Regulation of Heat Shock Factor Pathways by γ-aminobutyric Acid (GABA) Associated with Thermotolerance of Creeping Bentgrass
Abstract
:1. Introduction
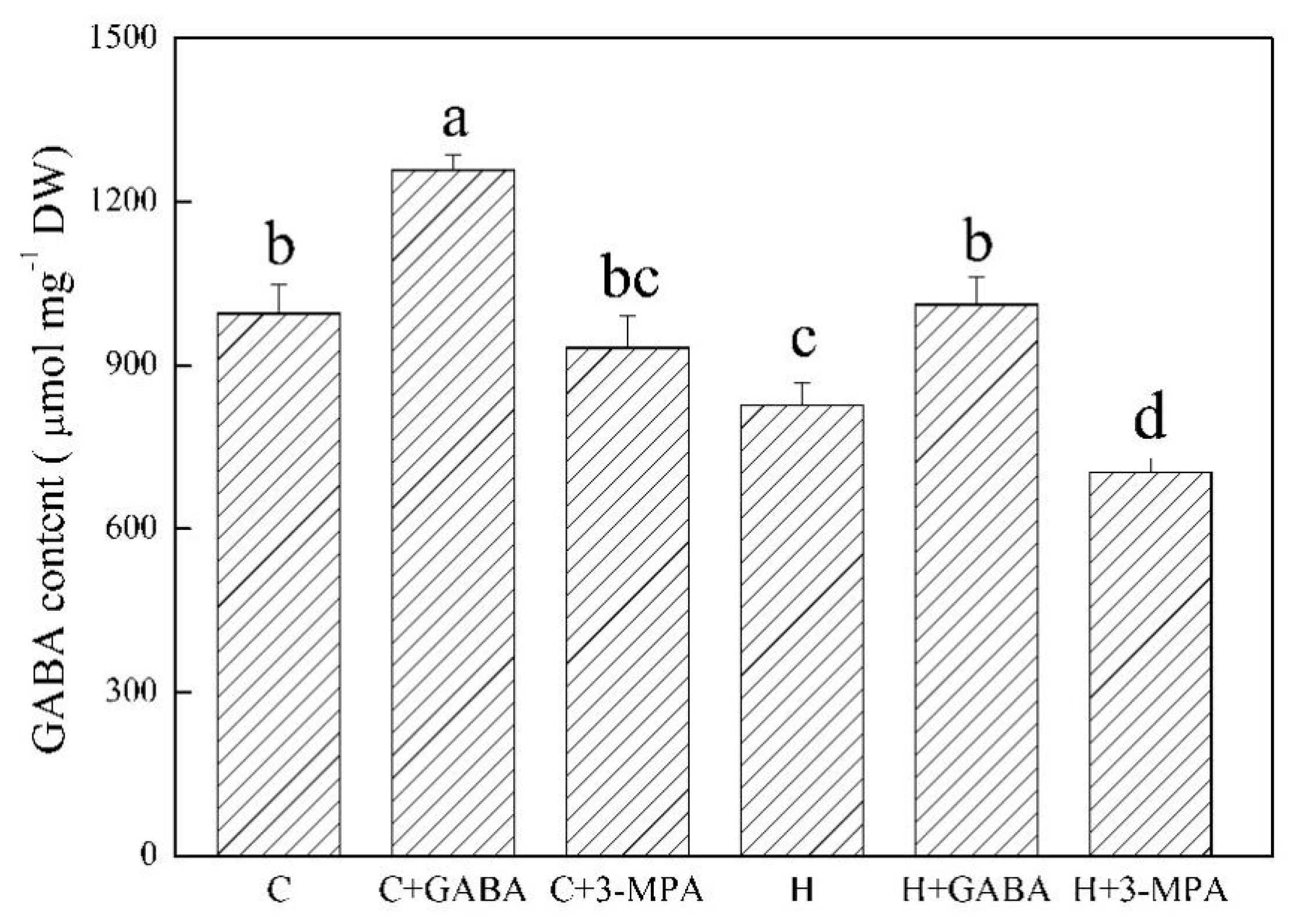
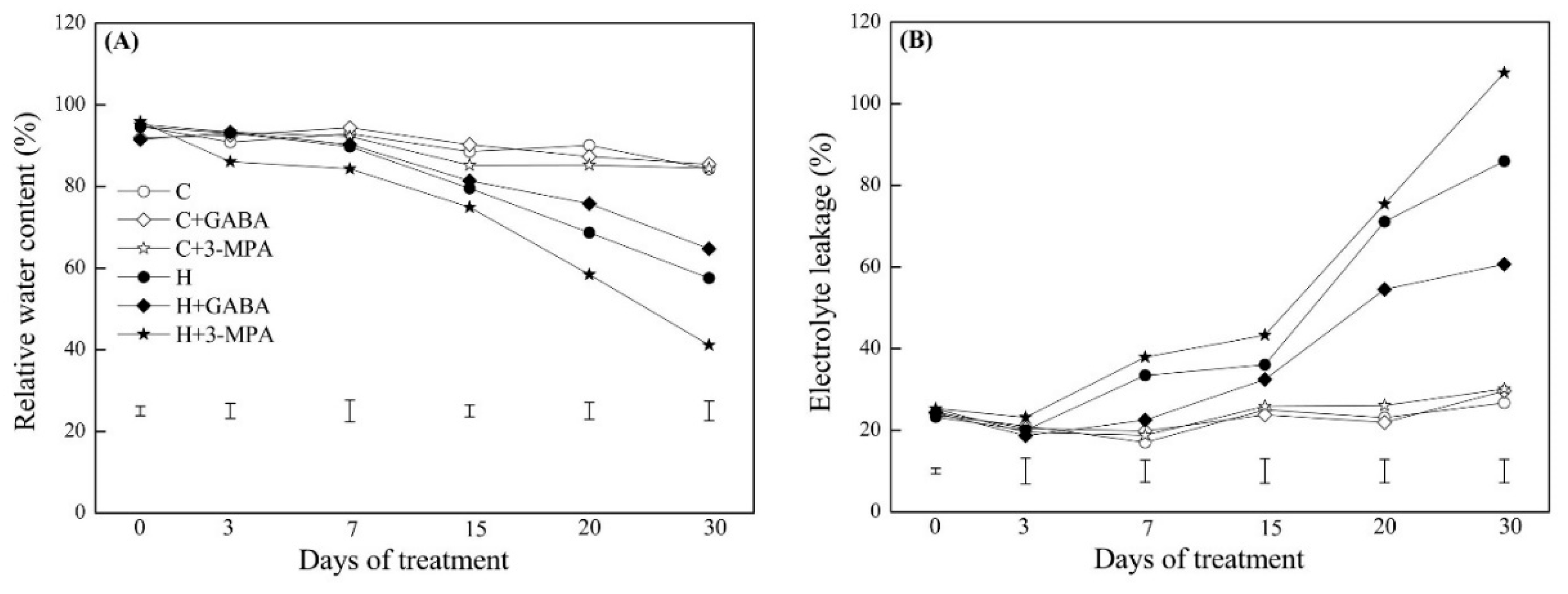
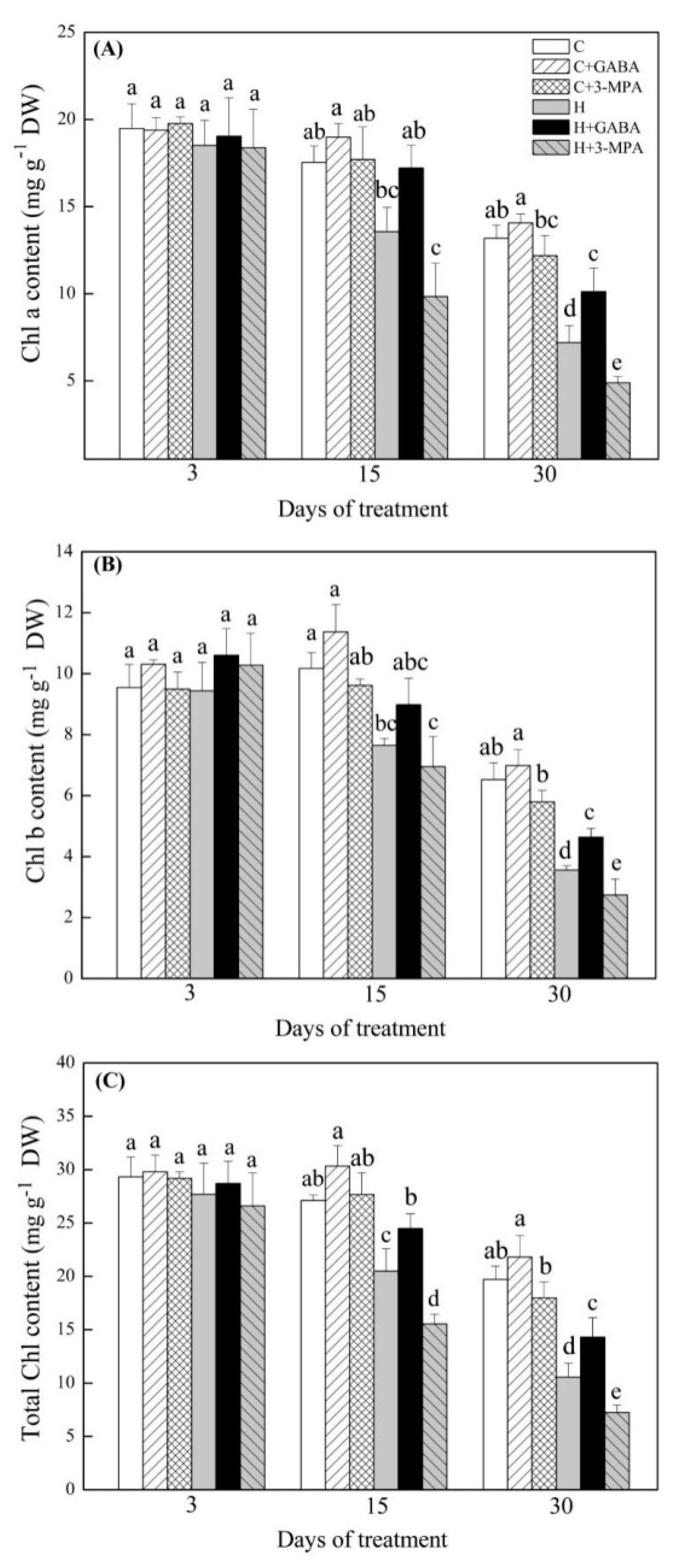
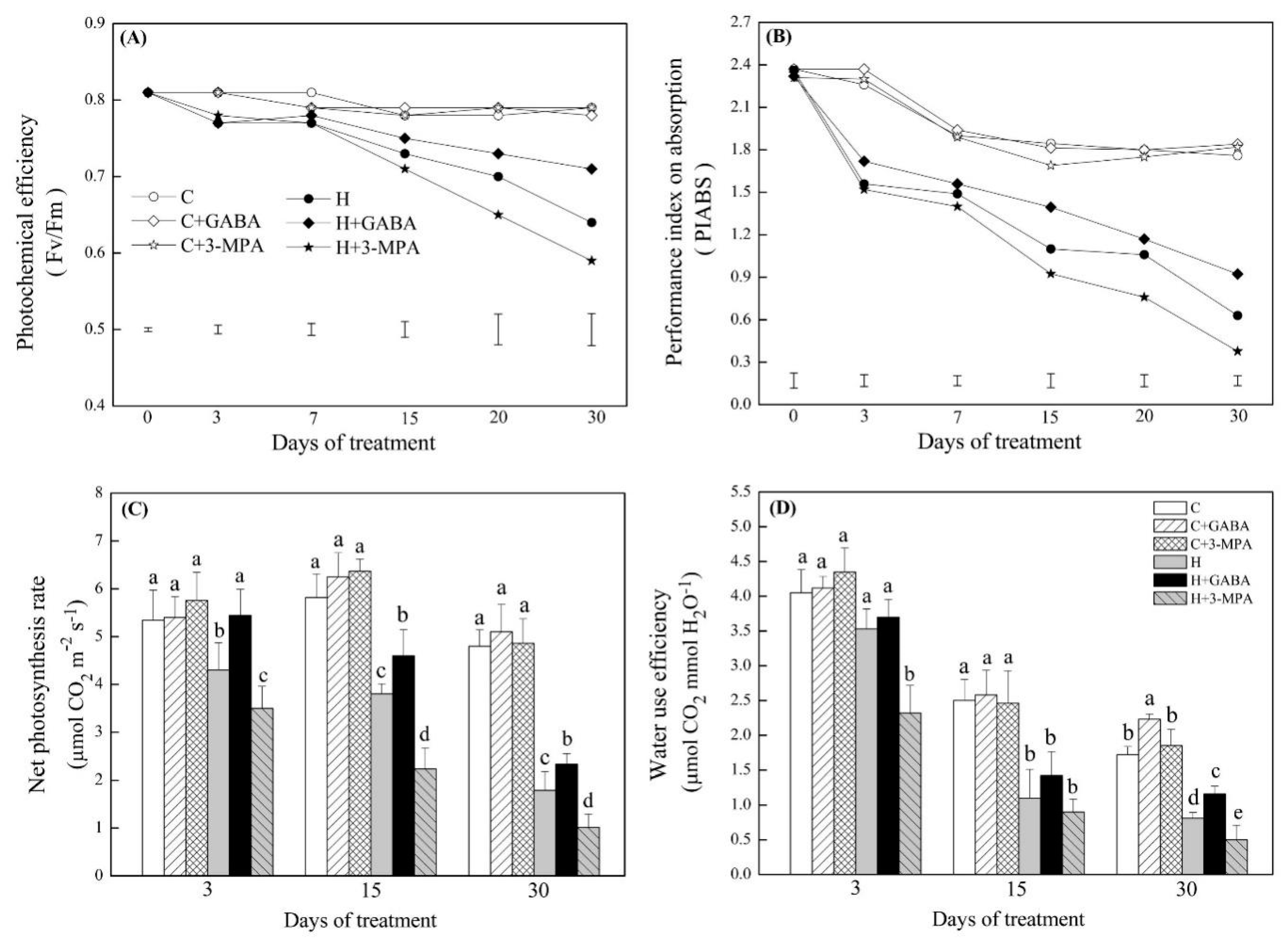
2. Results
2.1. The Mediation of Endogenous GABA Level Affects Water Status and Photosynthesis during Heat Stress
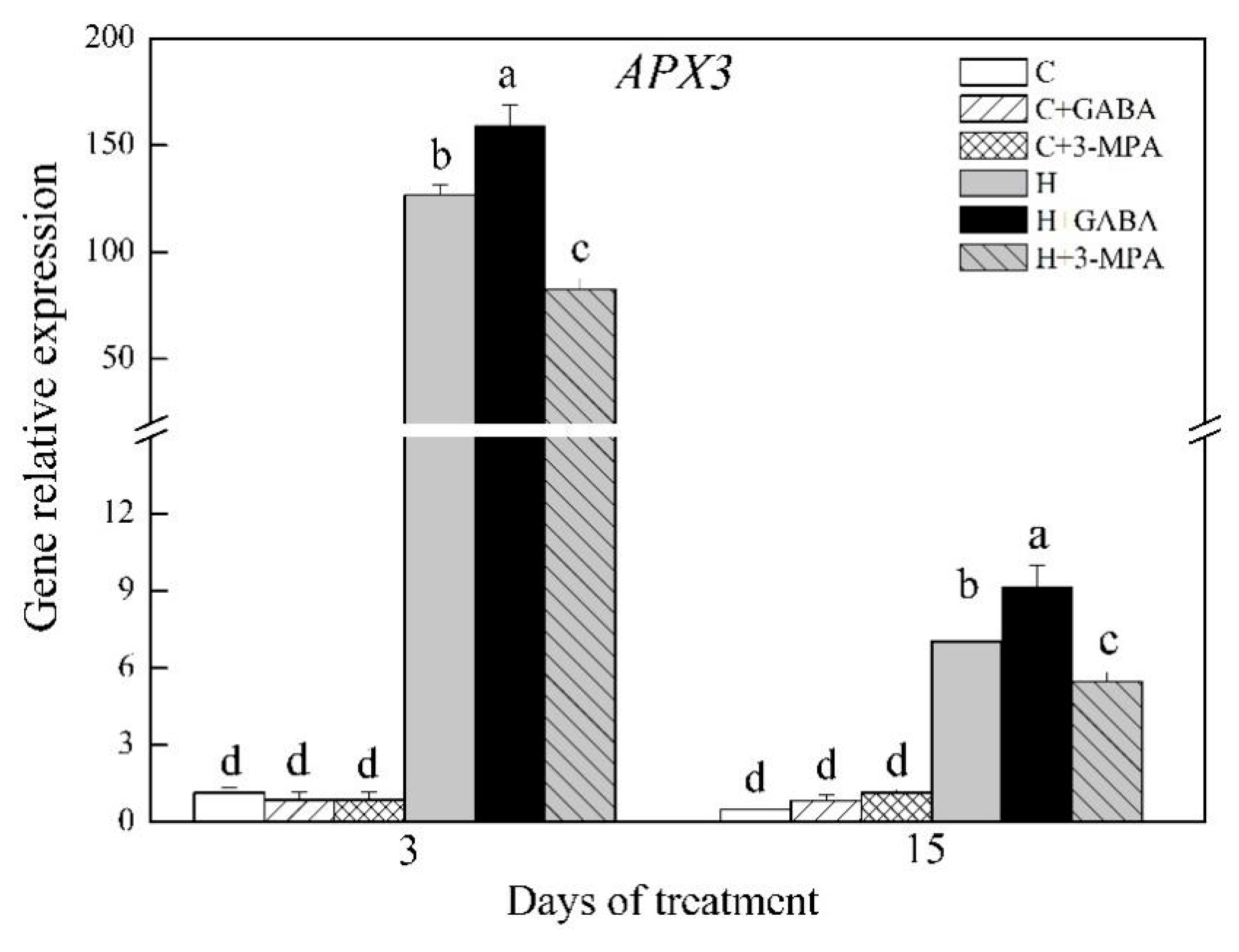
2.2. The Mediation of Endogenous GABA Level Affects Antioxidant Capacity and Oxidative Damage under Heat Stress
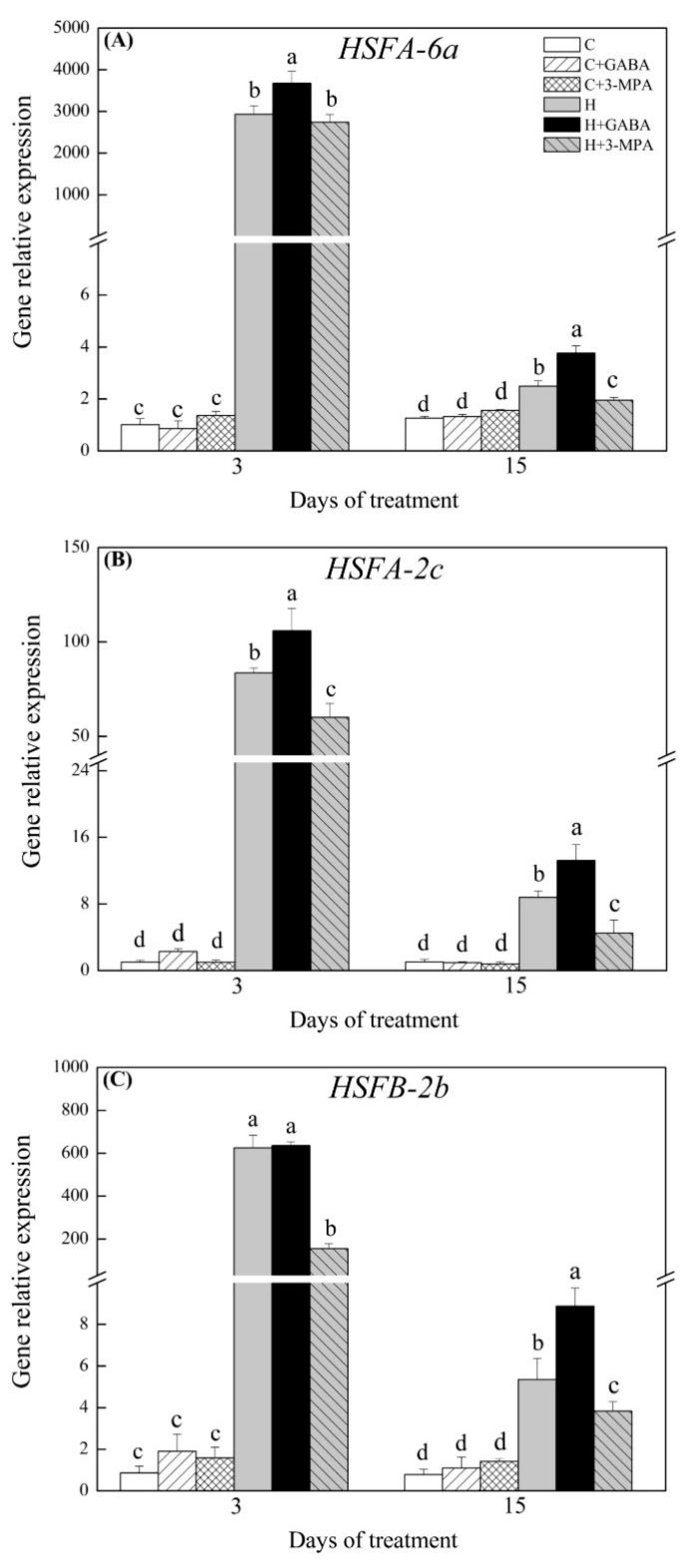
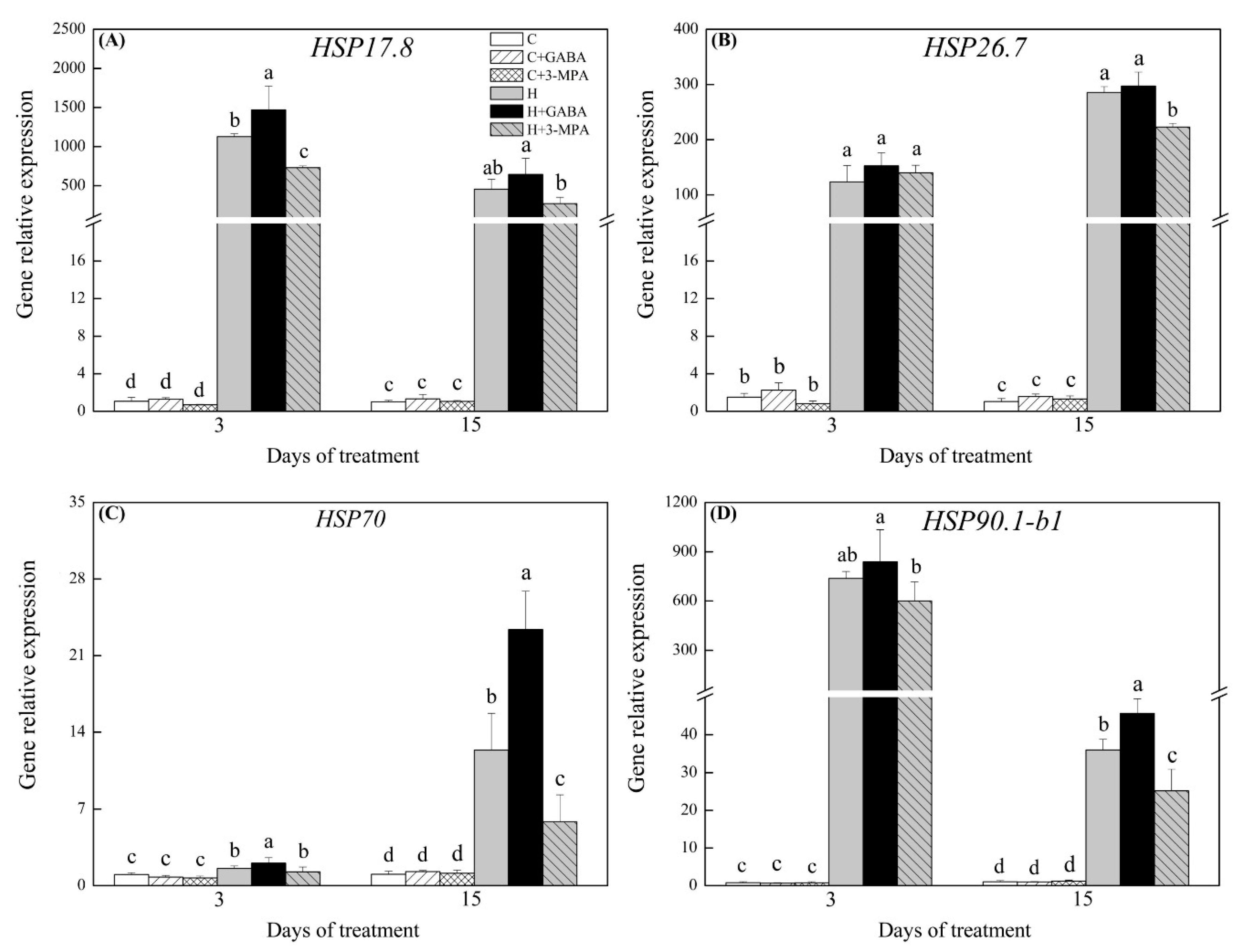
2.3. The Mediation of Endogenous GABA Level Affects HSFs Pathways during Heat Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. The Measurement of Endogenous GABA, Water Status, and Photosynthesis
4.3. The Measurement of Oxidative Damage and Total Antioxidant Capacity
4.4. Genes Expression Analyses
4.5. Statistical Analysis
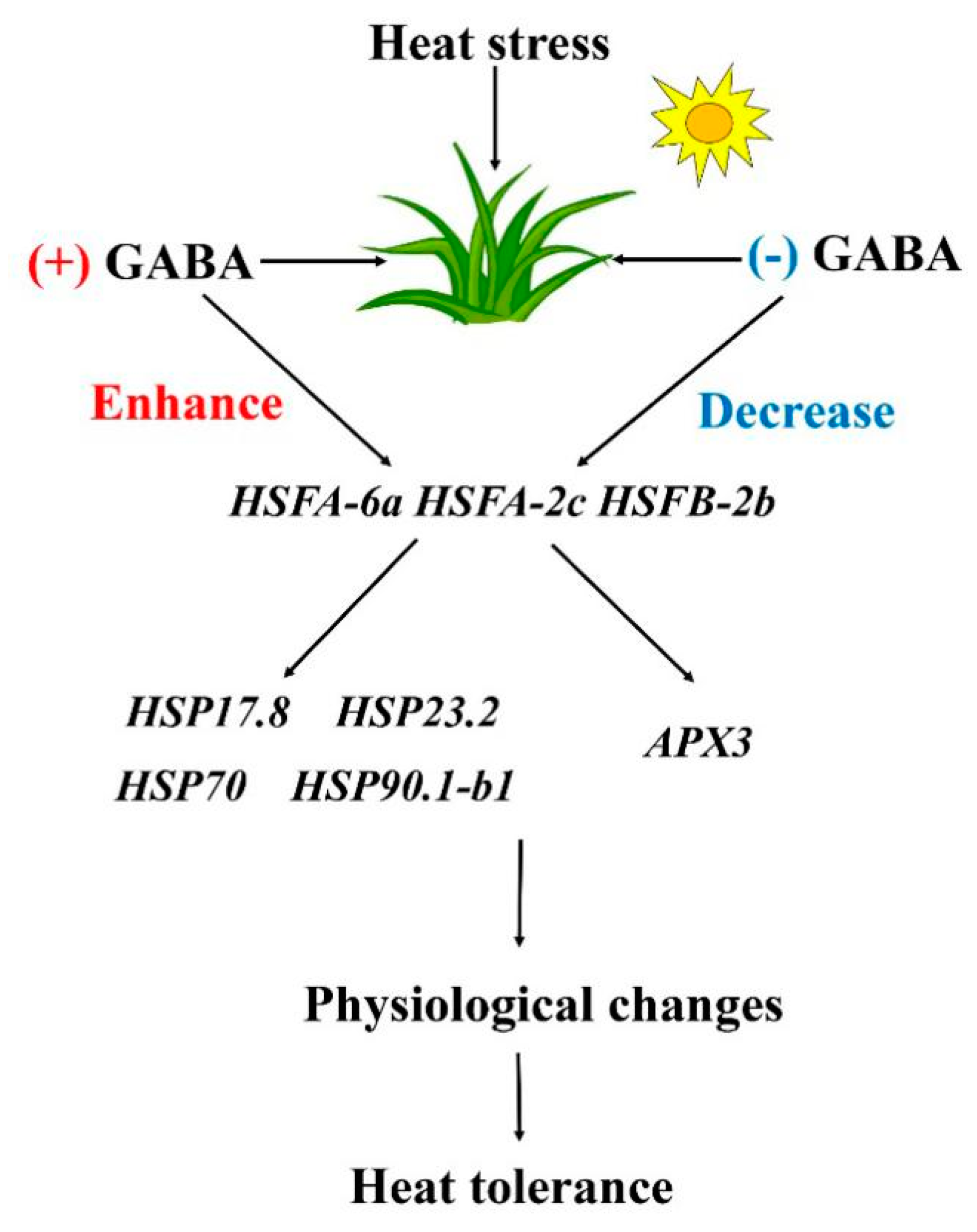
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wahid:, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Liu, J.M.; Feng, Z.J.; Chen, M.; Zhou, Y.B.; Chen, J.; Zhaoshi, X.U.; Guo, C.H. The response to heat and screening of the interacting proteins of zinc finger protein Gm Di19–5 in soybean. Sci. Agric. Sin. 2017, 50, 2389–2398. [Google Scholar]
- Christine, K.; Joshua, H.; Bonos, S.A. Characterization of 215 simple sequence repeat markers in creeping bentgrass (Agrostis stolonifera L.). Mol. Ecol. Resour. 2011, 11, 872–876. [Google Scholar]
- Hacisalihoglu, G.; Ross, Z. Effectiveness of seed matriconditioning on germination and storability parameters of creeping bentgrass. Proc. Meet. Fla. State Hortic. Soc. 2009, 122, 414–415. [Google Scholar]
- Chakraborty, N.; Bae, J.; Warnke, S.; Chang, T.; Jung, G. Linkage map construction in allotetraploid creeping bentgrass (Agrostis stolonifera L.). Tag.Theor. Appl. Genet. Theor. Und Angew. Genet. 2005, 111, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: mission possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Munshaw, G.; Stewart, B.R.; Massey, J.H.; Lemus, R.W. The effect of exogenous fructose on creeping bentgrass heat tolerance; Mississippi State University: Starkville, MS, USA, 2010. [Google Scholar]
