Vein Pattern Locating Technology for Cannulation: A Review of the Low-Cost Vein Finder Prototypes Utilizing near Infrared (NIR) Light to Improve Peripheral Subcutaneous Vein Selection for Phlebotomy
Abstract
:1. Introduction
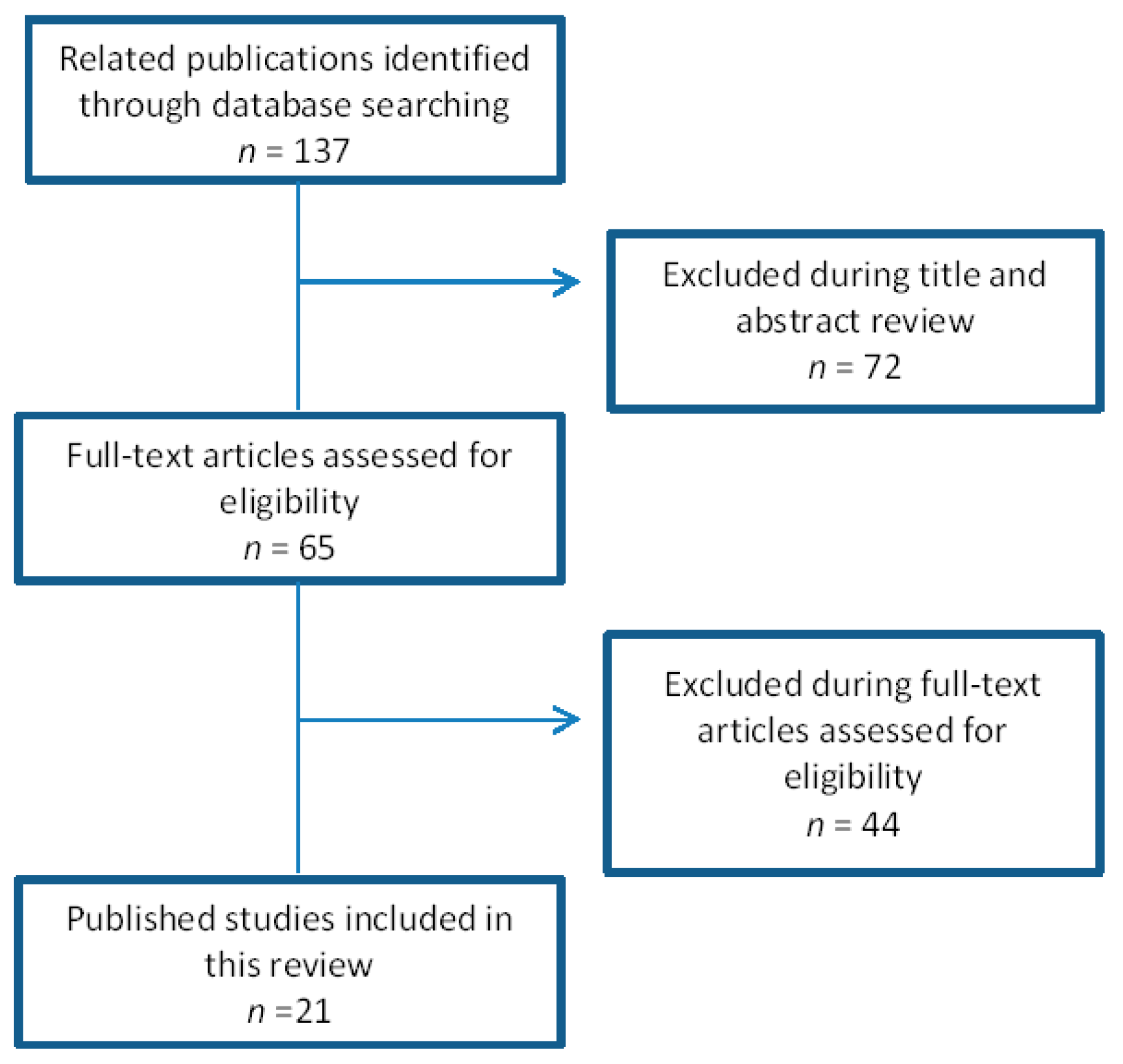
2. Methods
3. Results and Analysis
3.1. Errors in the Pre-Analytical Phase in Clinical Laboratory Testing
3.2. Studies Regarding the Assessment on Vein Visualization by Conventional or Standard Method (Visualization or Palpation) and with the Use of Infrared Vein Finder Device
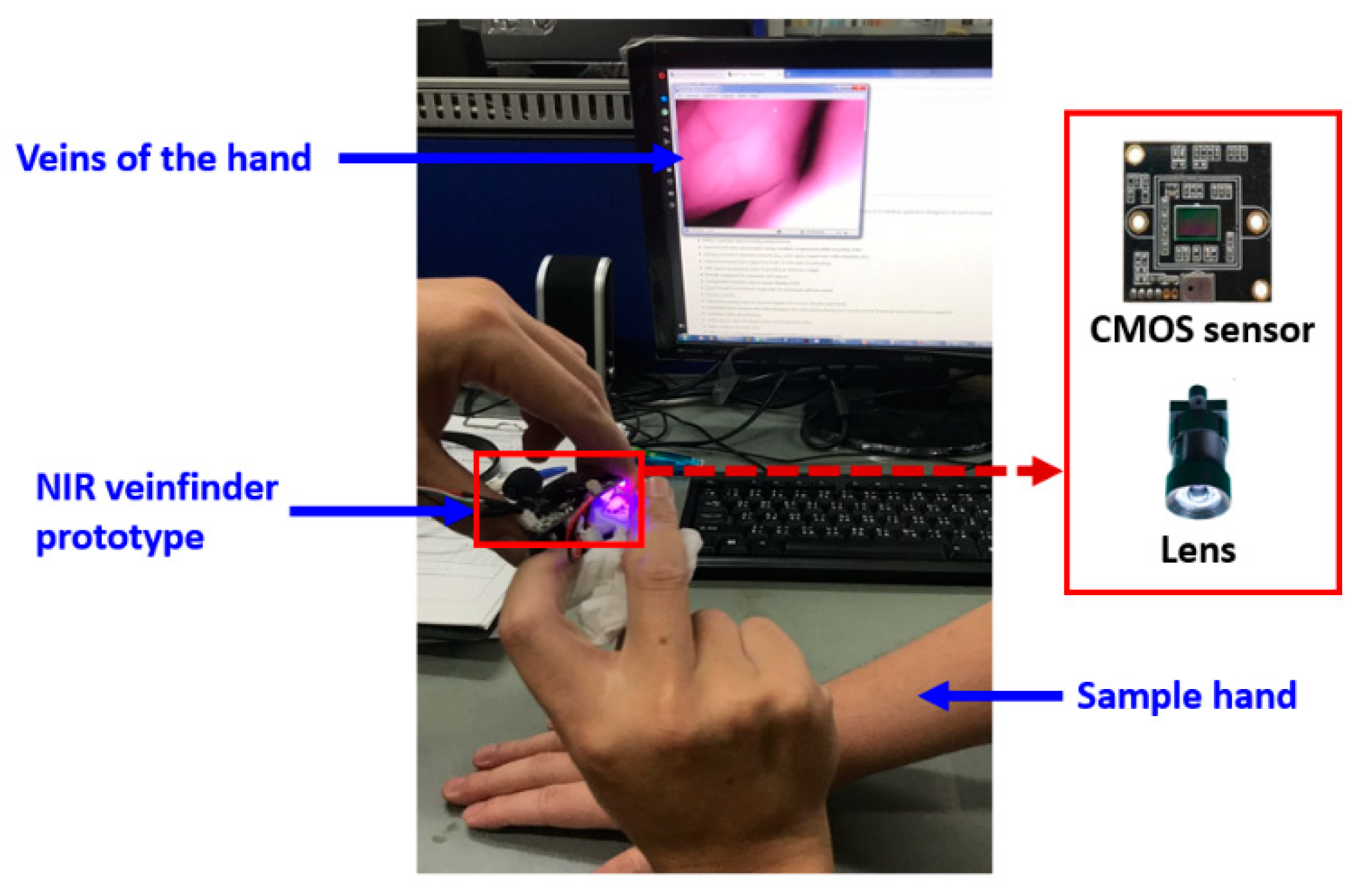
3.3. Basic Composition, Its Principle and Designs of a Vein Finder Prototype
3.4. Characteristics of the Different Vein Finder Prototypes
4. Discussion
4.1. Wavelength
4.2. Types of Camera, Sensors: Charge-Coupled Device (CCD) and Complementary Metal Oxide Semiconductor (CMOS) and Filters
4.3. The Common Parameters Used in the Assessment of the Prototypes Were the Following: Body Mass Index (BMI), Skin Color/Tone, Age, and Site for Venipuncture
4.3.1. Body Mass Index (BMI)
| BMI | |
| Underweight | Below 18.5 |
| Normal | 18.5–24.9 |
| Overweight | 25.0–29.9 |
| Obesity | 30.0 and Above [60] |
4.3.2. Skin color/tone
| Skin Type | Description |
| I | Very light complexion |
| II | Light complexion |
| III | Medium complexion |
| IV | Darker complexion |
| V | Dark complexion |
| VI | Black complexion [65] |
4.3.3. Age
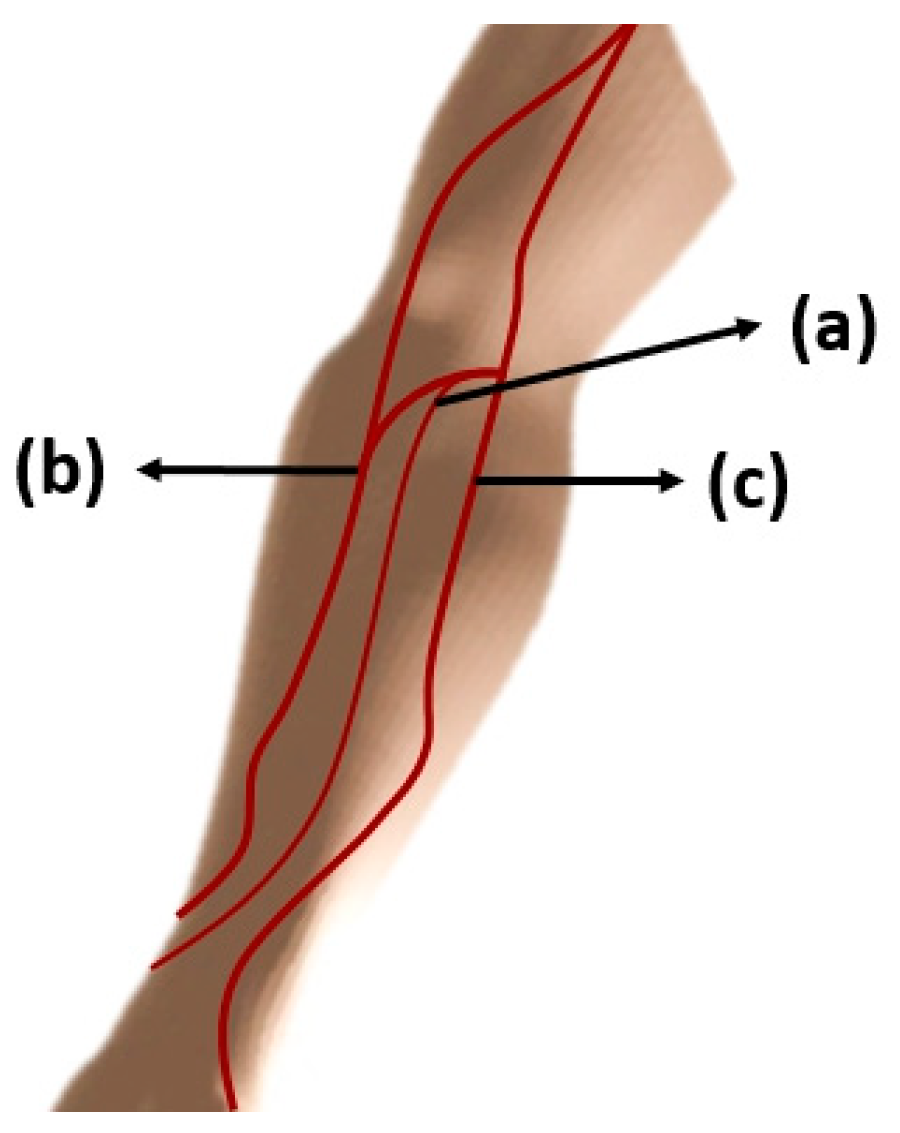
4.3.4. Site for venipuncture
4.4. Assessment of the Vein Finder Prototype
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lippi, G.; Baird, G.S.; Banfi, G.; Bölenius, K.; Cadamuro, J.; Church, S.; Cornes, M.P.; Dacey, A.; Guillon, A.; Hoffmann, G.; et al. Improving quality in the preanalytical phase through innovation, on behalf of the European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) Working Group for Preanalytical Phase (WG-PRE). Clin. Chem. Lab. Med. (CCLM) 2017, 55, 489–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippi, G.; Banfi, G.; Church, S.; Cornes, M.; De Carli, G.; Grankvist, K.; Kristensen, G.B.; Ibarz, M.; Panteghini, M.; Plebani, M.; et al. Preanalytical quality improvement. In pursuit of harmony, on behalf of European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) Working group for Preanalytical Phase (WG-PRE). Clin. Chem. Lab. Med. (CCLM) 2015, 53, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Giavarina, D.; Lippi, G. Blood venous sample collection: Recommendations overview and a checklist to improve quality. Clin. Biochem. 2017, 50, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Lamperti, M.; Pittiruti, M. Difficult peripheral veins: Turn on the lights. Br. J. Anaesth. 