Susceptibility of Winter Wheat and Triticale to Yellow Rust Influenced by Complex Interactions between Vernalisation, Temperature, Plant Growth Stage and Pathogen Race
Abstract
1. Introduction
2. Materials and Methods
2.1. Pathogen Isolates and Host Varieties
2.2. Experimental Procedure
2.2.1. Vernalised and Non-Vernalised Seedling Test
2.2.2. Vernalised Adult Plant Test
2.3. Data Analysis
3. Results
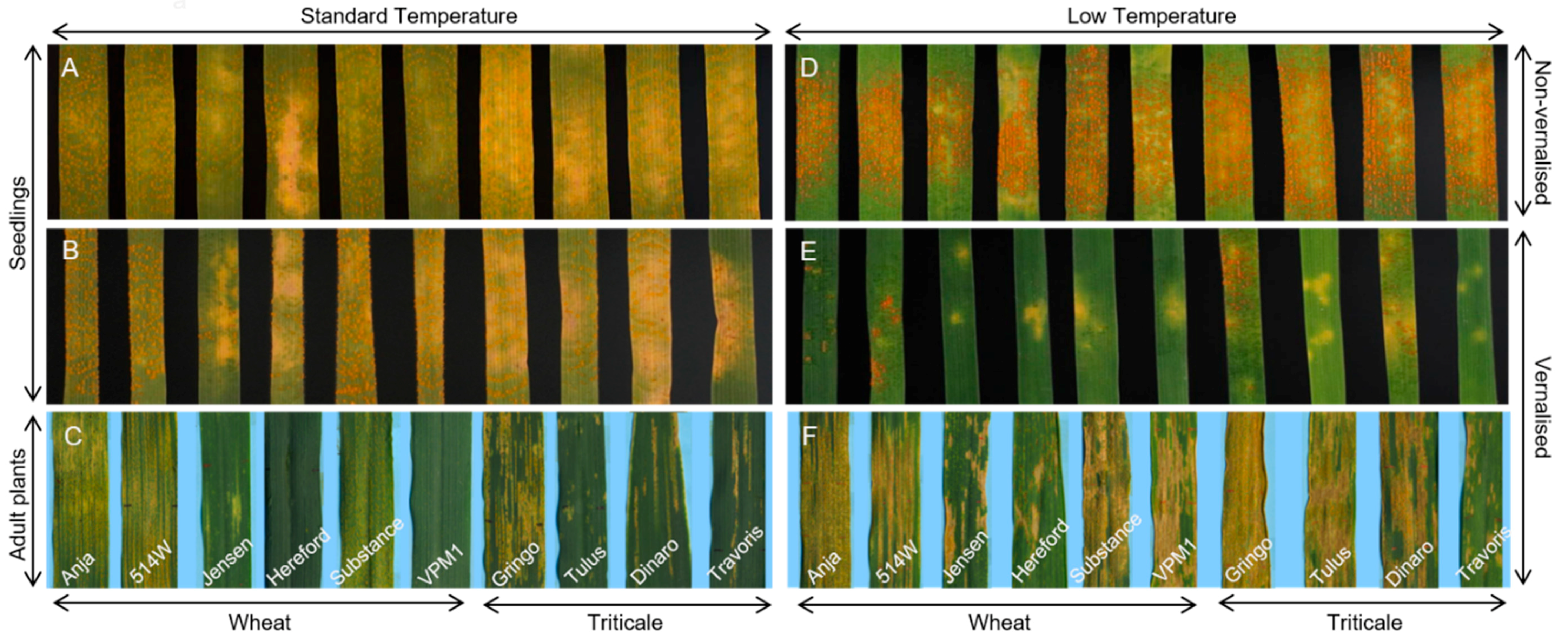
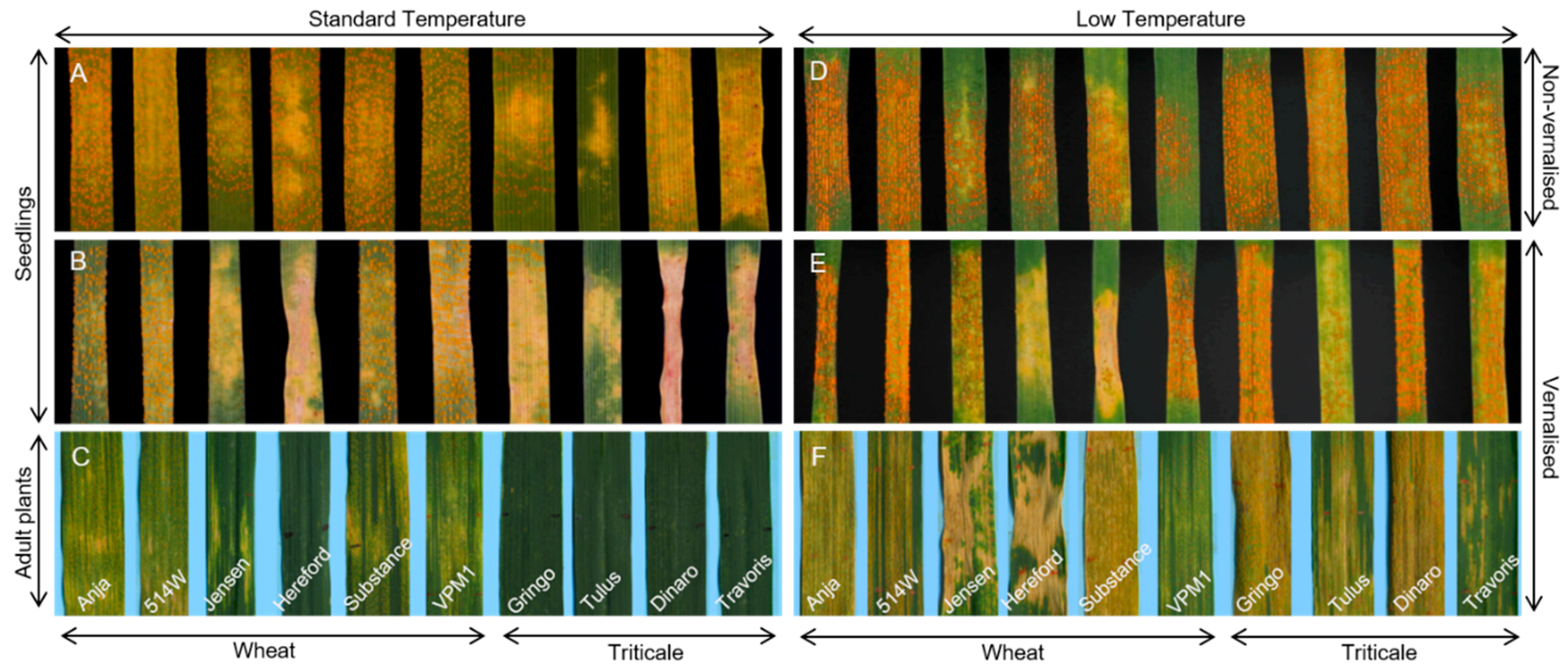
3.1. Experimental System
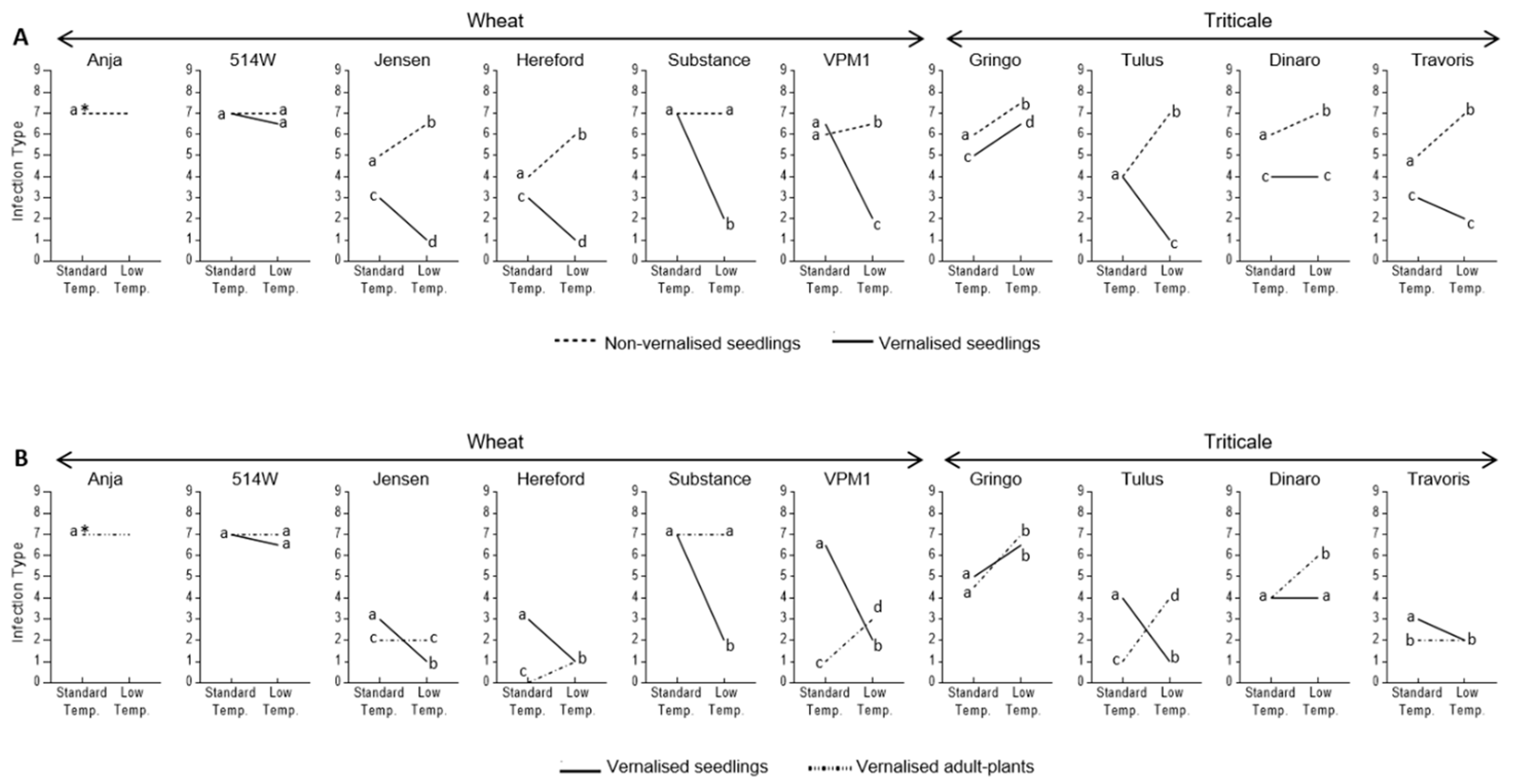
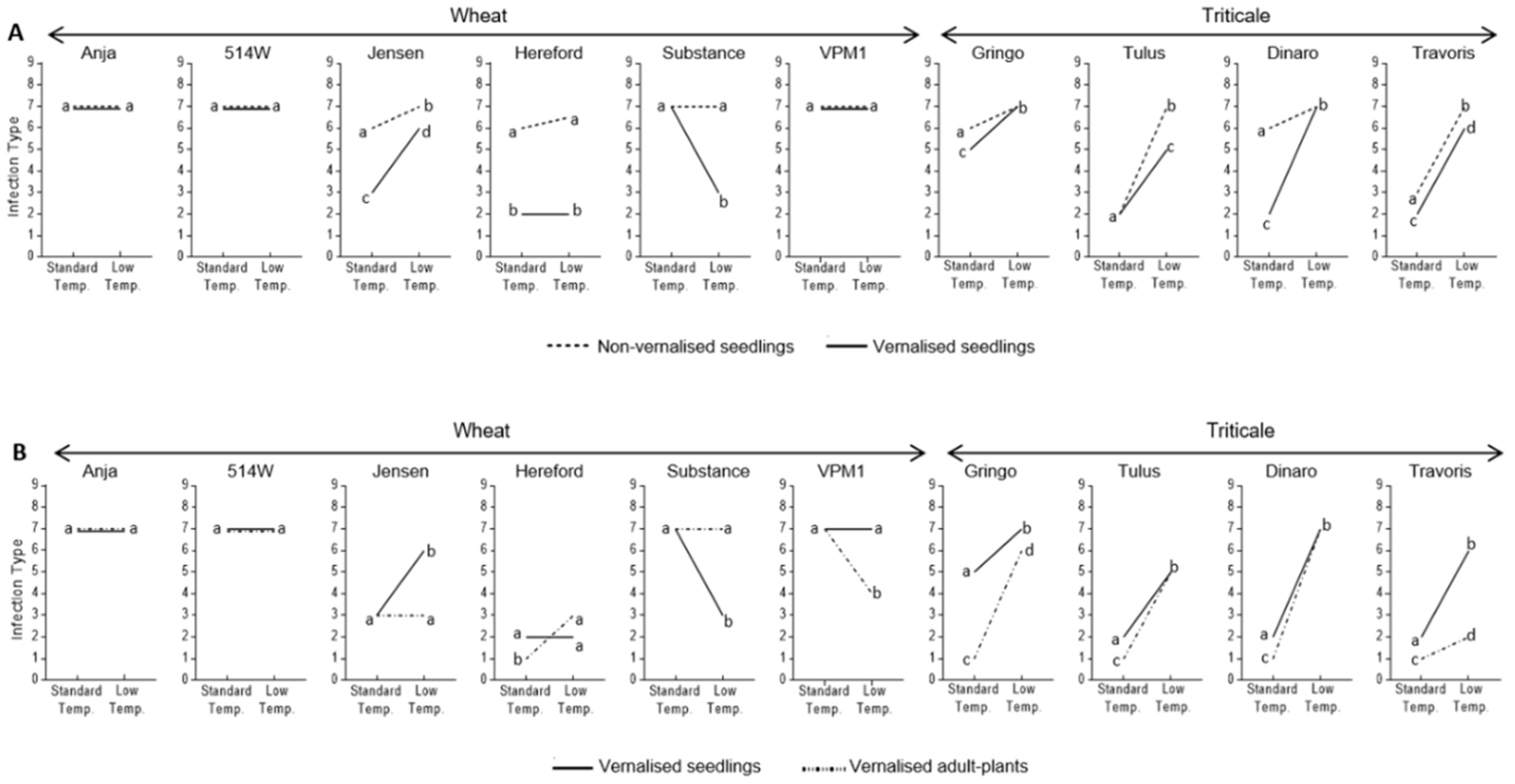
3.2. Effect of Vernalisation and Temperature on Seedling Susceptibility
3.3. Effect of Plant Growth Stage and Temperature on Host Susceptibility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chouard, P. Vernalization and its relations to dormancy. Annu. Rev. Plant Physiol. 1960, 11, 191–238. [Google Scholar] [CrossRef]
- Amasino, R. Vernalization, Competence, and the Epigenetic Memory of Winter. Plant Cell 2004, 16, 2553–2559. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Plant cold cclimation: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant Physiol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Hon, W.; Griffith, M.; Mlynarz, A.; Kwok, Y.C.; Yang, D.S.C. Antifreeze Proteins in Winter Rye Are Similar to Pathogenesis-Related Proteins. Plant Physiol. 1995, 109, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Griffith, M.; Yaish, M.W. Antifreeze proteins in overwintering plants: A tale of two activities. Trends Plant Sci. 2004, 9, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, C.; Imai, R. Molecular Basis of Disease Resistance Acquired through Cold Acclimation in Overwintering Plants. J. Plant Biol. 2009, 52, 19–26. [Google Scholar] [CrossRef]
- Ergon, A.; Klemsdal, S.S.; Tronsmo, A.M. Interactions between cold hardening and Microdochium nivale infection on expression of pathogenesis-related genes in winter wheat. Physiol. Mol. Plant Pathol. 1998, 53, 301–310. [Google Scholar] [CrossRef]
