Phenolic Compounds and the Anti-Atherogenic Effect of Bee Bread in High-Fat Diet-Induced Obese Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bee Bread Sample
2.3. Liquid-Chromatography-Mass Spectrometry Analysis of Bee Bread
2.4. Animals and Diet
2.5. Experimental Design
2.6. Measurement of Lipid Profile and Atherogenic Index
2.7. Determination of Aortic Oxidant/Antioxidant Status Markers and Fatty Acid Synthase (FAS)
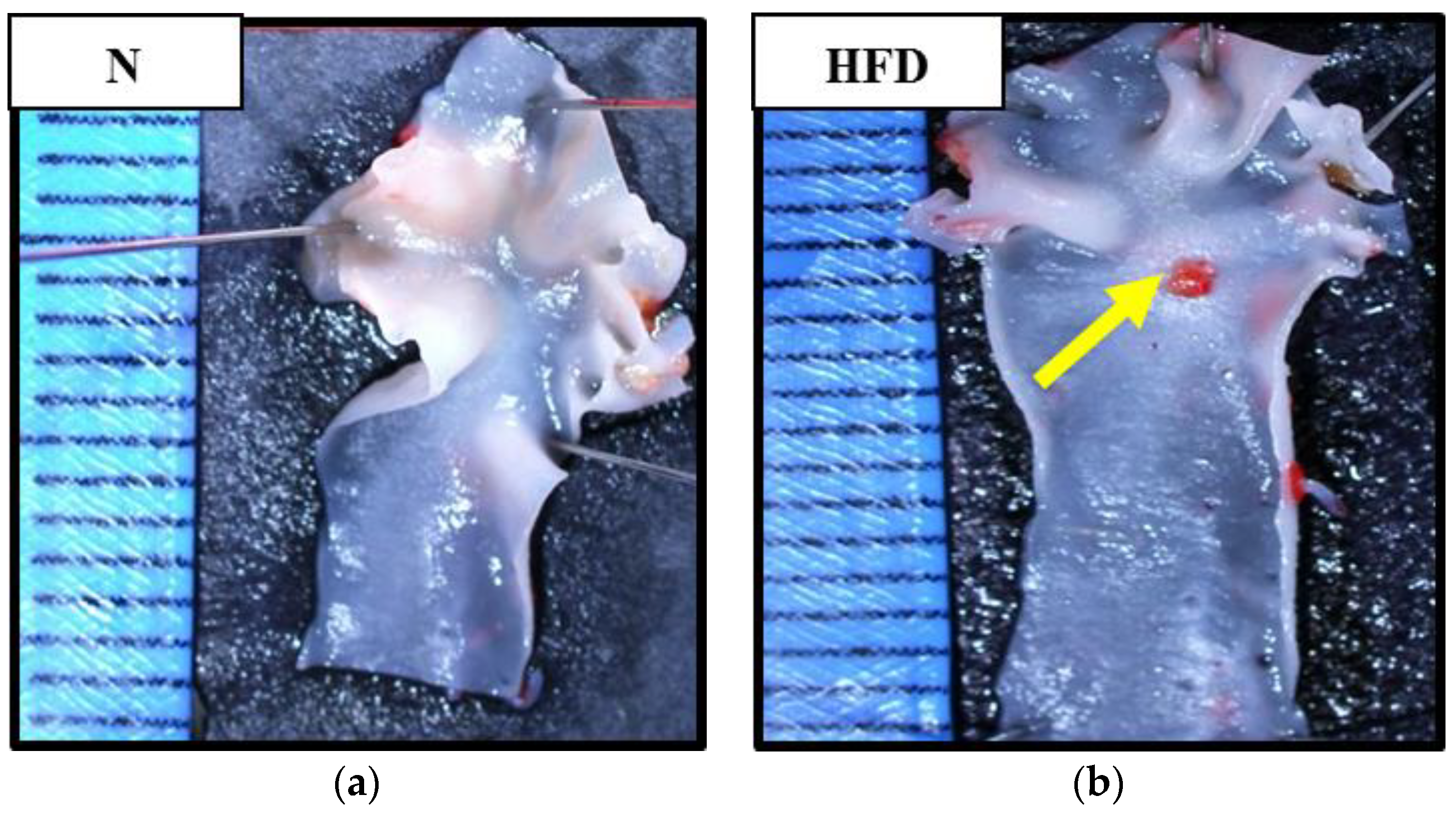
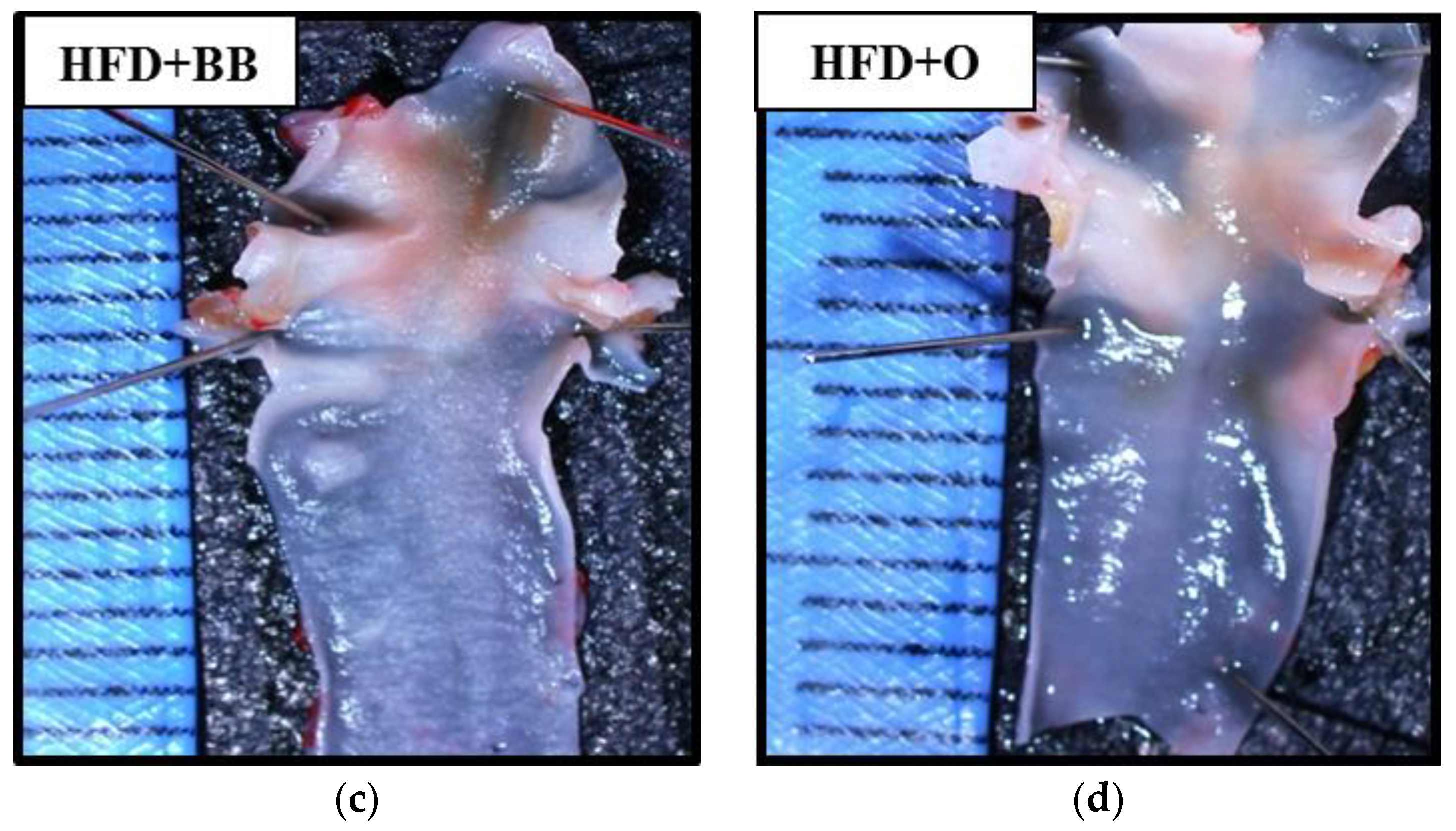
2.8. Assessment on the Presence of Atherosclerotic Plaque in Aortic Arch
2.9. Histology of Adipose Tissue
2.10. Statistical Analysis
3. Results
3.1. Liquid Chromatography-Mass Spectrophotometry Analysis of Bee Bread
3.2. Lee Obesity Index, Weight Gain, Food, and Calorie Intake
3.3. The Effects of Bee Bread on Lipid Profile and Atherogenic Index
3.4. The Effects of Bee Bread on Aortic Oxidant-Antioxidant Status and FAS Activity
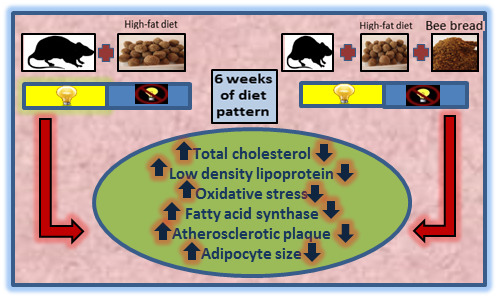
3.5. The Effects of Bee Bread on the Presence of Atherosclerotic Plaque
3.6. The Effects of Bee Bread on Histology of Adipose Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jean, N.; Somers, V.K.; Sochor, O.; Medina-Inojosa, J.; Liano, E.M.; Lopez-Jimenez, F. Normal-weight obesity: Implications for cardiovascular health. Curr. Atheroscler. Rep. 2014, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Hu, C.; Deng, X.; Bai, Y.; Cao, H.; Guo, J.; Su, Z. Therapeutic effect of Chitooligosaccharide tablets on lipids in high-fat diet induced hyperlipidemic rats. Moecules 2019, 24, 514. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Ma, D.; Wang, X.; Wang, Y.; Bi, Y.; Yang, J.; Wang, X.; Li, X. Astragaloside IV prevents obesity-associated hypertension by improving pro-inflammatory reaction and leptin resistance. Mol. Cells 2018, 41, 244–273. [Google Scholar]
- Nimmi, O.S.; George, P. Antiobesity and antioxidant effects of a new polyherbal formulation (PHF) in obesity induced wistar rats. Indian J. Tradit. Knowl. 2017, 16, 297–302. [Google Scholar]
