Systematic Detection and Identification of Bioactive Ingredients from Citrus aurantium L. var. amara Using HPLC-Q-TOF-MS Combined with a Screening Method
Abstract
1. Introduction
2. Results and Discussions
2.1. Establishment of the Screening Method
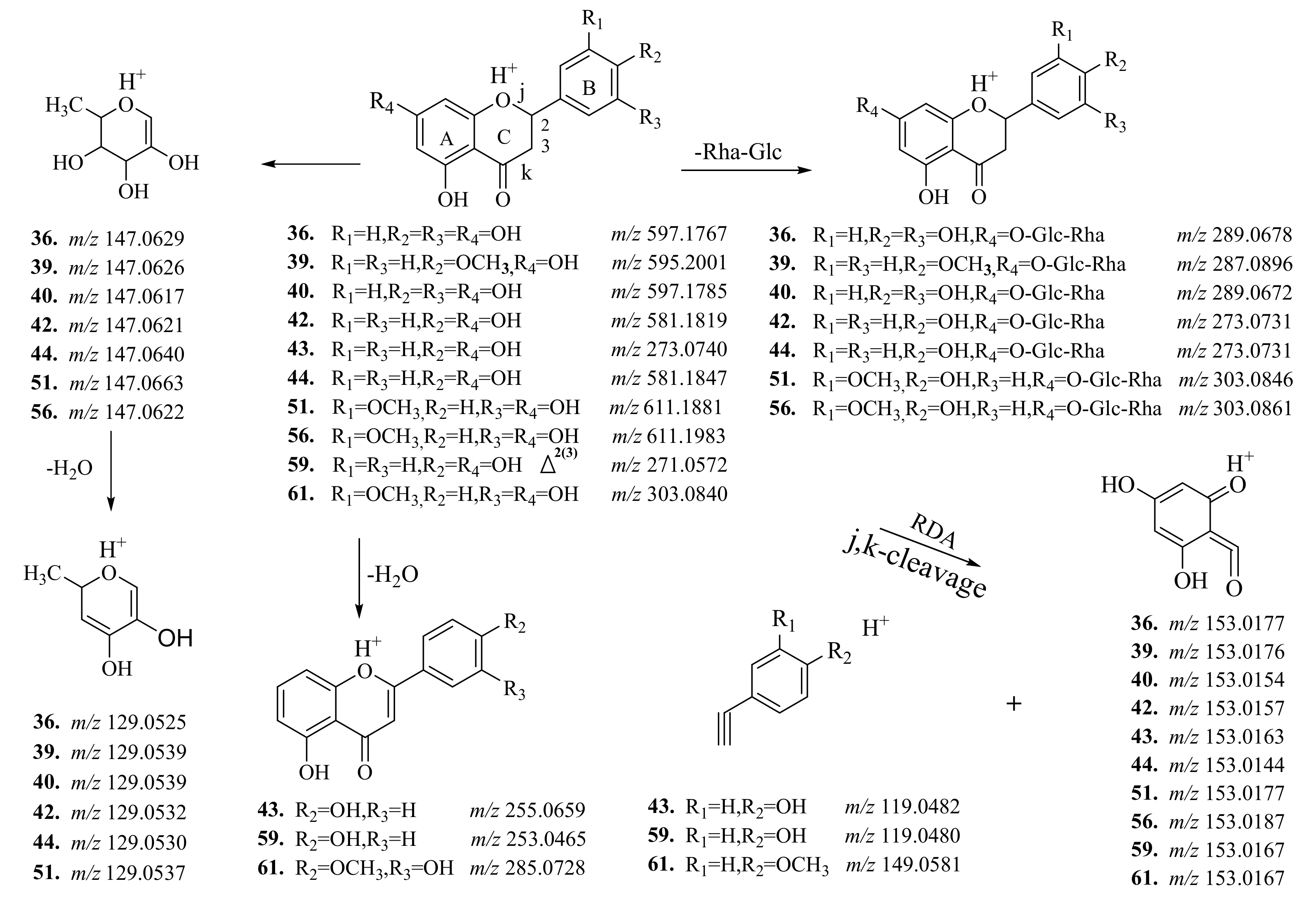
2.2. Screening and Identification of Flavonols and Flavonol Glycosides
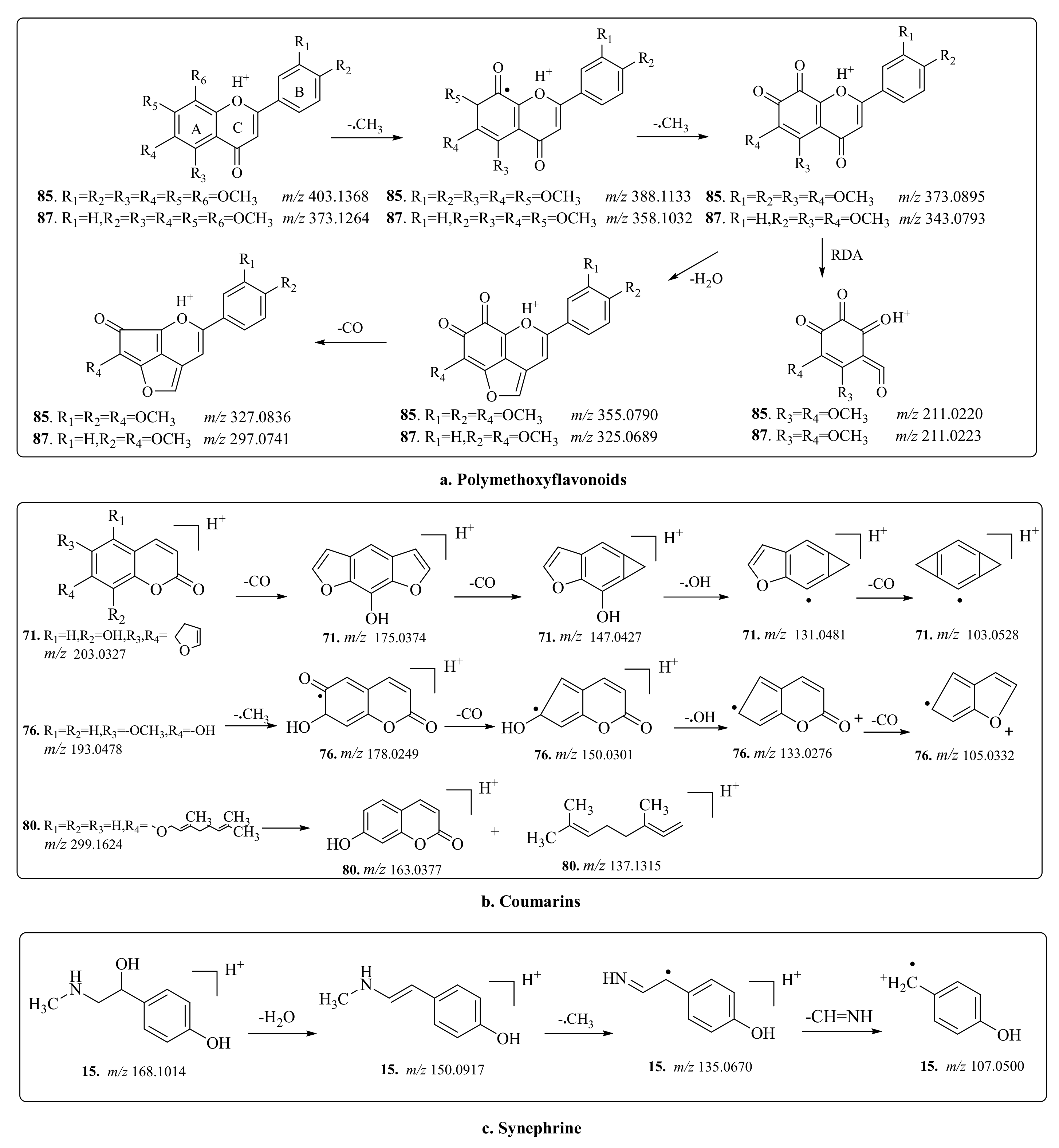
2.3. Screening and Identification of Coumarin
2.4. Screening and Identification of Alkaloids and Triterpenoid
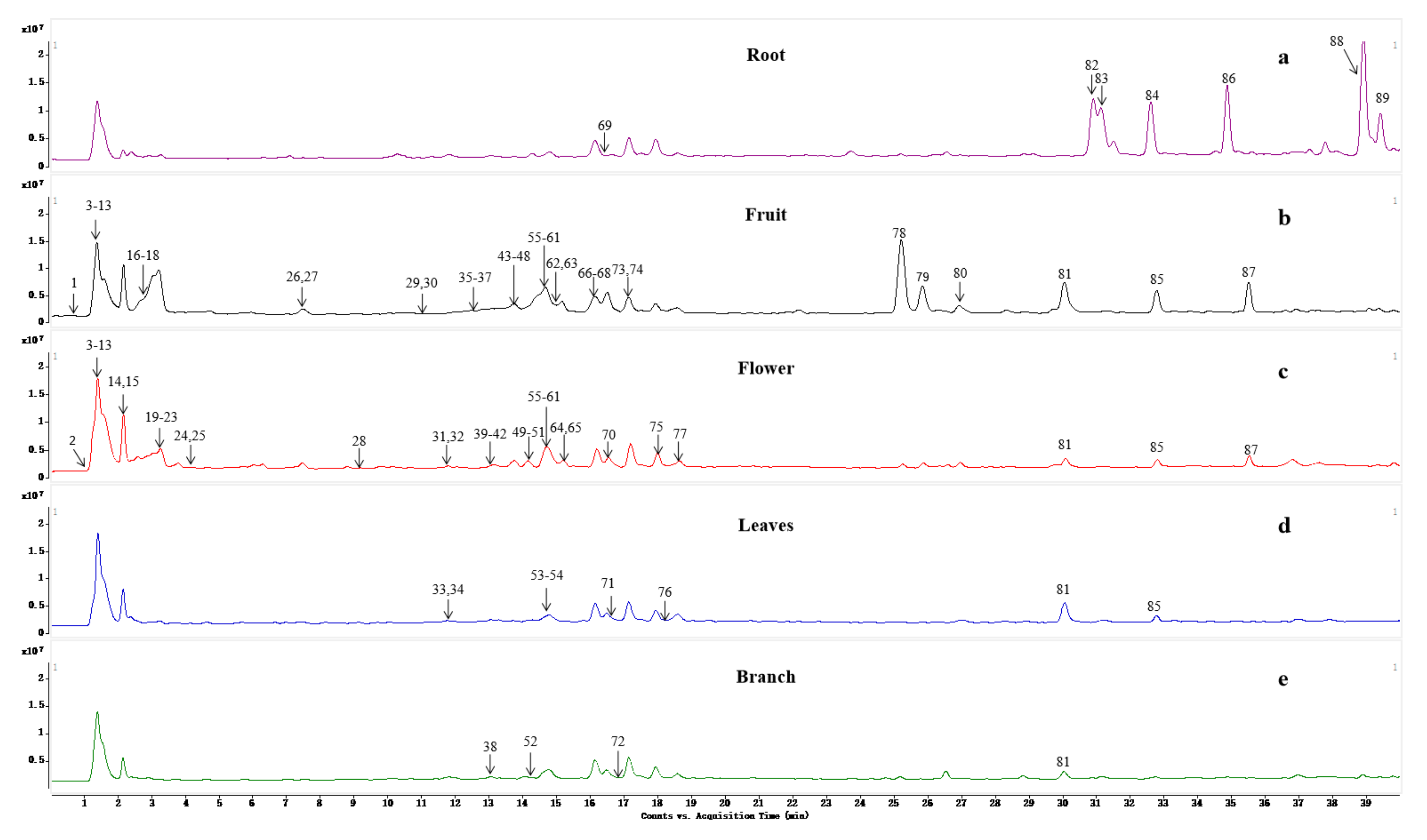
2.5. Distribution of Metabolites in CAVA
3. Conclusions
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Sample Collection and Preparation
4.3. HPLC-Q-TOF-MS Conditions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiao, P.G. New Chinese Medicine Record; Chemical Industry Press: Beijing, China, 2002; pp. 352–454. [Google Scholar]
- Xue, L.Y.; Gao, L.; Qin, X.M.; Du, G.H.; Zhou, Y.Z. A review of recent literature on anti-aging activity of medicinal and edible traditional Chinese herbs. Food Sci. 2017, 38, 302–309. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Yang, L.; Wei, J.; Huang, M.; Jiang, J.-G. Bioactivity evaluations of ingredients extracted from the flowers of Citrus aurantium L. var. amara Engl. Food Chem. 2012, 135, 2175–2181. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yang, L.; Zhao, H.-Y.; Jiang, J.-G.; Xu, X.-L. Protective effect of compounds from the flowers of Citrus aurantium L. var. amara Engl against carbon tetrachloride-induced hepatocyte injury. Food Chem. Toxicol. 2013, 62, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.-J.; Chen, D.; Huang, Q.-D.; Huang, Q.; Lian, Y.-F.; Cai, W.-W.; Zeng, H.-P.; Lin, Y.-L. Isolation of a new flavanone from Daidai fruit and hypolipidemic activity of total flavonoids extracts. Nat. Prod. Res. 2015, 29, 1521–1528. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Wang, T.-X.; Zhang, X.-M.; Jiang, J.-G. Various Antioxidant Effects Were Attributed to Different Components in the Dried Blossoms of Citrus aurantium L. var. amara Engl. J. Agric. Food Chem. 2017, 65, 6087–6092. [Google Scholar] [CrossRef]
- Karimi, E.; Oskoueian, E.; Hendra, R.; Oskoueian, A.; Jaafar, H.Z.E. Phenolic Compounds Characterization and Biological Activities of Citrus aurantium Bloom. Molecules 2012, 17, 1203–1218. [Google Scholar] [CrossRef]
- Shen, C.Y.; Jiang, J.G.; Zhu, W.; Ou-Yang, Q. Anti-inflammatory effect of essential oil from Citrus aurantium L. var. amara Engl. J. Agric. Food Chem. 2017, 65, 8586–8594. [Google Scholar] [CrossRef]
