Carbon and Nitrogen Dynamics Affected by Drip Irrigation Methods and Fertilization Practices in a Pomegranate Orchard
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Measurements
2.3. Plant Measurements
3. Results and Discussion
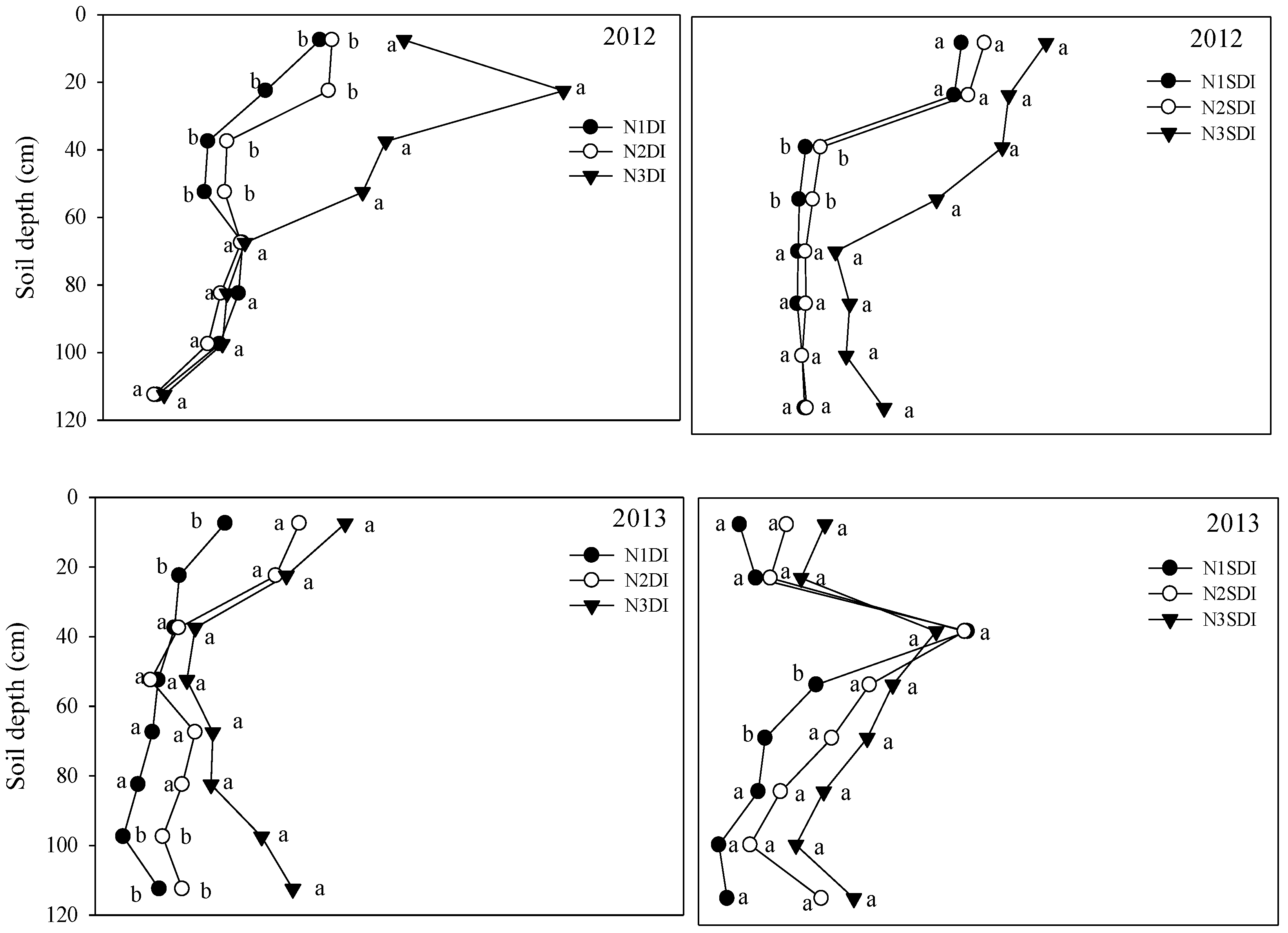
3.1. Soil Total C and N in Different Irrigation and N Treatments
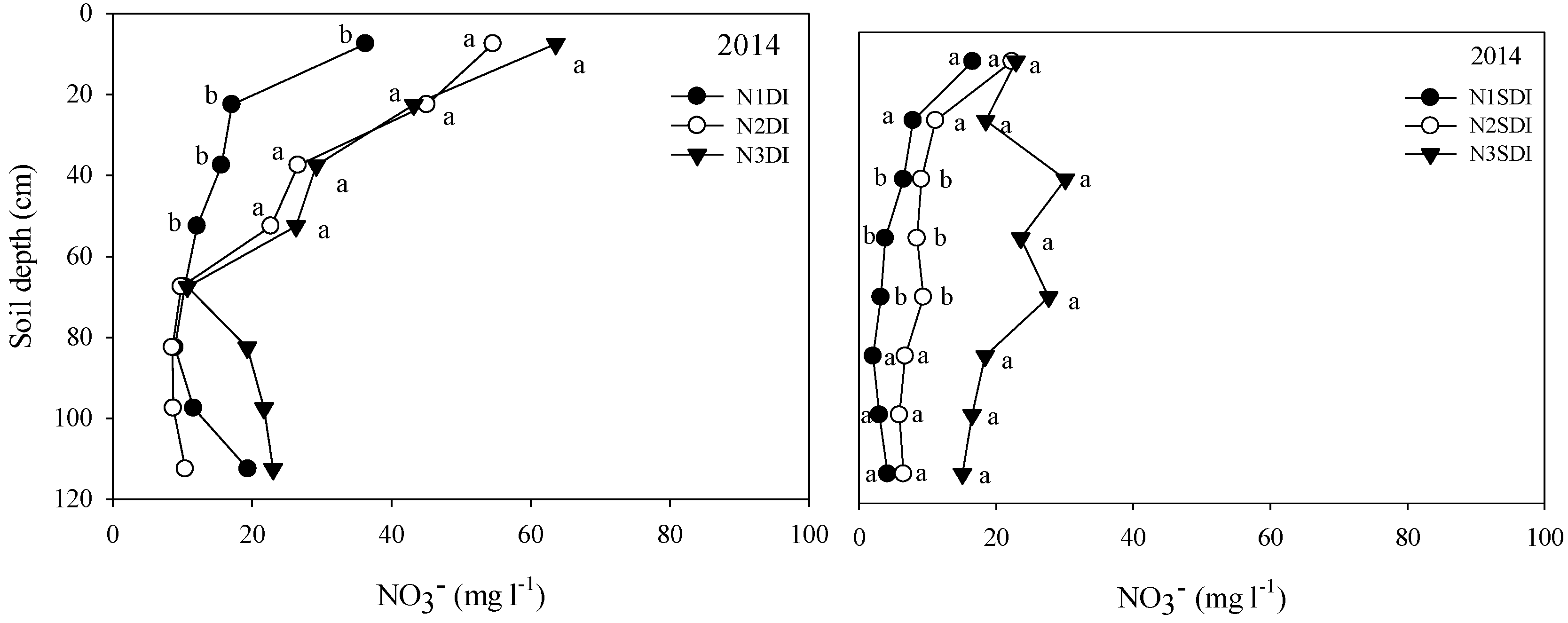
3.2. Soil DOC and NO3− in Different Irrigation and N Treatments
3.3. Total N Concentration and C/N Ratio in Leaves
3.4. Total N Uptake by Fruit
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, Z.H.; Wilkins, E.; Wang, W. Proceedings of the Third International Symposium on Pomegranate and Minor Mediterranean Fruits; International Society of Horticultural Science: Leuven, Belgium, 2015; p. 493. [Google Scholar]
- Wang, D.; Ayars, J.E.; Tirado-Corbalá, R.; Makus, D.; Phene, C.J.; Phene, R. Water and Nitrogen Management of Young and Maturing Pomegranate Trees. Acta Hort. 2015, 1089, 395–401. Available online: http://www.actahort.org/books/1089/1089_53.htm (accessed on 3 August 2019). [CrossRef]
- Gao, S.; Hendrata, A.; Zejiang, C.; Yinghua, D.; Qin, R.; Tirado-Corbalá, R. Subsurface Drip Irrigation Reduced Nitrous Oxide Emissions in Pomegranate Orchard. Int. J. Environ. Sci. Dev. 2019, 10, 79–85. [Google Scholar] [CrossRef] [Green Version]
- California Department of Food and Agriculture (CDFA). Agricultural Statistics Review; CDFA: Sacramento, CA, USA, 2017. [Google Scholar]
- Day, K.R.; Wilkins, E.D. Commercial Pomegranate (Punica granatum L.) production in California. In Proceedings of the IInd International Symposium on Pomegranate and Minor, including Mediterranean Fruits (ISPMMF—2009), Dharwar, India, 23–27 June 2009; Sheikh, M.K., Mokashi, A.N., Rokhade, A.K., Eds.; ISHS: Leuven, Belgium, 2011. [Google Scholar]
- Holland, D.; Hatib, K.; Bar-Yaakov, I. Pomegranate: Botany, horticulture and breeding. Hortic. Rev. 2009, 35, 127–191. [Google Scholar]
- Intrigliolo, D.S.; Nicolas, E.; Bonet, L.; Ferrer, P.; Alarcón, J.J.; Bartual, J. Water relations of field grown Pomegranate trees (Punica granatum) under different drip irrigation regimes. Agric. Water Manag. 2011, 98, 691–696. [Google Scholar] [CrossRef]