- Bais, S.; Singh, G.S.; Sharma, R. Antiobesity and Hypolipidemic Activity of Moringa oleifera Leaves against High Fat Diet-Induced Obesity in Rats. Adv. Biol. 2014, 2014, 162914. [Google Scholar] [CrossRef]
- Jeevangi, S.; Manjunath, S.; Sakhare, P. A study of anti-hyperlipidemia, hypolipedimic and anti-atherogenic activity of fruit of emblica officinalis (Amla) in high fat fed albino rats. Int. J. Med. Res. Health Sci. 2013, 2, 70–77. [Google Scholar]
- Gasparotto, F.M.; Palozi, R.A.C.; da Silva, C.H.F.; Pauli, K.B.; Donadel, G.; Lourenço, B.H.L.B.; Nunes, B.C.; Lívero, F.A.D.R.; de Souza, L.M.; Lourenço, E.L.B.; et al. Antiatherosclerotic Properties of Echinodorus grandiflorus (Cham. & Schltdl.) Micheli: From Antioxidant and Lipid-Lowering Effects to an Anti-Inflammatory Role. J. Med. Food 2019, 22. [Google Scholar] [CrossRef]
- Zuluaga, C.M.; Serrato, J.C.; Quicazan, M.C. Chemical, nutritional and bioactive characterization of Colombian bee-bread. Chem. Eng. Trans. 2015, 43, 175–180. [Google Scholar]
- Mutsaers, M.; Blitterswijk, H.V.; Leven, L.V.; Kerkvliet, J.; Waerdt, J.V.D. Bee Products, 1st ed.; Agromisa Foundation: Wageningen, The Netherlands, 2005; pp. 33–35. ISBN 90-8573-028-7. [Google Scholar]
- Isidorov, V.A.; Isidorova, A.G.; Sczczepaniak, L.; Czyzewska, U. Gas chromatographic–mass spectrometric investigation of the chemical composition of beebread. Food Chem. 2009, 115, 1056–1063. [Google Scholar] [CrossRef]
- Sobral, F.; Calhelha, R.C.; Barros, L.; Duenas, M.; Tomas, A.; Santos-Buelga, C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Flavonoid composition and antitumour activity of bee bread collected in northeast Portugal. Molecules 2017, 22, 248. [Google Scholar] [CrossRef]
- Markiewicz-Zukowska, R.; Naliwajko, S.K.; Bartosiuk, E.; Moskwa, J.; Isidorov, V.; Soroczynska, J.; Borawska, M.H. Chemical composition and antioxidant activity of beebread, and its influence on the glioblastoma cell line (U87MG). J. Apic. Sci. 2013, 57, 147–157. [Google Scholar] [CrossRef]