- Siddique, S. Volatile components and antimicrobial activity of Citrus sinensis var. mosammi leaves oil. J. Med. Plants Res. 2012, 6, 2184–2187. [Google Scholar] [CrossRef]
- Liu, L.; Shan, S.; Zhang, K.; Ning, Z.Q.; Lu, X.P.; Cheng, Y.Y. Naringenin and hesperetin, two flavonoids derived from Citrus aurantium up-regulate transcription of adiponectin. Phytother. Res. 2010, 22, 1400–1403. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Shabanian, G.; Rafieian-Kopaei, M.; Parvin, N.; Saadat, M.; Akhlaghi, M. Citrus aurantium Blossom and Preoperative Anxiety. Braz. J. Anesthesiol. 2011, 61, 702–712. [Google Scholar] [CrossRef]
- Rahnama, S.; Rabiei, Z.; Alibabaei, Z.; Mokhtari, S.; Rafieian-Kopaei, M.; Deris, F. Anti-amnesic activity of Citrus aurantium flowers extract against scopolamine-induced memory impairments in rats. Neurol. Sci. 2014, 36, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Mol. 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cao, Y.L.; Jiang, J.G.; Lin, Q.S.; Chen, J.; Zhu, L. Response surface optimization of ultrasound-assisted flavonoids extraction from the flower of Citrus aurantium L. var. amara Engl. J. Sep. Sci. 2015, 33, 1349–1355. [Google Scholar] [CrossRef]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, Z.Y.; Ma, Y.L.; Ma, S.C. Progress in research of traditional Chinese medicine Citrus aurantium. China J. Chin. Mater. Med. 2015, 40, 185–190. [Google Scholar] [CrossRef]
- Percy, D.W.; Adcock, J.L.; Conlan, X.A.; Barnett, N.W.; Gange, M.E.; Noonan, L.K.; Henderson, L.C.; Francis, P.S. Determination of Citrus aurantium protoalkaloids using HPLC with acidic potassium permanganate chemiluminescence detection. Talanta 2010, 80, 2191–2195. [Google Scholar] [CrossRef]
- Jiang, M.-H.; Yang, L.; Zhu, L.; Piao, J.-H.; Jiang, J.-G. Comparative GC/MS Analysis of Essential Oils Extracted by 3 Methods from the Bud of Citrus aurantium L. var. amara Engl. J. Food Sci. 2011, 76, C1219–C1225. [Google Scholar] [CrossRef]
- Liu, W.-Y.; Zhou, C.; Yan, C.-M.; Xie, S.-L.; Feng, F.; Wu, C.-Y.; Xie, N. Characterization and simultaneous quantification of multiple constituents in Aurantii Fructus Immaturus extracts by HPLC-DAD-ESI-MS/MS. Chin. J. Nat. Med. 2012, 10, 456–463. [Google Scholar] [CrossRef]
- Wang, T.X. Study on Isolation and Identification of Active Ingredients from Citrus aurantium L. var. amara Engl and Their Bioactivities. Master’s Thesis, South China University of Technology, Guangzhou, China, 2018. [Google Scholar]