- Ayars, J.E. Adapting Irrigated Agriculture to Drought in the San Joaquin Valley of California. In Drought in Arid and Semi-Arid Regions: A Multi-Disciplinary and Cross-Country Perspective; Springer: New York, NY, USA, 2013; pp. 25–39. [Google Scholar] [CrossRef]
- Scudiero, E.; Skaggs, T.H.; Corwin, D.L. Regional scale soil salinity evaluation using Landsat 7, western San Joaquin Valley, California, USA. Geoderma Reg. 2014, 2, 82–90. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Jilma-Stohlawetz, P.; Rios, J.; Hingorani, L.; Derenorf, H. Absorption, metabolism, and antioxidant effects of Pomegranate (Punica granatum L.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J. Agric. Food Chem. 2006, 54, 8956–8961. [Google Scholar] [CrossRef] [PubMed]
- Galindo, A.; Calín-Sánchez, Á.; Collado-González, J.; Odoño, S.; Hernández, F.; Torrecillas, A.; Carbonell-Barrachina, Á.A. Phytochemical and quality attributes of pomegranate fruits for juice consumption as affect by ripening stage and deficit irrigation. J. Sci. Food Agric. 2014, 94, 2259–2265. [Google Scholar] [CrossRef]
- Chauhan, S.K. Pomegranate grown in drip and furrow irrigation system in saline water condition of semi-arid areas. Technofame 2013, 2, 124–126. [Google Scholar]
- Glozer, K.; Ferguson, L. Pomegranate production in Afghanistan. UCDAVIS Coll. Agric. Environ. Sci. 2008, 32, 1–32. [Google Scholar]
- Parvizi, H.; Sepaskhah, A.R.; Ahmadi, S.H. Effect of drip irrigation and fertilizer regimes on fruit yields and water productivity of a pomegranate (Punica granatum (L.) cv. Rabab) orchard. Agric. Water Manag. 2014, 146, 45–56. [Google Scholar] [CrossRef]
- Parvizi, H.; Sepaskhah, A.R.; Ahmadi, S.H. Physiological and growth responses of pomegranate tree (Punica granatum (L.) cv. Rabab) under partial root zone drying and deficit irrigation regimes. Agric. Water Manag. 2016, 163, 146–158. [Google Scholar] [CrossRef]
- Parvizi, H.; Sepaskhah, A.R. Effect of drip irrigation and fertilizer regimes on fruit quality of a pomegranate (Punica granatum (L.) cv. Rabab) orchard. Agric. Water Manag. 2015, 156, 70–78. [Google Scholar] [CrossRef]