- Eswaran, V.U.; Bhargava, H.R. Chemical analysis and anti-microbial activity of Karnataka bee bread of Apis species. World Appl. Sci. J. 2014, 32, 379–385. [Google Scholar]
- Ceksteryte, V.; Balzekas, J. The use of beebread—Honey mixture in the treatment of liver diseases in alcohol-dependent patients. J. Chem. Technol. 2012, 2, 62–66. [Google Scholar]
- Kas’ianenko, V.I.; Komisarenko, I.A.; Dubtsova, E.A. Correction of atherogenic dyslipidemia with honey, pollen and bee bread in patients with different body mass. Ter. Arkh. 2011, 83, 58–62. [Google Scholar] [PubMed]
- Ceksteryte, V.; Kazlauskas, S.; Racys, J. Composition of flavonoids in Lithuanian honey and beebread. Biologija 2006, 2, 28–33. [Google Scholar]
- Duran, X.A.; Mardones, I.Q.; Gutierrez, M.M.; Ulloa, D.M. Total polyphenols in bee bread (Apis mellifera L.) from hives the Araucania Region. Idesia 2014, 32, 107–111. [Google Scholar]
- Ivanisova, E.; Kacaniova, M.; Francakova, H.; Petrova, J.; Hutkova, J.; Brovarskyi, V.; Velychko, S.; Adamchuk, L.; Schubertova, Z.; Musilova, J. Bee bread—Perspective source of bioactive compounds for future. Potravinarstvo 2015, 9, 592–598. [Google Scholar]
- Cocan, O.; Marghitas, L.A.; Dezmirean, D. Total polyphenols, flavonoids and radical scavenging activity of beepollen and bee bread collected from Transylvania area. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2009, 62, 1–5. [Google Scholar]
- Tavdidishvili, D.; Khutsidze, T.; Pkhakadze, M.; Vanidze, M.; Kalandia, A. Flavonoids in Georgian bee bread and bee pollen. J. Chem. Chem. Eng. 2014, 8, 676–681. [Google Scholar]
- Urcan, A.; Al-Marghitas, L.; Dezmirean, D.S.; Bobis, O.; Bonta v Murusen, C.L.; Margaoan, R. Chemical Composition and Biological Activities of Beebread—Review. Bull. UASVM Anim. Sci. Biotechnol. 2017, 74, 250–255. [Google Scholar] [CrossRef]
- Rason, N.; Ramli, N.; Safuan, S.; Noordin, L.; Ahmad, W.A.N. Histopathological Alteration in Organ Structures of Induced-Obese Rats Fed with High-Fat Diet. Ann. Microsc. 2016, 15, 38–48. [Google Scholar]
