Impact of Dietary Supplementation of Lactic Acid Bacteria Fermented Rapeseed with or without Macroalgae on Performance and Health of Piglets Following Omission of Medicinal Zinc from Weaner Diets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Formulation of Lactic Acid Bacteria Pre-Fermented Feeds
2.2. Study Animals and Experimental Design
2.3. Feeding and Recording of Piglets Performance
2.4. Blood and Intestinal Samplings
2.5. Intestinal Morphometry and Histopathology
2.6. Blood Hematology, Blood Biochemistry and Serum Immunoglobulin Analyses
2.7. DNA Extraction and Sequencing of 16S rRNA Genes for the Colon Microbiome
2.8. Data Analysis
3. Results
3.1. Piglet Performance
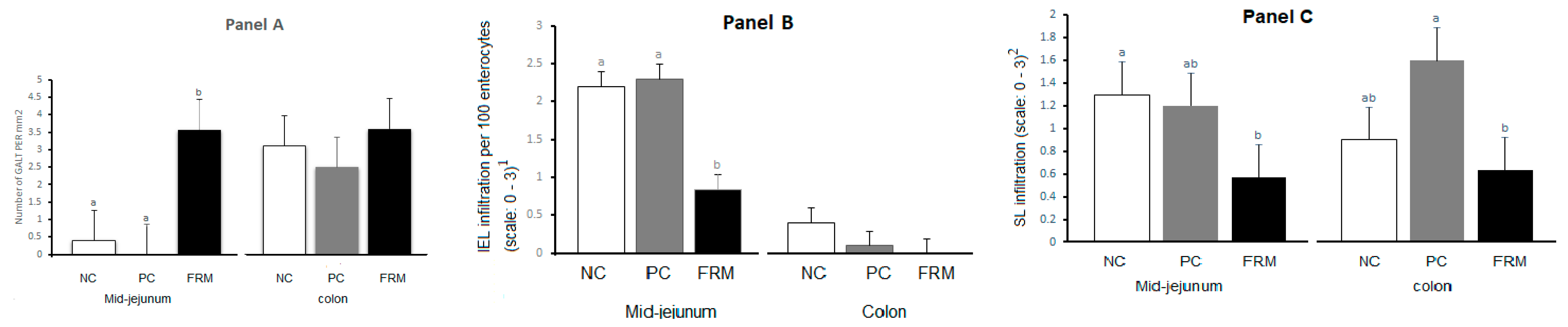
3.2. Gut Development and Morphology
3.3. Blood Haematology, Blood Biochemistry and Serum Immunoglobulin
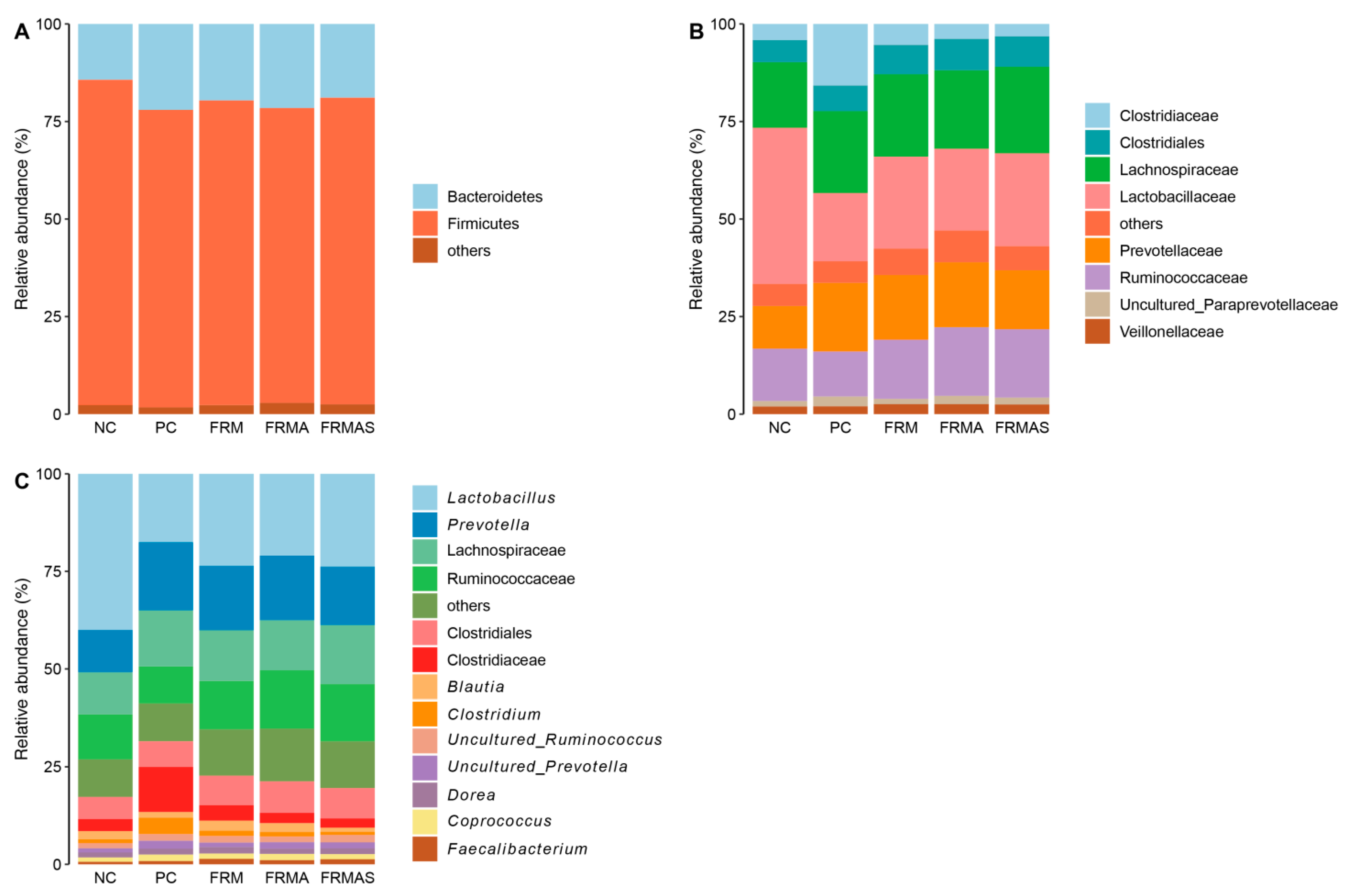
3.4. Colon Microbiome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pluske, J.R.; Hampson, D.J.; Williams, I.H. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 1997, 51, 215–236. [Google Scholar] [CrossRef]
- Lallès, J.P.; Boudry, G.; Favier, C.; Le Floc’h, N.; Luron, I.; Montagne, L.; Oswald, I.P.; Pié, S.; Piel, C.; Sève, B. Gut function and dysfunction in young pigs: Physiology. Anim. Res. 2004, 53, 301–316. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. Regulation (EC) No. 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Union. 2003, 50, 29–43. [Google Scholar]
- Jensen, J.; Larsen, M.M.; Bak, J. National monitoring study in Denmark finds increased 512 and critical levels of copper and zinc in arable soils fertilized with pig slurry. Environ. Pollut. 2016, 214, 334–340. [Google Scholar] [CrossRef]
- Ciesinski, L.; Guenther, S.; Pieper, R.; Kalisch, M.; Bednorz, C.; Wieler, L.H. High dietary zinc feeding promotes persistence of multi-resistant E. coli in the swine gut. PLoS ONE 2018, 13, e0191660. [Google Scholar] [CrossRef]