- Ayars, J.E.; Phene, C.J.; Phene, R.C.; Gao, S.; Wang, D.; Day, K.R.; Makus, D.J. Determining Pomegranate Water and Nitrogen Requirements with Drip Irrigation. Agric. Water Manag. 2017, 187, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.G.; Lo, M.H.; Famiglietti, J.M. Assessing surface water consumption using remotely-sensed groundwater, evapotranspiration, and precipitation. Geophys. Res. Lett. 2012, 39, L16401. [Google Scholar] [CrossRef] [Green Version]
- Chapelle, F.H.; Campbell, B.G.; Widowson, M.A.; Landon, M.K. Modeling the long-term fate of agricultural nitrate in groundwater in the San Joaquin Valley, California. In Current Perspectives in Contaminant Hydrology and Water Resources Sustainability; Paul, B., Ed.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef] [Green Version]
- Harter, T.; Onsoy, Y.S.; Heeren, K.; Denton, M.; Weismann, G.; Hopmans, J.W.; Horwath, W.R. Deep vadose zone hydrology demonstrates fate of nitrate in eastern San Joaquin Valley. Calif. Agric. 2005, 59, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Harter, T.; Lund, J.R.; Darby, J. Addressing nitrate in California’s drinking water with focus on Tulare Lake Basin and Salinas Valley groundwater. In Report for the State Water Resources Control Board Report to the Legislature; Center for Watershed Sciences, UC Davis, CA: Yolo County, CA, USA, 2012; p. 78. [Google Scholar]
- Jurgens, B.C.; Burow, K.R.; Dalgish, B.A.; Shelton, J.L. Hydrogeology, Water Chemistry, and Factors Affecting the Transport of Contaminants in the Zone of Contribution of Public-Supply Well in Modesto, Eastern San Joaquin Valley, California; U.S. Geological Survey: Reston, WV, USA, 2008; Volume 5156, p. 78.
- Muhammad, S.; Sanden, B.L.; Lampinen, B.D.; Saa, S.; Siddiqui, M.I.; Smart, D.R.; Olivos, A.; Shackel, K.A.; DeJong, T.; Brown, P.H.; et al. Seasonal changes in nutrient content and concentrations in a mature deciduous tree species: Studies in almond (Prunus dulcis (Mill.) D.A. Webb). Eur. J. Agron. 2015, 65, 52–68. [Google Scholar] [CrossRef]
- Chomycia, J.C.; Hernes, P.J.; Harter, T.; Bergamaschi, B.A. Land management impacts on dairy-derived dissolved organic carbon in ground water. J. Environ. Qual. 2008, 37, 333–343. [Google Scholar] [CrossRef]