- Mencherini, T.; Campone, L.; Piccinelli, A.L.; García Mesa, M.; Sánchez, D.M.; Aquino, R.P.; Rastrelli, L. HPLC-PDA-MS and NMR Characterization of a Hydroalcoholic Extract of Citrus aurantium L. var. amara, Peel with Antiedematogenic Activity. J. Agric. Food Chem. 2013, 61, 1686–1693. [Google Scholar] [CrossRef]
- Gatti, R.; Lotti, C.; Morigi, R.; Andreani, A. Determination of octopamine and tyramine traces in dietary supplements and phytoextracts by high performance liquid chromatography after derivatization with 2,5-dimethyl-1H-pyrrole-3,4-dicarbaldehyde. J. Chromatogr. A 2012, 1220, 92–100. [Google Scholar] [CrossRef]
- Qing, Z.-X.; Xu, Y.-Q.; Yang, P.; Yu, K.; Cheng, P.; Zeng, J.-G. Mass spectrometry-guided isolation of two new benzoquinoline alkaloids from Macleaya cordata. Nat. Prod. Res. 2016, 30, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.-X.; Liu, X.-B.; Cheng, P.; Wu, H.-M.; Zeng, J.-G. An improved separation method for classification of Macleaya cordata from different geographical origins. Anal. Methods 2015, 7, 1866–1871. [Google Scholar] [CrossRef]
- Qing, Z.-X.; Zhao, H.; Tang, Q.; Mo, C.-M.; Huang, P.; Cheng, P.; Yang, P.; Yang, X.-Y.; Liu, X.-B.; Zheng, Y.-J.; et al. Systematic identification of flavonols, flavonol glycosides, triterpene and siraitic acid glycosides from Siraitia grosvenorii using high-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry combined with a screening strategy. J. Pharm. Biomed. Anal. 2017, 138, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Tsimogiannis, D.; Samiotaki, M.; Panayotou, G.; Oreopoulou, V. Characterization of Flavonoid Subgroups and Hydroxy Substitution by HPLC-MS/MS. Molecules 2007, 12, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.B.; Li, J.J.; Xiong, H.Y.; Peng, C.S. Fragmentation Pathway Comparison of 5,6,7,4′-Tetrahydroxy-Flavone and 5,6,7,4′-Tetramethoxy-Flavone by High Resolution Electrospray Ionization Tandem Mass Spectroscopy. J. Chin. Mass Spectrom. Soc. 2016, 37, 385–392. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, Y.Q.; Taniguchi, M.; Baba, K. Studies on chemical constituents in roots of Peucedanum praeruptorum. China J. Chin. Mater. Med. 2009, 37, 1333–1335. [Google Scholar]
- Wan, J.-B.; Bai, X.; Cai, X.-J.; Rao, Y.; Wang, Y.-S.; Wang, Y.-T. Chemical differentiation of Da-Cheng-Qi-Tang, a Chinese medicine formula, prepared by traditional and modern decoction methods using UPLC/Q-TOFMS-based metabolomics approach. J. Pharm. Biomed. Anal. 2013, 83, 34–42. [Google Scholar] [CrossRef]
- Wu, T.; Cheng, D.; He, M.; Pan, S.; Yao, X.; Xu, X. Antifungal action and inhibitory mechanism of polymethoxylated flavones from Citrus reticulata Blanco peel against Aspergillus niger. Food Control 2014, 35, 354–359. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| PN | TR (min) | MS1 b (ppm) | Formula | Characteristic MS/MS Ions (m/z) | Identification | Screening Method | Plant Part |