- EMA. Questions and answers on veterinary medicinal products containing zinc oxide to be administered orally to food-producing species. In Outcome of a Referral Procedure under Article 35 of Directive 2001/82/EC (EMEA/V/A/118)- EMA/394961/2017; European Medicines Agency: London, UK, 2017. [Google Scholar]
- Chiang, G.; Lu, W.Q.; Piao, X.S.; Hu, J.K.; Gong, L.M.; Thacker, P.A. Effects of feeding solid-state fermented rapeseed meal on performance, nutrient digestibility, intestinal ecology and intestinal morphology of broiler chickens. Asian-Aust. J. Anim. Sci. 2010, 23, 263–271. [Google Scholar] [CrossRef]
- Sun, H.; Tang, J.W.; Yao, X.H.; Wu, Y.F.; Wang, X.; Feng, J. Effects of dietary inclusion of fermented cottonseed meal on growth, cecal microbial population, small intestinal morphology, and digestive enzyme activity of broilers. Trop. Anim. Health. Prod. 2013, 45, 987–993. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.J.; Wu, S.G.; Yu, S.H.; Yoon, I.; Moore, D.; Gao, Y.P.; Yan, H.J.; Qi, G.H. Effect of Saccharomyces cerevisiae fermentation product on immune functions of broilers challenged with Eimeria tenella. Poult. Sci. 2009, 88, 2141–2151. [Google Scholar] [CrossRef]
- Sugiharto, S.; Ranjitkar, S. Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses: A review. Anim. Nutr. 2018, 5, 1–10. [Google Scholar] [CrossRef]
- Canibe, N.; Jensen, B.B. Fermented liquid feed—Microbial and nutritional aspects and impact on enteric diseases in pigs. Anim. Feed Sci. Technol. 2012, 173, 17–40. [Google Scholar] [CrossRef]
- Missotten, J.A.; Michiels, J.; Degroote, J.; De Smet, S. Fermented liquid feed for pigs: An ancient technique for the future. J. Anim. Sci. Biotechnol. 2015, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Han, Y.; Zhao, J.Z.; Zhou, Z.J.; Fan, H. Consuming fermented distillers’ dried grains with solubles (DDGS) feed reveals a shift in the faecal microbiota of growing and fattening pigs using 454 pyrosequencing. J. Integr. Agric. 2017, 16, 900–910. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Su, W.; Zhang, Y.; Hao, L.; Wang, F.; Lu, Z.; Zhao, J.; Liu, X.; Wang, Y. Solid-state fermentation of distilled dried grain with solubles with probiotics for degrading lignocellulose and upgrading nutrient utilization. AMB Expr. 2018, 8, 188. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Wei, F.; Liu, X.; Chunxia, Y.; Zhang, Y. Improvement of the nutritional value, sensory properties and bioavailability of rapeseed meal fermented with mixed microorganisms. LWT 2019, 112, 108238. [Google Scholar] [CrossRef]
- Ashayerizadeh, A.; Dastar, B.; Shargh, M.S.; Mahoonak, A.S.; Zerehdaran, S. Fermented rapeseed meal is effective in controlling Salmonella enterica serovar Typhimurium infection and improving growth performance in broiler chicks. Vet. Microbiol. 2017, 201, 93–102. [Google Scholar] [CrossRef]
- Drażbo, A.; Ognik, K.; Zaworska, A.; Ferenc, K.; Jankowski, J. The effect of raw and fermented rapeseed cake on the metabolic parameters, immune status, and intestinal morphology of turkeys. Poult. Sci. 2018, 97, 3910–3920. [Google Scholar] [CrossRef]
- Grela, E.R.; Czech, A.; Kiesz, M.; Wlazło, Ł.; Nowakowicz-Dębek, B. A fermented rapeseed meal additive: Effects on production performance, nutrient digestibility, colostrum immunoglobulin content and microbial flora in sows. Anim. Nutr. 2019, 5, 373–379. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef] [Green Version]
- ZhaXi, Y.I.; Wang, W.; Zhang, W.; Gao, Q.; Guo, M.; Jia, S. Morphologic Observation of Mucosa-Associated Lymphoid Tissue in the Large Intestine of Bactrian Camels (Camelus bactrianus). Anat. Rec. 2014, 297, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Krych, Ł.; Kot, W.; Bendtsen, K.M.; Hansen, A.K.; Vogensen, F.K.; Nielsen, D.S. Have you tried spermine? A rapid and cost-effective method to eliminate dextran sodium sulfate inhibition of PCR and RT-PCR. J. Microbiol. Methods 2018, 144, 1–7. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 1 July 2019).
