in rats selectively bred for an infantile trait. Brain Behav. Immun. 2017, 59, 135–146. [Google Scholar] [CrossRef]
- Tremblay, S.; Miloudi, K.; Chaychi, S.; Favret, S.; Binet, F.; Polosa, A.; Lachapelle, P.; Chemtob, S.; Sapieha, P. Systemic inflammation perturbs developmental retinal angiogenesis and neuroretinal function. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8125–8139. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.; Dai, X.; Roller, A.; Carter, K.; Paul, I.; Bhatt, A.J.; Lin, R.C.S.; Fan, L.-W. Early Postnatal Lipopolysaccharide Exposure Leads to Enhanced Neurogenesis and Impaired Communicative Functions in Rats. PLoS ONE 2016, 11, 541–555. [Google Scholar] [CrossRef]
- Christensen, L.B.; Woods, T.A.; Carmody, A.B.; Caughey, B.; Peterson, K.E. Age-related differences in neuroinflammatory responses associated with a distinct profile of regulatory markers on neonatal microglia. J. Neuroinflamm. 2014, 11, 70. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.; Chen, C.-J.; Yan, X.-X.; Li, Z.; Deng, X.-H. Early-life lipopolysaccharide exposure potentiates forebrain expression of NLRP3 inflammasome proteins and anxiety-like behavior in adolescent rats. Brain Res. 2017, 1671, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Berkiks, I.; Benmhammed, H.; Mesfioui, A.; Ouichou, A.; El Hasnaoui, A.; Mouden, S.; Touil, T.; Bahbiti, Y.; Nakache, R.; El Hessni, A. Postnatal melatonin treatment protects against affective disorders induced by early-life immune stimulation by reducing the microglia cell activation and oxidative stress. Int. J. Neurosci. 2018, 128, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Sominsky, L.; Walker, A.K.; Ong, L.K.; Tynan, R.J.; Walker, F.R.; Hodgson, D.M. Increased microglial activation in the rat brain following neonatal exposure to a bacterial mimetic. Behav. Brain Res. 2012, 226, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-C.; Fan, L.-W.; Kaizaki, A.; Pang, Y.; Cai, Z.; Tien, L.-T. Neonatal lipopolysaccharide exposure induces long-lasting learning impairment, less anxiety-like response and hippocampal injury in adult rats. Neuroscience 2013, 234, 146–157. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.-W.; Tien, L.-T.; Zheng, B.; Pang, Y.; Lin, R.C.S.; Simpson, K.L.; Ma, T.; Rhodes, P.G.; Cai, Z. Dopaminergic neuronal injury in the adult rat brain following neonatal exposure to lipopolysaccharide and the silent neurotoxicity. Brain Behav. Immun. 2011, 25, 286–297. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.-W.; Pang, Y.; Lin, S.; Tien, L.-T.; Ma, T.; Rhodes, P.G.; Cai, Z. Minocycline reduces lipopolysaccharide-induced neurological dysfunction and brain injury in the neonatal rat. J. Neurosci. Res. 2005, 82, 71–82. [Google Scholar] [CrossRef]
- Cardoso, F.L.; Herz, J.; Fernandes, A.; Rocha, J.; Sepodes, B.; Brito, M.A.; McGavern, D.B.; Brites, D. Systemic inflammation in early neonatal mice induces transient and lasting neurodegenerative effects. J. Neuroinflamm. 2015, 12, 82. [Google Scholar] [CrossRef] [Green Version]
- Bilbo, S.D.; Biedenkapp, J.C.; Der-Avakian, A.; Watkins, L.R.; Rudy, J.W.; Maier, S.F. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J. Neurosci. 2005, 25, 8000–8009. [Google Scholar] [CrossRef] [Green Version]
- Bilbo, S.D.; Newsum, N.J.; Sprunger, D.B.; Watkins, L.R.; Rudy, J.W.; Maier, S.F. Differential effects of neonatal handling on early life infection-induced alterations in cognition in adulthood. Brain Behav. Immun. 2007, 21, 332–342. [Google Scholar] [CrossRef]
- Bland, S.T.; Beckley, J.T.; Young, S.; Tsang, V.; Watkins, L.R.; Maier, S.F.; Bilbo, S.D. Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain Behav. Immun. 2010, 24, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Bland, S.T.; Beckley, J.T.; Watkins, L.R.; Maier, S.F.; Bilbo, S.D. Neonatal Escherichia coli infection alters glial, cytokine, and neuronal gene expression in response to acute amphetamine in adolescent rats. Neurosci. Lett. 2010, 474, 52–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, L.L.; Sholar, P.W.; Mistry, R.S.; Smith, S.H.; Bilbo, S.D. Microglia and Memory: Modulation by Early-Life Infection. J. Neurosci. 2011, 31, 15511–15521. [Google Scholar] [CrossRef] [PubMed]