- Larkindale, J.; Huang, B. Effects of abscisic acid, salicylic acid, ethylene and hydrogen peroxide in thermotolerance and recovery for creeping bentgrass. Plant Growth Regul. 2005, 47, 17–28. [Google Scholar] [CrossRef]
- Seher, Y.; Filiz, O.; Melike, B. Gamma-amino butyric acid, glutamate dehydrogenase and glutamate decarboxylase levels in phylogenetically divergent plants. Plant Syst. Evol. 2013, 299, 403–412. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, J.; Sun, Y.Q.; Xie, J. Protective effect of γ-aminobutyric acid on antioxidation function in intestinal mucosa of Wenchang chicken induced by heat stress. J. Anim. Plant Sci. 2013, 23, 1634–1641. [Google Scholar]
- Cheng, J.B.; Bu, D.P.; Wang, J.Q.; Sun, X.Z.; Pan, L.; Zhou, L.Y.; Liu, W. Effects of rumen-protected γ-aminobutyric acid on performance and nutrient digestibility in heat-stressed dairy cows. J. Dairy Sci. 2014, 97, 5599–5607. [Google Scholar] [CrossRef]
- Bouche, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, J.; Peng, Y.; Huang, B. Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci. Rep. 2016, 6, 30338. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumari, K.; Puthur, J.T. γ-Aminobutyric acid (GABA) priming enhances the osmotic stress tolerance in Piper nigrum Linn. plants subjected to PEG-induced stress. Plant Growth Regul. 2015, 78, 1–11. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Naderi, R.; Jannatizadeh, A.; Babalar, M.; Sarcheshmeh, M.A.A.; Faradonbe, M.Z. Impact of exogenous GABA treatments on endogenous GABA metabolism in anthurium cut flowers in response to postharvest chilling temperature. Plant Physiol. Biochem. Ppb 2016, 106, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, H.; Kaur, R.; Kaur, S.; Singh, R. γ-Aminobutyric acid (GABA) imparts partial protection from heat stress injury to rice seedlings by improving leaf turgor and upregulating osmoprotectants and antioxidants. J. Plant Growth Regul. 2014, 33, 408–419. [Google Scholar] [CrossRef]