- Gaudet, D.A.; Laroche, A.; Frick, M.; Huel, R.; Puchalski, B. Plant development affects the cold-induced expression of plant defence-related transcripts in winter wheat. Physiol. Mol. Plant Pathol. 2003, 62, 175–184. [Google Scholar] [CrossRef]
- Nakajima, T.; Abe, J. Environmental factors affecting expression of resistance to pink snow mold caused by Microdochium nivale in winter wheat. Can. J. Bot. 1996, 74, 1783–1788. [Google Scholar] [CrossRef]
- White, N.; Jenkyn, J.F. Effects of sowing date and vernalisation on the growth of winter barley and its resistance to powdery mildew (Erysiphe graminis f.sp. hordei). Ann. Appl. Biol. 1995, 218, 269–283. [Google Scholar] [CrossRef]
- Qayoum, A.; Line, R.F. High-Temperature, Adult Plant Resistance to Stripe Rust of Wheat. Phytopathology 1985, 75, 1121–1125. [Google Scholar] [CrossRef]
- Bryant, R.R.M.; McGrann, G.R.D.; Mitchell, A.R.; Schoonbeek, H.; Boyd, L.A.; Uauy, C.; Dorling, S.; Ridout, C.J. A change in temperature modulates defence to yellow (stripe) rust in wheat line UC1041 independently of resistance gene Yr36. BMC Plant Biol. 2014, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Fetch, T.G., Jr. Effect of temperature on the expression of seedling resistance to Puccinia graminis f. sp. avenae in oat. Can. J. Plant Pathol. 2006, 28, 558–565. [Google Scholar] [CrossRef]
- Brown, G.N. The inheritance and expression of leaf chlorosis associated with gene Sr2 for adult plant resistance to wheat stem rust. Euphytica 1997, 95, 67–71. [Google Scholar] [CrossRef]
- Broers, L.H.M.; Wallenburg, S.C. Influence of post-infection temperature on three components of partial resistance in wheat to wheat leaf rust. Euphytica 1989, 44, 215–224. [Google Scholar] [CrossRef]
- Dyck, P.L.; Johnson, R. Temperature sensitive of genes for resistance in wheat to Puccinia recondita. Can. J. Plant Pathol. 1983, 5, 229–234. [Google Scholar] [CrossRef]
- Stubbs, R.W. Stripe Rust, The Cereal Rusts Vol. II: Diseases, Distribution, Epidemiology, and Control; Roelfs, A.P., Bushnell, W.R., Eds.; Academic Press: Orlando, FL, USA, 1985; pp. 77–115. [Google Scholar]
