Oral Administration of Okara Soybean By-Product Attenuates Cognitive Impairment in a Mouse Model of Accelerated Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Animal Protocol
2.3. Barnes Maze Test
2.4. Passive-Avoidance Task
2.5. Microbiota Sequencing and Analysis
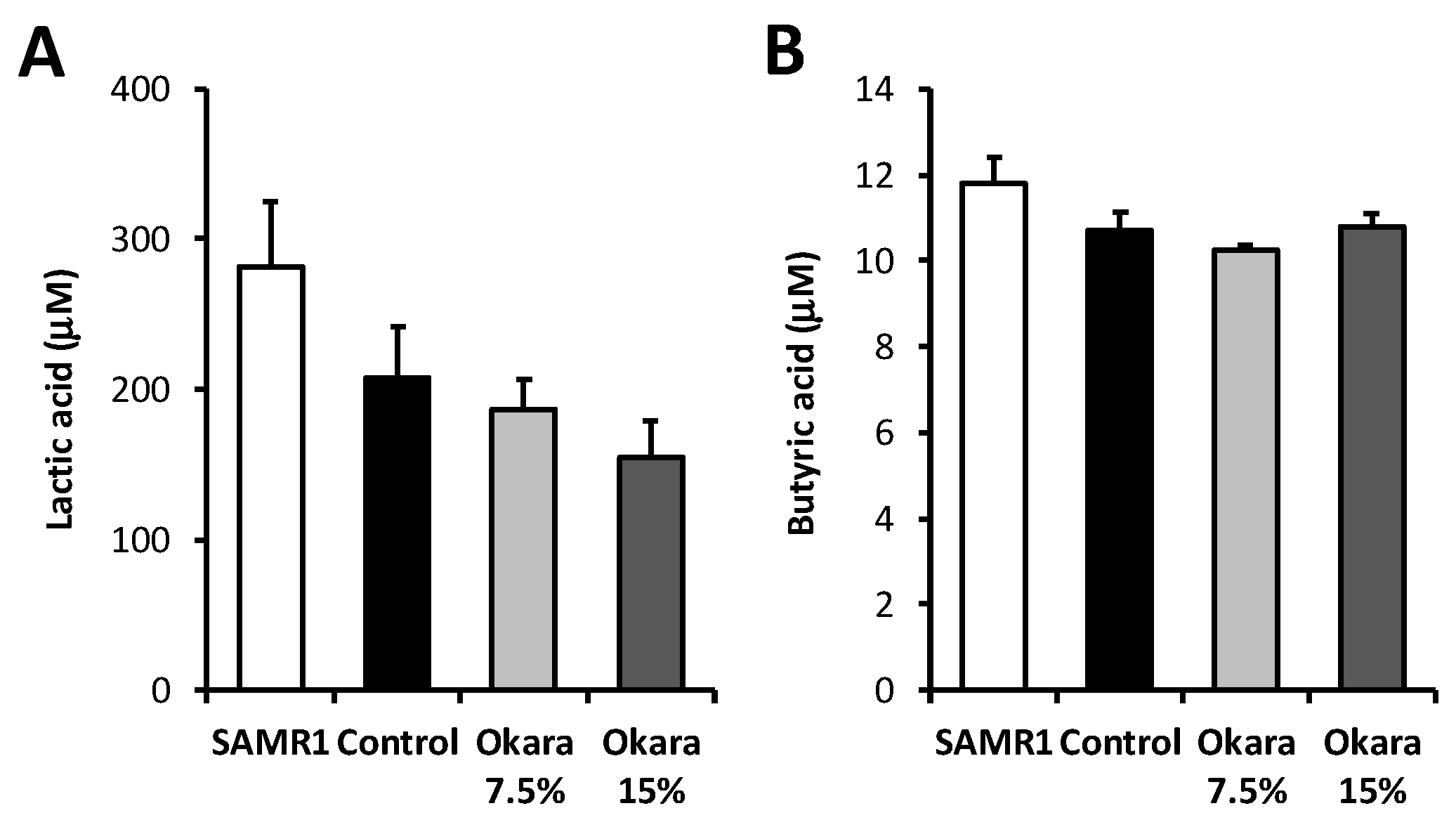
2.6. Measurement of Lactic Acid and Butyric Acid Concentration
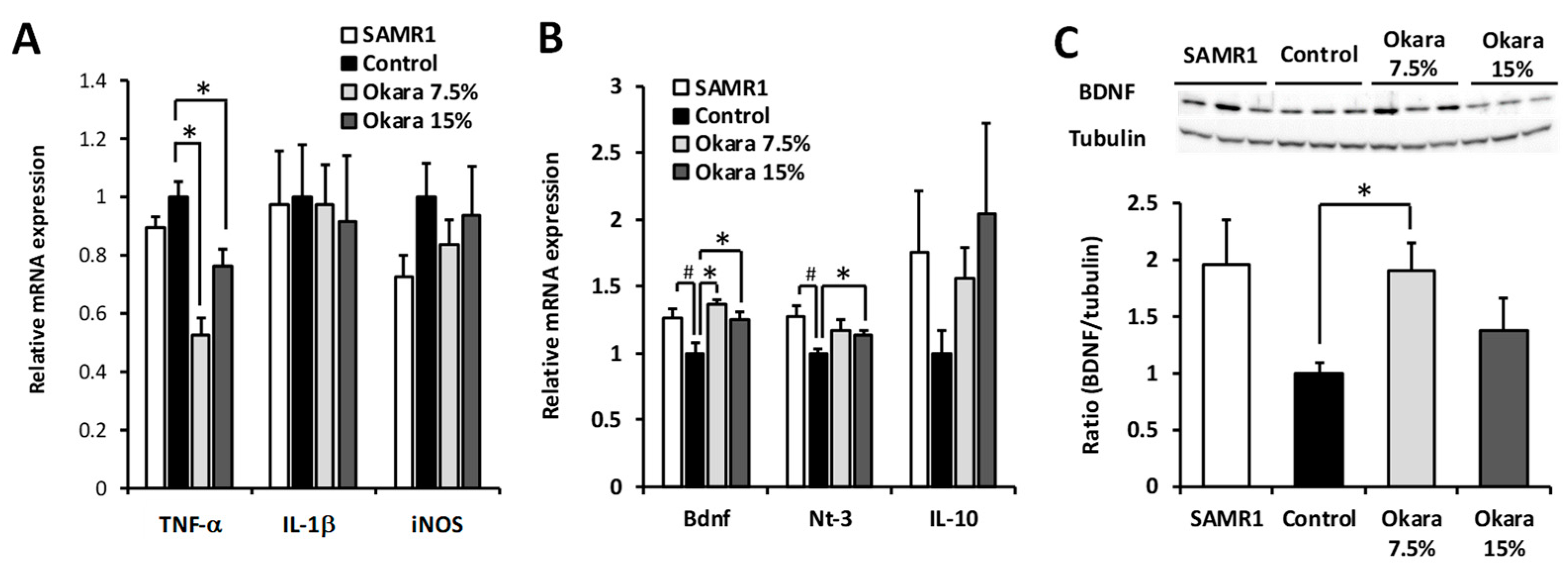
2.7. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.8. Western Blotting Analysis
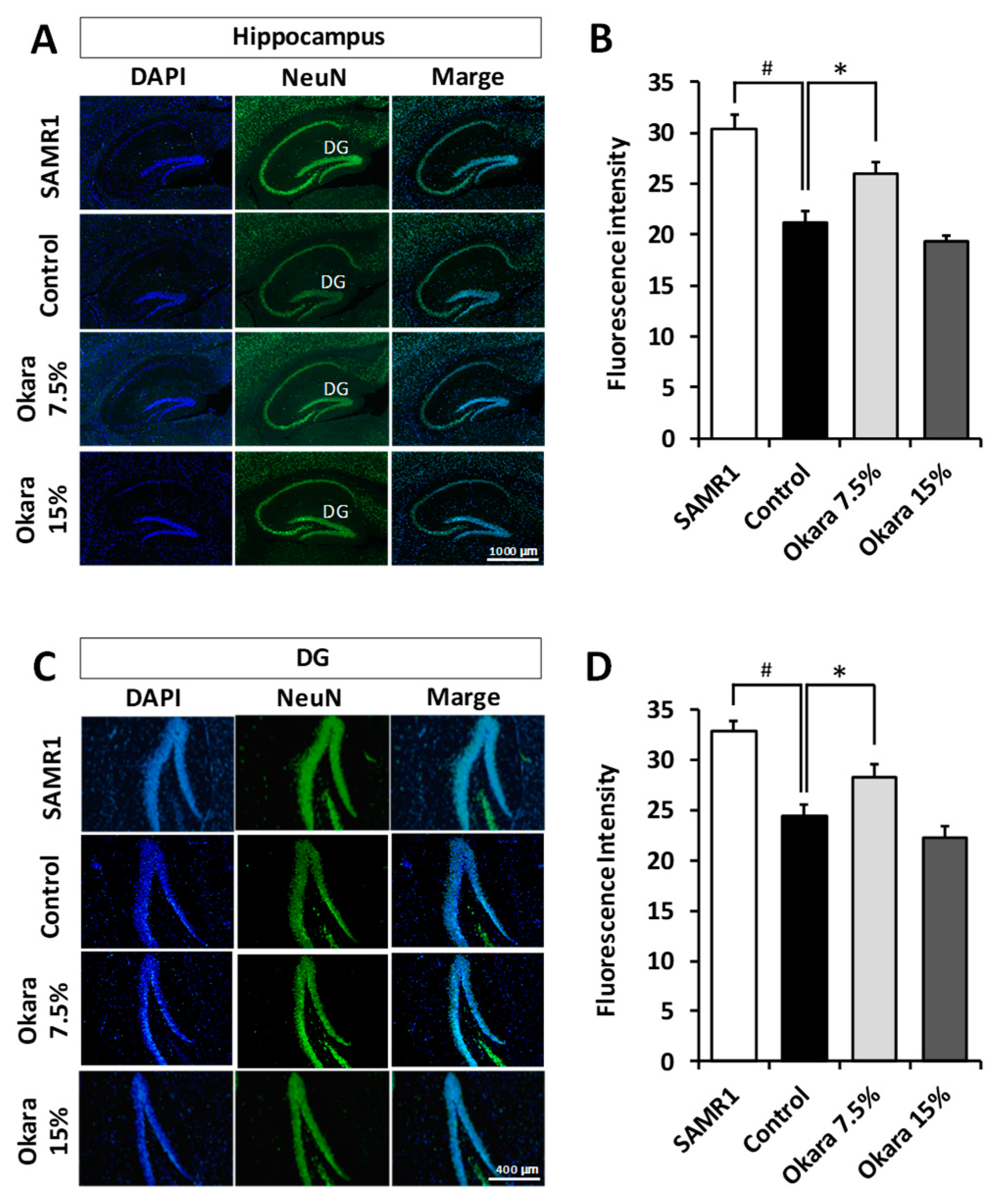
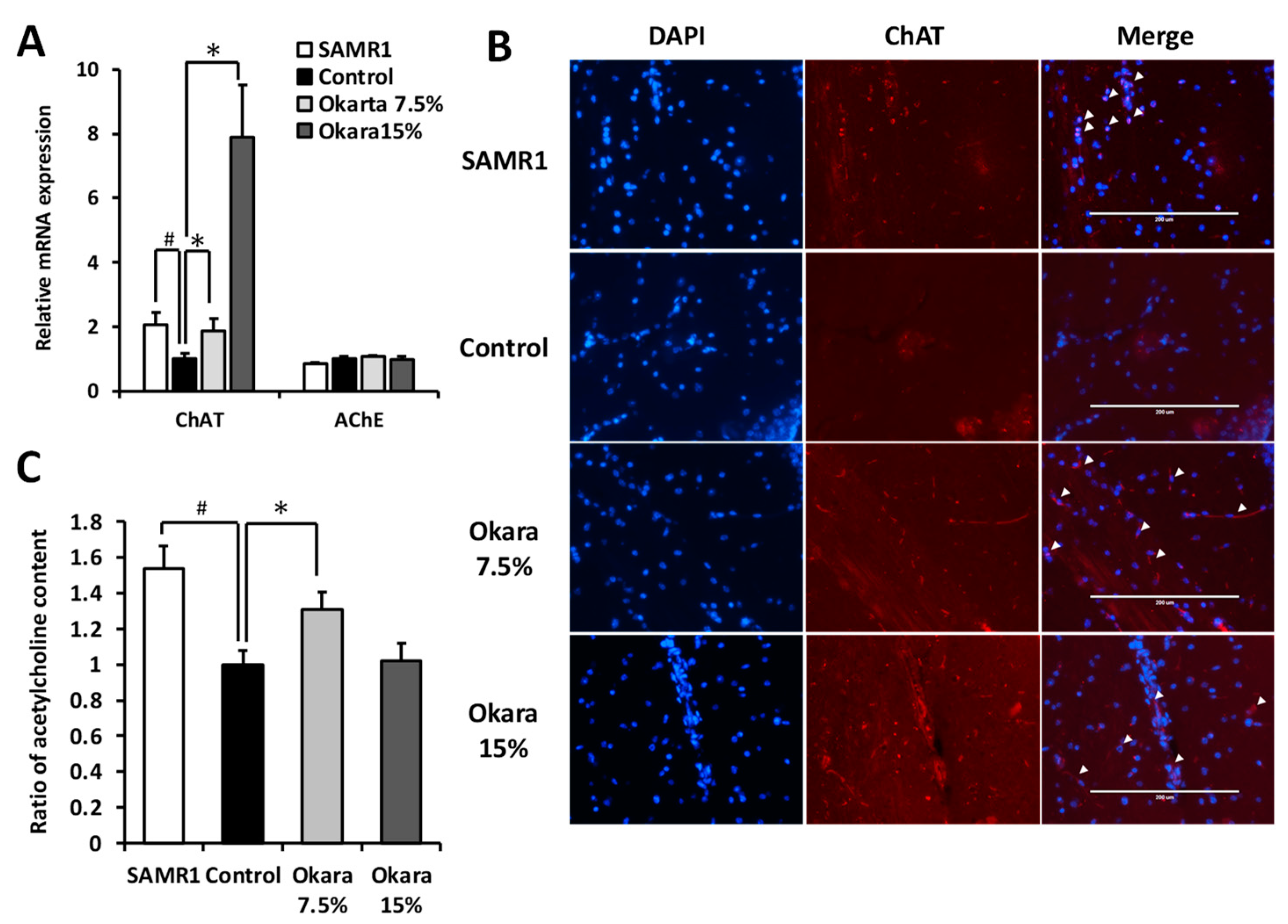
2.9. Immunohistochemical Analysis
2.10. Measurement of Acetylcholine Concentration
2.11. Statistical Analysis
3. Results
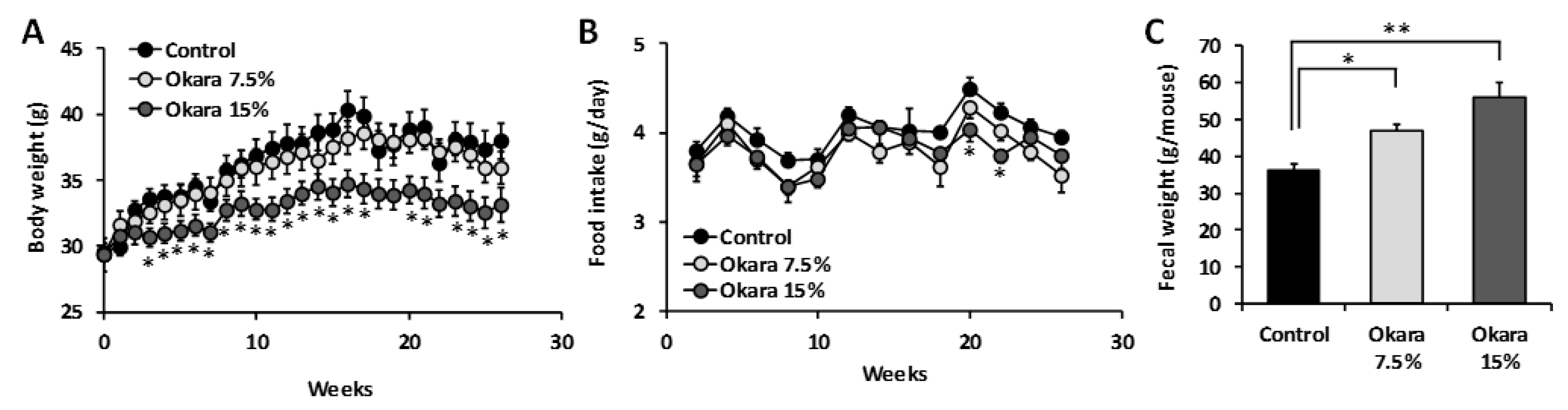
3.1. Effects of 26 Weeks of Okara Administration on the Body Weight, Food Intake, and Fecal Weight of SAMP8 Mice
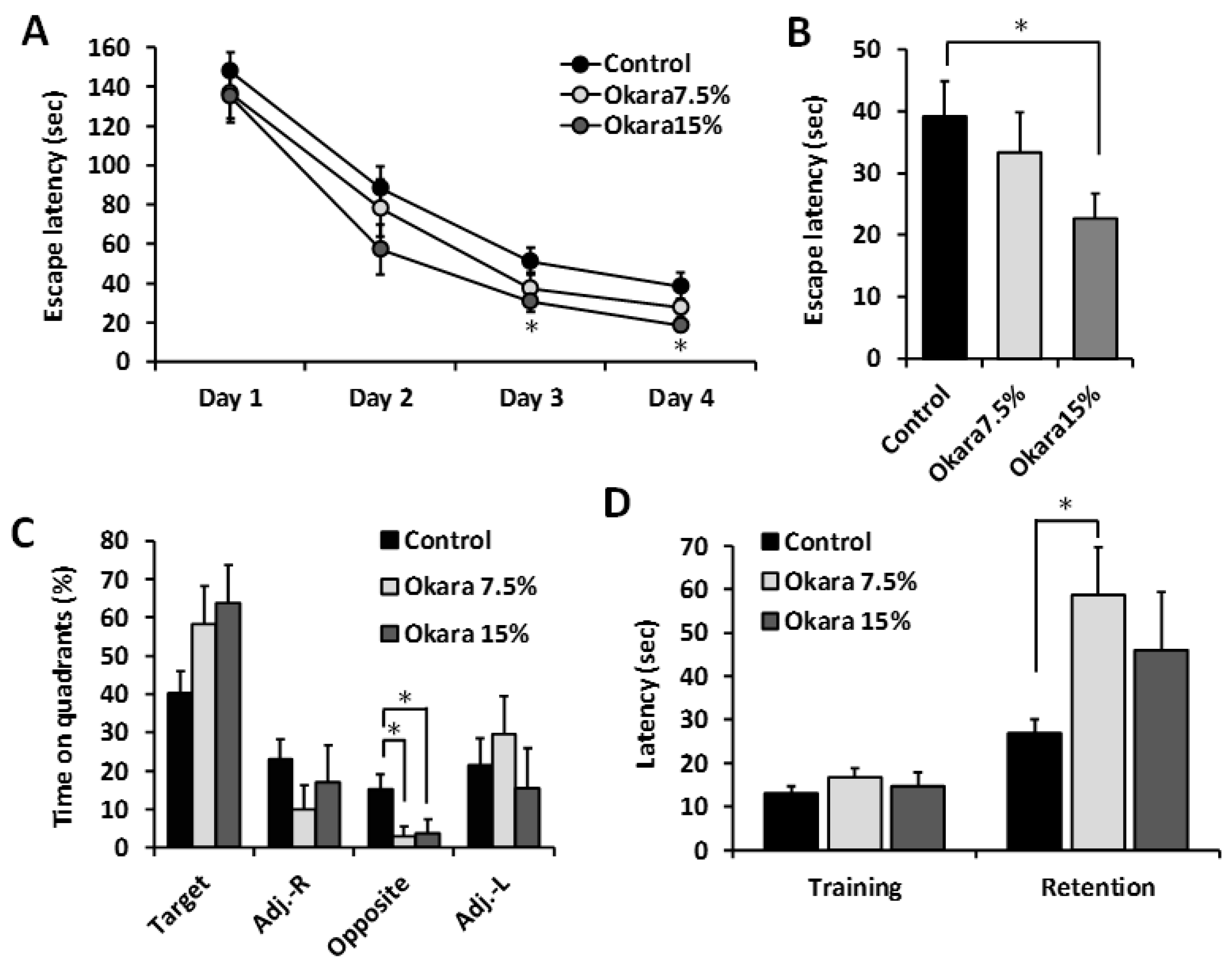
3.2. Oral Administration of Okara Suppressed Age-Related Cognitive Impairment in SAMP8 Mice
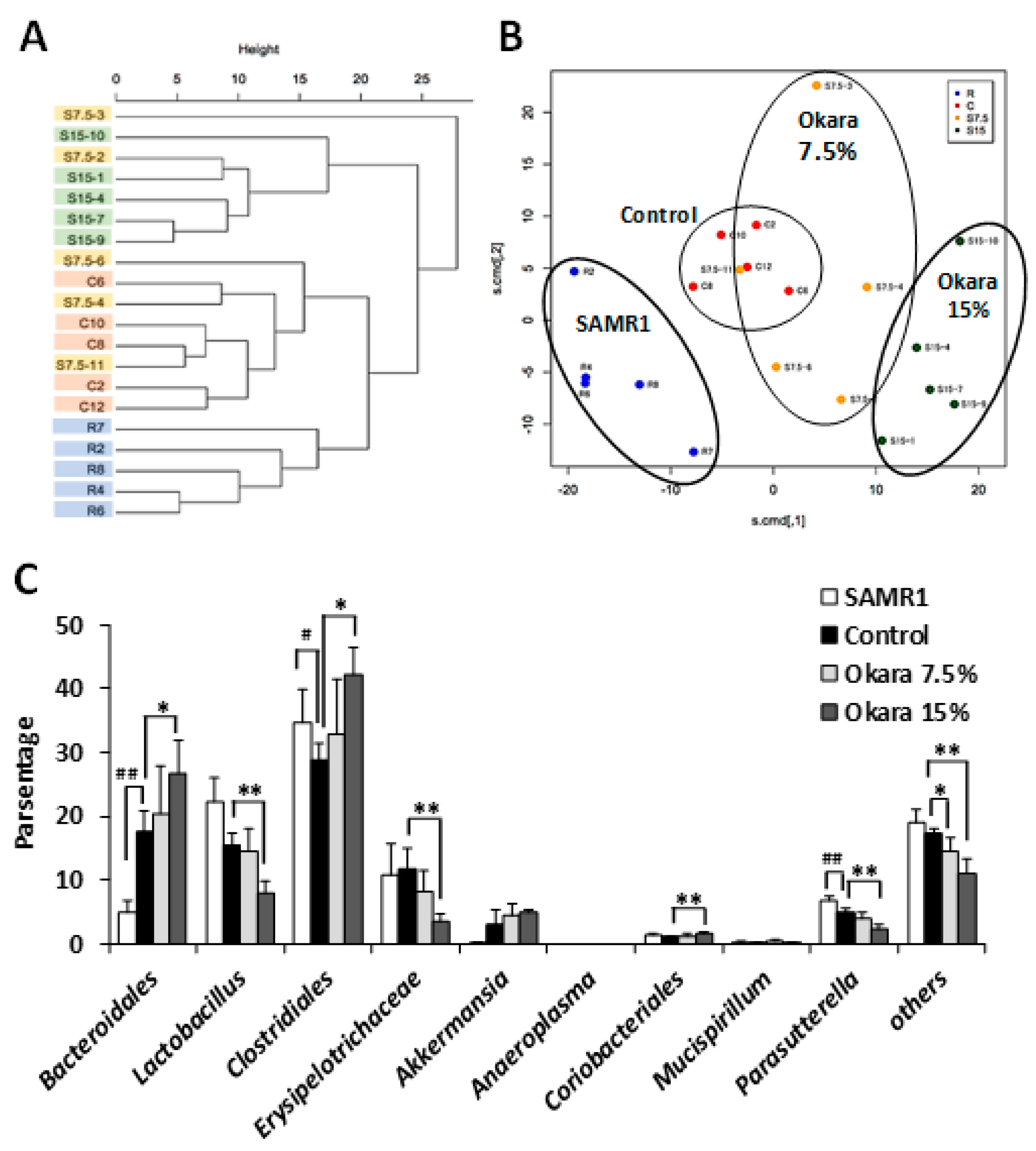
3.3. Administration of Okara at a Higher Dosage Altered the Cecal Microbial Composition of SAMP8 Mice
3.4. Neuroprotection in the Hippocampus and Dentate Gyrus (DG) of SAMP8 Mice by Administration of a Diet Supplemented with Okara
3.5. Effects of Okara Administration on the Cholinergic System in the Mouse Hippocampus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 203. [Google Scholar]