- Locy, R.D.; Wu, S.J.; Bisnette, J.; Barger, T.W.; Mcnabb, D.; Zik, M.; Fromm, H.; Singh, N.K.; Cherry, J.H. The Regulation of GABA Accumulation by Heat Stress in Arabidopsis. In Plant Tolerance to Abiotic Stresses in Agriculture: Role of Genetic Engineering; Springer: Dordrecht, The Netherlands, 2000; pp. 39–52. [Google Scholar]
- Liu, X.; Huang, B. Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci. 2000, 40, 503–510. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Osmotic adjustment and root growth associated with drought preconditioning-enhanced heat tolerance in Kentucky bluegrass. Crop Sci. 2001, 41, 1168–1173. [Google Scholar] [CrossRef]
- Yang, X.T.; Zhang, Z.Q.; Joyce, D.; Huang, X.M.; Xu, L.Y.; Pang, X.Q. Characterization of chlorophyll degradation in banana and plantain during ripening at high temperature. Food Chem. 2009, 114, 383–390. [Google Scholar] [CrossRef]
- Czarnecka-sVerner, E.; Yuan, C.-X.; Nover, L.; Scharf, K.-D.; Englich, G.; Gurley, W.B. Plant heat shock transcription factors: Positive and negative aspects of regulation. Acta Physiol. Plant. 1997, 19, 529–537. [Google Scholar] [CrossRef]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, W.; Yang, Z.; Liu, J.; Huang, B. Transcriptional regulation of heat shock proteins and ascorbate peroxidase by CtHsfA2b from African bermudagrass conferring heat tolerance in Arabidopsis. Sci. Rep. 2016, 6, 28021. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhan, C.; Huang, B. Heat shock proteins in association with heat tolerance in grasses. Int. J. Proteom. 2011, 2011. [Google Scholar] [CrossRef]
- Vierling, E. The Roles of Heat Shock Proteins in Plants. Annu. Rev. Plant Physiol.Plant Mol. Biol 2003, 42, 579–620. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Riadh, K.; Gopi, R.; Manivannan, P.; Inès, J.; Al-Juburi, H.J.; Zhao, C.X.; Shao, H.B.; Panneerselvam, R. Antioxidant defense responses: Physiological plasticity in higher plants under abiotic constraints. Acta Physiol. Plant. 2009, 31, 427–436. [Google Scholar] [CrossRef]