- Park, R.; Ash, G.; Rees, R. Effects of temperature on the response of some Australian wheat varieties to Puccinia striiformis f. sp. Tritici. Mycol. Res. 1992, 96, 166–170. [Google Scholar] [CrossRef]
- Bariana, H.S.; McIntosh, R.A. Characterisation and origin of rust and powdery mildew resistance genes in VPM1 wheat. Euphytica 1994, 76, 53–61. [Google Scholar] [CrossRef]
- Pretorius, Z.A.; Kloppers, F.J.; Drijepondt, S.C. Effects of inoculum density and temperature on 3 components of leaf rust resistance controlled by Lr34 in wheat. Euphytica 1994, 74, 91–96. [Google Scholar] [CrossRef]
- Flor, H.H. Host-parasite interaction in flax rust-its genetics and other implications. Phytopathology 1955, 45, 680–685. [Google Scholar]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Boyd, L.A. Can Robigus defeat an old enemy?—Yellow rust of wheat. J. Agric. Sci. 2005, 143, 233. [Google Scholar] [CrossRef]
- Johnson, R. A Critical Analysis of Durable Resistance. Annu. Rev. Phytopathol. 1984, 22, 309–330. [Google Scholar] [CrossRef]
- Pretorius, Z.A.; Pienaar, L.; Prins, R. Greenhouse and field assessment of adult plant resistance in wheat to Puccinia striiformis f. sp. Tritici. Australas. Plant Pathol. 2007, 36, 552–559. [Google Scholar] [CrossRef]
- Johnson, R. Past, present and future opportunities in breeding for disease resistance, with examples from wheat. Euphytica 1992, 63, 3–22. [Google Scholar] [CrossRef]
- Singh, R.P.; Herrera-Foessel, S.; Huerta-Espino, J.; Singh, S.; Bhavani, S.; Lan, C.; Basnet, B.R. Progress Towards Genetics and Breeding for Minor Genes Based Resistance to Ug99 and Other Rusts in CIMMYT High-Yielding Spring Wheat. J. Integr. Agric. 2014, 13, 255–261. [Google Scholar] [CrossRef]
- Ellis, J.G.; Lagudah, E.S.; Spielmeyer, W.; Dodds, P.N. The past, present and future of breeding rust resistant wheat. Front. Plant Sci. 2014, 5, 641. [Google Scholar] [CrossRef]
- Chen, X. High-Temperature Adult-Plant Resistance, Key for Sustainable Control of Stripe Rust. Am. J. Plant Sci. 2013, 4, 608–627. [Google Scholar] [CrossRef]