- Reagen-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. Life Sci. Forum 2018, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, L.L.; Bernardis, L.L. Effect of dorsomedial hypothalamic nuclei knife cuts on ingestive behavior. Am. J. Physiol. Integr. Comp. Physiol. 1999, 276, R1772–R1779. [Google Scholar] [CrossRef] [PubMed]
- Malafaia, A.B.; Nassif, P.A.N.; Ribas, C.A.P.M.; Ariede, B.L.; Sue, K.N.; Cruz, M.A. Obesity induction with high fat sucrose in rats. ABCD Arquiros Bras. Cir. Dig. 2013, 26, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.; Levy, R.; Fredrickson, D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar]
- Suanarunsawat, T.; Ayutthaya, W.D.N.; Songsak, T.; Thirawarapan, S.; Poungshompoo, S. Antioxidant activity and lipid-lowering effect of essential oils extracted from Ocimum sanctum L. leaves in rats fed with a high cholesterol diet. Clin. Biochem. 2009, 46, 52–59. [Google Scholar] [CrossRef]
- Andrés-manzano, M.J.; Andrés, V.; Dorado, B. Methods in Mouse Atherosclerosis in Methods in Molecular Biology; Springer Science + Business Media: New York, NY, USA, 2015; pp. 85–99. [Google Scholar]
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical Study of Oxidative Stress Markers in the Liver, Kidney and Heart of High Fat Diet Induced Obesity in Rats. Diabetol. Metab. Syndr. 2011, 3, 17. [Google Scholar] [CrossRef]
- Sacks, F.M. The crucial roles of apolipoproteins E and C-III in apoB lipoprotein metabolism in normolipidemia and hypertriglyceridemia. Curr. Opin. Lipidol. 2015, 26, 56–63. [Google Scholar] [CrossRef]
- Bougoulia, M.; Triantos, A.; Koliakos, G. Effect of weight loss with or without orlistat treatment on adipocytokines, inflammation, and oxidative markers in obese women. Hormones 2006, 5, 259–269. [Google Scholar] [CrossRef]
- Nwagha, U.; Ikekpeazu, E.J.; Ejezie, F.E.; Neboh, E.E.; Maduka, I.C. Atherogenic index of plasma as useful predictor of cardiovascular risk among postmenopausal women in Enugu, Nigeria. Afr. Health Sci. 2010, 10, 248–252. [Google Scholar]