|---|---|---|---|---|---|---|---|
| 1 b | 0.67 | 163.1116 (0.8) | C11H14O | 117.0720, 89.0605, 57.0691 | Citrus H | X | Flower, root, fruit, leaf, branch |
| 2 b | 1.08 | 130.0862 (0.4) | C6H11NO2 | 84.0784, 70.0643 | Pipecolic acid | X, Y | Flower, root, fruit, leaf, branch |
| 3 b | 1.21 | 133.0600 (5.8) | C4H8N2O3 | 116.0335, 87.0543, 74.0230 | Asparagine | Y | Flower, root, fruit, leaf, branch |
| 4 b | 1.23 | 277.1270 (4.2) | C12H20O7 | 259.0916, 211.0695, 133.0596 | Citrus I | X | Flower, root, fruit, leaf, branch |
| 5 b | 1.24 | 147.0764 (0.1) | C5H10N2O3 | 130.0494, 101.0701, 84.0436 | Glutamine | Y | Flower, fruit, leaf, branch |
| 6 b | 1.26 | 106.0500 (−1.2) | C3H7NO3 | 88.0389, 70.0286, 60.0437 | Citrus J | X | Flower, fruit, leaf, branch |
| 7 b | 1.29 | 156.0397 (4.3) | C5H5N3O3 | 110.0712, 83.0606 | Citrus A | X | Flower, root, fruit, leaf, branch |
| 8 b | 1.32 | 175.1193 (−2.0) | C6H15N4O2 | 158.0929, 130.0962, 116.0698, 70.0643, 60.0548 | Arginine | Y | Flower, fruit, leaf |
| 9 b | 1.38 | 247.1933 (−6.4) | C14H22N4 | 144.0948, 58.0704 | Citrus K | X | Flower, fruit, leaf |
| 10 b | 1.46 | 248.1993 (6.4) | C16H25NO | 202.0961, 104.1008 | Citrus B | X | Flower, root, fruit, leaf, branch |
| 11 b | 1.52 | 314.1586 (3.8) | C15H23NO6 | 152.1000, 121.0561, 91.0456 | N-acetylnorsynephrine-rhamnoside | X | Flower, root, fruit, leaf, branch |
| 12 b | 1.53 | 104.1067 (2.8) | C5H13NO | 60.0807, 58.0649 | Citrus M | X | Flower, fruit, leaf |
| 13 b | 1.58 | 116.0711 (−4.3) | C5H10NO2 | 86.0162, 70.0649 | Citrus N | X | Flower, fruit, leaf |
| 14 b | 1.93 | 170.1214 (−9.2) | C9H16NO2 | 152.0893, 124.0686, 97.0715, 91.0471 | Dihydro-synephrine | X | Flower, fruit |
| 15 a | 2.11 | 168.0973 (8.4) | C9H14NO2 | 150.0874, 135.0633, 119.0453, 91.0507 | Synephrine | X, Y | Flower, fruit |
| 16 b | 2.56 | 300.1432 (3.2) | C14H22NO6 | 282.1317, 138.0897, 121.0647 | Citrus C | X | Flower, fruit, leaf, branch |
| 17 b | 2.60 | 268.1039 (0.5) | C10H14N5O4 | 136.0605, 119.0328 | Adenosine | X, Y | Flower, fruit, leaf, branch |
| 18 b | 2.61 | 284.0973 (5.8) | C10H14N5O5 | 267.1405, 152.0561, 135.0262 | Hydroxyadenosine | X | Flower, fruit, leaf, branch |
| 19 b | 3.34 | 222.1120 (2.1) | C12H15NO3 | 205.1423, 87.0435 | Citrus D | X | Flower, fruit |
| 20 b | 3.35 | 240.1032 (−5.4) | C15H13NO2 | 222.1059, 208.0996, 195.0853, 149.0818, 121.0559, 105.0280 | Citrus E | X | Flower, fruit |
