Zirconia Reduced Graphene Oxide Nano-Hybrid Structure Fabricated by the Hydrothermal Reaction Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Graphene Oxide
2.2. Synthesis of ZrO2/rGO Nanocomposite Powders
2.3. Microstructural Characterization of the As-Prepared Nanocomposite Powders
2.4. X-ray Diffraction (XRD), Raman, X-ray Photoelectron Spectroscopy (XPS) and Fourier Transform Infrared (FTIR) Characterization
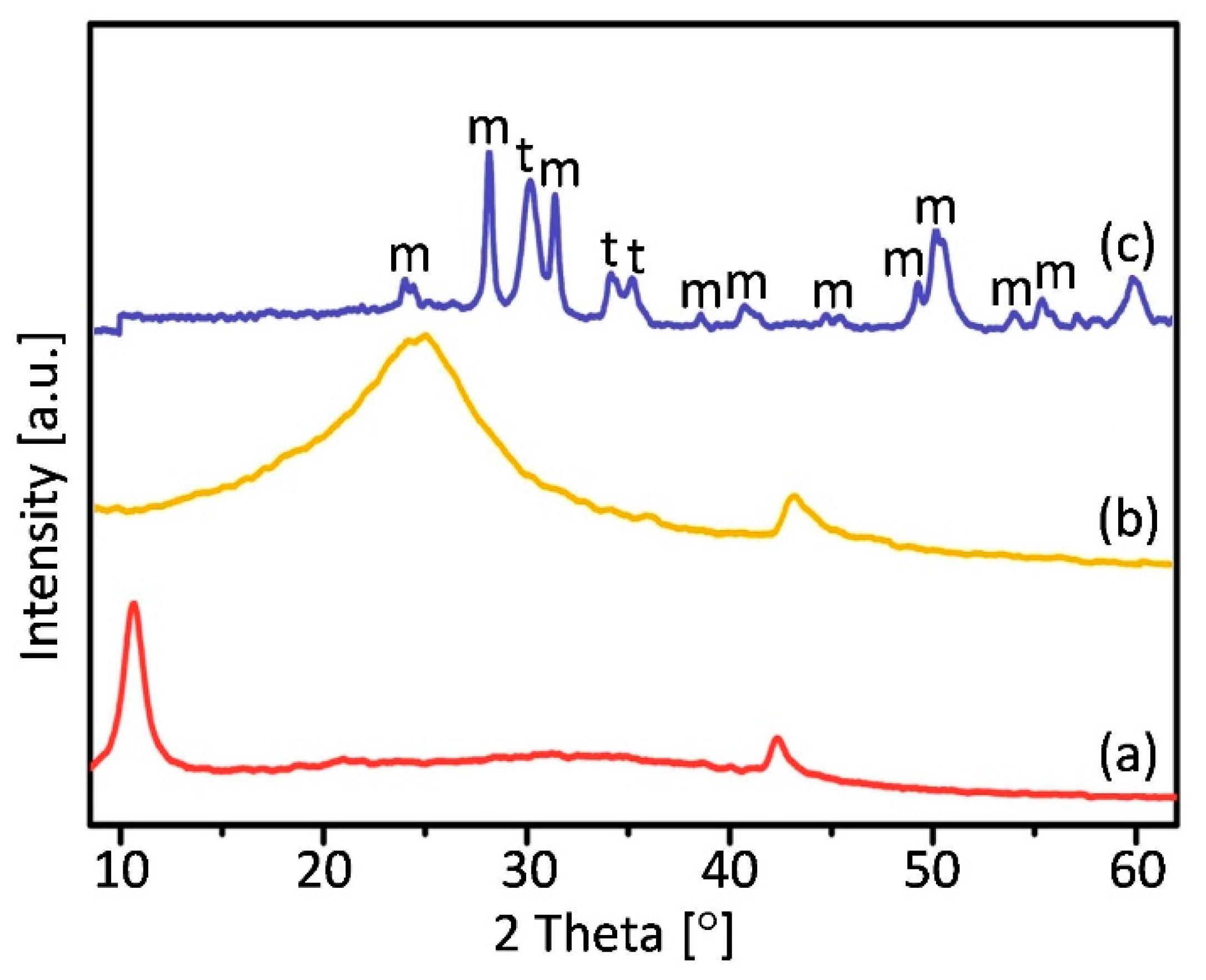
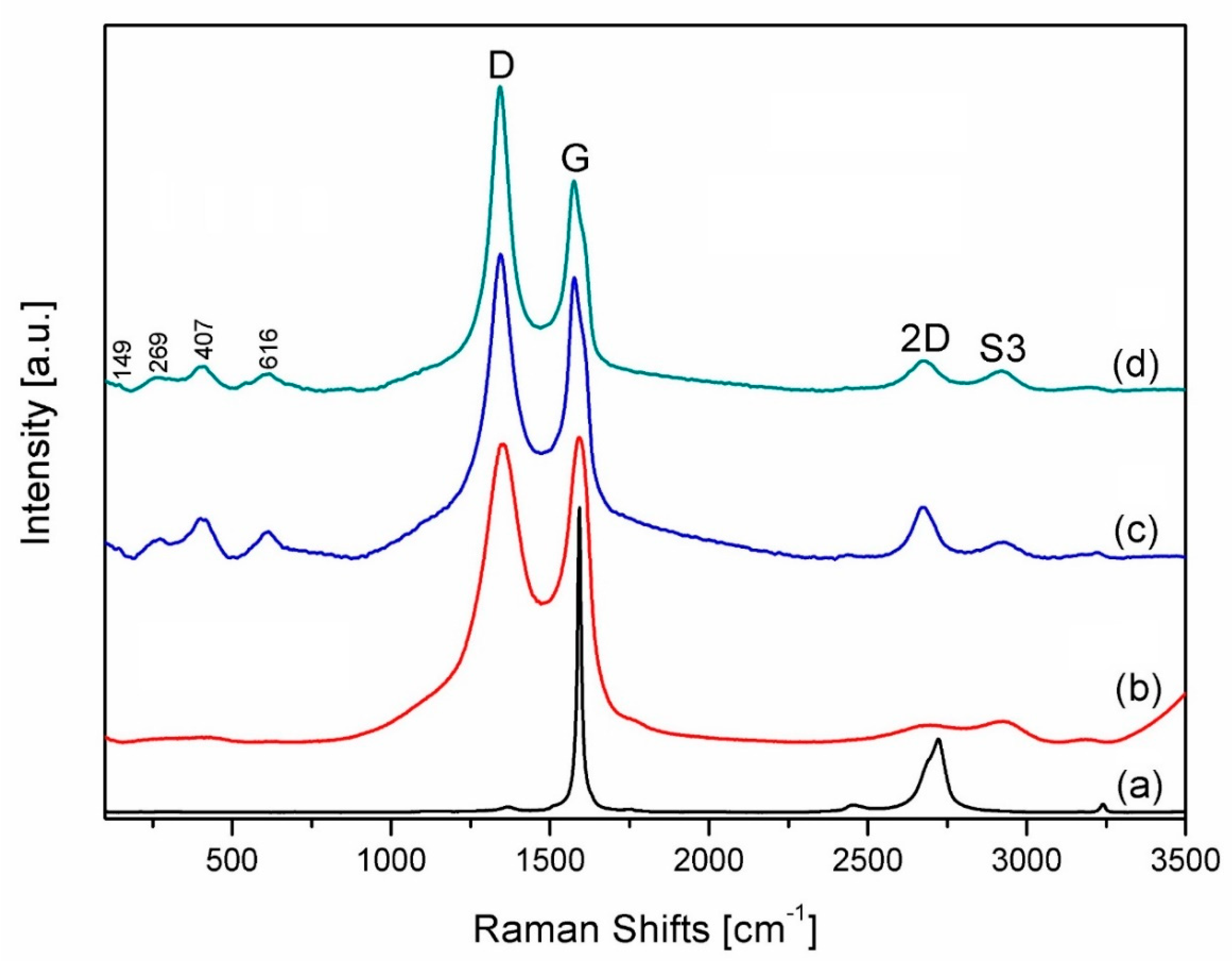
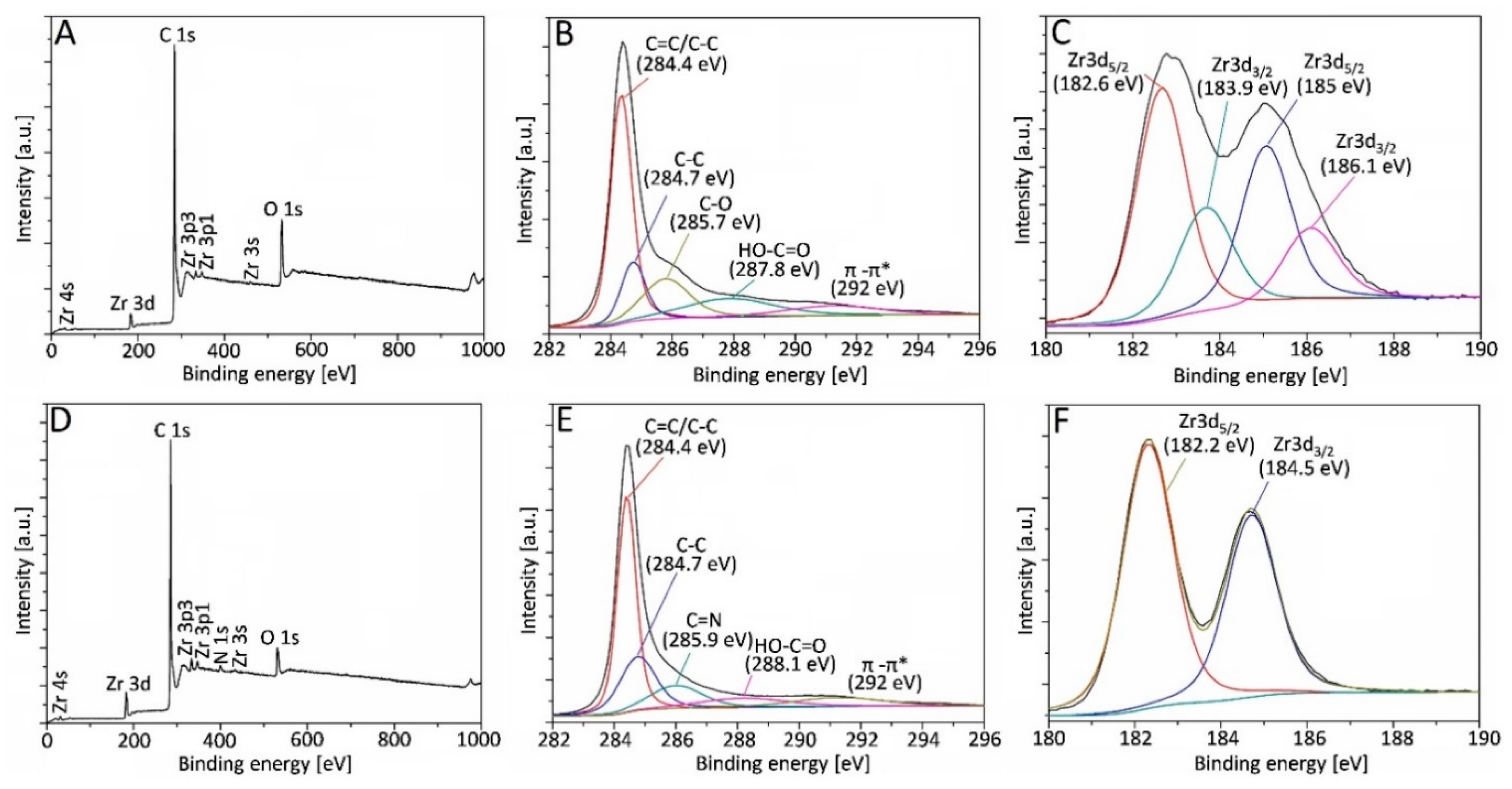
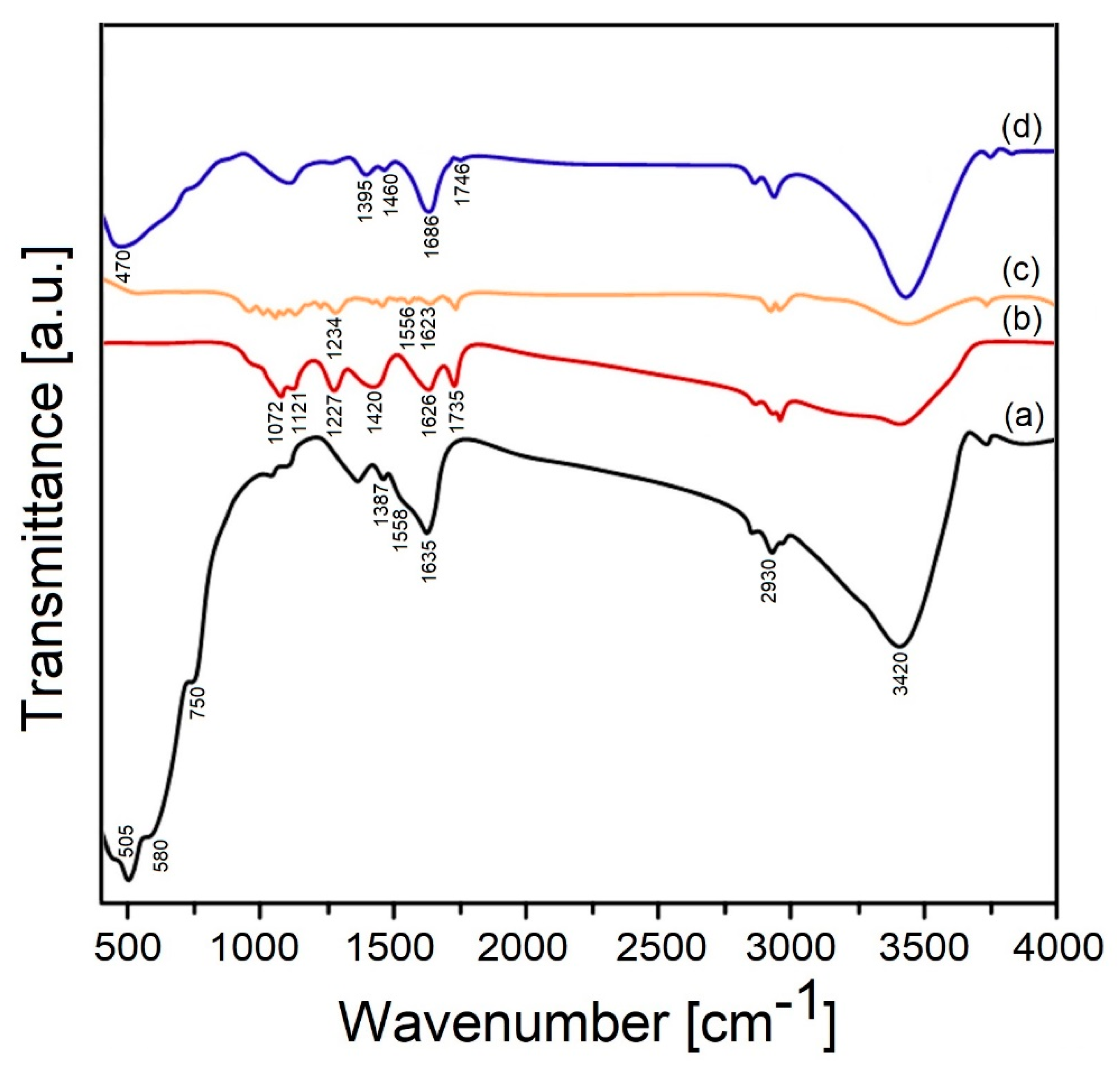
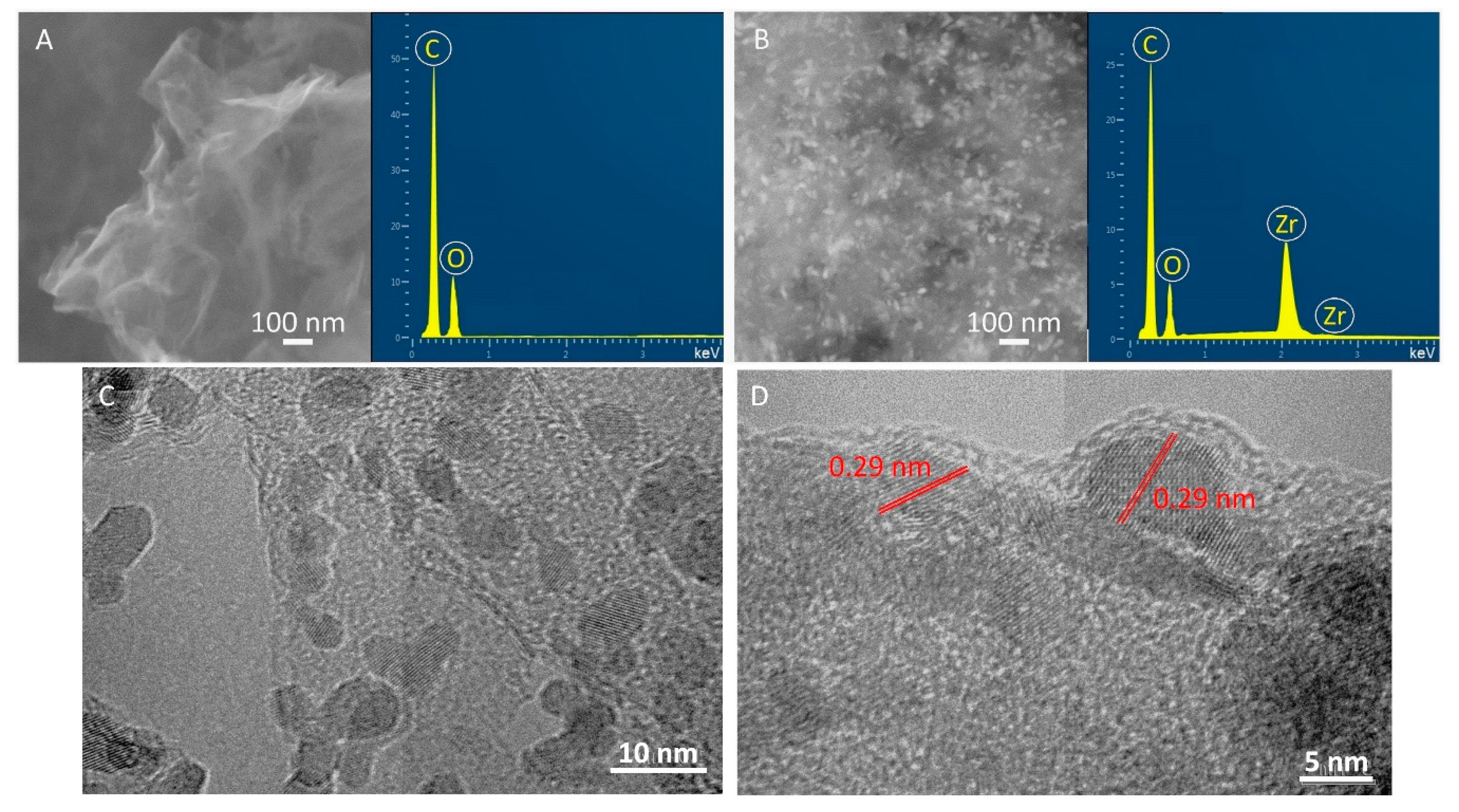
3. Results and Discussion
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigoriev, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of graphene and its applications: A review. Crit. Rev. Solid State Mater. Sci. 2012, 35, 52–71. [Google Scholar] [CrossRef]
- Wei, D.; Liu, T. Controllable synthesis of graphene and its applications. Adv. Mater. 2010, 22, 3225–3241. [Google Scholar] [CrossRef] [PubMed]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Jia, X.; Campos-Delgado, J.; Terrones, M.; Meuniere, V.; Dresselhaus, M.S. Graphene edges: A review of their fabrication and characterization. Nanoscale 2011, 3, 86–95. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Edwards, R.S.; Coleman, K.S. Graphene synthesis: Relationship to applications. Nanoscale 2013, 4, 38–51. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Zheng, C.; Zhou, X.; Cao, H.; Liu, Z. Applications of graphene in supercapacitors. In Graphene: Energy Storage and Conversion Applications; Liu, Z., Zhou, X., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 171–172. [Google Scholar]
- Bocanegra-Bernal, M.H.; De La Torre, S.D. Phase transitions in zirconium dioxide and related materials for high performance engineering ceramics. J. Mater. Sci. 2002, 37, 4947–4971. [Google Scholar] [CrossRef]
- Solís, N.W.; Peretyagin, P.; Torrecillas, R.; Fernández, A.; Menéndez, J.L.; Mallada, C.; Díaz, L.A.; Moya, J.S. Electrically conductor black zirconia ceramic by SPS using graphene oxide. J. Electroceram. 2017, 38, 119–124. [Google Scholar] [CrossRef]
- Kameo, K.; Friedrich, K.; Bartolome, J.F.; Diaz, M.; Lopez-Esteban, S.; Moya, J.S. Sliding wear of ceramics and cermets against steel. J. Eur. Ceram. Soc. 2003, 23, 2867–2877. [Google Scholar] [CrossRef]
- Pecharroman, C.; Lopez-Esteban, S.; Bartolome, J.F.; Moya, J.S. Evidence of nearest-neighbor ordering in wet-processed zirconia-nickel composites. J. Am. Ceram. Soc. 2001, 84, 2439–2441. [Google Scholar] [CrossRef]