- Lee, H.-J.; Lee, K.-E.; Kim, J.-K.; Kim, D.-H. Suppression of gut dysbiosis by Bifidobacterium longum alleviates cognitive decline in 5XFAD transgenic and aged mice. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundin, J.; Öhman, L.; Simrén, M. Understanding the gut microbiota in inflammatory and functional gastrointestinal diseases. Psychosom. Med. 2017, 79, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.; Keating, D.; Young, R.; Wong, M.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738. [Google Scholar] [CrossRef] [Green Version]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Jiménez-Escrig, A.; Tenorio, M.D.; Espinosa-Martos, I.; Rupérez, P. Health-promoting effects of a dietary fiber concentrate from the soybean byproduct okara in rats. J. Agric. Food Chem. 2008, 56, 7495–7501. [Google Scholar] [CrossRef]
- Kaczmarczyk, M.M.; Miller, M.J.; Freund, G.G. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism 2012, 61, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Proctor, C.; Thiennimitr, P.; Chattipakorn, N.; Chattipakorn, S.C. Diet, gut microbiota and cognition. Metab. Brain Dis. 2017, 32, 1–17. [Google Scholar] [CrossRef]
- Tarr, A.J.; Galley, J.D.; Fisher, S.E.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The prebiotics 3′ Sialyllactose and 6′ Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut–brain axis. Brain Behav. Immun. 2015, 50, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Cao, S.; Zhang, X. Modulation of gut microbiota–brain axis by probiotics, prebiotics, and diet. J. Agric. Food Chem. 2015, 63, 7885–7895. [Google Scholar] [CrossRef]
- Cho, S.S.; Qi, L.; Fahey Jr, G.C.; Klurfeld, D.M. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am. J. Clin. Nutr. 2013, 98, 594–619. [Google Scholar] [CrossRef]
- Huynh, K.; Schneider, M.; Gareau, M. Altering the Gut Microbiome for Cognitive Benefit? In The Gut-Brain Axis; Elsevier: Amsterdam, The Netherlands, 2016; pp. 319–337. [Google Scholar]
- Romo-Araiza, A.; Gutiérrez-Salmeán, G.; Galván, E.J.; Hernández-Frausto, M.; Herrera-López, G.; Romo-Parra, H.; García-Contreras, V.; Fernández-Presas, A.M.; Jasso-Chávez, R.; Borlongan, C.V. Probiotics and prebiotics as a therapeutic strategy to improve memory in a model of middle-aged rats. Front. Aging Neurosci. 2018, 10, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smriga, M.; Chen, J.; Zhang, J.-T.; NARUI, T.; SHIBATA, S.; HIRANO, E.; SAITO, H. Isolichenan, an α-glucan isolated from lichen Cetrariella islandica, repaires impaired learning behaviors and facilitates hippocampal synaptic plasticity. Proc. Jpn. Acad. Ser. B 1999, 75, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Han, H.S.; Jang, J.