2013, 110, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Lalongo, C.; Sergio Bernardini, S. Phlebotomy, a bridge between laboratory and patient. Biochem. Med. 2016, 26, 17–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, A. Venipuncture-related lateral antebrachial cutaneous nerve injury: What to know? Braz. J. Anesth. (Engl. Ed.) 2014, 64, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Saad, N.M.; Walter, N.; Malik, S.A.; Meriaudeau, F. Hyperspectral venous image quality assessment for optimum illumination range selection based on skin tone characteristics. Biomed. Eng. Line 2014, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.; Panditrao, A. Non-invasive optical sensor for hemoglobin determination. Int. J. Eng. Res. Appl. (Ijera) 2013, 3, 559–562. [Google Scholar]
- Thom, C.; Dickson, C.; Gell, D.; Weiss, M. Hemoglobin Variants: Biochemical Properties and Clinical Correlates. Cold Spring Harb. Perspect. Med. 2013, 3, 011858. [Google Scholar] [CrossRef]
- Sebbane, M.; Claret, P.G.; Lefebvre, S.; Mercier, G.; Rubenovitch, J.; Jreige, R.; Eledjam, J.J.; de La Coussaye, J.E. Predicting peripheral venous access difficulty in the emergency department using body mass index and a clinical evaluation of venous accessibility. J. Emerg. Med. 2012, 44, 299–305. [Google Scholar] [CrossRef]
- Parker, S.; Benzies, K.; Hayden, K.; Lang, E. Effectiveness of interventions for adult peripheral intravenous catheterization: A systematic review and meta-analysis of randomized controlled trials. Int. Emerg. Nurs. 2017, 31, 15–21. [Google Scholar] [CrossRef] [PubMed]
- West, J.; Atherton, J.; Costelloe, S.; Pourmahram, G.; Stretton, A.; and Cornes, M. Pre-analytical errors in medical laboratories: A review of the available methodologies of data collection and analysis. Ann. Clin. Biochem. 2017, 54, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Najat, D. Prevalence of Pre-Analytical Errors in Clinical Chemistry Diagnostic Labs in Sulaimani City of Iraqi Kurdistan. PLoS ONE 2017, 12, e0170211. [Google Scholar] [CrossRef] [PubMed]
- Salinas, M.; Garrigós, M.; Flores, E.; Quiles, A.; Gutierrez, M.; Lugo, J.; Lillo, R.; Salinas, C. Ten years of preanalytical monitoring and control: Synthetic Balance Score Card Indicator. Biochem. Med. 2015, 25, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Mukherjee, B.; Das, A. Pre-analytical errors in the clinical laboratory and how to minimize them. Int. J. Bioassays 2013, 2, 551–553. [Google Scholar]
- Hammerling, J. A Review of Medical Errors in Laboratory Diagnostics and Where We Are Today. Lab. Med. 2012, 43, 41–44. [Google Scholar] [CrossRef]
- Lippi, G.1; Chance, J.J.; Church, S.; Dazzi, P.; Fontana, R.; Giavarina, D.; Grankvist, K.; Huisman, W.; Kouri, T.; Palicka, V.; et al. Pre-analytical quality improvement: From dream to reality. Clin. Chem. Lab. Med. (CCLM) 2011, 49, 1113–1126. [Google Scholar] [CrossRef]
- Goswami, B.; Singha, B.; Chawla, R.; Mallika, V. Evaluation of errors in a clinical laboratory: A one-year experience. Clin Chem Lab. Med. 2010, 48, 63–66. [Google Scholar] [CrossRef]
- Oliveira, G.; Volanski, W.; Lippi, G.; Picheth, G.; Guidi, G. Pre-analytical phase management: A review of the procedures from patient preparation to laboratory analysis. Scand. J. Clin. Lab. Investig. 2017, 77, 153–163. [Google Scholar] [CrossRef]