- Sergo, V.; Lughi, V.; Pezzotti, G.; Lucchini, E.; Meriani, S.; Muraki, N.; Katagiri, G.; Nishida, T. The effect of wear on the tetragonal-to-monoclinic transformation and the residual stress distribution in zirconia-toughened alumina cutting tools. Wear 1998, 214, 264–270. [Google Scholar] [CrossRef]
- Chen, Z.D.; Myo, M.H.; Choy, C.M. Rapid manufacturing of Y-TZP ceramic punch using powder injection moulding technology. Mater. Sci. Forum 2003, 437, 415–418. [Google Scholar] [CrossRef]
- Bartolome, J.F.; Montero, I.; Diaz, M.; Lopez-Esteban, S.; Moya, J.S. Accelerated aging in 3-mol%-yttria-stabilized tetragonal zirconia ceramics sintered in reducing conditions. J. Am. Ceram. Soc. 2004, 12, 2282–2285. [Google Scholar] [CrossRef]
- Bartolomé, J.F.; Moya, J.S.; Couceiro, R.; Gutierrez, C.F.; Guitián, F.; Martínez-Insua, M. In vitro and in vivo evaluation of a new zirconia/niobium biocermet for hard tissue replacement. Biomaterials 2016, 76, 313–320. [Google Scholar] [CrossRef]
- Moya, J.S.; Sanchez-Herencia, J.A.; Bartolome, J.F.; Tanimoto, T. Elastic modulus in rigid Al2O3/ZrO2 ceramic laminates. Scripta Mater. 1997, 37, 1095–1103. [Google Scholar] [CrossRef]
- Smirnov, A.; Seleznev, A.; Solis Pinargote, N.W.; Pristinskiy, Y.; Peretyagin, P.; Bartolome, J.F. The influence of wire electrical discharge machining cutting parameters on the surface roughness and flexural strength of ZrO2/TiN ceramic nanocomposites obtained by Spark Plasma Sintering. Nanomaterials 2019, 9, 1391. [Google Scholar] [CrossRef]
- Smirnov, A.; Kurland, H.-D.; Grabow, J.; Müller, F.A.; Bartolome, J.F. Microstructure, mechanical properties and low temperature degradation resistance of 2Y-TZP ceramic materials derived from nanopowders prepared by laser vaporization. J. Eur. Ceram. Soc. 2015, 35, 2685–2691. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified Hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Centeno, A.; Rocha, V.G.; Alonso, B.; Fernández, A.; Gutierrez-Gonzalez, C.F.; Torrecillas, R.; Zurutuza, A. Graphene for tough and electroconductive alumina ceramics. J. Eur. Ceram. Soc. 2013, 33, 3201–3210. [Google Scholar] [CrossRef]
- Grigoriev, S.; Peretyagin, P.; Smirnov, A.; Solis, W.; Diaz, L.A.; Fernandez, A.; Torrecillas, R. Effect of graphene addition on the mechanical and electrical properties of Al2O3-SiCw ceramics. J. Eur. Ceram. Soc. 2017, 37, 2473–2479. [Google Scholar] [CrossRef]