- Morales, M.A. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar]
- Zhu, X.; Shen, H. Effect of high temperature stress on cell membrane in celery seedlings. North. Hortic. 2014, 7, 16–20. [Google Scholar]
- Rezaei-Chiyaneh, E.; Seyyedi, S.M.; Ebrahimian, E.; Moghaddam, S.S.; Damalas, C.A. Exogenous application of gamma-aminobutyric acid (GABA) alleviates the effect of water deficit stress in black cumin (Nigella sativa L.). Ind. Crops Prod. 2018, 112, 741–748. [Google Scholar] [CrossRef]
- Wang, C.Y.; Jing-Rui, L.I.; Xia, Q.P.; Xiao-Lei, W.U.; Gao, H.B. Influence of exogenous γ-aminobutyric acid (GABA) on GABA metabolism and amino acid contents in roots of melon seedling under hypoxia stress. Chin. J. Appl. Ecol. 2014, 25, 2011–2018. [Google Scholar]
- Gao, H.B.; Zhang, T.J.; Gui-Yun, L.; Xiao-Lei, W.U.; Zhou, Z.N. Effects of exogenous γ-aminobutyric acid on growth and reactive oxygen species metabolism of cucumber seedlings under NaCl stress. Acta Bot. Boreali-Occident. Sin. 2007, 27, 2046–2051. [Google Scholar]
- Wang, Y.; Gu, W.; Meng, Y.; Xie, T.; Li, L.; Li, J.; Wei, S. γ-Aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants. Sci. Rep. 2017, 7, 43609. [Google Scholar] [CrossRef]
- Liu, J.P.; Xiao-Feng, L.I.; Zhu, H.F.; Zhu, Y.Y.; Hou, X.L.; University, N.A. Effects of γ-aminobutyric acid on the growth and photosynthesis of Pakchoi under waterlogging stress. Acta Agric. Shanghai 2016, 32, 55–59. [Google Scholar]
- Li, Y.; Fan, Y.; Ma, Y.; Zhang, Z.; Yue, H.; Wang, L.; Li, J.; Jiao, Y. Effects of exogenous γ-aminobutyric acid (GABA) on photosynthesis and antioxidant system in pepper (Capsicum annuum L.) seedlings under low light stress. J. Plant Growth Regul. 2017, 36, 436–449. [Google Scholar] [CrossRef]
- Wang, X.; Huang, W.; Liu, J.; Yang, Z.; Huang, B. Molecular regulation and physiological functions of a novel FaHsfA2c cloned from tall fescue conferring plant tolerance to heat stress. Plant Biotechnol. J. 2017, 15, 237–248. [Google Scholar] [CrossRef]