- Edgar, R.C. UNOISE2: Improved error-correction for Illumina 16S and ITS amplicon sequencing. BioRxiv 2016, 081257. [Google Scholar] [CrossRef] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Shi, C.; He, J.; Wang, J.; Yu, J.; Yu, B.; Mao, X.; Zheng, P.; Huang, Z.; Chen, D. Effects of Aspergillus niger fermented rapeseed meal on nutrient digestibility, growth performance and serum parameters in growing pigs. Anim. Sci. J. 2016, 87, 557–563. [Google Scholar] [CrossRef]
- Zhu, J.; Gao, M.; Zhang, R.; Sun, Z.; Wang, C.; Yang, F.; Huang, T.; Qu, S.; Zhao, L.; Li, Y.; et al. Effects of soybean meal fermented by L. plantarum, B. subtilis and S. cerevisieae on growth, immune function and intestinal morphology in weaned piglets. Microb. Cell Fact. 2017, 16, 191. [Google Scholar] [CrossRef]
- Yuan, L.; Chang, J.; Yin, Q.; Lu, M.; Di, Y.; Wang, P.; Wang, Z.; Wang, E.; Lu, F. Fermented soybean meal improves the growth performance, nutrient digestibility, and microbial flora in piglets. Anim. Nutr. 2017, 3, 19–24. [Google Scholar] [CrossRef]
- Ashayerizadeh, A.; Dastar, B.; Shargh, M.S.; Mahoonak, A.S.; Zerehdaran, S. Effects of feeding fermented rapeseed meal on growth performance, gastrointestinal microflora population, blood metabolites, meat quality, and lipid metabolism in broiler chickens. Livest. Sci. 2018, 216, 183–190. [Google Scholar] [CrossRef]
- Dierick, N.; Ovyn, A.; De Smet, S. Effect of feeding intact brown seaweed Ascophyllum nodosum on some digestive parameters and on iodine content in edible tissues in pigs. J. Sci. Food Agric. 2009, 89, 584–594. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Bach, S.J.; McAllister, T.A. Effects of phlorotannins from Ascophyllum nodosum (brown seaweed) on in vitro ruminal digestion of mixed forage or barley grain. Anim. Feed Sci. Technol. 2008, 145, 375–395. [Google Scholar] [CrossRef]
- Gülzari, Ş.Ö.; Lind, V.; Aasen, I.M.; Steinshamn, H. Effect of supplementing sheep diets with macroalgae species on in vivo nutrient digestibility, rumen fermentation and blood amino acid profile. Animal 2019, 13, 2792–2801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chye, F.Y.; Ooi, P.W.; Ng, S.Y.; Sulaiman, M.R. Fermentation-Derived Bioactive Components from Seaweeds: Functional Properties and Potential Applications. J. Aquat. Food Prod. Technol. 2018, 27, 144–164. [Google Scholar] [CrossRef]
- Fazhi, X.; Lvmu, L.; Jiaping, X.; Kun, Q.; Zhide, Z.; Zhangyi, L. Effects of fermented rapeseed meal on growth performance and serum parameters in ducks. Asian-Aust. J. Anim. Sci. 2011, 24, 678–684. [Google Scholar] [CrossRef]
- Drażbo, A.; Kozłowski, K.; Ognik, K.; Zaworska, A.; Jankowski, J. The effect of raw and fermented rapeseed cake on growth performance, carcass traits, and breast meat quality in turkey. Poult. Sci. 2019, 98, 6161–6169. [Google Scholar] [CrossRef]
- Shi, C.; He, J.; Yu, J.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; Chen, D. Solid state fermentation of rapeseed cake with Aspergillus niger for degrading glucosinolates and upgrading nutritional value. J. Anim. Sci. Biotechnol. 2015, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Nabuurs, M.J.A.; Hoogendoorn, A.; Van der Molen, E.J.; Van Osta, A.L.M. Villus height and crypt depth in weaned and unweaned pigs, reared under various circumstances in the Netherlands. Res. Vet. Sci. 1993, 55, 78–84. [Google Scholar] [CrossRef]
