New Perspectives in Liver Transplantation: From Regeneration to Bioengineering
Abstract
1. Introduction
2. Liver Regeneration
2.1. Overview of Liver Development
2.2. Homeostasis and First Line of Response to Injury
2.3. Hepatic Stem Cells and Second Line of Response to Liver Injury
2.4. Liver Regeneration, Inflammation, and Gender
3. Alternatives to Liver Transplantation
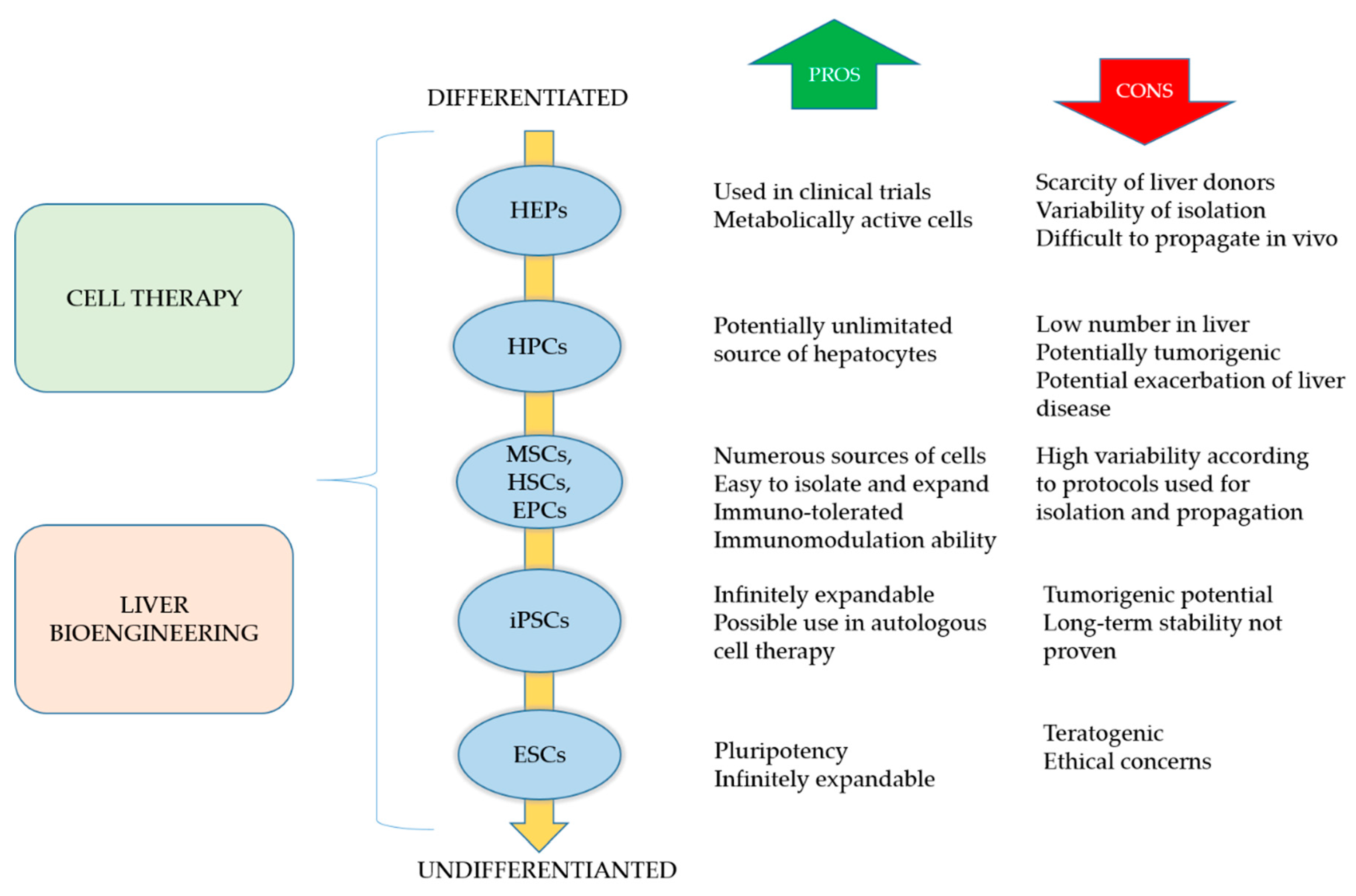
3.1. Cell-Based Regeneration Therapy
3.1.1. Hepatocytes
3.1.2. Macrophages
3.1.3. Pluripotent Stem Cells
3.1.4. Adult Stem Cells
3.1.5. Hepatic Organoids
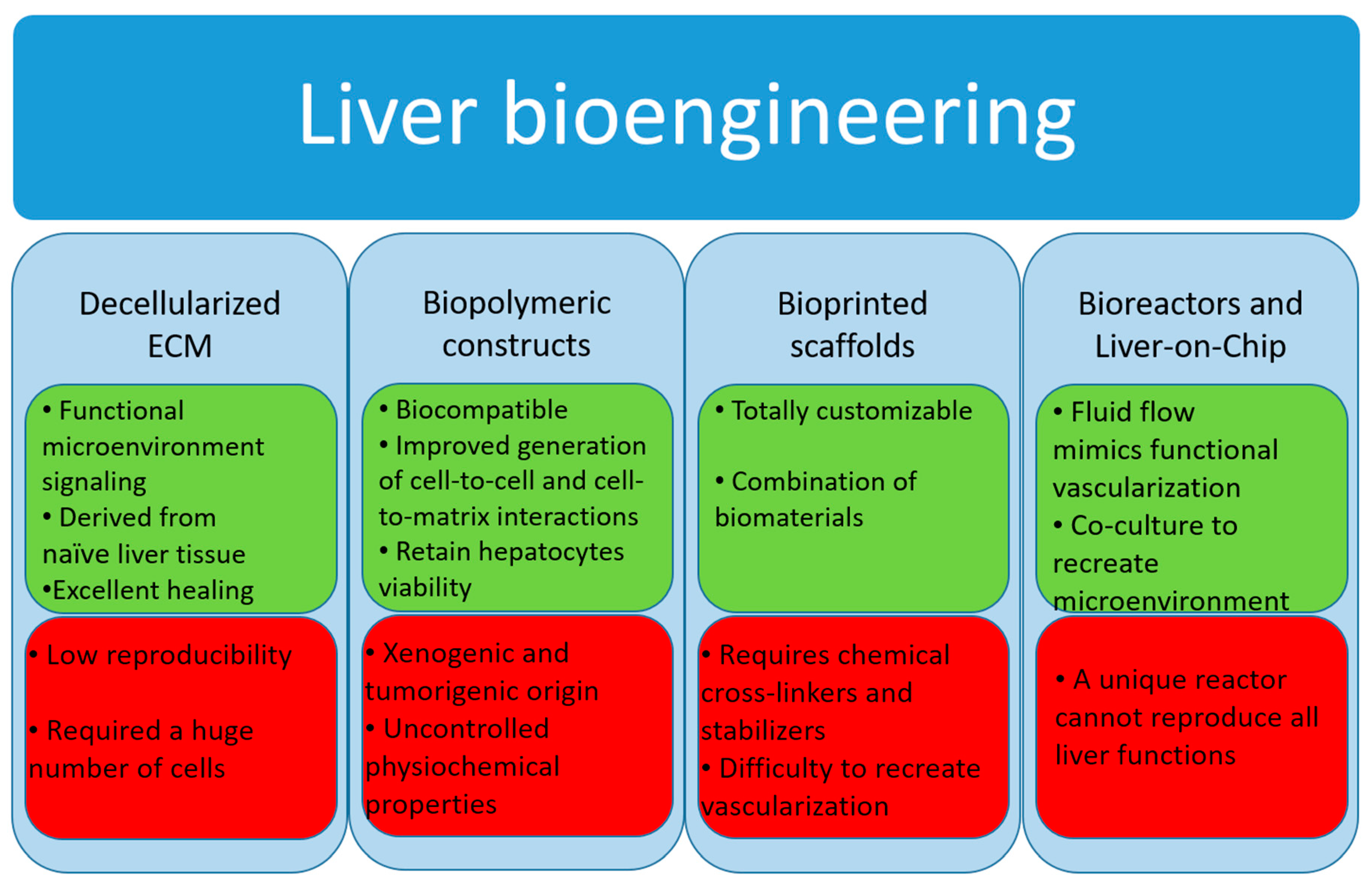
3.2. Liver Tissue Bioengineering
3.2.1. Decellularized Extracellular Matrix
3.2.2. Biopolymer Constructs
3.2.3. Bioprinted Scaffolds
3.3. Bioreactor Systems
3.4. Micro-Bioreactors and Liver-on-Chip
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manns, M.P.; Burra, P.; Sargent, J.; Horton, R.; Karlsen, T.H. The Lancet-EASL Commission on liver diseases in Europe: overcoming unmet needs, stigma, and inequities. Lancet 2018, 392, 621–622. [Google Scholar] [CrossRef]
- Germani, G.; Theocharidou, E.; Adam, R.; Karam, V.; Wendon, J.; O’Grady, J.; Burra, P.; Senzolo, M.; Mirza, D.; Castaing, D.; et al. Liver transplantation for acute liver failure in Europe: Outcomes over 20 years from the ELTR database. J. Hepatol. 2012, 57, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Burra, P.; Berenguer, M.; Pomfret, E. The ILTS Consensus Conference on NAFLD/NASH and liver transplantation. Transplantation 2018. [Google Scholar] [CrossRef]
- Pang, Y.; Horimoto, Y.; Sutoko, S.; Montagne, K.; Shinohara, M.; Mathiue, D.; Komori, K.; Anzai, M.; Niino, T.; Sakai, Y. Novel integrative methodology for engineering large liver tissue equivalents based on three-dimensional scaffold fabrication and cellular aggregate assembly. Biofabrication 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.C.; Li, P.S.; Wu, B.G.; Ma, H.; Wang, Y.C.; Liu, G.X.; Zeng, H.L.; Li, Z.Z.; Wei, X. PHBVHHx scaffolds loaded with umbilical cord-derived mesenchymal stem cells or hepatocyte-like cells differentiated from these cells for liver tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 374–382. [Google Scholar] [CrossRef]
- Tremblay, K.D.; Zaret, K.S. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev. Biol. 2005, 280, 87–99. [Google Scholar] [CrossRef]
- Houssaint, E. Differentiation of the mouse hepatic primordium. I. An analysis of tissue interactions in hepatocyte differentiation. Cell Differ. 1980, 9, 269–279. [Google Scholar] [CrossRef]
- Medlock, E.S.; Haar, J.L. The liver hemopoietic environment: I. Developing hepatocytes and their role in fetal hemopoiesis. Anat. Rec. 1983, 207, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Lemaigre, F.P. Development of the biliary tract. Mech. Dev. 2003, 120, 81–87. [Google Scholar] [CrossRef]
- Kung, J.W.C.; Currie, I.S.; Forbes, S.J.; Ross, J.A. Liver Development, Regeneration, and Carcinogenesis. J. Biomed. Biotechnol. 2010. [Google Scholar] [CrossRef]
- Tanimizu, N.; Miyajima, A. Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. J. Cell Sci. 2004, 117, 3165–3174. [Google Scholar] [CrossRef] [PubMed]
- McCright, B.; Lozier, J.; Gridley, T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development 2002, 129, 1075–1082. [Google Scholar] [PubMed]
- Suzuki, A.; Iwama, A.; Miyashita, H.; Nakauchi, H.; Taniguchi, H. Role for growth factors and extracellular matrix in controlling differentiation of prospectively isolated hepatic stem cells. Development 2003, 130, 2513–2524. [Google Scholar] [CrossRef] [PubMed]
- Jochheim, A.; Cieslak, A.; Hillemann, T.; Cantz, T.; Scharf, J.; Manns, M.P.; Ott, M. Multi-stage analysis of differential gene expression in BALB/C mouse liver development by high-density microarrays. Differentiation 2003, 71, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Duncan, S.A. Embryonic development of the liver. Hepatology 2005, 41, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.J.; Rosenthal, N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat. Med. 2014, 20, 857–869. [Google Scholar] [CrossRef]