- Scharf, K.-D.; Berberich, T.; Ebersberger, I.; Nover, L. The plant heat stress transcription factor (Hsf) family: Structure, function and evolution. Biochim. et Biophys. Acta (BBA)-Gene Regul. Mech. 2012, 1819, 104–119. [Google Scholar] [CrossRef]
- Pascal, K.D.R.; Klaus-Dieter, S.; Lutz, N. The diversity of plant heat stress transcription factors. Trends Plant Sci. 2007, 12, 452–457. [Google Scholar]
- Nover, L.; Bharti, K.; D.?Ring, P.; Mishra, S.K.; Ganguli, A.; Scharf, K.D. Arabidopsis and the heat stress transcription factor world: How many heat stress transcription factors do we need? Cell Stress Chaperones 2001, 6, 177–189. [Google Scholar] [CrossRef]
- Hwang, S.M.; Kim, D.W.; Woo, M.S.; Jeong, H.S.; Son, Y.S.; Akhter, S.; Choi, G.J.; Bahk, J.D. Functional characterization of Arabidopsis HsfA6a as a heat-shock transcription factor under high salinity and dehydration conditions. Plant Cell Environ. 2014, 37, 1202–1222. [Google Scholar] [CrossRef]
- Ma, H.; Wang, C.; Yang, B.; Cheng, H.; Wang, Z.; Mijiti, A.; Ren, C.; Qu, G.; Zhang, H.; Ma, L. CarHSFB2, a Class B Heat shock transcription factor, is involved in different developmental processes and various stress responses in Chickpea (Cicer Arietinum L.). Plant Mol. Biol. Report. 2015, 34, 1–14. [Google Scholar] [CrossRef]
- Baniwal, S.K.; Bharti, K.; Chan, K.Y.; Fauth, M.; Ganguli, A.; Kotak, S.; Mishra, S.K.; Nover, L.; Port, M.; Scharf, K.D. Heat stress response in plants: A complex game with chaperones and more than twenty heat stress transcription factors. J. Biosci. 2004, 29, 471–487. [Google Scholar] [CrossRef]
- Timperio, A.M.; Egidi, M.G.; Zolla, L. Proteomics applied on plant abiotic stresses: Role of heat shock proteins (HSP). J. Proteom. 2008, 71, 391–411. [Google Scholar] [CrossRef]
- Huang, B.X. Chenping. Identification and characterization of proteins associated with plant tolerance to heat stress. J. Integr. Plant Biol. 2010, 50, 1230–1237. [Google Scholar] [CrossRef]
- Schramm, F.; Ganguli, A.; Kiehlmann, E.; Englich, G.; Walch, D.; von Koskull-Döring, P. The heat stress transcription factor HsfA2 serves as a regulatory amplifier of a subset of genes in the heat stress response in Arabidopsis. Plant Mol. Biol. 2006, 60, 759–772. [Google Scholar] [CrossRef]