- Oliveira, G.; Lippi, G.; Salvagno, G.; Montagnana, M.; Picheth, G.; Guidi, G. The effective reduction of tourniquet application time after minor modification of the CLSI H03-A6 blood collection procedure. Croatian Society of Medical Biochemistry and Laboratory Medicine. Biochem. Med. 2013, 23, 308–315. [Google Scholar] [CrossRef]
- Buowari, O. Complications of venipuncture. Adv. Biosci. Biotechnol. 2013, 4, 126–128. [Google Scholar] [CrossRef]
- Miyake, R.; Zeman, H.; Duarte, F.; Kikuchi, R.; Ramacciotti, E.; Lovhoiden, G.; Vrancken, C. Vein imaging: A new method of near infrared imaging, where a processed image is projected onto the skin for the enhancement of vein treatment. Am. Soc. Derm. Surg. 2006, 32, 1031–1038. [Google Scholar] [CrossRef]
- Huang, K.; Chang, C.; Chang, H. The Image Analysis of Skin Tissue Irradiated with Difference Wavelengths of LED Sources. In Proceedings of the 2012 IEEE International Instrumentation and Measurement Technology Conference Proceedings, Graz, Austria, 13–16 May 2012. [Google Scholar]
- Juric, S.; Flis, V.; Debevc, M.; Holzinger, A.; Zalik, B. Towards a Low-Cost Mobile Subcutaneous Vein Detection Solution Using Near-Infrared Spectroscopy. Hindawi Publ. Corp. Sci. World J. 2014, 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Chiao, F.B.; Resta-Flarer, F.; Lesser, J.; Ng, J.; Ganz, A.; Pino-Luey, D.; Bennett, H.; Perkins, C. Jr.; Witek, B. Vein visualization: Patient characteristic factors and efficacy of a new infrared vein finder technology. Br. J. Anaesth. 2013, 110, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Phipps, K.; Modic, A.; O’Riordan, M.A.; Walsh, M. A randomized trial of the Vein Viewer versus standard technique for placement of peripherally inserted central catheters (PICCs) in neonates. J. Perinatol. 2012, 32, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Barreras, J.; Chang, T. Using a Near Infrared Device to Improve Successful Venous Access in Children with Special Health Care Needs. J. Assoc. Vasc. Access 2016, 22, 75–80. [Google Scholar] [CrossRef]
- Fukuroku, K.; Narita, Y.; Taneda, Y.; Kobayashi, S.; Gayle, A. Does infrared visualization improve selection of venipuncture sites for indwelling needle at the forearm in second-year nursing students? Nurse Educ. Pr. 2016, 18, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuper, N.; Verdaasdonk, R.; de Roode, R.; de Vooght, K.; Viergever, M.; Kalkman, C.; de Graaff, J. Visualizing Veins with Near- Infrared Light to Facilitate Blood Withdrawal in Children. Clin. Pediatrics 2011, 50, 508–512. [Google Scholar] [CrossRef]
- Liukui, C.; Zuojin, L.; Ying, W.; Yi, X. A Design of Infrared Finger Vein Image Acquisition Terminal. In Proceedings of the 2011 International Conference on Business Management and Electronic Information, Guangzhou, China, 13–15 May 2011. [Google Scholar]