| 21 | 3.37 | 152.1065(3.2) | C9H13NO | 121.1639, 103.0538, 77.0378 | N-acetylnorsynephrine | X, Y | Flower, fruit |
| 22 b | 3.38 | 367.1830 (−6.8) | C25H22N2O | 322.1387, 229.1012, 58.0654 | Citrus L | X | Flower, fruit |
| 23 b | 3.41 | 163.0384 (3.5) | C9H6O3 | 89.0585, 57.0698 | Citrus O | X | Flower, fruit |
| 24 b | 3.76 | 120.0808(−0.2) | C8H9N | 103.0533, 91.0532, 77.0381 | Citrus F | X | Flower, fruit |
| 25 b | 3.78 | 166.0856(3.9) | C9H12NO2 | 131.0488, 120.0799, 103.0535 | Phenylalanine | X, Y | Flower, fruit |
| 26 b | 7.55 | 463.1550 (8.2) | C23H26O10 | 313.0728, 185.0925, 153.0174 | 3′,4′,5′-trimethoxyflavone-O-arabinoside | X | Fruit |
| 27 b | 7.59 | 180.1013 (3.3) | C10H13NO2 | 163.1293, 107.0683, 89.0588 | Citrus G | X | Flower, fruit |
| 28 | 9.18 | 595.1657 (0.1) | C27H30O15 | 449.1104, 287.0537, 147.0639 | Luteolin-O-glucoside-O-rhamnoside | Y, Z | Flower, fruit |
| 29 b | 10.86 | 627.1564(−1.3) | C27H30O17 | 465.0947, 303.0482, 145.0516 | 3′,4′,5′,5-hydroxy-flavone-O-glucoside-O-glucoside | X | Flower, fruit |
| 30 b | 11.17 | 447.1252 (7.5) | C22H22O10 | 285.0702, 121.0957 | Acacetin-O-glucoside | Z | Flower, fruit |
| 31 b | 11.74 | 449.1104 (−5.7) | C21H20O11 | 287.0521, 147.0512, 129.0525 | Luteolin-O-glucoside | X, Y, Z | Flower, fruit |
| 32 b | 11.75 | 611.1636 (−4.8) | C27H30O16 | 449.1117, 287.0532 | Luteolin-O-glucoside-O-glucoside | Y, Z | Flower, fruit |
| 33 b | 11.79 | 451.1244 (−2.0) | C21H22O11 | 289.0704, 153.0186, 107.0429 | Eriodictyol-O-glucoside | Y, Z | Leaf, branch |
| 34 a | 11.88 | 289.0700 (2.3) | C15H12O6 | 163.0365, 153.0167, 145.0281 | Eriodictyol | X, Y, Z | Leaf, branch |
| 35 b | 12.25 | 595.2077 (−8.4) | C28H34O14 | 449.1455, 303.0851, 153.0231 | Hesperitin-O-rhamnoside-O-rhamnoside | Z | Flower, fruit |
| 36 a | 12.30 | 597.1835 (−3.5) | C27H32O15 | 451.1220, 289.0698, 153.0161, 147.0624, 129.0518 | Neoeriocitrin | X, Y, Z | Flower, fruit |
| 37 b | 12.31 | 597.1861 (−9.8) | C27H32O15 | 449.1391, 287.0606 | 4′-hydroxyl-flavanone-O-glucoside -O-rhamnoside | X | Flower, fruit |
| 38 b | 13.11 | 463.1244 (−1.9) | C22H22O11 | 377.9540, 301.0700, 121.1001 | Luteolin-O-glucoside | X, Y, Z | Flower, fruit, leaf, branch |
| 39 ab | 13.20 | 595.2018 (0.5) | C28H34O14 | 449.1436, 287.0897, 129.0540 | Poncirin | X, Y | Flower, fruit, root |
| 40 a | 13.27 | 597.1818(1.2) | C27H32O15 | 331.07, 9289.0709, 147.0259 | Eriocitrin | Y, Z | Flower, fruit, root |
| 41 b | 13.29 | 419.1186 (−0.4) | C17H22O12 | 273.0757, 153.0152, 129.0556 | Naringenin-O-rhamnoside | X, Y, Z | Flower, fruit, root |