- Riehle, K.J.; Dan, Y.Y.; Campbell, J.S.; Fausto, N. New concepts in liver regeneration. J. Gastroenterol. Hepatol. 2011, 26, 203–212. [Google Scholar] [CrossRef]
- MacDonald, R.A. Lifespan of liver cells: autoradiographic study using tritiated thymidine in normal, cirrhotic, and partially hepatectomized rats. Arch. Int. Med. 1961, 107, 335–343. [Google Scholar] [CrossRef]
- Gilgenkrantz, H.; Collin de l’Hortet, A. New insights into liver regeneration. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 623–629. [Google Scholar] [CrossRef]
- Michalopoulos, G.K. Hepatostat: Liver Regeneration and Normal Liver Tissue Maintenance. Hepatology 2017, 65, 1384–1392. [Google Scholar] [CrossRef]
- Taub, R. Liver regeneration: From myth to mechanism. Nat. Rev. Mol. Cell Biol. 2004, 5, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Aravinthan, A.; Scarpini, C.; Tachtatzis, P.; Verma, S.; Penrhyn-Lowe, S.; Harvey, R.; Davies, S.E.; Allison, M.; Coleman, N.; Alexander, G. Hepatocyte senescence predicts progression in non-alcohol-related fatty liver disease. J. Hepatol. 2013, 58, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Fausto, N.; Campbell, J.S.; Riehle, K.J. Liver regeneration. J. Hepatol. 2012, 57, 692–694. [Google Scholar] [CrossRef]
- Farber, E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3’-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956, 16, 142–148. [Google Scholar] [PubMed]
- Lazaro, C.A.; Rhim, J.A.; Yamada, Y.; Fausto, N. Generation of hepatocytes from oval cell precursors in culture. Cancer Res. 1998, 58, 5514–5522. [Google Scholar] [PubMed]
- Dunsford, H.A.; Karnasuta, C.; Hunt, J.M.; Sell, S. Different lineages of chemically-induced hepatocellular-carcinoma in rats defined by monoclonal-antibodies. Cancer Res. 1989, 49, 4894–4900. [Google Scholar] [PubMed]
- Theise, N.D.; Saxena, R.; Portmann, B.C.; Thung, S.N.; Yee, H.; Chiriboga, L.; Kumar, A.; Crawford, J.M. The canals of Hering and hepatic stem cells in humans. Hepatology 1999, 30, 1425–1433. [Google Scholar] [CrossRef]
- Stueck, A.E.; Wanless, I.R. Hepatocyte Buds Derived From Progenitor Cells Repopulate Regions of Parenchymal Extinction in Human Cirrhosis. Hepatology 2015, 61, 1696–1707. [Google Scholar] [CrossRef]
- Roskams, T.A.; Theise, N.D.; Balabaud, C.; Bhagat, G.; Bhathal, P.S.; Bioulac-Sage, P.; Brunt, E.M.; Crawford, J.M.; Crosby, H.A.; Desmet, V.; et al. Nomenclature of the finer branches of the biliary tree: Canals, ductules, and ductular reactions in human livers. Hepatology 2004, 39, 1739–1745. [Google Scholar] [CrossRef]
- Zajicek, G.; Oren, R.; Weinreb, M. THE STREAMING LIVER. Liver 1985, 5, 293–300. [Google Scholar] [CrossRef]
- Roskams, T.; Yang, S.Q.; Koteish, A.; Durnez, A.; DeVos, R.; Huang, X.W.; Achten, R.; Verslype, C.; Diehl, A.M. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am. J. Pathol. 2003, 163, 1301–1311. [Google Scholar] [CrossRef]
- Kuwahara, R.; Kofman, A.V.; Landis, C.S.; Swenson, E.S.; Barendswaard, E.; Theise, N.D. The hepatic stem cell niche: Identification by label-retaining cell assay. Hepatology 2008, 47, 1994–2002. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Mehal, W.Z. Sterile Inflammation in the Liver. Gastroenterology 2012, 143, 1158–1172. [Google Scholar] [CrossRef] [PubMed]
- Schattenberg, J.M.; Galle, P.R.; Schuchmann, M. Apoptosis in liver disease. Liver Int. 2006, 26, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.; Terui, K.; Zhang, H.Q.; Enosawa, S.; Ogawa, W.; Inoue, H.; Okuyama, T.; Takeda, K.; Akira, S.; Ogino, T.; et al. Stat3 protects against Fas-induced liver injury by redox-dependent and -independent mechanisms. J. Clin. Investig. 2003, 112, 989–998. [Google Scholar] [CrossRef]
- Li, W.; Liang, X.P.; Kellendonk, C.; Poli, V.; Taub, R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J. Biol. Chem. 2002, 277, 28411–28417. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.I.; Park, O.; Radaeva, S.; Gao, B. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology 2006, 44, 1441–1451. [Google Scholar] [CrossRef]