- Marathe, M.; Bhatt, N.; Sundararajan, R. A Novel Wireless Vein Finder. In Proceedings of the International Conference on Circuits, Communication, Control and Computing (I4C 2014) MSRIT, Bangalore, India, 21–22 November 2014. [Google Scholar]
- Chakravorty, T.; Sonawane, D.; Sharma, S.; Patil, T. Low-Cost Subcutaneous Vein Detection System using ARM9 based Single Board Computer. In Proceedings of the 2011 3rd International Conference on Electronics Computer Technology, Kanyakumari, India, 8–10 April 2011. [Google Scholar]
- Ayoub, Y.; Serhal, S.; Farhat, B.; Ali, A.; Amatoury, J.; Nasser, H.; Ali, M. Diagnostic Superficial Vein Scanner. In Proceedings of the 2018 International Conference on Computer and Applications (ICCA), Beirut, Lebanon, 25–26 August 2018. [Google Scholar]
- Kauba, C.; Uhl, A. Shedding Light on the Veins Reflected Light or Transillumination in Hand-Vein Recognition. In Proceedings of the 2018 International Conference on Biometrics, Gold Coast, QLD, Australia, 20–23 February 2018. [Google Scholar]
- Dai, X.; Zhoua, Y.; Hub, X.; Liua, M.; Zhua, X.; Wua, Z. A Fast Vein Display Device Based on the Camera-projector System. In Proceedings of the 2013 IEEE International Conference on Imaging Systems and Techniques (IST), Beijing, China, 22–23 October 2013. [Google Scholar]
- Agnalt, S.; Canfield, D.; Perreault, K.; Legris, J.; McPheron, B. Vein Detection using Vein Transillumination and Contrast Differentiation for Practitioner Aid. In Proceedings of the 2016 IEEE MIT Undergraduate Research Technology Conference (URTC), Cambridge, MA, USA, 4–6 November 2016. [Google Scholar]
- Anupongongarch, P.; Khaosomboon, K.; Keawgun, T. Design and Construction of Median Cubital Vein Transillumination Device by Using LED. In Proceedings of the 2015 Biomedical Engineering International Conference (BMEiCON-2015), Pattaya, Thailand, 25–27 November 2015. [Google Scholar]
- Peled, G.; Halak, M.; Blechman, Z. Peripheral vein locating techniques. Imaging Med. 2016, 8. [Google Scholar] [CrossRef]
- Ghozali, H.; Setiawardhana; Sigit, R. Vein Detection System using Infrared Camera. In Proceedings of the 2016 International Electronics Symposium (IES), Denpasar, Indonesia, 29–30 September 2016. [Google Scholar]
- de Graaff, J.; Cuper, N.; Dijk, T.; Timmers-Raaijmaakers, B.; van der Werff, D.; Kalkman, C. Evaluating NIR vascular imaging to support intravenous cannulation in awake children difficult to cannulate; a randomized clinical trial. Pediatric Anesth. 2014, 24, 1174–1179. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.; Lee, D. A Phantom Study on the Propagation of NIR Rays under the Skin for Designing a Novel Vein-Visualizing Device. In Proceedings of the 13th International Conference on Control, Automation and Systems (ICCAS 2013), Gwangju, South Korea, 20–23 October 2013. [Google Scholar]