- Miranzo, P.; Belmonte, M.; Osendi, I. From bulk to cellular structures: A review on ceramic/graphene filler composites. J. Eur. Ceram. Soc. 2017, 37, 3649–3672. [Google Scholar] [CrossRef]
- Smirnov, A.; Peretyagin, P.; Bartolome, J.F. Processing and mechanical properties of new hierarchical metal-graphene flakes reinforced ceramic matrix composites. J. Eur. Ceram. Soc. 2019, 39, 3491–3497. [Google Scholar] [CrossRef]
- Liu, J.; Yan, H.; Reece, M.J.; Jiang, K. Toughening of zirconia/alumina composites by the addition of graphene platelets. J. Eur. Ceram. Soc. 2012, 32, 4185–4193. [Google Scholar] [CrossRef]
- Walker, L.S.; Marotto, V.R.; Rafiee, M.A.; Koratkar, N.; Corral, E.L. Toughening in graphene ceramic composites. ACS Nano 2011, 5, 3182–3190. [Google Scholar] [CrossRef]
- Shin, J.H.; Hong, S.H. Fabrication and properties of reduced graphene oxide reinforced yttria-stabilized zirconia composite ceramics. J. Eur. Ceram. Soc. 2014, 34, 1297–1302. [Google Scholar] [CrossRef]
- Kwon, S.M.; Lee, S.J.; Shon, I.J. Enhanced properties of nanostructured ZrO2–graphene composites rapidly sintered via high-frequency induction heating. Ceram. Int. 2015, 41, 835–842. [Google Scholar] [CrossRef]
- Chen, F.; Jin, D.; Tyeb, K.; Wang, B.; Han, Y.-Y.; Kim, S.; Schoenung, J.M.; Shen, Q.; Zhang, L. Field assisted sintering of graphene reinforced zirconia ceramics. Ceram. Int. 2015, 41, 6113–6116. [Google Scholar] [CrossRef]
- Rincón, A.; Moreno, R.; Gutiérrez-González, C.F.; Sainz, R.; Salvador, M.D.; Borrell, A. Colloidal processing of fully stabilized zirconia laminates comprising graphene oxide-enriched layers. J. Eur. Ceram. Soc. 2016, 36, 1797–1804. [Google Scholar] [CrossRef]
- Khan Rao, R.A.; Singh, S.; Singh, B.R.; Khan, W.; Naqvi, A.H. Synthesis and characterization of surface modified graphene–zirconium oxide nanocomposite and its possible use for the removal of chlorophenol from aqueous solution. J. Environ. Chem. Eng. 2014, 2, 199–210. [Google Scholar]
- Teymourian, H.; Salimi, A.; Firoozi, S.; Korani, A.; Soltanian, S. One-pot hydrothermal synthesis of zirconium dioxide nanoparticles decorated reduced graphene oxide composite as high performance electrochemical sensing and biosensing platform. Electrochim. Acta 2014, 143, 196–206. [Google Scholar] [CrossRef]
- Li, H.; Xie, Y.; Li, K.; Huang, S.; Zhao, B.; Zheng, X. Microstructure and wear behavior of graphene nanosheets-reinforced zirconia coating. Ceram. Int. 2014, 40, 12821–12829. [Google Scholar] [CrossRef]