- Lee, H.Y.; Oh, M.R.; Jung, E.S.; Lee, Y.S.; Kim, D.S.; Kang, S.S.; Chae, H.J.; Chae, S.W. Mulberry and its main components protect against oxidized low-density lipoprotein-induced endothelial nitric oxide synthase uncoupling. J. Funct. Foods 2017, 29, 295–302. [Google Scholar] [CrossRef]
- Sunil, V.; Shree, N.; Venkataranganna, M.V.; Bhonde, R.R.; Majumdar, M. The anti-diabetic and anti-obesity effect of Memecylon umbellatum extract in high fat diet induced obese mice. Biomed. Pharmacother. 2017, 89, 880–886. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Horbury, M.D.; Baker, L.A.; Quan, W.D.; Greenough, S.E.; Stavros, V.G. Photodynamics of potent antioxidants: Ferulic and caffeic acids. Phys. Chem. Chem. Phys. 2016, 26, 17691–17697. [Google Scholar] [CrossRef] [PubMed]
- Barene, I.; Daberte, I.; Siksna, S. Investigation of bee bread and development of its dosage forms. Proteins 2015, 24, 20–30. [Google Scholar] [CrossRef]
- Othman, Z.A.; Wan Ghazali, W.S.; Nordin, L.; Omar, N.; Mohamed, M. Nutritional, Phytochemical and Antioxidant Analysis of Bee Bread from Different Regions of Malaysia. Indian J. Pharm. Sci. 2019, 81, 955–960. [Google Scholar] [CrossRef]
- Galaly, S.R.; Hozayen, W.G.; Amin, K.A.; Ramadan, S.M. Effects of Orlistat and herbal mixture extract on brain, testes functions and oxidative stress biomarkers in a rat model of high fat diet. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 93–105. [Google Scholar] [CrossRef]
- Ueshima, K.; Akihisa-umeno, H.; Nagayoshi, A.; Takakura, S.; Matsuo, M.; Mutoh, S. A gastrointestinal lipase inhibitor reduces progression of atherosclerosis in mice fed a western-type diet. Eur. J. Pharmacol. 2004, 501, 137–142. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Pingeesakikul, T.; Tirawanchai, N.; Tuntipopipat, S.; Lilitchan, S.; Komindr, S. Effects of Ferulic Acid Supplementation on Lipid Profiles, Oxidative Stress and Inflammatory Status in Hypercholesterolemic Subjects. FASEB J. 2012, 26, 263–267. [Google Scholar]