- Bussone, S.; Lo Iacono, L. The “systems approach” to treating the brain: Opportunities in developmental psychopharmacology. Dialogues Clin. Neurosci. 2019, 21, 211–215. [Google Scholar] [PubMed]
- Francis, D.D.; Diorio, J.; Plotsky, P.M.; Meaney, M.J. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J. Neurosci. 2002, 22, 7840–7843. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Yang, Y.; Yang, J.; Zhang, J.; Han, H.; Ma, W.; Li, H.; Mao, R.; Xu, L.; Hao, W.; et al. Enriched environment experience overcomes the memory deficits and depressive-like behavior induced by early life stress. Neurosci. Lett. 2006, 404, 208–212. [Google Scholar] [CrossRef]
- Hui, J.; Zhang, Z.; Liu, S.; Xi, G.; Zhang, X.; Teng, G.-J.; Chan, K.C.; Wu, E.X.; Nie, B.; Shan, B.; et al. Hippocampal neurochemistry is involved in the behavioural effects of neonatal maternal separation and their reversal by post-weaning environmental enrichment: A magnetic resonance study. Behav. Brain Res. 2011, 217, 122–127. [Google Scholar] [CrossRef]
- Vivinetto, A.L.; Suárez, M.M.; Rivarola, M.A. Neurobiological effects of neonatal maternal separation and post-weaning environmental enrichment. Behav. Brain Res. 2013, 240, 110–118. [Google Scholar] [CrossRef]
- Laviola, G.; Rea, M.; Morley-Fletcher, S.; Carlo, S.D.; Bacosi, A.; Simone, R.D.; Bertini, M.; Pacifici, R. Beneficial effects of enriched environment on adolescent rats from stressed pregnancies. Eur. J. Neurosci. 2004, 20, 1655–1664. [Google Scholar] [CrossRef]
- Prado, C.H.; Narahari, T.; Holland, F.H.; Lee, H.-N.; Murthy, S.K.; Brenhouse, H.C. Effects of early adolescent environmental enrichment on cognitive dysfunction, prefrontal cortex development, and inflammatory cytokines after early life stress. Dev. Psychobiol. 2016, 58, 482–491. [Google Scholar] [CrossRef]
- Ehninger, D.; Wang, L.-P.; Klempin, F.; Römer, B.; Kettenmann, H.; Kempermann, G. Enriched environment and physical activity reduce microglia and influence the fate of NG2 cells in the amygdala of adult mice. Cell Tissue Res. 2011, 345, 69–86. [Google Scholar] [CrossRef] [Green Version]
- Okuda, H.; Tatsumi, K.; Makinodan, M.; Yamauchi, T.; Kishimoto, T.; Wanaka, A. Environmental enrichment stimulates progenitor cell proliferation in the amygdala. J. Neurosci. Res. 2009, 87, 3546–3553. [Google Scholar] [CrossRef] [PubMed]
- Buschert, J.; Sakalem, M.E.; Saffari, R.; Hohoff, C.; Rothermundt, M.; Arolt, V.; Zhang, W.; Ambrée, O. Prenatal immune activation in mice blocks the effects of environmental enrichment on exploratory behavior and microglia density. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2016, 67, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Ehninger, D.; Kempermann, G. Regional Effects of Wheel Running and Environmental Enrichment on Cell Genesis and Microglia Proliferation in the Adult Murine Neocortex. Cereb. Cortex 2003, 13, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Williamson, L.L.; Chao, A.; Bilbo, S.D. Environmental enrichment alters glial antigen expression and neuroimmune function in the adult rat hippocampus. Brain Behav. Immun. 2012, 26, 500–510. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, M.C.; Yamaguchi, N.; Ogawa, S. Early life stress disrupts peripubertal development of aggression in male mice. Neuroreport 2011, 22, 259. [Google Scholar] [CrossRef]
- Myers, B.; Carvalho-Netto, E.; Wick-Carlson, D.; Wu, C.; Naser, S.; Solomon, M.B.; Ulrich-Lai, Y.M.; Herman, J.P. GABAergic Signaling within a Limbic-Hypothalamic Circuit Integrates Social and Anxiety-Like Behavior with Stress Reactivity. Neuropsychopharmacology 2016, 41, 1530–1539. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Liu, S.; Bai, X.; Gao, Y.; Liu, G.; Wang, X.; Liu, D.; Li, T.; Hao, A.; Wang, Z. Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J. Neuroinflamm. 2016, 13, 77. [Google Scholar] [CrossRef] [Green Version]