| 42 a | 13.39 | 581.1818 (8.0) | C27H32O14 | 435.1291, 273.0727, 153.0179, 147.0641, 129.0526 | Naringin | Y, Z | Flower, fruit, root |
| 43 a | 13.49 | 273.0808 (3.0) | C8H16O10 | 153.0187, 147.0390, 119.0456 | Naringenin | X, Y, Z | Flower, fruit |
| 44 ab | 13.67 | 581.1841 (4.1) | C27H32O14 | 419.1302, 273.0723, 147.0623, 129.0532 | Narirutin | X, Y, Z | Flower, fruit |
| 45 | 13.69 | 609.1805 (1.4) | C28H32O13 | 463.1229, 301.0701, 129.0644, 85.0277 | Diosmetin-O-glucoside-O-rhamnoside | X, Y, Z | Flower, fruit, leaf, branch |
| 46 b | 13.76 | 419.1391(−11.6) | C21H22O9 | 383.1107,285.0716,129.0534 | 4′-Methoxy-flavanone-O-arabinose | X, Y | Flower, fruit |
| 47 b | 13.82 | 727.2451 (−0.9) | C33H42O18 | 527.1484, 419.1294, 315.0925129.0538 | 4′,5′-Methoxy-flavanone-O-rhamnoside-O-arabinose-O-arabinose | X | Flower, fruit |
| 48 b | 13.98 | 565.1954 (−6.7) | C16H12O5 | 419.1351, 285.0881, 147.0503 | 4′-Methoxy-flavanone-O-rhamnoside-O-arabinoside | X | Flower, fruit |
| 49 b | 14.15 | 449.1458 (−3.5) | C22H24O10 | 413.1228, 303.0833 | Hesperitin-O-rhamnoside | Y, Z | Flower, fruit, Root, leaf, branch |
| 50 b | 14.17 | 593.1495 (1.0) | C28H32O14 | 447.1265, 285.0711 | Acacetin-O-glucuronic acid-O-arabinoside | Z | Flower, fruit, Leaf, branch |
| 51 a | 14.19 | 611.1937 (5.4) | C28H34O15 | 449.1412, 303.0835, 129.0524 | Neohesperidin | X, Y, Z | Flower, fruit, Leaf, branch |
| 52 b | 14.22 | 579.1715 (−1.5) | C27H30O14 | 271.0599, 153.0153, 129.0525 | Apigenin-O-glucoside-O-rhamnoside | Y,Z | Flower, fruit, Root, leaf, branch |
| 53 b | 14.23 | 449.1441 (0.2) | C22H24O10 | 303.0855, 153.0199,129.0541 | Hesperitin-O-rhamnoside | X, Z | Flower, fruit, Root, leaf, branch |
| 54 b | 14.44 | 345.0951 (5.1) | C18H16O7 | 303.0801, 195.0277, 153.0201 | 3′,4′,5′-trimethoxyflavone | Y | Leaf, branch |
| 55 b | 14.57 | 593.1861 (6.5) | C28H32O14 | 447.1259, 315.0863, 153.0152 | Cirsimaritin-O-arabinose | Z | Flower, fruit, branch |
| 56 a | 14.58 | 611.1961 (1.5) | C28H34O15 | 449.1416, 303.0826, 129.0536 | Hesperidin | X, Y, Z | Flower, fruit, root, leaf, branch |
| 57 b | 14.61 | 435.1295 (−2.1) | C21H22O10 | 273.0742, 153.0175 | Naringenin-O-glucoside | Z | Flower, fruit, root |
| 58 b | 14.65 | 757.2226 (−5.3) | C39H50O25 | 611.1763, 287.0477, 129.0537 | Luteolin-O-glucoside-O-rhamnoside-O-glucoside | X, Z | Flower, fruit, branch |
| 59 ab | 14.68 | 271.0581 (6.7) | C15H10O5 | 243.0623, 153.0167, 119.0479 | Apigenin | X, Y, Z | Flower, fruit, branch |
| 60 b | 14.70 | 757.2214 (−3.7) | C33H40O20 | 449.1448, 303.0853, 129.0528 | Hesperitin-O-glucuronic acid-O-arabinoside-O-rhamnoside | Z | Flower, fruit, Root, leaf, branch |