- Hovmøller, M.S.; Walter, S.; Bayles, R.A.; Hubbard, A.; Flath, K.; Sommerfeldt, N.; Leconte, M.; Czembor, P.; Rodriguez-Algaba, J.; Thach, T.; et al. Replacement of the European wheat yellow rust population by new races from the centre of diversity in the near-Himalayan region. Plant Pathol. 2016, 65, 402–411. [Google Scholar] [CrossRef]
- Hovmøller, M.S.; Justesen, A.F. Appearance of atypical Puccinia striiformis f. sp. tritici phenotypes in north-western Europe. Aust. J. Agric. Res. 2007, 58, 518. [Google Scholar] [CrossRef]
- Thach, T.; Ali, S.; Justesen, A.F.; Rodriguez-Algaba, J.; Hovmøller, M.S. Recovery and virulence phenotyping of the historic ‘Stubbs collection’ of the yellow rust fungus Puccinia striiformis from wheat. Ann. Appl. Biol. 2015, 167, 314–326. [Google Scholar] [CrossRef]
- Bariana, H.S.; McIntosh, R.A. Cytogenetic studies in wheat. XV. Location of rust resistance genes in VPM1 and their genetic linkage with other disease resistance genes in Chromosome 2A. Genome 1993, 36, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Hovmøller, M.S. Sources of seedling and adult plant resistance to P. striiformis f. sp. tritici in European wheats. Plant Breed. 2007, 126, 225–233. [Google Scholar]
- Milus, E.A.; Moon, D.E.; Lee, K.D.; Mason, R.E. Race-Specific Adult-Plant Resistance in Winter Wheat to Stripe Rust and Characterization of Pathogen Virulence Patterns. Phytopathology 2015, 105, 1114–1122. [Google Scholar] [CrossRef]
- Sørensen, C.K.; Thach, T.; Hovmøller, M.S. Evaluation of Spray and Point Inoculation Methods for the Phenotyping of Puccinia striiformis on Wheat. Plant Dis. 2016, 100, 1064–1070. [Google Scholar] [CrossRef]
- Milus, E.A.; Kristensen, K.; Hovmoller, M.S. Evidence for increased aggressiveness in a recent widespread strain of Puccinia striiformis f. sp. tritici causing stripe rust of wheat. Phytopathology 2009, 99, 89–94. [Google Scholar] [CrossRef]
- McNeal, F.H.; Konzak, C.F.; Smith, E.P.; Tate, W.S.; Russel, T.S. A uniform system for recording and processing cereal research data. USDA Agric. Res. Serv. 1971, 42, 34–121. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models; Chapman and Hall: London, UK, 1989. [Google Scholar]