- Amini-Khoei, H.; Mohammadi-Asl, A.; Amiri, S.; Hosseini, M.-J.; Momeny, M.; Hassanipour, M.; Rastegar, M.; Haj-Mirzaian, A.; Mirzaian, A.H.-; Sanjarimoghaddam, H.; et al. Oxytocin mitigated the depressive-like behaviors of maternal separation stress through modulating mitochondrial function and neuroinflammation. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2017, 76, 169–178. [Google Scholar] [CrossRef]
- Scott, L.J.; Goa, K.L. Galantamine: A review of its use in Alzheimer’s disease. Drugs 2000, 60, 1095–1122. [Google Scholar] [CrossRef]
- Guan, Y.-Z.; Jin, X.-D.; Guan, L.-X.; Yan, H.-C.; Wang, P.; Gong, Z.; Li, S.-J.; Cao, X.; Xing, Y.-L.; Gao, T.-M. Nicotine inhibits microglial proliferation and is neuroprotective in global ischemia rats. Mol. Neurobiol. 2015, 51, 1480–1488. [Google Scholar] [CrossRef]
- Furukawa, S.; Yang, L.; Sameshima, H. Galantamine, an acetylcholinesterase inhibitor, reduces brain damage induced by hypoxia-ischemia in newborn rats. Int. J. Dev. Neurosci. 2014, 37, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Farlow, M.R. Clinical pharmacokinetics of galantamine. Clin. Pharmacokinet. 2003, 42, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Giunta, B.; Ehrhart, J.; Townsend, K.; Sun, N.; Vendrame, M.; Shytle, D.; Tan, J.; Fernandez, F. Galantamine and nicotine have a synergistic effect on inhibition of microglial activation induced by HIV-1 gp120. Brain Res. Bull. 2004, 64, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Benetti, F.; Mello, P.B.; Bonini, J.S.; Monteiro, S.; Cammarota, M.; Izquierdo, I. Early postnatal maternal deprivation in rats induces memory deficits in adult life that can be reversed by donepezil and galantamine. Int. J. Dev. Neurosci. 2009, 27, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Kitamura, Y.; Saeki, M.; Terada, M.; Kagitani, S.; Kitamura, R.; Fujikawa, Y.; Maelicke, A.; Tomimoto, H.; Taniguchi, T.; et al. Galantamine-induced Amyloid-β Clearance Mediated via Stimulation of Microglial Nicotinic Acetylcholine Receptors. J. Biol. Chem. 2010, 285, 40180–40191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, K.S.; Pocivavsek, A.; Wu, H.-Q.; Pershing, M.L.; Schwarcz, R.; Bruno, J.P. Early Developmental Elevations of Brain Kynurenic Acid Impair Cognitive Flexibility in Adults: Reversal with Galantamine. Neuroscience 2013, 238, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, Y.; Zheng, X.; Fang, T.; Yang, X.; Luo, X.; Guo, A.; Newell, K.A.; Huang, X.-F.; Yu, Y. Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in mice. J. Neuroinflamm. 2018, 15, 112. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G.; Vaillancourt, C.; Maes, M.; Reiter, R.J. Breast Feeding and Melatonin: Implications For Improving Perinatal Health. J. Breastfeed. Biol. 2016, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Shrestha, S.; Li, J.; Yu, X.; Chen, J.; Yan, F.; Ying, G.; Gu, C.; Wang, L.; Chen, G. Melatonin-mediated mitophagy protects against early brain injury after subarachnoid hemorrhage through inhibition of NLRP3 inflammasome activation. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Lee, D.-E.; Jang, H.; Byeon, Y.; Kim, Y.-S.; Back, K. Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress. J. Pineal Res. 2013, 54, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Arioz, B.I.; Tastan, B.; Tarakcioglu, E.; Tufekci, K.U.; Olcum, M.; Ersoy, N.; Bagriyanik, A.; Genc, K.; Genc, S. Melatonin Attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 Inflammasome Activation Through the SIRT1/Nrf2 Pathway. Front. Immunol. 2019, 10, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, S.; Wen, H.; Liu, T.; Cai, J.; Du, D.; Zhu, D.; Chen, F.; Xia, C. Melatonin decreases M1 polarization via attenuating mitochondrial oxidative damage depending on UCP2 pathway in prorenin-treated microglia. PLoS ONE 2019, 14, 653–754. [Google Scholar] [CrossRef] [PubMed]