- Haslbeck, M. sHsps and their role in the chaperone network. Cell. Mol. Life Sci. CMLS 2002, 59, 1649–1657. [Google Scholar] [CrossRef]
- Chen, X.; Lin, S.; Liu, Q.; Jian, H.; Zhang, W.; Lin, J.; Wang, Y.; Ke, Y.; He, H. Expression and interaction of small heat shock proteins (sHsps) in rice in response to heat stress. BB-Proteins Proteom. 2014, 1844, 818–828. [Google Scholar] [CrossRef]
- Kim, K.H.; Alam, I.; Kim, Y.G.; Sharmin, S.A.; Lee, K.W.; Lee, S.H.; Lee, B.H. Overexpression of a chloroplast-localized small heat shock protein OsHSP26 confers enhanced tolerance against oxidative and heat stresses in tall fescue. Biotechnol. Lett. 2012, 34, 371–377. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Goatley, M.; Ervin, E. Heat shock proteins in relation to heat stress tolerance of creeping bentgrass at different N levels. PLoS ONE 2014, 9, e102914. [Google Scholar] [CrossRef]
- Jinyan, X.; Chenchen, X.; Dong, X.; Jinming, Z.; Junyi, G.; Na, G.; Han, X. Overexpression of GmHsp90s, a heat shock protein 90 (Hsp90) gene family cloning from soybean, decrease damage of abiotic stresses in Arabidopsis thaliana. PLoS ONE 2013, 8, e69810. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margispinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef]
- Shi, W.M.; Muramoto, Y.; Ueda, A.; Takabe, T. Cloning of peroxisomal ascorbate peroxidase gene from barley and enhanced thermotolerance by overexpressing in Arabidopsis thaliana. Gene 2001, 273, 23–27. [Google Scholar] [CrossRef]
- Yoshimura, K. Expression of spinach ascorbate peroxidase isoenzymes in response to oxidative stresses. Plant Physiol. 2000, 123, 223–234. [Google Scholar] [CrossRef]
- Agrawal, G.K.; Jwa, N.-S.; Iwahashi, H.; Rakwal, R. Importance of ascorbate peroxidases OsAPX1 and OsAPX2 in the rice pathogen response pathways and growth and reproduction revealed by their transcriptional profiling. Gene 2003, 322, 93–103. [Google Scholar] [CrossRef]