- Jørgensen, B.; Labouriau, R.; Lundbye-Christensen, S. Linear growth curve analysis based on exponential dispersion models. J. R. Stat. Soc. 1996, 58, 573–592. [Google Scholar] [CrossRef]
- Lehmann, E. Nonparametrics: Statistical Methods Based on Ranks; Springer: New York, NY, USA, 2006. [Google Scholar]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Walters, D.; Walsh, D.; Newton, A.; Lyon, G. Induced Resistance for Plant Disease Control: Maximizing the Efficacy of Resistance Elicitors. Phytopathology 2005, 95, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Hofgaard, I.S.; Wanner, L.A.; Tronsmo, A.M. The effect of age and cold hardening on resistance to pink snow mould (Microdochium nivale) in perennial ryegrass (Lolium perenne L.). Acta Agric. Scand. Sect. B Plant Soil Sci. 2006, 56, 315–323. [Google Scholar]
- Tronsmo, A.M. Host water potentials may restrict development of snow mould fungi in low temperature-hardened grasses. Physiol. Plant. 1986, 68, 175–179. [Google Scholar] [CrossRef]
- Tronsmo, A.M. Resistance to the rust fungus Puccinia poae-nemoralis in Poa pratensis induced by low-temperature hardening. Can. J. Bot. 1984, 62, 2891–2892. [Google Scholar] [CrossRef]
- Gaudet, D.A.; Laroche, A.; Frick, M.; Davoren, J.; Puchalski, B.; Ergon, Å. Expression of plant defence-related (PR-protein) transcripts during hardening and dehardening of winter wheat. Physiol. Mol. Plant Pathol. 2000, 57, 15–24. [Google Scholar] [CrossRef]
- Liu, J.J.; Sturrock, R.; Ekramoddoullah, A.K. The superfamily of thaumatin-like proteins: Its origin, evolution, and expression towards biological function. Plant Cell Rep. 2010, 29, 419–436. [Google Scholar] [CrossRef]
- Lopez-Sanchez, A.; Stassen, J.H.; Furci, L.; Smith, L.M.; Ton, J. The role of DNA (de)methylation in immune responsiveness of Arabidopsis. Plant J. 2016, 88, 361–374. [Google Scholar] [CrossRef]
- Tuan-Ngoc, L.; Schumann, N.U.; Smith, N.A.; Tiwari, S.; Au, P.C.K.; Zhu, Q.; Taylor, J.M.; Kazan, K.; Llewellyn, D.J.; Zhang, R.; et al. DNA demethylases target promoter transposable elements to positively regulate stress responsive genes in Arabidopsis. Genome Biol. 2014, 15, 458. [Google Scholar]