- Carlsen, R.; Zyhier, S.; Sirinterlikci, A. Project-based Learning: Engaging Biomedical Engineering Sophomores Through a Collaborative Vein-finder Device Project with Nursing. In Proceedings of the 2018 ASEE Annual Conference Exposition, Salt Lake City, UT, USA, 23 June 2018. [Google Scholar]
- Chandra, F.; Wahyudianto, A.; Yasin, M. Design of vein finder with multi tuning wavelength using RGB. Led J. Phys. Conf. Ser. Conf. Ser. 2017, 853, 012019. [Google Scholar] [CrossRef]
- Fernández, R.; Armada, M. Multisensory System for the Detection and Localization of Peripheral Subcutaneous Veins. Sens. (Basel) 2017, 17, 897. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, Y.; Yoon, S.; Lee, D. Preliminary Study for Designing a Novel Vein-Visualizing Device. Sensors 2017, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Kimori, K.; Sugama, J.; Nakatani, T.; Nakayama, K.; Miyati, T.; Sanada, H. An observational study comparing the prototype device with the existing device for the effective visualization of invisible veins in elderly patients in Japan. Sage Open Med. 2015, 3, 2050312115615365. [Google Scholar] [CrossRef] [PubMed]
- Dhakshayani, M.; Yacin, S. Economically Affordable and Clinically Reliable Vein Finder. In Proceedings of the 30th Indian Engineering Congress, the 21st Century Engineering: The Make in India Pathway, Guwahati, India, 17–20 December 2015. [Google Scholar]
- Meng, G.; Shahzad, A.; Saad, N.; Malik, A.; Meriaudeau, F. Prototype Design for Wearable Veins Localization System Using Near Infrared Imaging Technique. In Proceedings of the IEEE Xplore 11th International Colloquium on Signal Processing its Applications (CSPA2015), Kuala Lumpur, Malaysia, 6–8 March 2015. [Google Scholar]
- Chen, A.; Nikitczuk, K.; Nikitczuk, J.; Maguire, T.; Yarmush, M. Portable robot for autonomous venipuncture using 3D near infrared image guidance. Technol. (Singap World Sci) 2013, 1, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Behrooz, A.; Morris, M.; Adibia, A. High-contrast subcutaneous vein detection and localization using multispectral imaging. J. Biomed. Opt. 2013, 18, 050504–1. [Google Scholar] [CrossRef]
- Jin, Y.; Jing, Z.; Cui-ying, H. Near-Infrared Imaging Approach for in vivo Detecting the Distribution of Human Blood Vessels. In Proceedings of the 2012 International Conference on Biomedical Engineering and Biotechnology, Macao, China, 28–30 May 2012. [Google Scholar]
- Nundy, K.; Sanyal, S. A Low Cost Vein Detection System using Integrable Mobile Camera Devices. In Proceedings of the 2010 Annual IEEE India Conference (INDICON), Kolkata, India, 17–19 December 2010. [Google Scholar]
- Crisan, S.; Tarnovan, I.; Crisan, T. Vein Pattern Recognition. Image Enhancement and feature Extraction Algorithms. In Proceedings of the 15th IMEKO TC4 Symposium on Novelties in Electrical Measurements and Instrumentation, Iaşi, Romania, 19–21 September 2007. [Google Scholar]
- Electronics.howstuffworks.com. Cameras photography/digital/question362. 2000. Available online: htmHowStuffWorks.com (accessed on 1 December 2018).