- Jazi, V.; Ashayerizadeh, A.; Toghyani, M.; Shabani, A.; Tellez, G.; Toghyani, M. Fermented soybean meal exhibits probiotic properties when included in Japanese quail diet in replacement of soybean meal. Poult. Sci. 2019, 97, 2113–2122. [Google Scholar] [CrossRef]
- Pessione, E.; Cirrincione, S. Bioactive molecules released in food by lactic acid bacteria: Encrypted peptides and biogenic amines. Front. Microbiol. 2016, 7, 876. [Google Scholar] [CrossRef] [Green Version]
- Garrote, G.L.; Abraham, A.G.; Rumbo, M. Is lactate an undervalued functional component of fermented food products? Front. Microbiol. 2015, 6, 629. [Google Scholar] [CrossRef]
- Esquivel-Elizondo, S.; Ilhan, Z.E.; Garcia-Peña, E.I.; Krajmalnik-Brown, R. Insights into butyrate production in a controlled fermentation system via gene predictions. MSystems 2017, 2, e00051-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suiryanrayna, M.V.; Ramana, J.V. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Nićiforović, N.; Abramovič, H. Sinapic acid and its derivatives: Natural sources and bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Yang, S.C.; Arasu, M.V.; Chun, J.H.; Jang, Y.S.; Lee, Y.H.; Kim, I.H.; Lee, K.T.; Hong, S.T.; Kim, S.J. Identification and determination of phenolic compounds in rapeseed meals (Brassica napus L.). J. Agric. Chem. Environ. 2015, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Olukomaiya, O.; Fernando, C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Solid-state fermented plant protein sources in the diets of broiler chickens: A review. Anim. Nutr. 2019, 5, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P.; Shin, D.H.; Jung, S.J.; Chae, S.W. Functional properties of microorganisms in fermented foods. Front. Microbial. 2016, 7, 578. [Google Scholar] [CrossRef] [Green Version]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [Green Version]
- Reilly, P.; O’doherty, J.V.; Pierce, K.M.; Callan, J.J.; O’sullivan, J.T.; Sweeney, T. The effects of seaweed extract inclusion on gut morphology, selected intestinal microbiota, nutrient digestibility, volatile fatty acid concentrations and the immune status of the weaned pig. Animal 2008, 2, 1465–1473. [Google Scholar] [CrossRef] [Green Version]
- Leonard, S.G.; Sweeney, T.; Bahar, B.; Lynch, B.P.; O’Doherty, J.V. Effects of dietary seaweed extract supplementation in sows and post-weaned pigs on performance, intestinal morphology, intestinal microflora and immune status. Br. J. Nutr. 2011, 106, 688–699. [Google Scholar] [CrossRef] [Green Version]
- Michiels, J.; Skrivanova, E.; Missotten, J.; Ovyn, A.; Mrazek, J.; De Smet, S.; Dierick, N. Intact brown seaweed (Ascophyllum nodosum) in diets of weaned piglets: Effects on performance, gut bacteria and morphology and plasma oxidative status. J. Anim. Physiol. Anim. Nutr. 2012, 96, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Jiang, F.; Xu, Q.; Chen, D.; He, J. Alginic acid oligosaccharide accelerates weaned pig growth through regulating antioxidant capacity, immunity and intestinal development. RSC Adv. 2016, 6, 87026–87035. [Google Scholar] [CrossRef]