- Yu, A.; Lepere, G.; Jay, F.; Wang, J.; Bapaume, L.; Wang, Y.; Abraham, A.L.; Penterman, J.; Fischer, R.L.; Voinnet, O.; et al. Dynamics and biological relevance of DNA demethylation in Arabidopsis antibacterial defense. Proc. Natl. Acad. Sci. USA 2013, 110, 2389–2394. [Google Scholar] [CrossRef]
- Steward, N.; Ito, M.; Yamaguchi, Y.; Koizumi, N.; Sano, H. Periodic DNA methylation in maize nucleosomes and demethylation by environmental stress. J. Biol. Chem. 2002, 277, 37741–37746. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhang, J. Plant genomic DNA methylation in response to stresses: Potential applications and challenges in plant breeding. Prog. Nat. Sci. 2009, 19, 1037–1045. [Google Scholar] [CrossRef]
- Sherman, J.D.; Talbert, L.E. Vernalization-induced changes of the DNA methylation pattern in winter wheat. Genome 2002, 45, 253–260. [Google Scholar] [CrossRef]
- Finnegan, E.J.; Genger, R.K.; Peacock, W.J.; Dennis, E.S. DNA METHYLATION IN PLANTS. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 223–247. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, K.; Katakami, H.; Kim, H.J.; Ogawa, E.; Sano, C.M.; Wada, Y.; Sano, H. Epigenetic inheritance in rice plants. Ann. Bot. 2007, 100, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, L.J.; Quinton-Tulloch, M.; Olohan, L.; Price, J.; Hall, N.; Hall, A. A genome-wide survey of DNA methylation in hexaploid wheat. Genome Biol. 2015, 16, 273. [Google Scholar] [CrossRef]
- Brown, J.F.; Sharp, E.L. Interactions of minor host genes for resistance to Puccinia striiformis with changing temperatures. Phytopathology 1969, 59, 999–1001. [Google Scholar]
- Zadoks, J.C. Yellow rust on wheat, studies in epidemiology and physiologic specialization. Tijdschrift Over Plantenziekten 1961, 67, 69–256. [Google Scholar] [CrossRef]