- Viers, J.H.; Liptzin, D.; Rosenstock, T.S.; Jensen, V.B.; Hollander, A.D.; McNally, A.D.; King, A.; Kourakos, A.M.; Lopez, G.; De La Mora, E.M.; et al. Nitrogen sources and loading to groundwater. Technical report 2. In Addressing Nitrate in California’s Drinking Water Resources Control Board Report to the Legislature; Center for Watershed Sciences, University of California: Davis, CA, USA, 2012. [Google Scholar]
- Ayars, J.E.; Phene, C.J.; Hutmacher, R.B.; Davis, K.R.; Schoneman, R.A.; Vail, S.S.; Mead, R.M. Subsurface drip irrigation of row crops: A review of 15 years of research at the Water Management Research Laboratory. Agric. Water Manag. 1999, 42, 1–27. [Google Scholar] [CrossRef]
- Baram, S.; Couvreur, V.; Harter, T.; Read, M.; Brown, P.H.; Kandelous, M.; Smart Hopmans, J.W. Estimating nitrate leaching to groundwater from orchards: Comparing crop nitrogen excess, deep vadose zone data-driven estimates, and HYDRUS modeling. Vadose Zone J. 2016, 15, 11. [Google Scholar] [CrossRef] [Green Version]
- Phene, C.J.; Ayars, J.E.; Phene, R.C.; Schoneman, R.S. Irrigation and fertigation with high frequency subsurface drip irrigation can prevent drainage and groundwater contamination. In Proceedings of the 2015 ASABE/IA Irrigation Symposium: Emerging Technologies for Sustainable Irrigation—A Tribute to the Career of Terry Howell, Sr. Conference Proceedings, Long Beach, CA, USA, 10–12 November 2015. [Google Scholar]
- Zhang, H.; Wang, D.; Ayars, J.E.; Phene, C.J. Biophysical response of young pomegranate tree to surface and sub-surface drip irrigation and deficit irrigation. Irrig. Sci. 2017, 35, 425–435. [Google Scholar] [CrossRef]
- Phene, C.J.; Hoffman, G.J.; Howell, T.A.; Clark, D.A.; Mead, R.M.; Johnson, R.S.; Williams, L.E. Automated Lysimeter for Irrigation and Drainage Control. Lysimeters for Evapotranspiration and Environmental Measurements; ASCE: New York, NY, USA, 1991. [Google Scholar]
- Ayars, J.E.; Johnson, R.S.; Phene, C.J.; Trout, T.J.; Clark, D.A.; Mead, R.M. Crop water use by late season drip irrigated peaches. Irrig. Sci. 2003, 22, 187–194. [Google Scholar] [CrossRef]