- Sun, Z.L.; Klein, A.S.; Radaeva, S.; Hong, F.; El-Assal, O.; Pan, H.N.; Jaruga, B.; Batkai, S.; Hoshino, S.; Tian, Z.G.; et al. In vitro interleukin-6 treatment prevents mortality associated with fatty liver transplants in rats. Gastroenterology 2003, 125, 202–215. [Google Scholar] [CrossRef]
- Bizzaro, D.; Crescenzi, M.; Di Liddo, R.; Arcidiacono, D.; Cappon, A.; Bertalot, T.; Amodio, V.; Tasso, A.; Stefani, A.; Bertazzo, V.; et al. Sex-dependent differences in inflammatory responses during liver regeneration in a murine model of acute liver injury. Clin. Sci. (Lond.) 2018, 132, 255–272. [Google Scholar] [CrossRef]
- Yates, F.E.; Herbst, A.L.; Urquhart, J. Sex difference in rate of ring A reduction of delta 4–3-keto-steroids in vitro by rat liver. Endocrinology 1958, 63, 887–902. [Google Scholar] [CrossRef]
- Marcos, R.; Lopes, C.; Malhao, F.; Correia-Gomes, C.; Fonseca, S.; Lima, M.; Gebhardt, R.; Rocha, E. Stereological assessment of sexual dimorphism in the rat liver reveals differences in hepatocytes and Kupffer cells but not hepatic stellate cells. J. Anat. 2016, 228, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, I.; Kojo, S. The sex difference in the regulation of liver regeneration after partial hepatectomy in the rat. Biochim. Biophys. Acta 1990, 1033, 287–290. [Google Scholar] [CrossRef]
- Imamura, H.; Shimada, R.; Kubota, M.; Matsuyama, Y.; Nakayama, A.; Miyagawa, S.; Makuuchi, M.; Kawasaki, S. Preoperative portal vein embolization: an audit of 84 patients. Hepatology 1999, 29, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, A.; Eagon, P.K.; DiLeo, A.; Polimeno, L.; Panella, C.; Aquilino, A.M.; Ingrosso, M.; Van Thiel, D.H.; Starzl, T.E. Sex hormone-related functions in regenerating male rat liver. Gastroenterology 1986, 91, 1263–1270. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yu, L.; Nazmy El-Assal, O.; Satoh, T.; Kumar Dhar, D.; Yamanoi, A.; Nagasue, N. Androgen metabolism in regenerating liver of male rats: evidence for active uptake and utilization of testosterone. Hepatol. Res. 2001, 20, 114–127. [Google Scholar] [CrossRef]
- Starzl, T.E.; Marchioro, T.L.; Porter, K.A.; Brettschneider, L. Homotransplantation of the liver. Transplantation 1967, 5, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Karam, V.; Delvart, V.; O’Grady, J.; Mirza, D.; Klempnauer, J.; Castaing, D.; Neuhaus, P.; Jamieson, N.; Salizzoni, M.; et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J. Hepatol. 2012, 57, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Dutkowski, P.; Linecker, M.; DeOliveira, M.L.; Mullhaupt, B.; Clavien, P.A. Challenges to Liver Transplantation and Strategies to Improve Outcomes. Gastroenterology 2015, 148, 307–323. [Google Scholar] [CrossRef]
- Kim, W.R.; Therneau, T.M.; Benson, J.T.; Kremers, W.K.; Rosen, C.B.; Gores, G.J.; Dickson, E.R. Deaths on the liver transplant waiting list: An analysis of competing risks. Hepatology 2006, 43, 345–351. [Google Scholar] [CrossRef]
- Toniutto, P.; Zanetto, A.; Ferrarese, A.; Burra, P. Current challenges and future directions for liver transplantation. Liver Int. 2017, 37, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.C.; Newsome, P.N. Hepatocyte cell therapy in liver disease. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1261–1272. [Google Scholar] [CrossRef]
- Fisher, R.A.; Strom, S.C. Human hepatocyte transplantation: Worldwide results. Transplantation 2006, 82, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Huebert, R.C.; Rakela, J. Cellular Therapy for Liver Disease. Mayo Clin. Proc. 2014, 89, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J.R.; Chokechanachaisakul, A.; Wertheim, J.A. New Tools in Experimental Cellular Therapy for the Treatment of Liver Diseases. Curr. Transplant. Rep. 2015, 2, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Duffield, J.S.; Forbes, S.J.; Constandinou, C.M. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Investig. 2005, 115, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Fallowfield, J.A.; Mizuno, M.; Kendall, T.J. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J. Immunol. 2007, 178, 5288–5295. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Pellicoro, A.; Vernon, M.A.; Boulter, L.; Aucott, R.L.; Ali, A.; Hartland, S.N.; Snowdon, V.K.; Cappon, A.; Gordon-Walker, T.T.; et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl. Acad. Sci. USA 2012, 109, E3186–E3195. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; Pope, C.; Wojtacha, D.; Robson, A.J.; Gordon-Walker, T.T.; Hartland, S.; Ramachandran, P.; Van Deemter, M.; Hume, D.A.; Iredale, J.P.; et al. Macrophage Therapy for Murine Liver Fibrosis Recruits Host Effector Cells Improving Fibrosis, Regeneration, and Function. Hepatology 2011, 53, 2003–2015. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Reubinoff, B.E.; Pera, M.F.; Fong, C.Y.; Trounson, A.; Bongso, A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 2000, 18, 399–404. [Google Scholar] [CrossRef]
- Brolen, G.; Sivertsson, L.; Bjorquist, P.; Eriksson, G.; Ek, M.; Semb, H.; Johansson, I.; Andersson, T.B.; Ingelman-Sundberg, M.; Heins, N. Hepatocyte-like cells derived from human embryonic stem cells specifically via definitive endoderm and a progenitor stage. J. Biotechnol. 2010, 145, 284–294. [Google Scholar] [CrossRef]
- Hay, D.C.; Fletcher, J.; Payne, C.; Terrace, J.D.; Gallagher, R.C.J.; Snoeys, J.; Black, J.R.; Wojtacha, D.; Samuel, K.; Hannoun, Z.; et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 12301–12306. [Google Scholar] [CrossRef] [PubMed]
- Woo, D.H.; Kim, S.K.; Lim, H.J.; Heo, J.; Park, H.S.; Kang, G.Y.; Kim, S.E.; You, H.J.; Hoeppner, D.J.; Kim, Y.; et al. Direct and Indirect Contribution of Human Embryonic Stem Cell-Derived Hepatocyte-Like Cells to Liver Repair in Mice. Gastroenterology 2012, 142, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.Y.; Catana, A.; Meng, Y.; Yamamoto, N.; He, S.Q.; Gupta, S.; Gambhir, S.S.; Zerna, M.A. Differentiation and enrichment of hepatocyte-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells 2007, 25, 3058–3068. [Google Scholar] [CrossRef] [PubMed]
- Lavon, N.; Yanuka, O.; Benvenisty, N. Differentiation and isolation of hepatic-like cells from human embryonic stem cells. Differentiation 2004, 72, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Basma, H.; Soto-Gutierrez, A.; Yannam, G.R.; Liu, L.P.; Ito, R.; Yamamoto, T.; Ellis, E.; Carson, S.D.; Sato, S.; Chen, Y.; et al. Differentiation and Transplantation of Human Embryonic Stem Cell-Derived Hepatocytes. Gastroenterology 2009, 136, 990–999. [Google Scholar] [CrossRef]
- Yamamoto, H.; Quinn, G.; Asari, A.; Yamanokuchi, H.; Teratani, T.; Terada, M.; Ochiya, T. Differentiation of embryonic stem cells into hepatocytes: Biological functions and therapeutic application. Hepatology 2003, 37, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, L.; Caron, J.; Hannoun, Z.; Antoni, M.; Lopez, S.; Burks, D.; Castell, J.V.; Weber, A.; Gomez-Lechon, M.J.; Dubart-Kupperschmitt, A. Transplantation of hESC-derived hepatocytes protects mice from liver injury. Stem Cell Res. Ther. 2015, 6. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Rezvani, M.; Harbell, J.; Mattis, A.N.; Wolfe, A.R.; Benet, L.Z.; Willenbring, H.; Ding, S. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature 2014, 508, 93–97. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Kia, R.; Sison, R.L.; Heslop, J.; Kitteringham, N.R.; Hanley, N.; Mills, J.S.; Park, B.K.; Goldring, C.E. Stem cell-derived hepatocytes as a predictive model for drug-induced liver injury: are we there yet? Br. J. Clin. Pharmacol. 2013, 75, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lechon, M.J.; Tolosa, L. Human hepatocytes derived from pluripotent stem cells: a promising cell model for drug hepatotoxicity screening. Arch. Toxicol. 2016, 90, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.B.; Zhang, Z.N.; Rong, Z.L.; Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 2011, 474, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, L.; Pareja, E.; Gomez-Lechon, M. Clinical Application of Pluripotent Stem Cells: An Alternative Cell-Based Therapy for Treating Liver Diseases? Transplantation 2016, 100, 2548–2557. [Google Scholar] [CrossRef] [PubMed]