-H.; Jang, J.H.; Choi, J.S.; Kim, Y.J.; Lee, C.; Lim, S.H.; Lee, H.-K.; Lee, J. Water extract of Triticum aestivum L. and its components demonstrate protective effect in a model of vascular dementia. J. Med. Food 2010, 13, 572–578. [Google Scholar] [CrossRef]
- Takeda, T.; Hosokawa, M.; Higuchi, K.; Hosono, M.; Akiguchi, I.; Katoh, H. A novel murine model of aging, Senescence-Accelerated Mouse (SAM). Arch. Gerontol. Geriatr. 1994, 19, 185–192. [Google Scholar] [CrossRef]
- Flood, J.F.; Morley, J.E. Learning and memory in the SAMP8 mouse. Neurosci. Biobehav. Rev. 1997, 22, 1–20. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Poon, H.F. The senescence-accelerated prone mouse (SAMP8): A model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp. Gerontol. 2005, 40, 774–783. [Google Scholar] [CrossRef]
- Corpuz, H.; Ichikawa, S.; Arimura, M.; Mihara, T.; Kumagai, T.; Mitani, T.; Nakamura, S.; Katayama, S. Long-term diet supplementation with Lactobacillus paracasei K71 prevents age-related cognitive decline in senescence-accelerated mouse prone 8. Nutrients 2018, 10, 762. [Google Scholar] [CrossRef] [Green Version]
- Katayama, S.; Sugiyama, H.; Kushimoto, S.; Uchiyama, Y.; Hirano, M.; Nakamura, S. Effects of sesaminol feeding on brain Aβ accumulation in a senescence-accelerated mouse-prone 8. J. Agric. Food Chem. 2016, 64, 4908–4913. [Google Scholar] [CrossRef]