- De Ferreira, E.V.O.; Novais, R.F.; Médice, B.M.; de Barros, N.F.; Silva, I.R. Leaf total nitrogen concentration as an indicator of nitrogen status for plantlets and young plants of eucalyptus clones. Revista Brasileira de Ciência do Solo 2015, 39, 1127–1140. [Google Scholar] [CrossRef] [Green Version]
- Decock, C.; Garland, G.; Suddick, C.E.; Six, J. Season and location–specific nitrous oxide emissions in an almond orchard in California. Nutr. Cycl. Agroecosyst. 2017, 107, 139–155. [Google Scholar] [CrossRef]
- Wolf, K.; Herrera, I.; Tomich, T.; Scow, K. Long-term agricultural experiments inform the development of climate-smart agricultural practices. Calif. Agric. 2017, 71, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.; Xu, H.; Ji, M.; Jiang, y. Characteristics of Soil Organic Carbon, Total Nitrogen, and C/N Ratio in Chinese Apple Orchards. Open J. Soil Sci. 2015, 3, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Bangroo, S.; Itoo, H.; Tasneem, M.; Malik, A.R. Soil organic carbon and total nitrogen in temperate apple orchards of south Kashmir. Int. J. Ecol. Environ. Sci. 2018, 44, 287–292. Available online: https://file.scirp.org/Html/2-1660139_36404.htm (accessed on 3 August 2019).
- Gullickson, G. Here’s Why the Carbon-Nitrogen Ratio Matters. Available online: https://www.agriculture.com/crops/cover-crops/heres-why-carbonnitrogen-ratio-matters_568-ar48014 (accessed on 3 August 2019).
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Luo, Y.; van Groenigen, K.J.; Hungate, B.A.; Cao, J.; Zhou, X.; Wang, R.W. A keystone microbial enzyme for nitrogen control of soil carbon storage. Sci. Adv. 2018, 4, eaaq1689. [Google Scholar] [CrossRef] [Green Version]
- Jian, S.; Li, J.; Chen, J.; Wang, G.; Mayes, M.; Dzantor, K.; Hui, D. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Chen, J.; Chen, Y.; Feng, W. Data-driven microbial modeling for soil carbon decomposition and stabilization. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 23–28 April 2017; Available online: https://meetingorganizer.copernicus.org/EGU2017/EGU2017-4548.pdf (accessed on 3 August 2019).