- Araki, R.; Uda, M.; Hoki, Y.; Sunayama, M.; Nakamura, M.; Ando, S.; Sugiura, M.; Ideno, H.; Shimada, A.; Nifuji, A.; et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature 2013, 494, 100. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.J.; Newsome, P.N. New horizons for stem cell therapy in liver disease. J. Hepatol. 2012, 56, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Wang, Y.; Wei, L.; Chen, H.; Cong, X.; Fei, R.; Gao, Y.; Liu, F. Differentiation of hematopoietic stem cells into hepatocytes in liver fibrosis in rats. Transplant. Proc. 2006, 38, 3082–3085. [Google Scholar] [CrossRef] [PubMed]
- Terai, S.; Takami, T.; Yamamoto, N.; Fujisawa, K.; Ishikawa, T.; Urata, Y.; Tanimoto, H.; Iwamoto, T.; Mizunaga, Y.; Matsuda, T.; et al. Status and Prospects of Liver Cirrhosis Treatment by Using Bone Marrow-Derived Cells and Mesenchymal Cells. Tissue Eng. Part B Rev. 2014, 20, 206–210. [Google Scholar] [CrossRef]
- Nakamura, T.; Torimura, T.; Sakamoto, M.; Hashimoto, O.; Taniguchi, E.; Inoue, K.; Sakata, R.; Kumashiro, R.; Murohara, T.; Ueno, T.; et al. Significance and therapeutic potential of endothelial progenitor cell transplantation in a cirrhotic liver rat model. Gastroenterology 2007, 133, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Dollé, L.; Best, J.; Mei, J.; Al Battah, F.; Reynaert, H.; van Grunsven, L.A.; Geerts, A. The quest for liver progenitor cells: A practical point of view. J. Hepatol. 2010, 52, 117–129. [Google Scholar] [CrossRef]
- Rossi, L.; Challen, G.A.; Sirin, O.; Lin, K.K.-Y.; Goodell, M.A. Hematopoietic stem cell characterization and isolation. Methods Mol. Biol. 2011, 750, 47–59. [Google Scholar] [CrossRef]
- Lagasse, E.; Connors, H.; Al-Dhalimy, M.; Reitsma, M.; Dohse, M.; Osborne, L.; Wang, X.; Finegold, M.; Weissman, I.L.; Grompe, M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 2000, 6, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Yannaki, E.; Athanasiou, E.; Xagorari, A.; Constantinou, V.; Batsis, L.; Kaloyannidis, P.; Proya, E.; Anagnostopoulos, A.; Fassas, A. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp. Hematol. 2005, 33, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, J.M.; Kabarriti, R.; Mehta, K.J.; Roy-Chowdhury, J.; Guha, C. Bone Marrow-Derived Stromal Cell Therapy in Cirrhosis: Clinical Evidence, Cellular Mechanisms, and Implications for the Treatment of Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 786–803. [Google Scholar] [CrossRef] [PubMed]
- Austin, T.W.; Lagasse, E. Hepatic regeneration from hematopoietic stem cells. Mech. Dev. 2003, 120, 131–135. [Google Scholar] [CrossRef]
- Thorgeirsson, S.S.; Grisham, J.W. Hematopoietic cells as hepatocyte stem cells: A critical review of the evidence. Hepatology 2006, 43, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Larrivée, B.; Karsan, A. Involvement of Marrow-Derived Endothelial Cells in Vascularization. In Bone Marrow-Derived Progenitors; Handbook of Experimental Pharmacology; Kauser, K., Zeiher, A.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Asahara, T.; Masuda, H.; Takahashi, T.; Kalka, C.; Pastore, C.; Silver, M.; Kearne, M.; Magner, M.; Isner, J.M. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999, 85, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; vanderZee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.D.; Xie, G.H.; Hill, C.K.; DeLeve, L.D. Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats. J. Clin. Investig. 2012, 122, 1567–1573. [Google Scholar] [CrossRef]
- Taniguchi, E.; Kin, M.; Torimura, T.; Nakamura, T.; Kumemura, H.; Hanada, S.; Hisamoto, T.; Yoshida, T.; Kawaguchi, T.; Baba, S.; et al. Endothelial progenitor cell transplantation improves the survival following liver injury in mice. Gastroenterology 2006, 130, 521–531. [Google Scholar] [CrossRef]