- Walsh, A.M.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effects of supplementing dietary laminarin and fucoidan on intestinal morphology and the immune gene expression in the weaned pig. J. Anim. Sci. 2012, 90, 284–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Shea, C.J.; O’Doherty, J.V.; Callanan, J.J.; Doyle, D.; Thornton, K.; Sweeney, T. The effect of algal polysaccharides laminarin and fucoidan on colonic pathology, cytokine gene expression and Enterobacteriaceae in a dextran sodium sulfate-challenged porcine model. J. Nutr. Sci. 2016, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rupérez, P.; Ahrazem, O.; Leal, J.A. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef]
- Vijayabaskar, P.; Vaseela, N. In vitro antioxidant properties of sulfated polysaccharide from brown marine algae Sargassum tenerrimum. Asian Pac. J. Trop. Dis. 2012, 2, S890–S896. [Google Scholar] [CrossRef]
- Sauerwein, H.; Schmitz, S.; Hiss, S. The acute phase protein haptoglobin and its relation to oxidative status in piglets undergoing weaning-induced stress. Redox Rep. 2005, 10, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, T.; Meredith, H.; Vigors, S.; McDonnell, M.J.; Ryan, M.; Thornton, K.; O’Doherty, J.V. Extracts of laminarin and laminarin/fucoidan from the marine macroalgal species Laminaria digitata improved growth rate and intestinal structure in young chicks, but does not influence Campylobacter jejuni colonisation. Anim. Feed Sci. Technol. 2017, 232, 71–79. [Google Scholar] [CrossRef]
- McDonnell, P.; Figat, S.; O’doherty, J.V. The effect of dietary laminarin and fucoidan in the diet of the weanling piglet on performance, selected faecal microbial populations and volatile fatty acid concentrations. Animal 2010, 4, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Walsh, A.M.; Sweeney, T.; O’shea, C.J.; Doyle, D.N.; O’doherty, J.V. Effect of dietary laminarin and fucoidan on selected microbiota, intestinal morphology and immune status of the newly weaned pig. Br. J. Nutr. 2013, 110, 1630–1638. [Google Scholar] [CrossRef] [Green Version]
- Pabst, O.; Herbrand, H.; Worbs, T.; Friedrichsen, M.; Yan, S.; Hoffmann, M.W.; Körner, H.; Bernhardt, G.; Pabst, R.; Förster, R. Cryptopatches and isolated lymphoid follicles: Dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur. J. Immunol. 2005, 35, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Burkey, T.E.; Skjolaas, K.A.; Minton, J.E. Board-invited review: Porcine mucosal immunity of the gastrointestinal tract. J. Anim. Sci. 2009, 87, 1493–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnardel, J.; Da Silva, C.; Henri, S.; Tamoutounour, S.; Chasson, L.; Montañana-Sanchis, F.; Gorvel, J.P.; Lelouard, H. Innate and adaptive immune functions of peyer’s patch monocyte-derived cells. Cell Rep. 2015, 11, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Tokuhara, D.; Kurashima, Y.; Kamioka, M.; Nakayama, T.; Ernst, P.; Kiyono, H. A comprehensive understanding of the gut mucosal immune system in allergic inflammation. Allergol. Int. 2019, 68, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, E.; Roos, S.; Liu, H.Y.; Lindberg, J.E. Fermentable non-starch polysaccharides increase the abundance of Bacteroides-Prevotella-Porphyromonas in ileal microbial community of growing pigs. Animal 2014, 8, 1777–1787. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.; Follador, R. Metabolism of oligosaccharides and starch in lactobacilli: A review. Front. Microbiol. 2012, 3, 340. [Google Scholar] [CrossRef] [Green Version]
- Leblois, J.; Massart, S.; Li, B.; Wavreille, J.; Bindelle, J.; Everaert, N. Modulation of piglets’ microbiota: Differential effects by a high wheat bran maternal diet during gestation and lactation. Sci. Rep. 2017, 7, 7426. [Google Scholar] [CrossRef] [Green Version]