- Gong, S.; Guo, R.; Zhang TGuo, J. Warming and nitrogen addition increase litter decomposition in a temperate meadow ecosystem. PLoS ONE 2015, 10, e0116013. [Google Scholar] [CrossRef]
- Talbot, J.M.; Treseder, K.K. Interactions among lignin, cellulose, and nitrogen drive litter chemistry–decay relationships. Ecology 2012, 93, 345–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, C.; Liu, Y.; Mohamed, I.; Zhang, R.; Wang, X.; Nie, X.; Jiang, M.; Brooks, M.; Chen, F.; Li, Z. Dynamic Changes of Soil Surface Organic Carbon under Different Mulching Practices in Citrus Orchards on Sloping Land. PLoS ONE 2016, 11, e0168384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebastian, S.; Muhammad, S.; Brown, P.H. Development of Leaf Sampling and Interpretation Methods and Nutrient Budget Approach to Nutrient Management in Almond (Prunus dulcis (Mill.) D.A. Webb). Acta Hortic. 2013, 984, 291–296. [Google Scholar] [CrossRef]


| Soil Depth | Treatments (2012) | Treatments (2013) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drip Irrigation | Subsurface Drip Irrigation | Drip Irrigation | Subsurface Drip Irrigation | |||||||||
| (cm) | N1 z | N2 | N3 | N1 | N2 | N3 | N1 | N2 | N3 | N1 | N2 | N3 |
| (%) | (%) | |||||||||||
| 0–15 | 0.02 b y | 0.04 b | 0.82 a | 0.02 a | 0.08 a | 0.03 a | 1.11 b | 1.42 b | 3.73 a | 0.1b | 0.1 b | 1.03 a |
| 15–30 | ND b | 0.02 b | 0.78 a | 0.01 a | 0.03 a | 0.01 a | 1.15 b | 1.27 b | 3.26 a | 0.24 b | 0.15 b | 1.19 a |
| 30–45 | ND b | 0.02 b | 0.74 a | 0.01 a | 0.02 a | 0.01 a | 1.13 b | 1.18 b | 1.87 a | 0.36 b | 0.2 b | 1.19 a |
| 45–60 | ND b | 0.02 b | 0.46 a | 0.01 b | 0.03 b | 0.24 a | 1.1 a | 1.06 a | 1.06 a | 1.27 b | 1.02 b | 3.73 a |
| 60–75 | ND b | 0.02 b | 0.28 a | 0.01 b | 0.06 b | 0.76 a | 0.48 b | 0.9 a | 0.06 c | 0.9 b | 0.03 b | 2.38 a |
| 75–90 | ND | ND | ND | 0.01 a | 0.01 a | 0.68 a | 0.18 a | 0.11 a | 0.06 a | 0.48 b | 0.04 b | 2.1 a |
| 90–105 | ND | ND | ND | 0.01 b | ND b | 0.54 a | 0.1 a | 0.06 a | 0.02 a | 0.18 b | 0.04 b | 2.1 a |
| 105–120 | ND | ND | ND | ND b | ND b | 0.43 a | 0.03 a | 0.05 a | 0.02 a | 0.00 b | 0.03 b | 1.06 a |
| Soil Depth | Treatments (2012) | Treatments (2013) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drip Irrigation | Subsurface Drip Irrigation | Drip Irrigation | Subsurface Drip Irrigation | |||||||||
| (cm) | N1 z | N2 | N3 | N1 | N2 | N3 | N1 | N2 | N3 | N1 | N2 | N3 |
| (%) | (%) | |||||||||||
| 0–15 | 0.12 c y | 0.38 b | 0.82 a | 0.01 a | 0.11 a | 0.11 a | 0.27 b | 0.35 b | 0.91 a | 0.11 a | 0.06 a | 0.09 a |