- Ueno, T.; Nakamura, T.; Torimura, T.; Sata, M. Angiogenic cell therapy for hepatic fibrosis. Med. Mol. Morphol. 2006, 39, 16–21. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Riminucci, M.; Gronthos, S.; Robey, P.G. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells 2001, 19, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.M.; Elabd, C.; Amri, E.Z.; Ailhaud, G.; Dani, C. The human adipose tissue is a source of multipotent stem cells. Biochimie 2005, 87, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.B.; Furlan, J.M.; Salton, G.D.; Schmalfuss, T.; Rohsig, L.M.; Silla, L.M.R.; Passos, E.P.; Paz, A.H. Isolation of human mesenchymal stem cells from amnion, chorion, placental decidua and umbilical cord: comparison of four enzymatic protocols. Biotechnol. Lett. 2018, 40, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Burra, P.; Arcidiacono, D.; Bizzaro, D.; Chioato, T.; Di Liddo, R.; Banerjee, A.; Cappon, A.; Bo, P.; Conconi, M.T.; Parnigotto, P.P.; et al. Systemic administration of a novel human umbilical cord mesenchymal stem cells population accelerates the resolution of acute liver injury. BMC Gastroenterol. 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Bizzaro, D.; Burra, P.; Di Liddo, R.; Pathak, S.; Arcidiacono, D.; Cappon, A.; Bo, P.; Conconi, M.T.; Crescenzi, M.; et al. Umbilical cord mesenchymal stem cells modulate dextran sulfate sodium induced acute colitis in immunodeficient mice. Stem Cell Res. Ther. 2015, 6. [Google Scholar] [CrossRef]
- Christ, B.; Bruckner, S.; Winkler, S. The Therapeutic Promise of Mesenchymal Stem Cells for Liver Restoration. Trends Mol. Med. 2015, 21, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells 2019, 37. [Google Scholar] [CrossRef]
- Huch, M.; Gehart, H.; van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.A.; Ellis, E.; van Wenum, M.; Fuchs, S.A.; de Ligt, J.; et al. Long-Term Culture of Genome-Stable Bipotent Stem Cells from Adult Human Liver. Cell 2015, 160, 299–312. [Google Scholar] [CrossRef]
- Lanzoni, G.; Cardinale, V.; Carpino, G. The Hepatic, Biliary, and Pancreatic Network of Stem/Progenitor Cell Niches in Humans: A New Reference Frame for Disease and Regeneration. Hepatology 2016, 64, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, E.; Zhang, L.; Bruce, A.; Wauthier, E.; Ludlow, J.; Yao, H.L.; Moss, N.; Melhem, A.; McClelland, R.; Turner, W.; et al. Human hepatic stem cells from fetal and postnatal donors. J. Exp. Med. 2007, 204, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Libbrecht, L. Hepatic progenitor cells in human liver tumor development. World J. Gastroenterol. 2006, 12, 6261–6265. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Dorrell, C.; Boj, S.F.; van Es, J.H.; Li, V.S.; van de Wetering, M.; Sato, T.; Hamer, K.; Sasaki, N.; Finegold, M.J.; et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013, 494, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Boj, S.F.; Clevers, H. Lgr5(+) liver stem cells, hepatic organoids and regenerative medicine. Regen Med. 2013, 8, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Willyard, C. The boom in mini stomachs, brains, breasts, kidneys and more. Nat. News 2015, 523, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Koo, B.K. Modeling mouse and human development using organoid cultures. Development 2015, 142, 3113–3125. [Google Scholar] [CrossRef]
- Shafiee, A.; Atala, A. Tissue Engineering: Toward a New Era of Medicine. Annu. Rev. Med. 2017, 68, 29–40. [Google Scholar] [CrossRef]
- Ehrbar, M.; Sala, A.; Lienemann, P.; Ranga, A.; Mosiewicz, K.; Bittermann, A.; Rizzi, S.C.; Weber, F.E.; Lutolf, M.P. Elucidating the role of matrix stiffness in 3D cell migration and remodeling. Biophys. J. 2011, 100, 284–293. [Google Scholar] [CrossRef]