- Limaye, A.U.; Helble, J.J. Effect of precursor and solvent on morphology of zirconia nanoparticles produced by combustion aerosol synthesis. J. Am. Ceram. Soc. 2003, 86, 273–278. [Google Scholar] [CrossRef]
- Heshmatpour, F.; Aghakhanpour, R.B. Synthesis and characterization of nanocrystalline zirconia powder by simple sol-gel method with glucose and fructose as organic additives. Powder Tech. 2011, 205, 193–200. [Google Scholar] [CrossRef]
- Keskinen, H.; Moravec, P.; Smolík, J.; Levdansky, V.V.; Mäkelä, J.M.; Keskinen, J. Preparation of ZrO2 fine particles by CVD process: Thermal decomposition of zirconium tert-butoxide vapor. J. Mater. Sci. 2004, 39, 4923–4929. [Google Scholar] [CrossRef]
- Nimmo, W.; Hind, D.; Ali, N.J.; Hampartsoumian, E.; Milne, S.J. The production of ultrafine zirconium oxide powders by spray pyrolysis. J. Mater. Sci. 2002, 37, 3381–3387. [Google Scholar] [CrossRef]
- Tai, C.Y.; Hsiao, B.-Y.; Chiu, H.-Y. Preparation of spherical hydrous-zirconia nanoparticles by low temperature hydrolysis in a reverse microemulsion. Colloids Surf. A: Physicochem. Eng Asp. 2004, 237, 105–111. [Google Scholar] [CrossRef]
- Dittmar, A.; Hoang, D.L.; Martin, A. TPR and XPS characterization of chromia-lanthana-zirconia catalyst prepared by impregnation and microwave plasma enhanced chemical vapour deposition methods. Thermochim Acta 2008, 470, 40–46. [Google Scholar] [CrossRef]
- Piticescu, R.R.; Monty, C.; Taloi, D.; Motoc, A.; Axinte, S. Hydrothermal synthesis of zirconia nanomaterials. J. Eur. Ceram. Soc. 2001, 21, 2057–2060. [Google Scholar] [CrossRef]
- Sagadevan, S.; Podder, J.; Das, I. Hydrothermal synthesis of zirconium oxide nanoparticles and its characterization. J. Mater. Sci.: Mater. Electr. 2016, 27, 5622–5627. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Botas, C.; Pérez-Mas, A.M.; Álvarez, P.; Santamaría, R.; Granda, M.; Blanco, C.; Menéndez, R. Optimization of the size and yield of graphene oxide sheets in the exfoliation step. Carbon 2013, 63, 576–578. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Volosova, M.A.; Peretyagin, P.Y.; Seleznev, A.E.; Okunkova, A.A.; Smirnov, A. The effect of TiC additive on mechanical and electrical properties of Al2O3 ceramic. Appl. Sci. 2018, 8, 2385. [Google Scholar] [CrossRef]
- Bartolomé, J.F.; Smirnov, A.; Kurland, H.-D.; Grabow, J.; Müller, F.A. New ZrO2/Al2O3 nanocomposite fabricated from hybrid nanoparticles prepared by CO2 laser co-vaporization. Sci. Rep. 2016, 6, 20589. [Google Scholar] [CrossRef]