- Zhong, X.; Wang, S.; Zhang, Z.; Cao, L.; Zhou, L.; Sun, A.; Zhong, Z.; Nabben, M. Microbial-driven butyrate regulates jejunal homeostasis in piglets during the weaning stage. Front. Microbiol. 2018, 9, 3335. [Google Scholar] [CrossRef]
- Guevarra, R.B.; Lee, J.H.; Lee, S.H.; Seok, M.J.; Kim, D.W.; Kang, B.N.; Johnson, T.J.; Isaacson, R.E.; Kim, H.B. Piglet gut microbial shifts early in life: Causes and effects. J. Anim. Sci. Biotechnol. 2019, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Lindberg, A.E. Fiber effects in nutrition and gut health in pigs. J. Anim. Sci. Biotechnol. 2014, 5, 15. [Google Scholar] [CrossRef]
- Umu, Ö.C.O.; Fauske, A.K.; Åkesson, C.P.; de Nanclares, M.P.; Sørby, R.; Press, C.M.; Øverland, M.; Sørum, H. Gut microbiota profiling in Norwegian weaner pigs reveals potentially beneficial effects of a high-fiber rapeseed diet. PLoS ONE 2018, 13, e0209439. [Google Scholar]
- Gardiner, G.E.; Campbell, A.J.; O’Doherty, J.V.; Pierce, E.; Lynch, P.B.; Leonard, F.C.; Stanton, C.; Ross, R.P.; Lawlor, P.G. Effect of Ascophyllum nodosum extract on growth performance, digestibility, carcass characteristics and selected intestinal microflora populations of grower-finisher pigs. Anim. Feed Sci. Technol. 2008, 141, 259–273. [Google Scholar] [CrossRef]
- Sweeney, T.; Dillon, S.; Fanning, J.; Egan, J.; O’Shea, C.J.; Figat, S.; Gutierrez, J.J.M.; Mannion, C.; Leonard, F.; O’Doherty, J.V. Evaluation of seaweed-derived polysaccharides on indices of gastrointestinal fermentation and selected populations of microbiota in newly weaned pigs challenged with Salmonella Typhimurium. Anim. Feed Sci. Technol. 2011, 165, 85–94. [Google Scholar] [CrossRef]


| Parameters | NC | PC | FRM | FRMA | FRMAS | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TG | Body Weight at Weaning | 3 Weeks Post-Weaning | |||||||||
| L | Q | L | Q | ||||||||
| Weaning weight (age 28 days), kg | 6.07 ± 0.33 | 5.86 ± 0.98 | 6.16 ± 0.54 | 6.21 ± 0.79 | 5.75 ± 1.03 | NS | |||||
| Body weight, kg, at age (days): | |||||||||||
| 42 | 7.11 a | 6.99 a | 6.34 b | 6.79 ab | 6.97 a | 0.30 | 0.050 | 0.000 | 0.033 | ||
| 49 | 8.21 | 9.35 | 8.58 | 8.13 | 8.02 | 0.42 | 0.191 | 0.002 | 0.860 | ||
| 77 | 20.13 | 20.73 | 20.29 | 20.30 | 19.19 | 0.80 | 0.317 | 0.012 | 0.432 | ||
| 85 | 22.42 ac | 24.04 ab | 24.97 b | 23.87 bc | 22.26 c | 0.72 | 0.015 | 0.020 | 0.134 | ||
| ADFI, kg/day, in age interval (days): | |||||||||||
| 28–42 | 0.161 | 0.177 | 0.170 | 0.186 | 0.165 | 0.018 | 0.577 | 0.252 | 0.732 | ||
| 28–49 | 0.223 | 0.248 | 0.235 | 0.238 | 0.208 | 0.067 | 0.464 | 0.512 | 0.620 | ||
| 28–85 | 0.528 ab | 0.527 ab | 0.573 a | 0.526 ab | 0.469 b | 0.025 | 0.030 | 0.529 | 0.522 | ||
| 50–85 | 0.691 ab | 0.698 ab | 0.753 a | 0.697 ab | 0.629 b | 0.035 | 0.033 | 0.689 | 0.322 | ||
| ADG, kg/day, in the age interval (days): | |||||||||||
| 28–42 | 0.078 | 0.075 | 0.032 | 0.058 | 0.067 | 0.025 | 0.224 | 0.616 | 0.116 | ||
| 28–49 | 0.106 | 0.160 | 0.123 | 0.102 | 0.097 | 0.020 | 0.204 | 0.636 | 0.859 | ||
| 28–85 | 0.293 ac | 0.322 ab | 0.339 b | 0.319 bc | 0.290 c | 0.013 | 0.030 | 0.960 | 0.135 | ||
| 50–85 | 0.413 | 0.426 | 0.466 | 0.444 | 0.393 | 0.025 | 0.077 | 0.217 | 0.155 | ||
| FCR in the age interval (days): | |||||||||||
| 28–42 | 3.37 | 2.39 | 3.79 | 2.85 | 3.13 | 0.81 | 0.147 | 0.009 | 0.042 | ||