- Vatteroni, M.; Covi, D.; Stoppa, D.; Crespi, B.; Sartori, A. High dynamic range CMOS image sensors in biomedical applications. In Proceedings of the 29th Annual International Conference of the IEEE EMBS, Lyon, France, 22–26 August 2007. [Google Scholar]
- Kaur, A.; Mishra, D.; Sarkar, M. An on-chip interpolation based readout scheme for low-power, high-speed CMOS image sensors. In Proceedings of the 2018 IEEE SENSORS, New Delhi, India, 28–31 October 2018. [Google Scholar]
- Shishido, S.; Oguro, Y.; Noda, T.; Sasagawa, K.; Tokuda, T.; Ohta, J. CMOS image sensor for recording of intrinsicoptical-signal of the brain. In Proceedings of the 2009 International SoC Design Conference (ISOCC), Busan, South Korea, 22–24 November 2009. [Google Scholar]
- Juric, S.; Zalik, B. An innovative approach to near-infrared spectroscopy using a standard mobile device and its clinical application in the real-time visualization of peripheral veins. BMC Med. Inf. Decis. Mak. 2014, 25, 100. [Google Scholar] [CrossRef]
- Ahmed, K.; Habaebi, M.; Islam, M.; Zainal, N. Enhanced Vision Based Vein Detection System. In Proceedings of the 4th IEEE International Conference on Smart Instrumentation, Measurement and Applications (ICSIMA), Putrajaya, Malaysia, 28–30 November 2017. [Google Scholar]
- National Institute of Health. Aim for a Healthy Weight; U.S. Department of Health Human Services 9000 Rockville Pike: Bethesda, MD, USA, 2018.
- Canadian Diabetes Association. 2019. Available online: www.diabetes.ca/diabetes-and-you/healthy-living-resources/weight-management/body-mass-index-bmi-calculator (accessed on 1 March 2019).
- Kam, J.; Taylor, D. Obesity significantly increases the difficulty of patient management in theemergency department. Emerg. Med. Australas. 2010, 22, 316–323. [Google Scholar] [CrossRef]
- Ramachandran, A.; Snehalatha, C. Rising Burden of Obesity in Asia. J. Obes. 2010, 2010, 8. [Google Scholar] [CrossRef]
- Hales, C.; Carroll, M.; Fryar, C.; Ogden, C.; Centers for Disease Control and Prevention (CDC). Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. 2017. Available online: www.cdc.gov/nchs/data/databriefs/db288_table.pdf#1 (accessed on 1 March 2019).
- Ash, C.; Town, G.; Bjerring, P.; Webster, S. Evaluation of a novel skin tone meter and the correlation between Fitzpatrick skin type and skin color. Photonics Lasers Med. 2015, 4, 177–186. [Google Scholar] [CrossRef]
- van der Woude, O.; Cuper, N.; Getrouw, C.; Kalkman, C.; de Graaff, J. The Effectiveness of a Near-Infrared Vascular Imaging Device to Support Intravenous Cannulation in Children with Dark Skin Color: A Cluster Randomized Clinical Trial. Int. Anesth. Res. Soc. 2013, 116, 6. [Google Scholar] [CrossRef] [PubMed]
- Keohane, E.; Smith, L.; Walenga, J. Rodak’s Hematology Clinical Principles and Applications, 5th ed.; Elsivier: Amsterdam, The Nederland, 2016. [Google Scholar]
- The Clinical Laboratory Standards Institute (CLSI). NEW CLSI Venipuncture Guidelines-CLSI, Collection of Diagnostic Venous Blood Specimens, 7th ed.; Standard GP41; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Grable, H.; Gill, G. Phlebotomy Puncture Juncture Preventing Phlebotomy Errors—Potential for Harming Your Patients. Labmedicine 2005, 36. [Google Scholar] [CrossRef]
- Ganesh, S. Depth and Size Limits for the Visibility of Veins Using the Vein Viewer Imaging System. Thesis and Dissertations (ETD), University of Tennessee Health Science Center, Knoxville, TN, USA2007, 2007. [Google Scholar]