- Welin, A.-K.; Svedin, P.; Lapatto, R.; Sultan, B.; Hagberg, H.; Gressens, P.; Kjellmer, I.; Mallard, C. Melatonin Reduces Inflammation and Cell Death in White Matter in the Mid-Gestation Fetal Sheep Following Umbilical Cord Occlusion. Pediatr. Res. 2007, 61, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.-Y.; Han, S.-H. Melatonin attenuates kainic acid-induced hippocampal neurodegeneration and oxidative stress through microglial inhibition. J. Pineal Res. 2003, 34, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, T.; Layé, S. Food for Mood: Relevance of Nutritional Omega-3 Fatty Acids for Depression and Anxiety. Front. Physiol. 2018, 9, 1047. [Google Scholar] [CrossRef]
- De Smedt-Peyrusse, V.; Sargueil, F.; Moranis, A.; Harizi, H.; Mongrand, S.; Layé, S. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J. Neurochem. 2008, 105, 296–307. [Google Scholar] [CrossRef]
- Pettit, L.K.; Varsanyi, C.; Tadros, J.; Vassiliou, E. Modulating the inflammatory properties of activated microglia with Docosahexaenoic acid and Aspirin. Lipids Health Dis. 2013, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.P.-C.; Su, K.-P.; Mondelli, V.; Pariante, C.M. Omega-3 Polyunsaturated Fatty Acids in Youths with Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Clinical Trials and Biological Studies. Neuropsychopharmacology 2018, 43, 534–545. [Google Scholar] [CrossRef] [Green Version]
- Fourrier, C.; Remus-Borel, J.; Greenhalgh, A.D.; Guichardant, M.; Bernoud-Hubac, N.; Lagarde, M.; Joffre, C.; Layé, S. Docosahexaenoic acid-containing choline phospholipid modulates LPS-induced neuroinflammation in vivo and in microglia in vitro. J. Neuroinflamm. 2017, 14, 170. [Google Scholar] [CrossRef]
- Dang, R.; Zhou, X.; Xu, P.; Guo, Y.; Gong, X.; Wang, S.; Yuan, F.; Yao, J.; Jiang, P. ω-3 polyunsaturated fatty acid supplementation ameliorates lipopolysaccharide-induced behavioral deficits and modulates neurotrophic factors in rats: Focus on tPA/PAI-1 system and BDNF-TrkB signaling. J. Funct. Food. 2017, 30, 74–80. [Google Scholar] [CrossRef]
- Ferraz, A.C.; Delattre, A.M.; Almendra, R.G.; Sonagli, M.; Borges, C.; Araujo, P.; Andersen, M.L.; Tufik, S.; Lima, M.M.S. Chronic ω-3 fatty acids supplementation promotes beneficial effects on anxiety, cognitive and depressive-like behaviors in rats subjected to a restraint stress protocol. Behav. Brain Res. 2011, 219, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Réus, G.Z.; Maciel, A.L.; Abelaira, H.M.; de Moura, A.B.; de Souza, T.G.; Dos Santos, T.R.; Darabas, A.C.; Parzianello, M.; Matos, D.; Abatti, M.; et al. ω-3 and folic acid act against depressive-like behavior and oxidative damage in the brain of rats subjected to early- or late-life stress. Nutrition 2018, 53, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Madore, C.; Nadjar, A.; Delpech, J.-C.; Sere, A.; Aubert, A.; Portal, C.; Joffre, C.; Layé, S. Nutritional n-3 PUFAs deficiency during perinatal periods alters brain innate immune system and neuronal plasticity-associated genes. Brain Behav. Immun. 2014, 41, 22–31. [Google Scholar] [CrossRef]
- Dahoun, T.; Calcia, M.A.; Veronese, M.; Bloomfield, P.; Reis Marques, T.; Turkheimer, F.; Howes, O.D. The association of psychosocial risk factors for mental health with a brain marker altered by inflammation: A translocator protein (TSPO) PET imaging study. Brain Behav. Immun. 2019, 80, 742–750. [Google Scholar] [CrossRef]
- Horti, A.G.; Naik, R.; Foss, C.A.; Minn, I.; Misheneva, V.; Du, Y.; Wang, Y.; Mathews, W.B.; Wu, Y.; Hall, A.; et al. PET imaging of microglia by targeting macrophage colony-stimulating factor 1 receptor (CSF1R). Proc. Natl. Acad. Sci. USA 2019, 116, 1686–1691. [Google Scholar] [CrossRef] [Green Version]
- Hahn-Holbrook, J.; Fish, A.; Glynn, L.M. Human Milk Omega-3 Fatty Acid Composition is Associated with Infant Temperament. Nutrients 2019, 11, 2964. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, H.C.; Holton, K.F.; Anderson, A.N.; Nousen, E.K.; Sullivan, C.A.; Loftis, J.M.; Nigg, J.T.; Sullivan, E.L. Increased Maternal Prenatal Adiposity, Inflammation, and Lower Omega-3 Fatty Acid Levels Influence Child Negative Affect. Front. Neurosci. 2019, 13, 1035. [Google Scholar] [CrossRef]