| 61 a | 14.71 | 303.0850 (4.3) | C16H14O4 | 177.0538, 153.0365, 145.0269 | Hesperitin | X, Y, Z | Flower, fruit, root |
| 62 b | 14.86 | 739.2450 (−0.8) | C34H42O18 | 575.1642, 413.1240, 315.0863 | Cirsimaritin-O-arabinoside-O-rhamnoside-O-rhamnoside | Z | Flower, fruit, branch |
| 63 b | 15.17 | 653.1725 (−1.9) | C29H32O17 | 347.0759, 129.0522 | 3′,3,5-hydroxy-4′,5′-Methoxy-flavone-O-glucoside-O-rhamnoside | X | Flower, fruit |
| 64 b | 15.23 | 435.1251 (8.0) | C20H18O11 | 273.0730, 153.0193, 147.0478 | Naringenin-O-glucoside | X, Z | Flower, fruit |
| 65 b | 15.23 | 609.1797 (2.7) | C28H32O15 | 301.0690, 463.1244, 153.0151 | Diosmetin-O-glucoside-O-rhamnoside | X, Y, Z | Flower, fruit |
| 66 b | 15.96 | 579.1723 (−2.5) | C22H22O10 | 433.1139, 271.0590, 129.0534 | Apigenin-O-glucoside-O-rhamnoside | Z | Fruit |
| 67 | 16.12 | 463.1213 (4.7) | C22H22O11 | 445.0233, 301.0707 | Diosmetin-O-glucoside | X, Y, Z | Flower, fruit, Root, leaf, branch |
| 68 | 16.16 | 465.1389 (0.5) | C22H24O11 | 345.1045, 303.0839, 153.0100 | Hesperitin-O-glucoside | Y, Z | Flower, fruit |
| 69 b | 16.36 | 667.2219 (2.0) | C31H38O16 | 521.1088, 273.0693 | Naringenin-O-arabinoside-O-rhamnoside-O-arabinoside | Z | Leaf, root branch |
| 70 b | 16.52 | 725.2224 (9.1) | C33H40O18 | 461.1186, 315.0884, 129.0551 | Cirsimaritin-O-rhamnoside-O-arabinoside-O-arabinoside | X, Z | Flower, fruit, Root, leaf, branch |
| 71 a | 16.83 | 203.0337 (0.9) | C11H6O4 | 175.0382, 147.0439, 119.0479 | Xanthotoxol | X, Y | Flower, root, Fruit, leaf, branch |
| 72 b | 16.86 | 491.1511 (7.5) | C24H26O11 | 345.0871, 153.0143 | 3′,4′,5′-trimethoxyflavone-O-rhamnoside | X | Root, branch |
| 73 b | 17.12 | 755.2379 (1.8) | C34H42O19 | 597.1865, 271.0806, 127.0386 | Apigenin-O-glucuronic acid-O-arabinoside-O-rhamnoside | Z | Flower, fruit, root, leaf, branch |
| 74 b | 17.37 | 465.1427 (−6.5) | C22H24O11 | 303.0861, 153.0165 | Hesperitin-O-glucoside | Y, Z | Flower, fruit, Root, leaf, branch |
| 75 b | 17.88 | 579.1971 (8.3) | C28H34O10 | 301.1401, 245.0759, 153.0158 | 4′-Methoxy-flavanone-O-rhamnoside-O-arabinoside | X, Y | Flower, fruit, Root, leaf, branch |
| 76 a | 18.24 | 193.0476 (7.8) | C10H8O4 | 178.0247, 150.0302, 133.0275 | Scopoletin | X, Y | Flower, root, fruit, leaf, branch |
| 77 b | 18.72 | 755.2382 (1.4) | C34H42O19 | 609.1823, 303.0831, 153.0207 | Hesperitin-O-glucosideacid-O-rhamnoside-O-rhamnoside | Z | Flower, fruit |
| 78 b | 25.46 | 217.0483 (5.7) | C12H9O4 | 202.0250, 174.0299, 161.0581146.0345, 131.0486, 115.0532 | Bergapten | X, Y | Flower, fruit |