- Katayama, S.; Imai, R.; Sugiyama, H.; Nakamura, S. Oral administration of soy peptides suppresses cognitive decline by induction of neurotrophic factors in SAMP8 mice. J. Agric. Food Chem. 2014, 62, 3563–3569. [Google Scholar] [CrossRef]
- Vong, W.C.; Liu, S.-Q. Biovalorisation of okara (soybean residue) for food and nutrition. Trends Food Sci. Technol. 2016, 52, 139–147. [Google Scholar] [CrossRef]
- Li, B.; Qiao, M.; Lu, F. Composition, nutrition, and utilization of okara (soybean residue). Food Rev. Int. 2012, 28, 231–252. [Google Scholar] [CrossRef]
- Young-Bernier, M.; Kamil, Y.; Tremblay, F.; Davidson, P.S. Associations between a neurophysiological marker of central cholinergic activity and cognitive functions in young and older adults. Behav. Brain Funct. 2012, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Keogh, J.; Clifton, P. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr. Res. Rev. 2018, 31, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narushima, S.; Sugiura, Y.; Oshima, K.; Atarashi, K.; Hattori, M.; Suematsu, M.; Honda, K. Characterization of the 17 strains of regulatory T cell-inducing human-derived Clostridia. Gut Microbes 2014, 5, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Nagano, Y.; Itoh, K.; Honda, K. The induction of Treg cells by gut-indigenous Clostridium. Curr. Opin. Immunol. 2012, 24, 392–397. [Google Scholar] [CrossRef]