- Prince, S.M.P.; Senthil Kumaran, K. Preventive effects of caffeic acid on lipids, lipoproteins and glycoproteins in isoproterenol induced myocardial infarcted rats. Food Res. Int. 2012, 45, 155–160. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Witztum, J.L.; Steinberg, D. Role of oxidized low density lipoprotein in atherogenesis. J. Clin. Investig. 1991, 88, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Luo, C.; Li, X.; Zhou, Y.; He, H. The anti-inflammatory effect of kaempferol on early atherosclerosis in high cholesterol fed rabbits. Lipids Health Dis. 2013, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chirala, S.S.; Chang, H.; Matzuk, M.; Abu-Elheiga, L.; Mao, J.; Mahon, K.; Finegold, M.; Wakil, S.J. Fatty acid synthesis is essential in embryonic development: Fatty acid synthase null mutants and most of the heterozygotes die in utero. Proc. Natl. Acad. Sci. USA 2003, 100, 6358–6363. [Google Scholar] [CrossRef]

| Nutrient Composition | Normal Diet (g/100) | High-Fat Diet (g/100 g) |
|---|---|---|
| Carbohydrate | 64 | 46 |
| Protein | 24 | 12 |
| Fat | 12 | 31 |
| Ash | 6.9 | 3.8 |
| Energy (kcal/100 g) | 318.8 | 516.5 |
| Compounds | Molecular Formula | Molecular Weight (g/mol) | Mass Spectrum (m/z) | Retention Time (min) |
|---|---|---|---|---|
| Apigenin | C15H10O5 | 270.05 | 271.06 | 17.76 |
| Caffeic acid | C9H8O4 | 180.16 | 181.12 | 17.37 |
| Ferulic acid | C10H10O4 | 194.18 | 195.09 | 12.45 |
| Isorhamnetin | C16H12O7 | 316.26 | 317.07 | 17.49 |
| Kaempferol | C15H10O6 | 286.23 | 287.06 | 17.59 |
| N | HFD | HFD + BB | HFD + O | |
|---|---|---|---|---|
| Lee obesity index | 312.37 ± 5.66 | 337.83 ± 13.01 a | 316.83 ± 7.70 b | 322.27 ± 13.97 |
| Weight gain (g) | 112.73 ± 42.22 | 123.20 ± 21.84 | 98.70 ± 35.11 | 104.73 ± 21.99 |
| Food intake (g) | 20.23 ± 3.61 | 17.94 ± 1.58 | 18.73 ± 2.82 | 20.90 ± 1.06 |
| Calorie intake (kcal) | 64.32 ± 11.47 | 92.41 ± 8.13 a | 96.46 ± 14.50 a | 107.61 ± 5.45 a |
| Serum Lipid Profile and Atherogenic Index | N | HFD | HFD + BB | HFD + O |
|---|---|---|---|---|
| TC (mmol/L) | 1.77 ± 0.18 | 2.29 ± 0.20 a | 1.89 ± 0.23 b | 1.93 ± 0.23 |