- Goh, C.; Subramaniam, R.; Saad, N.; Ali, S.; Meriaudeau, F. Subcutaneous veins depth measurement using diffuse reflectance images. Opt. Express 2017, 25, 25741–25759. [Google Scholar] [CrossRef] [PubMed]
- Leitch, A.; McCormick, I.; Gunn, I.; Gillespie, T. Reducing the potential for phlebotomy tourniquets to act as a reservoir for meticillin-resistant Staphylococcus aureus. J. Hosp. Infect. 2006, 63, 428–431. [Google Scholar] [CrossRef] [PubMed]
- The Clinical Laboratory Standards Institute (CLSI). CLSI H3-A6 Procedures for the Collection of Diagnostic Blood Specimens by Venipuncture, Approved Standard-6th Ed.; CLSI: Wayne, PA, USA, 2007. [Google Scholar]


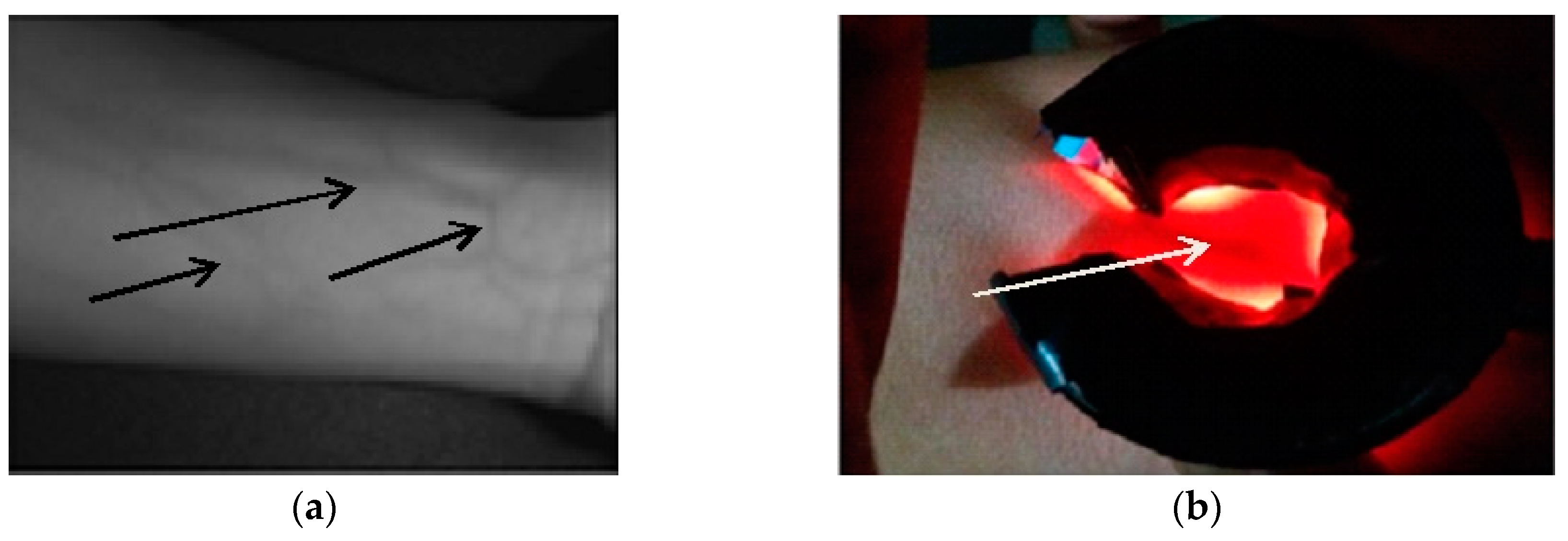
| STUDY (First Author, Year of Publication) | PRE-ANALYTICAL ERROR (%) |
|---|---|
| West J et al., 2017 [12] | up to 68.20 |
| Najat D, 2017 [13] | up to 70.00 |
| Salinas M et al., 2015 [14] | 60.00 to 70.00 |
| Patra S, 2013 [15] | 46.00 to 68.20 |
| Hammerling J, 2012 [16] | 46.00 to 68.20 |
| Lippi G et al., 2011 [17] | 60.00 to 70.00 |
| Goswami B et al., 2010 [18] | 77.10 |
| Prototype Study First Author (Year) | Light Source Wave Length (nm) | Camera, Sensor and Filters | Considered Parameters and Site for Evaluation | Type of Evaluation (Human Testing) |
|---|---|---|---|---|
| Ayoub, Y. et al. (2018) [33] | 850 and 940 | Nikon D810 camera (resolution 36.6 MP), Zomei 720 IR filter | Body Temperature, Site: arm | 10 subjects |
| Carlsen, R. et al. (2018) [42] | 850 | 8 MP NoIR Camera (8 megapixel image sensor), Plastic sheet inside floppy disks or negative films, with diffusers, such as tissue paper and frosted window films. | NA | NA—No actual testing done |
| Chandra, F. et al. (2017) [43] | 600–696 | NA | (*) BMI, age, and skin color | Tested to 10 patients |
| Fernandez, R. et al. (2017) [44] | 940 | GoldEye P-032 SWIR camera (AlliedVision, Stradtroda, Germany), a Swiss Ranger SR-400011 TOF 3D camera (MesaImaging, Zürich, Switzerland) | NA | NA—No patient testing done |
| Kim, D. et al. (2017) [45] | 850 | NIR CCD camera (Grasshopper3 GS3-U3-41C6NIR-C, Point Grey Inc., Richmond, BC, Canada) and a high-resolution lens (GMTHR48014MCN, Goyo Optical Inc., Asaka, Japan) 850 nm band-pass filter (BP850-S44.5, Midwest Optical System Inc., Palatine, IL, USA) | (*) NA | NA—No patient testing done |
| Anupongongarch et al. (2015) [37] | 700–1000 | NA | Patient’s skin color | 17 pale skin, 13 color skin |
| Kimori, K. et al. (2015) [46] | 850 | Compact IR-sensitive charged-coupled device (CIS) CCD | BMI, age, hemoglobin, skin color Sites: Cubital fossa and forearm | 72 patients |
| Dhakshayani, M. et al. (2015) [47] | Multispectral imaging IR 740, 765, 770, 780 | Web camera with CMOS sensors, IR pass filters—Kodak wratten 87 IR filter infrared (IR) photographic film | Age, body mass, skin color Sites: antecubital vein, cephalic vein, forearm, and dorsal hand | 25 dark skinned people, 25 obese subjects, 25 paediatrics, and 2 elderly |
| Meng, G. et al. (2015) [48] | 830 and 850 | Vuzix STAR 1200XL eyewear system IR CCD camera | NA | NA—No patient testing done |
| Marathe, M. et al. (2014) [31] | 920 | CMOS camera, captured image is compressed in Joint Photographic Experts Group (JPEG) format, IR filter | NA Site: wrist | NA—No patient testing done |
| Juric, S. et al. (2014) [24] | 740 | Standard (Universal Serial Bus) USB camera Pass-through filter (exposed and developed empty 35 mm camera film | BMI | 72 subjects |