- Wang, Z.L.; Quan, Z.W.; Lin, J. Remarkable changes in the optical properties of CeO2 nanocrystals induced by lanthanide ions doping. Inorg. Chem. 2007, 46, 5237. [Google Scholar] [CrossRef]
- Phoka, S.; Laokul, P.; Swatsitang, E.; Promarak, V.; Seraphin, S.; Maensiri, S. Synthesis, structural and optical properties of CeO2 nanoparticles synthesized by a simple polyvinyl pyrrolidone (PVP) solution route. Mater. Chem. Phys. 2009, 115, 423. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, J.G.; Maji, S.; Malhotra, B.D. Nanostructured zirconia decorated reduced graphene oxide based efficient biosensing platform for non-invasive oral cancer detection. Biosens. Bioelectron. 2016, 15, 497. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pruvost, S.; Livi, S.; Duchet-Rumeau, J. The Role of fluorinated IL as an interfacial agent in P(VDF-CTFE)/Graphene composite films. Nanomaterials 2019, 9, 1181. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 032183. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.-W.; Jung, W.-G. Facile and safe graphene preparation on solution-based platform. J. Industr. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef]
- Bugrov, A.N.; Smyslov, R.Y.; Zavialova, A.Y.; Kopitsa, G.P. The influence of chemical prehistory on the structure, photoluminescent properties, surface and biological characteristics of Zr0.98Eu0.02O1.99 nanophosphors. Nanosyst. Phys. Chem. Math. 2019, 10, 164–175. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, Y.; Chen, W.; Li, X.; Zheng, Q.; Li, K.; Guo, R. Fabrication and properties of in situ reduced graphene oxide-toughened zirconia composite ceramics. J. Am. Ceram. Soc. 2018, 101, 3498–3507. [Google Scholar] [CrossRef]
- Gurushantha, K.; Anantharaju, K.S.; Renuka, L.; Sharma, S.C.; Nagaswarupa, H.P.; Prashantha, S.C.; Vidya, Y.S.; Nagabhushana, H. One pot synthesized reduced graphene oxide–ZrO2 composite as high performance photocatalyst under sunlight. RSC Adv. 2017, 21, 12690–12703. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Y.; Yang, Y.; Cui, J.; Huang, Z.; Wang, Y.; Yang, L.; Wang, H.; Xiaob, Y.; Rong, J. One-step preparation of nitrogen-doped graphene quantum dots from oxidized debris of graphene oxide. J. Mater. Chem. 2013, B1, 39–42. [Google Scholar] [CrossRef]