| TG (mmol/L) | 0.79 ± 0.33 | 1.09 ± 0.35 | 0.73 ± 0.23 | 1.03 ± 0.36 |
| LDL (mmol/L) | 0.16 ± 0.11 | 0.43 ± 0.20 a | 0.14 ± 0.10 b | 0.16 ± 0.10 b |
| HDL (mmol/L) | 1.20 ± 0.15 | 1.29 ± 0.13 | 1.40 ± 0.14 | 1.30 ± 0.15 |
| Atherogenic index | 0.44 ± 0.02 | 0.55 ± 0.16 | 0.32 ± 0.08 b | 0.33 ± 0.12 b |
| Oxidant-Antioxidant Status | Group | |||
|---|---|---|---|---|
| N | HFD | HFD + BB | HFD + O | |
| OxLDL(pg/mL) | 272.08 ± 41.73 | 468.23 ± 113.94 a | 287.19 ± 92.19 b | 310.83 ± 93.59 b |
| MDA(nmol/mg protein) | 0.18 ± 0.03 | 0.24 ± 0.05 a | 0.16 ± 0.02 b | 0.21 ± 0.05 |
| SOD(Umg/protein) | 3.29 ± 0.77 | 2.82 ± 0.43 | 3.86 ± 0.46 b | 3.48 ± 0.67 |
| GPx(Umg/protein) | 245.87 ± 12.74 | 232.28 ± 4.38 a | 269.23 ± 11.45 a,b | 311.53 ± 6.23 a,b,c |
| CAT(Umg/protein) | 1.13 ± 0.15 | 0.76 ± 0.42 a | 0.93 ± 0.63 | 1.08 ± 0.41 |
| FAS (pg/mL) | 763.07 ± 226.23 | 1580.47 ± 239.19 a | 754.09 ± 183.93 b | 831.70 ± 126.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, Z.A.; Wan Ghazali, W.S.; Noordin, L.; Mohd. Yusof, N.A.; Mohamed, M. Phenolic Compounds and the Anti-Atherogenic Effect of Bee Bread in High-Fat Diet-Induced Obese Rats. Antioxidants 2020, 9, 33. https://doi.org/10.3390/antiox9010033
Othman ZA, Wan Ghazali WS, Noordin L, Mohd. Yusof NA, Mohamed M. Phenolic Compounds and the Anti-Atherogenic Effect of Bee Bread in High-Fat Diet-Induced Obese Rats. Antioxidants. 2020; 9(1):33. https://doi.org/10.3390/antiox9010033
Chicago/Turabian StyleOthman, Zaidatul Akmal, Wan Syaheedah Wan Ghazali, Liza Noordin, Nurul Aiman Mohd. Yusof, and Mahaneem Mohamed. 2020. "Phenolic Compounds and the Anti-Atherogenic Effect of Bee Bread in High-Fat Diet-Induced Obese Rats" Antioxidants 9, no. 1: 33. https://doi.org/10.3390/antiox9010033
APA StyleOthman, Z. A., Wan Ghazali, W. S., Noordin, L., Mohd. Yusof, N. A., & Mohamed, M. (2020). Phenolic Compounds and the Anti-Atherogenic Effect of Bee Bread in High-Fat Diet-Induced Obese Rats. Antioxidants, 9(1), 33. https://doi.org/10.3390/antiox9010033