- Esch, M.B.; Prot, J.M.; Wang, Y.I.; Miller, P.; Llamas-Vidales, J.R.; Naughton, B.A.; Applegate, D.R.; Shuler, M.L. Multi-cellular 3D human primary liver cell culture elevates metabolic activity under fluidic flow. Lab Chip 2015, 15, 2269–2277. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Shupe, T.; Atala, A. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov. Today 2016, 21, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Liu, X.H. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudlou, P.; Georgiades, F.; Smith, H.; Milan, A.; Shangaris, P.; Urbani, L.; Loukogeorgakis, S.P.; Lombardi, B.; Mazza, G.; Hagen, C.; et al. Optimization of Liver Decellularization Maintains Extracellular Matrix Micro-Architecture and Composition Predisposing to Effective Cell Seeding. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Andrews, E. Damage of Porcine Aortic-Valve Tissue Caused by the Surfactant Sodiumdodecylsulphate. Thorac. Cardiovasc. Surg. 1986, 34, 340–341. [Google Scholar] [CrossRef]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.L.; Guzzardi, M.A.; Shulman, C.; Milwid, J.; Kobayashi, N.; Tilles, A.; Berthiaume, F.; et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010, 16, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Kadota, Y.; Yagi, H.; Inomata, K.; Matsubara, K.; Hibi, T.; Abe, Y.; Kitago, M.; Shinoda, M.; Obara, H.; Itano, O.; et al. Mesenchymal stem cells support hepatocyte function in engineered liver grafts. Organogenesis 2014, 10, 268–277. [Google Scholar] [CrossRef]
- Park, K.M.; Hussein, K.H.; Hong, S.H.; Ahn, C.; Yang, S.R.; Park, S.M.; Kweon, O.K.; Kim, B.M.; Woo, H.M. Decellularized Liver Extracellular Matrix as Promising Tools for Transplantable Bioengineered Liver Promotes Hepatic Lineage Commitments of Induced Pluripotent Stem Cells. Tissue Eng. Part A 2016, 22, 449–460. [Google Scholar] [CrossRef]
- Ogiso, S.; Yasuchika, K.; Fukumitsu, K.; Ishii, T.; Kojima, H.; Miyauchi, Y.; Yamaoka, R.; Komori, J.; Katayama, H.; Kawai, T.; et al. Efficient recellularisation of decellularised whole-liver grafts using biliary tree and foetal hepatocytes. Sci. Rep. 2016, 6, 35887. [Google Scholar] [CrossRef]
- Devalliere, J.; Chen, Y.B.; Dooley, K.; Yarmush, M.L.; Uygun, B.E. Improving functional re-endothelialization of acellular liver scaffold using REDV cell-binding domain. Acta Biomater. 2018, 78, 151–164. [Google Scholar] [CrossRef]
- Wu, Q.; Bao, J.; Zhou, Y.J.; Wang, Y.J.; Du, Z.G.; Shi, Y.J.; Li, L.; Bu, H. Optimizing Perfusion-Decellularization Methods of Porcine Livers for Clinical-Scale Whole-Organ Bioengineering. BioMed Res. Int. 2015. [Google Scholar] [CrossRef]
- Verstegen, M.M.A.; Willemse, J.; van den Hoek, S.; Kremers, G.J.; Luider, T.M.; van Huizen, N.A.; Willemssen, F.; Metselaar, H.J.; Ijzermans, J.N.M.; van der Laan, L.J.W.; et al. Decellularization of Whole Human Liver Grafts Using Controlled Perfusion for Transplantable Organ Bioscaffolds. Stem Cells Dev. 2017, 26, 1304–1315. [Google Scholar] [CrossRef]
- Mattei, G.; Magliaro, C.; Pirone, A.; Ahluwalia, A. Decellularized Human Liver Is Too Heterogeneous for Designing a Generic Extracellular Matrix Mimic Hepatic Scaffold. Artif. Organs 2017, 41, E347–E355. [Google Scholar] [CrossRef]
- Turner, W.S.; Schmelzer, E.; McClelland, R.; Wauthier, E.; Chen, W.; Reid, L.M. Human hepatoblast phenotype maintained by hyaluronan hydrogels. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 82B, 156–168. [Google Scholar] [CrossRef]
- Richert, L.; Binda, D.; Hamilton, G.; Viollon-Abadie, C.; Alexandre, E.; Bigot-Lasserre, D.; Bars, R.; Coassolo, P.; LeCluyse, E. Evaluation of the effect of culture configuration on morphology, survival time, antioxidant status and metabolic capacities of cultured rat hepatocytes. Toxicol. In Vitro 2002, 16, 89–99. [Google Scholar] [CrossRef]
- Fu, R.H.; Wang, Y.C.; Liu, S.P.; Huang, C.M.; Kang, Y.H.; Tsai, C.H.; Shyu, W.C.; Lin, S.Z. Differentiation of Stem Cells: Strategies for Modifying Surface Biomaterials. Cell Transplant. 2011, 20, 37–47. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Martin, G.R. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005, 15, 378–386. [Google Scholar] [CrossRef]
- Jain, E.; Damania, A.; Kumar, A. Biomaterials for liver tissue engineering. Hepatol. Int. 2014, 8, 185–197. [Google Scholar] [CrossRef]
- Wang, H.P.; Shi, Q.; Guo, Y.N.; Li, Y.N.; Sun, T.; Huang, Q.; Fukuda, T. Contact assembly of cell-laden hollow microtubes through automated micromanipulator tip locating. J. Micromech. Microeng. 2017, 27. [Google Scholar] [CrossRef]