- Canani, R.B.; Di Costanzo, M.; Leone, L. The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clin. Epigenetics 2012, 4, 4. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Komanduri, M.; Gondalia, S.; Scholey, A.; Stough, C. The microbiome and cognitive aging: A review of mechanisms. Psychopharmacology 2019, 236, 1559–1571. [Google Scholar] [CrossRef]
- Di Filippo, M.; Chiasserini, D.; Gardoni, F.; Viviani, B.; Tozzi, A.; Giampà, C.; Costa, C.; Tantucci, M.; Zianni, E.; Boraso, M. Effects of central and peripheral inflammation on hippocampal synaptic plasticity. Neurobiol. Dis. 2013, 52, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, M. Ngf, bdnf, nt3, and nt4. In Neurotrophic Factors; Springer: Berlin, Germany, 2014; pp. 3–15. [Google Scholar]
- AlFadly, E.D.; Elzahhar, P.A.; Tramarin, A.; Elkazaz, S.; Shaltout, H.; Abu-Serie, M.M.; Janockova, J.; Soukup, O.; Ghareeb, D.A.; El-Yazbi, A.F. Tackling neuroinflammation and cholinergic deficit in Alzheimer’s disease: Multi-target inhibitors of cholinesterases, cyclooxygenase-2 and 15-lipoxygenase. Eur. J. Med. Chem. 2019, 167, 161–186. [Google Scholar] [CrossRef] [PubMed]