- Rani, J.R.; Lim, J.; Oh, J.; Kim, J.-W.; Shin, H.S.; Kim, J.H.; Lee, S.; Jun, S.C. Epoxy to carbonyl group conversion in graphene oxide thin films: Effect on structural and luminescent characteristics. J. Phys. Chem. 2012, C116, 19010–19017. [Google Scholar] [CrossRef]
- Xu, B.; Yue, S.; Sui, Z.; Zhang, X.; Hou, S.; Cao, G.; Ya, Y. What is the choice for supercapacitors: Graphene or graphene oxide? Energy Environ. Sci. 2011, 4, 2826–2830. [Google Scholar] [CrossRef]
- Fan, X.; Peng, W.; Li, Y.; Li, X.; Wang, S.; Zhang, G.; Zhang, F. Deoxygenation of exfoliated graphite oxide under alkaline conditions: A green route to graphene preparation. Adv. Mater. 2008, 20, 4490–4493. [Google Scholar] [CrossRef]
- Takashi Ogi, T.; Iwasaki, H.; Aishima, K.; Iskandar, F.; Wang, W.-N.; Takimiya, K.; Okuyama, K. Transient nature of graphene quantum dot formation via a hydrothermal reaction. RSC Adv. 2014, 4, 55709–55715. [Google Scholar]
- Ju, J.; Zhang, R.; He, S.; Chen, W. Nitrogen-doped graphene quantum dots-based fluorescent probe for the sensitive turn-on detection of glutathione and its cellular imaging. RSC Adv. 2014, 4, 52583–52589. [Google Scholar] [CrossRef]
- Gong, P.; Hou, K.; Ye, X.; Ma, L.; Wang, J.; Yanga, S. Synthesis of highly luminescent fluorinated graphene quantum dots with tunable fluorine coverage and size. Mater. Lett. 2015, 143, 112–115. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Ge, L.; Jaroniec, M.; Qiao, S.Z. Two-Step Boron and Nitrogen Doping in Graphene for Enhanced Synergistic Catalysis. Angew. Chem. 2013, 125, 3192–3198. [Google Scholar] [CrossRef]
- Waltman, R.J.; Pacansky, J.; Bates, C.W., Jr. X-ray photoelectron spectroscopic studies on organic photoconductors: Evaluation of atomic charges on chlorodiane blue and p-(diethylamino)benzaldehyde diphenylhydrazone. Chem. Mater. 1993, 5, 1799–1804. [Google Scholar] [CrossRef]
- Liu, C.; Li, K.; Li, H.; Zhang, S.; Zhang, Y.; Wang, B. Synthesis, characterization and ceramization of a carbon-rich zirconium-containing precursor for ZrC ceramic. Ceram. Int. 2014, 40, 7285–7292. [Google Scholar] [CrossRef]
- Singh, R.R.K.; Singh, P. Electrical conductivity of LSGM–YSZ composite materials synthesized via coprecipitation route. J. Mater. Sci. 2014, 49, 5571–5578. [Google Scholar]
- Gao, Y.; Chen, K.; Tan, X.; Wang, X.; Alsaedi, A.; Hayat, T.; Chen, C. Interaction mechanism of Re(VII) with zirconium dioxide nanoparticles archored onto reduced graphene oxides. ACS Sustain. Chem. Eng. 2017, 5, 2163–2171. [Google Scholar] [CrossRef]