| 28–49 | 2.31 | 1.62 | 2.22 | 2.56 | 2.53 | 0.44 | 0.539 | 0.691 | 0.858 | ||
| 28–85 | 1.75 | 1.65 | 1.62 | 1.66 | 1.64 | 1.63 | 0.430 | 0.622 | 0.716 | ||
| 50–85 | 1.67 | 1.65 | 1.58 | 1.59 | 1.58 | 0.047 | 0.538 | 0.346 | 0.374 | ||
| Completion rate (%) in the age interval (days): | |||||||||||
| 28–42 | 94.63 | 96.11 | 94.9 | 96.74 | 96.13 | 3.06 | 0.977 | 0.739 | 0.665 | ||
| 28–49 | 92.0 | 95.4 | 93.7 | 97.6 | 96.4 | 2.9 | 0.845 | 0.743 | 0.190 | ||
| 28–77 * | 89.0 | 93.6 | 95.3 | 89.9 | 87.0 | 4.3 | 0.442 | 0.967 | 0.869 | ||
| 50–77 * | 98.3 | 96.9 | 97.7 | 94.4 | 94.5 | 3.2 | 0.714 | 0.960 | 0.833 | ||
| Parameters | Female | Male | SEM | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | FRM | FRMA | FRMAS | NC | PC | FRM | FRMA | FRMAS | TG | Sex | TG × Sex | Week | ||
| JVH | 398 a | 430 b | 401 a | 380 ac | 435 b | 364 c | 404 a | 391 a | 402 a | 448 b | 6.9 | 0.000 | 0.003 | 0.000 | 0.099 |
| JCD | 208 ab | 218 b | 221 b | 209 ab | 216 b | 191 cd | 202 ac | 218 b | 218 b | 180 d | 4.4 | 0.000 | 0.038 | 0.000 | 0.000 |
| VCR | 1.97 a | 2.18 b | 1.87 a | 1.84 a | 2.16 b | 1.99 a | 2.18 b | 1.95 a | 1.94 a | 2.48 c | 0.05 | 0.000 | 0.468 | 0.002 | 0.000 |
| CMT | 454 bd | 549 e | 478 c | 441 ad | 460 bc | 455 bd | 428 af | 514 g | 447 ab | 416 f | 5.7 | 0.000 | 0.000 | 0.000 | 0.000 |
| Alpha Diversity Indices | NC | PC | FRM | FRMA | FRMAS | p |
|---|---|---|---|---|---|---|
| Observed zOTUs | 3530.5 ± 47.5 | 3335.2 ± 55.4 | 3679.6 ± 66.4 | 3948.5 ± 70.7 | 3921.4 ± 57.8 | 0.092 |
| Shannon index | 8.65 a ± 0.08 | 9.29 ab ± 0.06 | 9.52 abc ± 0.09 | 9.79 c ± 0.10 | 9.53 bc ± 0.10 | 0.047 |
| Pielou’s evenness | 0.73 a ± 0.006 | 0.80 b ± 0.004 | 0.80 b ± 0.006 | 0.82 b ± 0.007 | 0.80 b ± 0.007 | 0.019 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satessa, G.D.; Tamez-Hidalgo, P.; Hui, Y.; Cieplak, T.; Krych, L.; Kjærulff, S.; Brunsgaard, G.; Nielsen, D.S.; Nielsen, M.O. Impact of Dietary Supplementation of Lactic Acid Bacteria Fermented Rapeseed with or without Macroalgae on Performance and Health of Piglets Following Omission of Medicinal Zinc from Weaner Diets. Animals 2020, 10, 137. https://doi.org/10.3390/ani10010137
Satessa GD, Tamez-Hidalgo P, Hui Y, Cieplak T, Krych L, Kjærulff S, Brunsgaard G, Nielsen DS, Nielsen MO. Impact of Dietary Supplementation of Lactic Acid Bacteria Fermented Rapeseed with or without Macroalgae on Performance and Health of Piglets Following Omission of Medicinal Zinc from Weaner Diets. Animals. 2020; 10(1):137. https://doi.org/10.3390/ani10010137
Chicago/Turabian StyleSatessa, Gizaw D., Paulina Tamez-Hidalgo, Yan Hui, Tomasz Cieplak, Lukasz Krych, Søren Kjærulff, Grete Brunsgaard, Dennis S. Nielsen, and Mette O. Nielsen. 2020. "Impact of Dietary Supplementation of Lactic Acid Bacteria Fermented Rapeseed with or without Macroalgae on Performance and Health of Piglets Following Omission of Medicinal Zinc from Weaner Diets" Animals 10, no. 1: 137. https://doi.org/10.3390/ani10010137
APA StyleSatessa, G. D., Tamez-Hidalgo, P., Hui, Y., Cieplak, T., Krych, L., Kjærulff, S., Brunsgaard, G., Nielsen, D. S., & Nielsen, M. O. (2020). Impact of Dietary Supplementation of Lactic Acid Bacteria Fermented Rapeseed with or without Macroalgae on Performance and Health of Piglets Following Omission of Medicinal Zinc from Weaner Diets. Animals, 10(1), 137. https://doi.org/10.3390/ani10010137