- Mitsushima, D.; Sano, A.; Takahashi, T. A cholinergic trigger drives learning-induced plasticity at hippocampal synapses. Nat. Commun. 2013, 4, 2760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.; Wang, Y.; Wang, D.; Zhang, L.; Lv, J.; Jiang, N.; Fan, B.; Liu, X.; Wang, F. Neuroprotective effects of soy isoflavones on scopolamine-induced amnesia in mice. Nutrients 2018, 10, 853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahmohammadi, A.; Rousta, A.-M.; Azadi, M.-R.; Fahanik-Babaei, J.; Baluchnejadmojarad, T.; Roghani, M. Soy isoflavone genistein attenuates lipopolysaccharide-induced cognitive impairments in the rat via exerting anti-oxidative and anti-inflammatory effects. Cytokine 2018, 104, 151–159. [Google Scholar]
- Ko, Y.-H.; Kwon, S.-H.; Ma, S.-X.; Seo, J.-Y.; Lee, B.-R.; Kim, K.; Kim, S.Y.; Lee, S.-Y.; Jang, C.-G. The memory-enhancing effects of 7, 8, 4′-trihydroxyisoflavone, a major metabolite of daidzein, are associated with activation of the cholinergic system and BDNF signaling pathway in mice. Brain Res. Bull. 2018, 142, 197–206. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Y.; Xu, T.; Li, Q.; Wang, D.; Zhang, L.; Fan, B.; Wang, F.; Liu, X. Genistein ameliorates scopolamine-induced amnesia in mice through the regulation of the cholinergic neurotransmission, antioxidant system and the ERK/CREB/BDNF signaling. Front. Pharmacol. 2018, 9, 1153. [Google Scholar] [CrossRef]
| Ingredients | Group | ||
|---|---|---|---|
| Control | Okara 7.5% | Okara 15% | |