- Jobst, A.; Padberg, F.; Mauer, M.-C.; Daltrozzo, T.; Bauriedl-Schmidt, C.; Sabass, L.; Sarubin, N.; Falkai, P.; Renneberg, B.; Zill, P.; et al. Lower Oxytocin Plasma Levels in Borderline Patients with Unresolved Attachment Representations. Front. Hum. Neurosci. 2016, 10, 125. [Google Scholar] [CrossRef] [Green Version]
- Müller, L.E.; Bertsch, K.; Bülau, K.; Herpertz, S.C.; Buchheim, A. Emotional neglect in childhood shapes social dysfunctioning in adults by influencing the oxytocin and the attachment system: Results from a population-based study. Int. J. Psychophysiol. 2019, 136, 73–80. [Google Scholar] [CrossRef]
- Fries, A.B.W.; Ziegler, T.E.; Kurian, J.R.; Jacoris, S.; Pollak, S.D. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc. Natl. Acad. Sci. USA 2005, 102, 17237–17240. [Google Scholar] [CrossRef] [Green Version]
- Julian, M.M.; Rosenblum, K.L.; Doom, J.R.; Leung, C.Y.Y.; Lumeng, J.C.; Cruz, M.G.; Vazquez, D.M.; Miller, A.L. Oxytocin and parenting behavior among impoverished mothers with low vs. high early life stress. Arch. Women’s Ment. Health 2018, 21, 375–382. [Google Scholar] [CrossRef]
- Bilbo, S.D.; Schwarz, J.M. The immune system and developmental programming of brain and behavior. Front. Neuroendocrinol. 2012, 33, 267–286. [Google Scholar] [CrossRef] [Green Version]
- De Biase, L.M.; Bonci, A. Region-Specific Phenotypes of Microglia: The Role of Local Regulatory Cues. Neuroscientist 2019, 25, 314–333. [Google Scholar] [CrossRef]
- Calcia, M.A.; Bonsall, D.R.; Bloomfield, P.S.; Selvaraj, S.; Barichello, T.; Howes, O.D. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology 2016, 233, 1637–1650. [Google Scholar] [CrossRef] [Green Version]
- Ajami, B.; Bennett, J.L.; Krieger, C.; Tetzlaff, W.; Rossi, F.M.V. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007, 10, 1538–1543. [Google Scholar] [CrossRef]
- Bordt, E.A.; Ceasrine, A.M.; Bilbo, S.D. Microglia and sexual differentiation of the developing brain: A focus on ontogeny and intrinsic factors. Glia 2019, 8, 581–589. [Google Scholar] [CrossRef]
- Bordeleau, M.; Carrier, M.; Luheshi, G.N.; Tremblay, M.-È. Microglia along sex lines: From brain colonization, maturation and function, to implication in neurodevelopmental disorders. Semin. Cell Dev. Biol. 2019, 94, 152–163. [Google Scholar] [CrossRef]
- Tan, Y.-L.; Yuan, Y.; Tian, L. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 2019, 75, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Cheng, Z.; Zhou, L.; Darmanis, S.; Neff, N.F.; Okamoto, J.; Gulati, G.; Bennett, M.L.; Sun, L.O.; Clarke, L.E.; et al. Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron 2019, 101, 207–223. [Google Scholar] [CrossRef] [Green Version]
- Hammond, T.R.; Dufort, C.; Dissing-Olesen, L.; Giera, S.; Young, A.; Wysoker, A.; Walker, A.J.; Gergits, F.; Segel, M.; Nemesh, J.; et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 2019, 50, 253–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Böttcher, C.; Amann, L.; Sagar Scheiwe, C.; Nessler, S.; Kunz, P.; van Loo, G.; Reinacher, P.C.; et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 2019, 566, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Stratoulias, V.; Venero, J.L.; Tremblay, M.-È.; Joseph, B. Microglial subtypes: Diversity within the microglial community. EMBO J. 2019, 38, e101997. [Google Scholar] [CrossRef]
- Gómez-González, B.; Escobar, A. Altered functional development of the blood-brain barrier after early life stress in the rat. Brain Res. Bull. 2009, 79, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, V.; Vernon, A.C. From early adversities to immune activation in psychiatric disorders: The role of the sympathetic nervous system. Clin. Exp. Immunol. 2019, 197, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordão, M.J.C.; Sankowski, R.; Brendecke, S.M.; Mai, D.; Locatelli, G.; Tai, Y.-H.; Tay, T.L.; Schramm, E.; Armbruster, S.; Hagemeyer, N.; et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 2019, 363, eaat7554. [Google Scholar] [CrossRef] [PubMed]