- Mergoum, M.; Pfeiffer, W.H.; Peña, R.J.; Ammar, K.; Rajaram, S. Triticale crop improvement: The CIMMYT programme. In Triticale Improvement and Production; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- Crespo-Herrera, L.A.; Garkava-Gustavsson, L.; Ahman, I. A systematic review of rye (Secale cereale L.) as a source of resistance to pathogens and pests in wheat (Triticum aestivum L.). Hereditas 2017, 154, 14. [Google Scholar] [CrossRef]
- Friebe, B.; Jiang, J.; Raupp, W.J.; Mclntosh, R.A.; Gill, B.S. Characterization of wheat-alien translocations conferring resistance to diseases and pests: Current status. Euphytica 1996, 91, 59–87. [Google Scholar] [CrossRef]
- Tyrka, M.; Chekowski, J. Enhancing the resistance of triticale by using genes from wheat and rye. J. Appl. Genet. 2004, 45, 283–295. [Google Scholar] [PubMed]
- Milus, E.A.; Lee, K.D.; Brown-Guedira, G. Characterization of Stripe Rust Resistance in Wheat Lines with Resistance Gene Yr17 and Implications for Evaluating Resistance and Virulence. Phytopathology 2015, 105, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Periyannan, S.; Aitken, E.; Hickey, L. A rapid phenotyping method for adult plant resistance to leaf rust in wheat. Plant Methods 2016, 12, 17. [Google Scholar] [CrossRef] [PubMed]
| Virulence Phenotype | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolates | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 15 | 17 | 24 | 25 | 27 | 32 | Sd | Su | Sp | AvS | Amb |
| DK09/11 | 1 | 2 | 3 | 4 | - | 6 | 7 | - | 9 | - | - | 17 | - | 25 | - | 32 | Sd | Su | Sp | AvS | Amb |
| DK28/12 | 1 | 2 | 3 | - | - | 6 | 7 | 8 | 9 | - | - | 17 | - | 25 | - | 32 | Sd | - | - | AvS | Amb |
| Variety | p-Value | Flag Leaf Standard Temp. | F-1 LeafStandard Temp. | Flag Leaf Low Temp. | F-1 Leaf Low Temp. |
|---|---|---|---|---|---|
| Anja | 0.106 | 0.39 | 0.78 | 0.78 | 0.72 |
| 514W | 0.062 | 0.83 | 0.83 | 0.72 | 0.39 |
| Jensen | 0.004 | 0.33c | 0.67b | 0.94a | 0.82b |
| Hereford | 0.098 | 0.72 | 0.67 | 0.61 | 0.69 |
| Substance | 0.699 | 0.50 | 0.61 | 0.78 | 0.56 |
| VPM1 | 0.098 | 0.61 | 0.61 | 0.56 | 0.53 |
| Gringo | 0.982 | 0.56 | 0.56 | 0.61 | 0.61 |
| Tulus | 0.098 | 0.61 | 0.61 | 0.61 | 0.56 |
| Dinaro | 0.982 | 0.72 | 0.61 | 0.78 | 0.72 |