| Okara | 0 | 7.5 | 15.0 |
| Casein | 14 | 12.2 | 10.4 |
| l-cystine | 0.18 | 0.18 | 0.18 |
| Cornstarch | 46.6 | 42.2 | 37.9 |
| α-cornstarch | 15.5 | 15.5 | 15.5 |
| Sucrose | 10 | 10 | 10 |
| Soybean oil | 4 | 2.6 | 1.2 |
| Cellulose | 5 | 5 | 5 |
| Mineral mix | 3.5 | 3.5 | 3.5 |
| Vitamin mix | 1 | 1 | 1 |
| Choline bitartrate | 0.25 | 0.25 | 0.25 |
| tert-Butylhydroquinone | 0.0008 | 0.0008 | 0.0008 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corpuz, H.M.; Arimura, M.; Chawalitpong, S.; Miyazaki, K.; Sawaguchi, M.; Nakamura, S.; Katayama, S. Oral Administration of Okara Soybean By-Product Attenuates Cognitive Impairment in a Mouse Model of Accelerated Aging. Nutrients 2019, 11, 2939. https://doi.org/10.3390/nu11122939
Corpuz HM, Arimura M, Chawalitpong S, Miyazaki K, Sawaguchi M, Nakamura S, Katayama S. Oral Administration of Okara Soybean By-Product Attenuates Cognitive Impairment in a Mouse Model of Accelerated Aging. Nutrients. 2019; 11(12):2939. https://doi.org/10.3390/nu11122939
Chicago/Turabian StyleCorpuz, Henry M., Misa Arimura, Supatta Chawalitpong, Keiko Miyazaki, Makoto Sawaguchi, Soichiro Nakamura, and Shigeru Katayama. 2019. "Oral Administration of Okara Soybean By-Product Attenuates Cognitive Impairment in a Mouse Model of Accelerated Aging" Nutrients 11, no. 12: 2939. https://doi.org/10.3390/nu11122939