| 79 a | 25.83 | 471.2025 (−2.4) | C26H31O8 | 425.1931, 161.0614 | Limonin | X, Y | Flower, fruit |
| 80 a | 26.55 | 299.1620 (7.2) | C19H22O3 | 163.0375, 137.1314 | Auraptene | X, Y | Flower, fruit |
| 81 b | 29.95 | 343.1168 (2.3) | C19H18O6 | 302.1577, 296.8757 | 3′,4′,6,7-tetramethoxyflavone | Y | Fruit |
| 82 b | 31.21 | 389.1226 (1.2) | C20H20O8 | 374.1078, 369.0821, 107.9603 | Hydroxy-4′,5′,6,7,8-pentamethoxyflavone | X | Root |
| 83 b | 31.57 | 375.1050 (6.5) | C19H18O8 | 303.1685 | 5,7-hydroxy-3′,4′,5′,6-tetramethoxyflavone | X | Root |
| 84 b | 32.60 | 373.1280 (0.4) | C20H20O7 | 358.0954, 343.0845 | 4′,5,6,7,8-pentamethoxy-flavone | X, Y | Flower, fruit, root |
| 85 a | 32.77 | 403.1389 (−0.3) | C21H22O8 | 388.1152, 373.0920, 355.070702, 327.0810,301.0723 | Nobiletin | X, Y | Flower, fruit, leaf, branch |
| 86 b | 35.26 | 343.1176 (0.0) | C19H18O6 | 328.0955, 313.0692, 285.0704 | 4′,5,6,7-tetramethoxyflavone | X, Y | Flower, fruit, root |
| 87 a | 35.48 | 373.1255 (6.7) | C20H20O7 | 358.1044, 343.0802, 328.07013250701 | Tangeretin | X, Y | Flower, fruit, Leaf, branch |
| 88 b | 38.87 | 373.1288 (−1.6) | C20H20O | 358.1046, 343.0811, 325.0664 | 4′,5′,6,7,8-pentamethoxy-flavone | X | Root |
| 89 b | 39.41 | 389.1222 (2.3) | C20H20O8 | 371.2897, 374.1026, 359.0627, 356.0858 | 5-hydroxy-3′,4′,6,7,8-pentamethoxyflavone | X | Root |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Chen, M.; Liu, J.; Huang, X.; He, W.; Qing, Z.; Zeng, J. Systematic Detection and Identification of Bioactive Ingredients from Citrus aurantium L. var. amara Using HPLC-Q-TOF-MS Combined with a Screening Method. Molecules 2020, 25, 357. https://doi.org/10.3390/molecules25020357
Yu L, Chen M, Liu J, Huang X, He W, Qing Z, Zeng J. Systematic Detection and Identification of Bioactive Ingredients from Citrus aurantium L. var. amara Using HPLC-Q-TOF-MS Combined with a Screening Method. Molecules. 2020; 25(2):357. https://doi.org/10.3390/molecules25020357
Chicago/Turabian StyleYu, Liuyi, Miaofen Chen, Jinghong Liu, Xiuqiong Huang, Wei He, Zhixing Qing, and Jianguo Zeng. 2020. "Systematic Detection and Identification of Bioactive Ingredients from Citrus aurantium L. var. amara Using HPLC-Q-TOF-MS Combined with a Screening Method" Molecules 25, no. 2: 357. https://doi.org/10.3390/molecules25020357
APA StyleYu, L., Chen, M., Liu, J., Huang, X., He, W., Qing, Z., & Zeng, J. (2020). Systematic Detection and Identification of Bioactive Ingredients from Citrus aurantium L. var. amara Using HPLC-Q-TOF-MS Combined with a Screening Method. Molecules, 25(2), 357. https://doi.org/10.3390/molecules25020357