- Wang, S.F.; Gu, F.; Lü, M.K.; Yang, Z.S.; Zhou, G.J.; Zhang, H.P.; Zhou, Y.Y.; Wang, S.M. Structure evolution and photoluminescence properties of ZrO2:Eu3+ nanocrystals. Opt. Mater. 2006, 28, 1222–1226. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Z.; Weng, J. Self-assembly of natural tripeptide glutathione triggered by graphene oxide. Soft Matter 2012, 8, 9855–9863. [Google Scholar] [CrossRef]
- Maity, S.K.; Rana, M.S.; Srinivas, B.N.; Bej, S.K.; Dhar, G.M.; Prasada Rao, T.S.R. Characterization and evaluation of ZrO2 supported hydrotreating catalysts. J. Mol. Catal. A Chem. 2000, 153, 121–127. [Google Scholar] [CrossRef]
- Song, H.J.; Jia, X.H.; Li, N.; Yang, X.F.; Tang, H. Synthesis of α-Fe2O3 nanorod/graphene oxide composites and their tribological properties. J. Mater. Chem. 2012, 22, 895–902. [Google Scholar] [CrossRef]
- Jang, S.; Sohn, H.; Ko, Y.K. Synthesis and characterization of soluble alkylalcohol-derivatized graphene oxide. Bull. Korean Chem. Soc. 2013, 34, 1237. [Google Scholar] [CrossRef][Green Version]
- Suneetha, R.B. Spectral, thermal and morphological characterization of biodegradable graphene oxide-chitosan nanocomposites. J. Nanosci. Technol. 2018, 4, 342–344. [Google Scholar] [CrossRef]
- Amer Al-Nafiey, A.; Al-Mamoori, M.H.K.; Alshrefi, S.M.; Shakir, A.K.; Ahmed, R.T. One step to synthesis (rGO/Ni NPs) nanocomposite and using to adsorption dyes from aqueous solution. Mater. Today Proceed. 2019, 19, 94–101. [Google Scholar] [CrossRef]
- Mohamadi, M.; Kowsari, E.; di-Asl, V.; Yousefzadeh, M.; Chinnappan, A.; Ramakrishna, S. Highly-efficient microwave absorptivity in reduced graphene oxide modified with PTA@ imidazolium based dicationic ionic liquid and fluorine atom. Compos. Sci. Technol. 2020, 188, 107960. [Google Scholar] [CrossRef]
- Lingaraju, K.; Naika, H.R.; Nagaraju, G.; Nagabhushana, H. Biocompatible synthesis of reduced graphene oxide from Euphorbia heterophylla (L.) and their in-vitro cytotoxicity against human cancer cell lines. Biotech. Rep. 2019, 24, e00376. [Google Scholar] [CrossRef]
- Walantina, E.; Dhineshbabu, N.R. Photonic band gap analysis of graphene oxide nanostructures using opti-FDTD. In Proceedings of the International Conference on Green Engineering and Technologies (IC-GET), Coimbatore, India, 19 November 2016. [Google Scholar]
- Verma, D.S.; Khan, L.U.; Kumar, S.; Khan, S.B. Fourier transform infrared spectroscopy: Fundamentals and applications in functional groups and nanomaterials characterization. In Handbook of Materials Characterization, 1st ed.; Springer International Publishing: New York, NY, USA, 2018. [Google Scholar]