- Menezes-Benavente, L.; Teixeira, F.K.; Kamei, C.L.A.; Margis-Pinheiro, M. Salt stress induces altered expression of genes encoding antioxidant enzymes in seedlings of a Brazilian indica rice (Oryza sativa L.). Plant Sci. 2004, 166, 323–331. [Google Scholar] [CrossRef]
- Fryer, M.J.; Ball, L.; Oxborough, K.; Karpinski, S.; Mullineaux, P.M.; Baker, N.R. Control of ascorbate peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J. 2010, 33, 691–705. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Allen, R.D. Overexpression of an Arabidopsis peroxisomal ascorbate peroxidase gene in tobacco increases protection against oxidative stress. Plant Cell Physiol. 1999, 40, 725–732. [Google Scholar] [CrossRef]
- Yan, J.; Jing, W.; Tissue, D.; Holaday, A.S.; Allen, R.; Hong, Z. Photosynthesis and seed production under water-deficit conditions in transgenic tobacco plants that overexpress an ascorbate peroxidase gene. Crop Sci. 2003, 43, 1477–1483. [Google Scholar] [CrossRef]
- Narendra, S.; Venkataramani, S.; Shen, G.; Wang, J.; Pasapula, V.; Lin, Y.; Kornyeyev, D.; Holaday, A.S.; Zhang, H. The Arabidopsis ascorbate peroxidase 3 is a peroxisomal membrane-bound antioxidant enzyme and is dispensable for Arabidopsis growth and development. J. Exp. Botany 2006, 57, 3033–3042. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 357–359. [Google Scholar]
- Yu, G.H.; Zou, J.; Feng, J.; Peng, X.B.; Wu, J.Y.; Wu, Y.L.; Palanivelu, R.; Sun, M.X. Exogenous γ-aminobutyric acid affects pollen tube growth via modulating putative Ca2+-permeable membrane channels and is coupled to negative regulation on glutamate decarboxylase. J. Exp. Botany 2014, 65, 3235–3248. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat 1. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Barr, H.D.; Weatherley, P.E. A reexamination of the relative turgidity technique for estimating water deficit in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumbdhindsa, P.; Thorpe, T.A. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Botany 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Xiao-Jian, X. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol. 2009, 2. [Google Scholar] [CrossRef]