| Shahzad, A. et al. (2014) [7] | 800–850 | Spectral Camera PS V10E | Skin tone: Fair, light brown, dark brown, and dark | 80 subjects/patients |
| Chen, A. et al. (2013) [49] | 940 | Two monochrome FireWire cameras with high sensitivity CCD sensors in the near-infrared range (Point Grey Firefly MV) 850–1060 nm band pass filters (Edmund Optics) | Age, gender, BMI and skin color (Fitzpatrick skin type) | 101 patients |
| Dai et al. (2013) [35] | 850 | Monochrome NIR CMOS camera (EO-0413BL Edmund) with DLP projector (DL3000 Texas Instruments) | Site: Hand | (1) Hand vein |
| Lee, S. et al. (2013) [41] | 740 | Real-time camera IR long-pass filter 695 nm | (*) NA | Vein model (plastic tube and dog’s blood) |
| Wang, F. et al. (2013) [50] | Multispectral imaging IR 850, 615, 570, 546, 475 | Spectrocam™ Multispectral Imaging Camera (Ocean Thin Films, Golden, Colorado) NIR enhanced CCD camera (a Sony ICX285 sensor) through a Carl Zeiss Distagon 2.8/25 mm ZF-IR lens With multi filters | Asian male, Caucasian male, skin tone, hairy forearm | 3 human subjects |
| Jin et al. (2012) [51] | 940 | Camera with CMOS high-transmittance imaging lens Filter passing wavelength of 940 nm | Sites: Hand and arm | (1) Hand and arm |
| Chakravorty, T. et al. (2011) [32] | 850 | Webcam with OV9650 sensor Liquid Crystal Display (LCD) screen ARM9 single board computer | Site: Finger | NA—No patient testing done |
| Cuper, N. et al. (2011) [29] | 850 | IR-sensitive camera with Video Graphics Array (VGA) resolution (640 × 480) Filter blocking all light less than 800 nm. | Children (0-6 years) male and female Dark skin, fat padding, | Children tested: 80 without NIR light, 45 with the NIR prototype |
| Nundy, K. et al. (2010) [52] | 740–760 | Ordinary camera phones with even VGA quality pictures Optical filter using butter paper and filter made from exposed and developed film strips | NA | NA—No patient testing done |
| Crisan, S. et al. (2007) [53] | 740–760 | Camera with CCD Polarizing filters and blank sheet made of polycarbonate | NA | NA—No patient testing done |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, C.-T.; Francisco, M.D.; Yen, C.-K.; Wang, S.-Y.; Shiue, Y.-L. Vein Pattern Locating Technology for Cannulation: A Review of the Low-Cost Vein Finder Prototypes Utilizing near Infrared (NIR) Light to Improve Peripheral Subcutaneous Vein Selection for Phlebotomy. Sensors 2019, 19, 3573. https://doi.org/10.3390/s19163573
Pan C-T, Francisco MD, Yen C-K, Wang S-Y, Shiue Y-L. Vein Pattern Locating Technology for Cannulation: A Review of the Low-Cost Vein Finder Prototypes Utilizing near Infrared (NIR) Light to Improve Peripheral Subcutaneous Vein Selection for Phlebotomy. Sensors. 2019; 19(16):3573. https://doi.org/10.3390/s19163573
Chicago/Turabian StylePan, Cheng-Tang, Mark D. Francisco, Chung-Kun Yen, Shao-Yu Wang, and Yow-Ling Shiue. 2019. "Vein Pattern Locating Technology for Cannulation: A Review of the Low-Cost Vein Finder Prototypes Utilizing near Infrared (NIR) Light to Improve Peripheral Subcutaneous Vein Selection for Phlebotomy" Sensors 19, no. 16: 3573. https://doi.org/10.3390/s19163573
APA StylePan, C.-T., Francisco, M. D., Yen, C.-K., Wang, S.-Y., & Shiue, Y.-L. (2019). Vein Pattern Locating Technology for Cannulation: A Review of the Low-Cost Vein Finder Prototypes Utilizing near Infrared (NIR) Light to Improve Peripheral Subcutaneous Vein Selection for Phlebotomy. Sensors, 19(16), 3573. https://doi.org/10.3390/s19163573