- Jammalamadaka, U.; Tappa, K. Recent Advances in Biomaterials for 3D Printing and Tissue Engineering. J. Funct. Biomater. 2018, 9. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhang, Y.S.; Heinrich, M.A.; De Ferrari, F.; Jang, H.L.; Bakht, S.M.; Alvarez, M.M.; Yang, J.Z.; Li, Y.C.; Trujillo-de Santiago, G.; et al. Rapid Continuous Multimaterial Extrusion Bioprinting. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Murphy, S.V.; Skardal, A.; Atala, A. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. Part A 2013, 101, 272–284. [Google Scholar] [CrossRef]
- Skardal, A.; Atala, A. Biomaterials for Integration with 3-D Bioprinting. Ann. Biomed. Eng. 2015, 43, 730–746. [Google Scholar] [CrossRef]
- Arslan-Yildiz, A.; El Assal, R.; Chen, P.; Guven, S.; Inci, F.; Demirci, U. Towards artificial tissue models: past, present, and future of 3D bioprinting. Biofabrication 2016, 8. [Google Scholar] [CrossRef]
- Li, X.; He, J.K.; Liu, Y.X.; Zhao, Q.; Wu, W.Q.; Li, D.C.; Jin, Z.M. Biomaterial Scaffolds with Biomimetic Fluidic Channels for Hepatocyte Culture. J. Bionic Eng. 2013, 10, 57–64. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Zavan, B.; Brun, P.; Vindigni, V.; Amadori, A.; Habeler, W.; Pontisso, P.; Montemurro, D.; Abatangelo, G.; Cortivo, R. Extracellular matrix-enriched polymeric scaffolds as a substrate for hepatocyte cultures: in vitro and in vivo studies. Biomaterials 2005, 26, 7038–7045. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, J.C.; Encke, J.; Hole, O.; Muller, C.; Ryan, C.J.; Neuhaus, P. BIOREACTOR FOR A LARGER SCALE HEPATOCYTE IN-VITRO PERFUSION. Transplantation 1994, 58, 984–988. [Google Scholar] [CrossRef]
- Lee, M.Y.; Kumar, R.A.; Sukumaran, S.M.; Hogg, M.G.; Clark, D.S.; Dordick, J.S. Three-dimensional cellular microarray for high-throughput toxicology assays. Proc. Natl. Acad. Sci. USA 2008, 105, 59–63. [Google Scholar] [CrossRef]
- Andria, B.; Bracco, A.; Cirino, G.; Chamuleau, R.A.F.M. Liver Cell Culture Devices. Cell Med. 2010, 1, 55–70. [Google Scholar] [CrossRef]
- Allen, J.W.; Khetani, S.R.; Bhatia, S.N. In vitro zonation and toxicity in a hepatocyte bioreactor. Toxicol. Sci. 2005, 84, 110–119. [Google Scholar] [CrossRef]
- Lee-Montiel, F.T.; George, S.M.; Gough, A.H.; Sharma, A.D.; Wu, J.F.; DeBiasio, R.; Vernetti, L.A.; Taylor, D.L. Control of oxygen tension recapitulates zone-specific functions in human liver microphysiology systems. Exp. Biol. Med. 2017, 242, 1617–1632. [Google Scholar] [CrossRef]
- Goulet, F.; Normand, C.; Morin, O. Cellular interactions promote tissue-specific function, biomatrix deposition and junctional communication of primary cultured hepatocytes. Hepatology 1988, 8, 1010–1018. [Google Scholar] [CrossRef]
- Baudoin, R.; Corlu, A.; Griscom, L.; Legallais, C.; Leclerc, E. Trends in the development of microfluidic cell biochips for in vitro hepatotoxicity. Toxicol. In Vitro 2007, 21, 535–544. [Google Scholar] [CrossRef]
- Banaeiyan, A.A.; Theobald, J.; Paukstyte, J.; Wolfl, S.; Adiels, C.B.; Goksor, M. Design and fabrication of a scalable liver-lobule-on-a-chip microphysiological platform. Biofabrication 2017, 9, 015014. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizzaro, D.; Russo, F.P.; Burra, P. New Perspectives in Liver Transplantation: From Regeneration to Bioengineering. Bioengineering 2019, 6, 81. https://doi.org/10.3390/bioengineering6030081
Bizzaro D, Russo FP, Burra P. New Perspectives in Liver Transplantation: From Regeneration to Bioengineering. Bioengineering. 2019; 6(3):81. https://doi.org/10.3390/bioengineering6030081
Chicago/Turabian StyleBizzaro, Debora, Francesco Paolo Russo, and Patrizia Burra. 2019. "New Perspectives in Liver Transplantation: From Regeneration to Bioengineering" Bioengineering 6, no. 3: 81. https://doi.org/10.3390/bioengineering6030081
APA StyleBizzaro, D., Russo, F. P., & Burra, P. (2019). New Perspectives in Liver Transplantation: From Regeneration to Bioengineering. Bioengineering, 6(3), 81. https://doi.org/10.3390/bioengineering6030081