| Target Gene | Chromosome | Forward Primer (5’-3’) | Reverse Primer (5’-3’) | Tm (◦C) |
|---|---|---|---|---|
| HSFA-2c | 3 | GCCGTCCAATGTGCCTCCATC | CGCTTCAGCCTGTCAATCTCTTCC | 62 |
| HSFA-6a | 1 | CACCTTCGAGGAGCTGGCATTG | TGTCTATCTCCGCCTGCTCATCC | 62 |
| HSFB-2b | 8 | GCTGGAGAACTCACGGCTAACG | CTGCTGAGTGTCGCTGTACTTGG | 62 |
| HSP17.8 | 5 | GGCGACATCAAGGTGCAGGTG | ACTTGGCGTCCTCCTTCTCCTC | 62 |
| HSP26.7 | 4 | GCGGCGAGCACAAGAAGGAG | GACCTGCACGTCGATGACCTTG | 64 |
| HSP70 | 2 | GCTGAGGATGACGAAGACGAAGAC | ATCGGCGTTGACGTGAACCATC | 60 |
| HSP90.1-b1 | 5 | ACGAAGTGATCTTCATGGTGGATG | CTGTCGCCGAGAATGTCCTTGAT | 64 |
| APX3 | 3 | GGCTCAAGATCGCAGTCG | TGGTAGAGGTCGGCATACG | 58 |
| β-Actin | 5 | CCTTTTCCAGCCATCTTTCA | GAGGTCCTTCCTGATATCCA | 58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Liu, Z.; Li, Z.; Peng, Y.; Zhang, X.; Ma, X.; Huang, L.; Liu, W.; Nie, G.; He, L. Regulation of Heat Shock Factor Pathways by γ-aminobutyric Acid (GABA) Associated with Thermotolerance of Creeping Bentgrass. Int. J. Mol. Sci. 2019, 20, 4713. https://doi.org/10.3390/ijms20194713
Liu T, Liu Z, Li Z, Peng Y, Zhang X, Ma X, Huang L, Liu W, Nie G, He L. Regulation of Heat Shock Factor Pathways by γ-aminobutyric Acid (GABA) Associated with Thermotolerance of Creeping Bentgrass. International Journal of Molecular Sciences. 2019; 20(19):4713. https://doi.org/10.3390/ijms20194713
Chicago/Turabian StyleLiu, Ting, Zhaoqiao Liu, Zhou Li, Yan Peng, Xinquan Zhang, Xiao Ma, Linkai Huang, Wei Liu, Gang Nie, and Liwen He. 2019. "Regulation of Heat Shock Factor Pathways by γ-aminobutyric Acid (GABA) Associated with Thermotolerance of Creeping Bentgrass" International Journal of Molecular Sciences 20, no. 19: 4713. https://doi.org/10.3390/ijms20194713
APA StyleLiu, T., Liu, Z., Li, Z., Peng, Y., Zhang, X., Ma, X., Huang, L., Liu, W., Nie, G., & He, L. (2019). Regulation of Heat Shock Factor Pathways by γ-aminobutyric Acid (GABA) Associated with Thermotolerance of Creeping Bentgrass. International Journal of Molecular Sciences, 20(19), 4713. https://doi.org/10.3390/ijms20194713