- Danese, A.; Lewis, S.J. Psychoneuroimmunology of Early-Life Stress: The Hidden Wounds of Childhood Trauma? Neuropsychopharmacology 2017, 42, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Hoogland, I.C.M.; Houbolt, C.; van Westerloo, D.J.; van Gool, W.A.; van de Beek, D. Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflamm. 2015, 12, 114. [Google Scholar] [CrossRef] [Green Version]
- Haage, V.; Semtner, M.; Vidal, R.O.; Hernandez, D.P.; Pong, W.W.; Chen, Z.; Hambardzumyan, D.; Magrini, V.; Ly, A.; Walker, J.; et al. Comprehensive gene expression meta-analysis identifies signature genes that distinguish microglia from peripheral monocytes/macrophages in health and glioma. Acta Neuropathol. Commun. 2019, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Fogelman, N.; Canli, T. Early Life Stress, Physiology, and Genetics: A Review. Front. Psychol. 2019, 10, 1668. [Google Scholar] [CrossRef]
- Madore, C.; Yin, Z.; Leibowitz, J.; Butovsky, O. Microglia, Lifestyle Stress, and Neurodegeneration. Immunity 2020. [Google Scholar] [CrossRef]
| Type of Stress | Time | Species and Sex | Early Effects on Microglia | Late Effects on Microglia | Brain Region | Microglial-Dependent Behavioral Effects | Ref | |
|---|---|---|---|---|---|---|---|---|
| Behavioral stressors | Maternal forced swim | E18–P21 | Rats, M F | P1: ↓lectin+cells and amoeboid morphology (in CC); ↑ lectin+ cells (other regions) | - | CC, PCtx, ECtx, PFC, Sp, BG, Th, Md, IC | - | [28] |
| Maternal sleep deprivation | E18–P1 | Rats, M | - | P21: ↑IBA-1+ cells and retracted thicker processes | HIP | ↓ spatial learning & memory | [29,30] | |
| Maternal restraint stress/LPS | E12–P21/P120 | Mouse, F | P120: ↑ IBA-1+ cells, ↑ soma size and thick processes/↑ IBA-1+ cells | - | HIP (CA1/DG) | - | [31] | |
| Environmental agents and infection | Maternal Poly I:C injection | E15 | Rat, M | - | P103: ↑ microglial IL-1β, TNFα | HIP | ↑ startle response in the PPI | [34] |
| E9 | Mouse, M F | - | P62: ↑ number, amoeboid IBA-1+ cells | Ctx, HIP, Th | ↑locomotor activity and startle response in the PPI, ↓ sociability | [36] | ||
| E9.5 | Mouse, M F | - | P90: ↑ IBA-1+ cells, ↓ arborization in M | PFC, HIP, Cb | - | [39] | ||
| E14.5 | Mouse, M F | P1: adult-like transcriptome | P28: no effect | WB | - | [49] | ||
| E15 | Mouse, M | P80: ↑IBA-1+ cells, ↓phagocytosis | HIP (DG) | ↑ startle response in the PPI, ↓working memory in novel object recognition | [50] | |||
| E15 | Rat, M F | - | P180:↑ OX-42+ cells (in CC), activated, ↓microglia arborization (in HIP) | CC, HIP | - | [35] | ||
| Maternal Poly I:C injection/unpredictable stress | E9/P30–40 | Mouse, M F | P41: ↑ CD68+ cells, ↑ soma size | - | PFC, HIP | ↑ startle response in the PPI, anxiety-like behavior, amphetamine-induced locomotion | [37] | |
| Maternal LPS injection | E9 | Mouse, M F | - | P15: ↓ CX3CR1 mRNA in M | HIP | - | [38] | |
| E13.5 | Mouse, M F | - | P7: = number of IBA-1+ cells | SCtx | - | [40] | ||
| Maternal high fat diet/limited nesting | E13–P21 | Mouse, M F | P21: ↑ CD11b+ and IBA-1+ cells | - | HIP | - | [43] | |
| Maternal high fat diet | E0–E17 | Mouse, M F | E17 (LPS in vitro): ↑ TNF-α release in M | - | WB, Plac | - | [44] | |
| Maternal low protein diet/IL-1β | E10–P1/P1-2 | Rat, M F | P4: ↑ microglial inflammatory genes; ↑ IBA-1+ amoeboid cells (in vitro) | --- | WB, Ctx WM | - | [45] | |
| Maternal alcohol exposure | E6–18 | Mouse, M F | E15.5: ↑ IBA-1+ amoeboid cells | P3:↑ IBA-1+ amoeboid cells, ↓ ramified | PFC | - | [46] | |
| Diesel exhaust particles exposure/high fat diet | E2,5,8,12,16 /P120–183 | Mouse, M F | P183: ↑ CD11b and CX3CR1 mRNA in M | - | HIP | - | [47] | |
| Diesel exhaust particles exposure | E2,5,8,12,16 | Mouse, M F | - | P30: = number of IBA-1+ cells, ↑ cell volume, microglia-neuron interaction | PCtx | - | [48] |