| Travoris | 0.063 | 0.67 | 0.67 | 0.56 | 0.22 |
| Variety | p-Value | Non-Vernalised Seedlings Standard Temp. | Vernalised Seedlings Standard Temp. | Non-Vernalised Seedlings Low Temp. | Vernalised Seedlings Low Temp. |
|---|---|---|---|---|---|
| Anja | 0.016 | 0.78b | 0.94a | 0.61b | 1.00a |
| 514W | 0.070 | 0.83 | 1.00 | 0.72 | 0.94 |
| Jensen | 0.420 | 0.67 | 0.78 | 0.61 | 0.94 |
| Hereford | 0.016 | 0.67b | 1.00a | 0.72b | 0.94a |
| Substance | 0.070 | 0.89 | 0.83 | 0.72 | 0.94 |
| VPM1 | 0.083 | 0.61 | 0.83 | 0.50 | 0.94 |
| Gringo | 0.069 | 0.72 | 0.89 | 0.83 | 1.00 |
| Tulus | 0.420 | 0.94 | 1.00 | 0.89 | 0.94 |
| Dinaro | 0.016 | 0.44b | 0.83a | 0.50b | 0.89a |
| Travoris | 0.070 | 0.72 | 1.00 | 0.89 | 0.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Algaba, J.; Sørensen, C.K.; Labouriau, R.; Justesen, A.F.; Hovmøller, M.S. Susceptibility of Winter Wheat and Triticale to Yellow Rust Influenced by Complex Interactions between Vernalisation, Temperature, Plant Growth Stage and Pathogen Race. Agronomy 2020, 10, 13. https://doi.org/10.3390/agronomy10010013
Rodriguez-Algaba J, Sørensen CK, Labouriau R, Justesen AF, Hovmøller MS. Susceptibility of Winter Wheat and Triticale to Yellow Rust Influenced by Complex Interactions between Vernalisation, Temperature, Plant Growth Stage and Pathogen Race. Agronomy. 2020; 10(1):13. https://doi.org/10.3390/agronomy10010013
Chicago/Turabian StyleRodriguez-Algaba, Julian, Chris K. Sørensen, Rodrigo Labouriau, Annemarie F. Justesen, and Mogens S. Hovmøller. 2020. "Susceptibility of Winter Wheat and Triticale to Yellow Rust Influenced by Complex Interactions between Vernalisation, Temperature, Plant Growth Stage and Pathogen Race" Agronomy 10, no. 1: 13. https://doi.org/10.3390/agronomy10010013
APA StyleRodriguez-Algaba, J., Sørensen, C. K., Labouriau, R., Justesen, A. F., & Hovmøller, M. S. (2020). Susceptibility of Winter Wheat and Triticale to Yellow Rust Influenced by Complex Interactions between Vernalisation, Temperature, Plant Growth Stage and Pathogen Race. Agronomy, 10(1), 13. https://doi.org/10.3390/agronomy10010013