- Huang, Q.; Gao, L. Immobilization of rutile TiO2 on multiwalled carbon nanotubes. J. Mater. Chem. 2003, 13, 1517–1519. [Google Scholar] [CrossRef]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.-C.; Kim, K.S. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 2010, 7, 3979–3986. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; An, J.; Jung, I.; Piner, R.D.; An, S.J.; Li, X.; Velamakanni, A.; Ruoff, R.S. Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett. 2009, 9, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.Y.; Wang, J.Q.; Yang, Z.G.; Wang, H.G.; Wang, Z.F.; Yang, S.R. Preparation of a highly effective lubricating oil additive–ceria/graphene composite. RSC Adv. 2014, 4, 47096–47105. [Google Scholar] [CrossRef]
- Kasatkin, I.; Girgsdies, F.; Ressler, T.; Caruso, R.A.; Schattka, J.; Urban, J.; Weiss, K. HRTEM observation of the monoclinic-to-tetragonal (m-t) phase transition in nanocrystalline ZrO2. J. Mater. Sci. 2004, 39, 2151–2157. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, C.F.; Smirnov, A.; Centeno, A.; Fernández, A.; Alonso, B.; Rocha, V.G.; Torrecillas, R.; Zurutuza, A.; Bartolome, J.F. Wear behavior of graphene/alumina composite. Ceram. Inter. 2015, 41, 7434–7438. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smirnov, A.; Solís Pinargote, N.W.; Peretyagin, N.; Pristinskiy, Y.; Peretyagin, P.; Bartolomé, J.F. Zirconia Reduced Graphene Oxide Nano-Hybrid Structure Fabricated by the Hydrothermal Reaction Method. Materials 2020, 13, 687. https://doi.org/10.3390/ma13030687
Smirnov A, Solís Pinargote NW, Peretyagin N, Pristinskiy Y, Peretyagin P, Bartolomé JF. Zirconia Reduced Graphene Oxide Nano-Hybrid Structure Fabricated by the Hydrothermal Reaction Method. Materials. 2020; 13(3):687. https://doi.org/10.3390/ma13030687
Chicago/Turabian StyleSmirnov, Anton, Nestor Washington Solís Pinargote, Nikita Peretyagin, Yuri Pristinskiy, Pavel Peretyagin, and José F. Bartolomé. 2020. "Zirconia Reduced Graphene Oxide Nano-Hybrid Structure Fabricated by the Hydrothermal Reaction Method" Materials 13, no. 3: 687. https://doi.org/10.3390/ma13030687
APA StyleSmirnov, A., Solís Pinargote, N. W., Peretyagin, N., Pristinskiy, Y., Peretyagin, P., & Bartolomé, J. F. (2020). Zirconia Reduced Graphene Oxide Nano-Hybrid Structure Fabricated by the Hydrothermal Reaction Method. Materials, 13(3), 687. https://doi.org/10.3390/ma13030687