| Type of Stress | Time | Species and Sex | Early Effects on Microglia | Late Effects on Microglia | Brain Region | Microglial-Dependent Behavioral Effects | Ref | |
|---|---|---|---|---|---|---|---|---|
| Behavioral stressors | Glucocorticoids exposure | P3–5 | Rat, M F | - | P12: ↓ number (Hortega method) | WM | - | [51] |
| P0 | Rat, M F | P2, 5: ↓ amoeboid microglia (Hortega method) | P10: ↑ branching | CC | - | [52] | ||
| P1,3,5 | Rat, M F | P4,7: ↓ amoeboid OX-42+ cells | P8: ↑ branching | CC | - | [53,54] | ||
| Limited nesting | P2–9 | Mouse, M | - | P245: ↑ CD68+ cells | HIP | Cognitive deficits | [55] | |
| P2–9 | Mouse, M | P9: ↓ IBA-1+ cells/processes complexity | - | ECtx/HIP | - | [56] | ||
| Maternal separation | P1-14 | Rat, M | P15: ↑ activated IBA-1+ cells | - | HIP (Hilus) | - | [57] | |
| P1–21 | Mouse, M | P14: ↑ number, activated IBA-1+ cells; P14, 28: transcriptomic alterations | - | HIP | - | [58] | ||
| P1–14 | Rat, M | P15: ↓ number, ↑ activated IBA-1+ cells | - | HIP (Hilus, CA3) | - | [59] | ||
| P3–12 | Rat, M F | P 14: ↑ number, soma size, ↓ arborization area of IBA-1+ cells | - | Md | - | [60] | ||
| P1–10 | Rat, M | P 10: ↑ IBA-1 IR | P20, 30, 60: ↑ IBA-1 IR and mRNA; P40,50: no effect | HIP, PFC | - | [61] | ||
| P2–14 | Rat, M F | P15: ↓ in CD11b+ apoptotic cells | - | VTA, SN (only M) | - | [62] | ||
| P1–21 | Rat, M | - | P100: ↑ IBA-1+ cells | DS, NAc, HIP (CA3) | - | [63] | ||
| P2–12 | Rat, M F | - | P84: ↓ IBA-1 mRNA | SC | - | [64] | ||
| P2–14 | Mouse, M | - | P60: ↑ motility of IBA-1+ cells | SCtx | - | [65] | ||
| Social isolation | P14–21 | Mouse, M F | - | P70: ↓ number, soma size and processes of IBA-1+ cells | HIP | ↑ depressive-like behavior | [66] | |
| P21–63 | Rat, M | P63: ↑IBA-1 IR, CD11b, ↓ CD200R mRNA | - | HIP | ↑ depressive-like behavior | [74] | ||
| Social defeat | P28–37 | Mouse, M | P28/38:↑ IBA-1 IR | P80: ↓ IBA-1 IR and IBA-1+ cells | PFC | Cognitive deficits | [67,68] | |
| Social stress | P14–21 | Mouse, M F | P22: ↑ IBA-1 IR and IBA-1+ cell number, soma size | P60: ↓ IBA-1 IR, ↑ soma size and processes complexity | VTA | ↑ cocaine CPP | [69] | |
| Social instability stress | P30–45 | Rats, M | P33,46: no effects on OX-42+ cells number | P75: no effects | HIP (DG) | - | [70] | |
| Maternal separation/restraint stress | P1–14/P42–56 | Mouse, M | P42: ↑ activated IBA-1+ cells, ↓ CX3CR1 mRNA/P56: ↑ pro-infl, ↓ anti-infl cytokines mRNA and IR | - | HIP | ↑ depressive-like and anxiety-like behavior | [71] | |
| Maternal separation/mild variable stress | P2–21/22–42 | Rat, F | P51: ↑ activated IBA-1+ cells and IBA-1 IR | - | PFC, HIP | - | [72] | |
| Maternal separation+food restriction/food restriction | P2–20+P36–55/P36-55 | Rat, M F | P55: ↑ IBA-1 IR both sexes/↑ IBA-1 IR and ramification only in F | - | PFC | - | [73] | |
| Maternal separation+handling | P2–20 | Rat, M F | - | P60: ↓ CX3CL1 mRNA | NAc, not HIP | ↓ morphine CPP and reinstatement | [75] | |
| Sleep deprivation | P35 | Mouse, M | P38: ↓ CD68 IR, CX3CR1, CD11b and P2Y12 mRNA, ↓ ramification and PSD95 engulfment, no effect on number and CSF1R mRNA | - | HIP | - | [76] |
| Type of Stress | Time | Species and Sex | Early Effects on Microglia | Late Effects on Microglia | Brain Region | Microglial-Dependent Behavioral Effects | Ref | |
|---|---|---|---|---|---|---|---|---|
| Environmental agents and infection | Alcohol exposure | P7 | Rat, M | P8: ↑ IBA-1 IR | - | Ctx | - | [77] |
| P2–6 | Rat, M F | P6: ↑CD11b+ cells, IL-6, TNF-α, CSF1R, TLR4 mRNA and epigenetic alterations | P90:↑ IBA-1+ and CD11b+ cells | HYP | - | [78] | ||
| P4–9 | Rat, M | P10: ↓ ramification of IBA-1+ cells | - | HIP | - | [79] | ||
| P35–39 | Rat, M F | P40: ↑ activated IBA-1+ cells | - | HIP | - | [80] | ||
| P50–71 | Rat, F | P71: ↑ activated OX-6 (CD74)+ cells | - | HIP | - | [81] | ||
| P3–5 | Rat, M | P6: ↑ amoeboid and ↓ resting IBA-1+ cells | - | HIP, Cb | - | [82] | ||
| P35–38 | Rat, M | P40: ↑ activated IBA-1+ cells, BrdU+ | P65: ↑ activated IBA-1+ cells | HIP | - | [83] | ||