| 15–30 | 0.11 c | 0.32 b | 0.76 a | 0.04 a | 0.09 a | 0.11 a | 0.26 b | 0.25 b | 0.72 a | 0.13 a | 0.08 a | 0.02 a |
| 30–45 | 0.12 c | 0.28 b | 0.37 a | 0.05 b | 0.1 b | 0.56 a | 0.23 b | 0.28 b | 0.54 a | 0.03 a | 0.08 a | 0.05 a |
| 45–60 | 0.15 a | 0.25 a | 0.22 a | 0.05 c | 0.19 b | 0.29 a | 0.12 b | 0.2 b | 0.54 a | ND a | 0.1 a | 0.02 a |
| 60–75 | 0.03 a | 0.11 a | 0.1 a | 0.08 b | 0.2 a | 0.25 a | 0.12 b | 0.1 b | 0.38 a | ND c | 0.11 b | 0.32 a |
| 75–90 | 0.03 a | 0.13 a | 0.07 a | 0.07 a | 0.22 a | 0.07 a | 0.06 a | 0.11 a | 0.02 a | ND b | 0.04 b | 0.27 a |
| 90–105 | ND a | 0.18 a | 0.07 a | 0.07 a | 0.2 a | 0.05 a | 0.04 a | 0.04 a | 0.02 a | ND b | 0.04 b | 0.15 a |
| 105–120 | ND a | 0.07 a | 0.06 a | 0.00 a | 0.21 a | 0.04 a | 0.02 a | 0.04 a | 0.00 a | ND a | 0.03 a | 0.00 a |
| Year | Total N (%) | C/N Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Irrigation | N Rate | Irrigation | N Rate | |||||||
| DI z | SDI | N1 y | N2 | N3 | DI | SDI | N1 | N2 | N3 | |
| 2012 | 1.70 a x | 1.65 b | 1.64 | 1.69 | 1.70 | 32.1 b | 32.4 a | 32.8 a | 31.3 b | 31.8 b |
| 2013 | 1.94 a | 1.93 a | 1.88 b | 1.95 a | 1.96 a | 28.1 a | 28.2 a | 28.2 a | 28.3 a | 28.4 a |
| 2014 | 1.96 a | 1.94 a | 1.88 a | 1.96 a | 2.01 a | 24.9 a | 25.1 a | 26.0 a | 24.7 b | 24.4 b |
| Year | Irrigation System | Fruit kg N/ha | N Treatment | Fruit kg N/ha |
|---|---|---|---|---|
| 2013 | ||||
| DI z | 105 a | N1 y | 95 b | |
| SDI | 113 a | N2 | 120 a | |
| N3 | 112 a | |||
| 2014 | ||||
| DI | 119 b x | N1 | 108 b | |
| SDI | 135 a | N2 | 138 a | |
| N3 | 135 a | |||
| 2015 | ||||
| DI | 141 b | N1 | 127 a | |
| SDI | 154 a | N2 | 159 a | |
| N3 | 156 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirado-Corbalá, R.; Gao, S.; Ayars, J.E.; Wang, D.; Phene, C.J.; Phene, R.C. Carbon and Nitrogen Dynamics Affected by Drip Irrigation Methods and Fertilization Practices in a Pomegranate Orchard. Horticulturae 2019, 5, 77. https://doi.org/10.3390/horticulturae5040077
Tirado-Corbalá R, Gao S, Ayars JE, Wang D, Phene CJ, Phene RC. Carbon and Nitrogen Dynamics Affected by Drip Irrigation Methods and Fertilization Practices in a Pomegranate Orchard. Horticulturae. 2019; 5(4):77. https://doi.org/10.3390/horticulturae5040077
Chicago/Turabian StyleTirado-Corbalá, Rebecca, Suduan Gao, James E. Ayars, Dong Wang, Claude J. Phene, and Rebecca C. Phene. 2019. "Carbon and Nitrogen Dynamics Affected by Drip Irrigation Methods and Fertilization Practices in a Pomegranate Orchard" Horticulturae 5, no. 4: 77. https://doi.org/10.3390/horticulturae5040077