| P4–9 | Mouse, M F | P10: ↑ IBA-1 IR, ↓ processes | - | PCtx, HIP, Cb | - | [84] | ||
| P3–5 | Mouse, M F | P6: ↓ isolectin+ cells | - | Cb | - | [85] | ||
| P4 | Rat, M | P5: ↑ CD11b in F, not M | - | HIP | - | [86] | ||
| P35–90 | Rat, M | P90: ↓ IBA-1+ cells | - | MCtx | - | [87] | ||
| P7 | Mouse, M F | P8: ↑ amoeboid CX3CR1-GFP/+ cells and CD68 IR; P9: return to control levels | - | SCtx | - | [88] | ||
| P4-9 | Mouse, M F | P5/10: no effects | P28: no effects | VCtx | - | [89] | ||
| P25–55 | Rat, M | - | P80: ↑ in IBA-1+/NF-κB+ cells | HIP | - | [90] | ||
| Morphine exposure | P37–42 | Rat, M | P43: ↑ microglial TLR4 mRNA and IR | P60: ↑ CD11b mRNA after morphine re-exposure | NAc | Reinstatement of morphine CPP | [91] | |
| Oxidative Insult (GBR12909) | P10-20 | Mouse, M F | - | P60:↑IBA-1+ and CD68+ cells | HIP | - | [92] | |
| Valproate exposure | P7 | Rat, M | P8: ↑ IBA-1+ cells, ↑ ramification | - | VCtx, HIP, AMY | ↑ depressive-like and anxiety-like behavior | [93] | |
| Methylphenidate exposure | P28–55 | Rats, M F | P57: ↓ ramification of IBA-1+ cells, ↓ CX3CR1 mRNA | - | PFC | - | [94] | |
| Manganese exposure | P20–34,90–150 | Mouse, M F | P35: ↑ameboid IBA-1+ cells, NOS2+; P150: no effect | - | DS, NAc, SN, GP | - | [95] | |
| Methylphenidate exposure | P21–111 | Rat, M | P112: ↑ [3H] PK 11195 binding | - | Ctx, Th, GP, SN | - | [96] | |
| Environmental agents and infection | LPS injection | P3–5 | Rat, M F | P7: ↑ IBA-1+ cells in F | - | HIP | [97] | |
| P4 | Mouse, M F | P7-10: ↑IBA-1 mRNA and IBA-1+ cells | - | Ret | - | [98] | ||
| P3 | Rat, M F | P6: ↑ IBA-1+ cells, amoeboid-like/rod soma, ↓ ED1, MHC-II, iNOS+ cells, ↑ TGF-β, CD206+ cells | P21: = number, ↑ soma size and ↓ processes | HIP, WM, PV | - | [99] | ||
| P2,21 | Mouse, M F | P21 (vs P2): ↑ microglial CD11a, CD172a IR, ↓ SLAMF7 IR | - | WB | - | [100] | ||
| P14,15 | Rat, M F | - | P35: ↑IBA-1+ hypertrophic, apoptotic cells | PFC, HIP | - | [101] | ||
| P6 | Rat, M | - | P90: ↑ IBA-1+ cells | PFC | - | [102] | ||
| P3,5 | Rat, M | - | P85: ↑IBA-1 soma expression | HIP (CA1, DG) | - | [103] | ||
| P5 | Rat, F | - | P71: ↑ OX-42+ cells | HIP (CA1) | - | [104] | ||
| P5 | Rat, M | - | P70: ↑ OX-42+ cells | SN | - | [105] | ||
| P5 | Rat, M | - | P21: ↑ activated OX-42+ cells | CC, PV | ↓ neurobehavioral performance | [106] | ||
| P4–6 | Mouse, M F | P7: ↑ proportion of amoeboid cells, P11: ↓ CX3CR1-GFP+ cells | P13-15: ↑ CX3CR1-GFP+ cells, ↑ proportion of amoeboid cells | Pons | - | [107] | ||
| E.Coli/LPS injection | P4/P60 | Rat, M | P5,6,7: ↑ CD11b expression | P60: ↑ CD11b mRNA, further ↑ by LPS | HIP | - | [108] | |
| P4/P60 | Rat, M | P6: ↑ number, active and proliferating IBA-1+ cells | P33: no changes/P60: ↑ IBA-1+ cells number and volumes, further ↑ by LPS | HIP, PCtx (not FC) | - | [110] | ||
| E.Coli/Handling/LPS | P4/P4–20/P60 | Rat, M | - | P60: ↑ CD11b mRNA, further ↑ by LPS, ↓ by handling | HIP | - | [109] | |
| E.Coli/Amphetamine | P4/P40 | Rat, M | - | P40: ↑ CD200 mRNA by amph, ↓ in E.Coli-amph | NAc | - | [111] | |
| E.Coli/LPS+Fear conditioning | P4/90 | Rat, M | - | P90: ↑ CD11b,↓ CD200, CD200R, CX3CR1 mRNA, ↑ microglial IL-1β mRNA (further ↑ by LPS) | HIP | ↓ memory performance | [112] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catale, C.; Gironda, S.; Lo Iacono, L.; Carola, V. Microglial Function in the Effects of Early-Life Stress on Brain and Behavioral Development. J. Clin. Med. 2020, 9, 468. https://doi.org/10.3390/jcm9020468
Catale C, Gironda S, Lo Iacono L, Carola V. Microglial Function in the Effects of Early-Life Stress on Brain and Behavioral Development. Journal of Clinical Medicine. 2020; 9(2):468. https://doi.org/10.3390/jcm9020468
Chicago/Turabian StyleCatale, Clarissa, Stephen Gironda, Luisa Lo Iacono, and Valeria Carola. 2020. "Microglial Function in the Effects of Early-Life Stress on Brain and Behavioral Development" Journal of Clinical Medicine 9, no. 2: 468. https://doi.org/10.3390/jcm9020468




