The Thermal and Mechanical Properties of Poly(ethylene-co-vinyl acetate) Random Copolymers (PEVA) and its Covalently Crosslinked Analogues (cPEVA)
Abstract
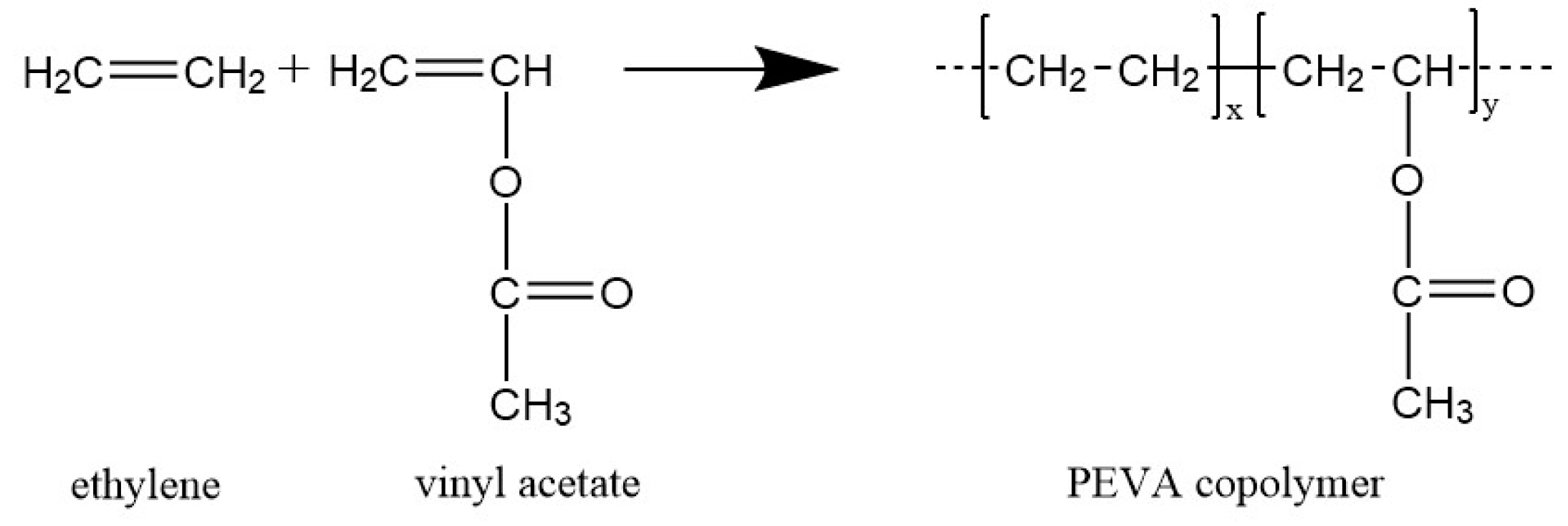
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PEVA Copolymer Networks (cPEVA)
2.3. Determination of Crosslink Density in cPEVAs
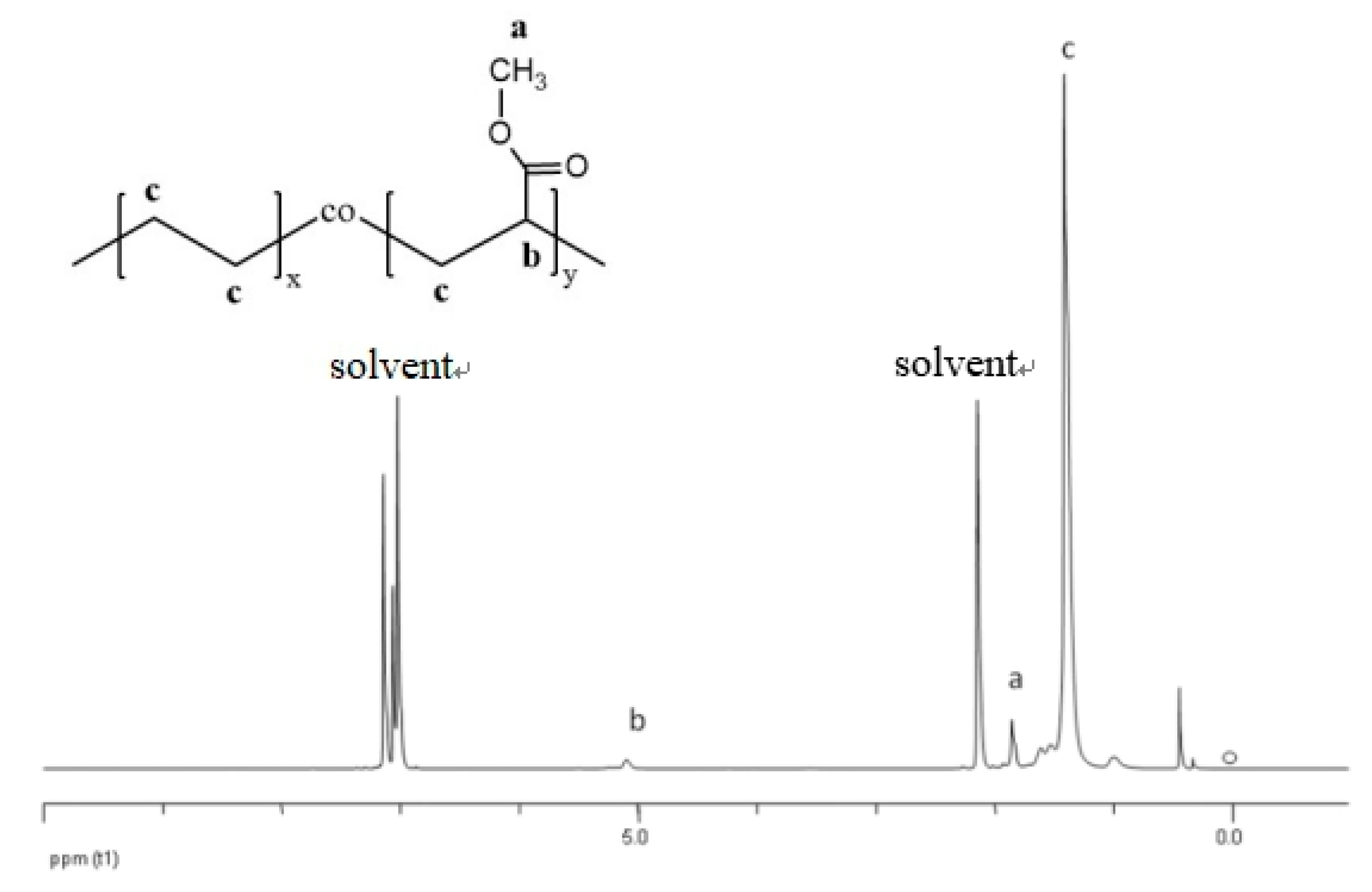
2.4. 1H-NMR Spectroscopy
2.5. Thermogravimetric (TG) and Derivative Thermogravimetric (DTG)
2.6. Wide Angle X-ray Scattering (WAXS)
2.7. Differential Scanning Calorimetry (DSC)
2.8. Dynamic Mechanical Analysis (DMA) at Varied Temperatures
2.9. Mechanical Testing
3. Results and Discussion
3.1. Composition of PEVA and cPEVA
3.1.1. Determination of VA-Content
3.1.2. Degree of Crystallinity
3.1.3. Crosslinking Density
3.2. Thermal Properties
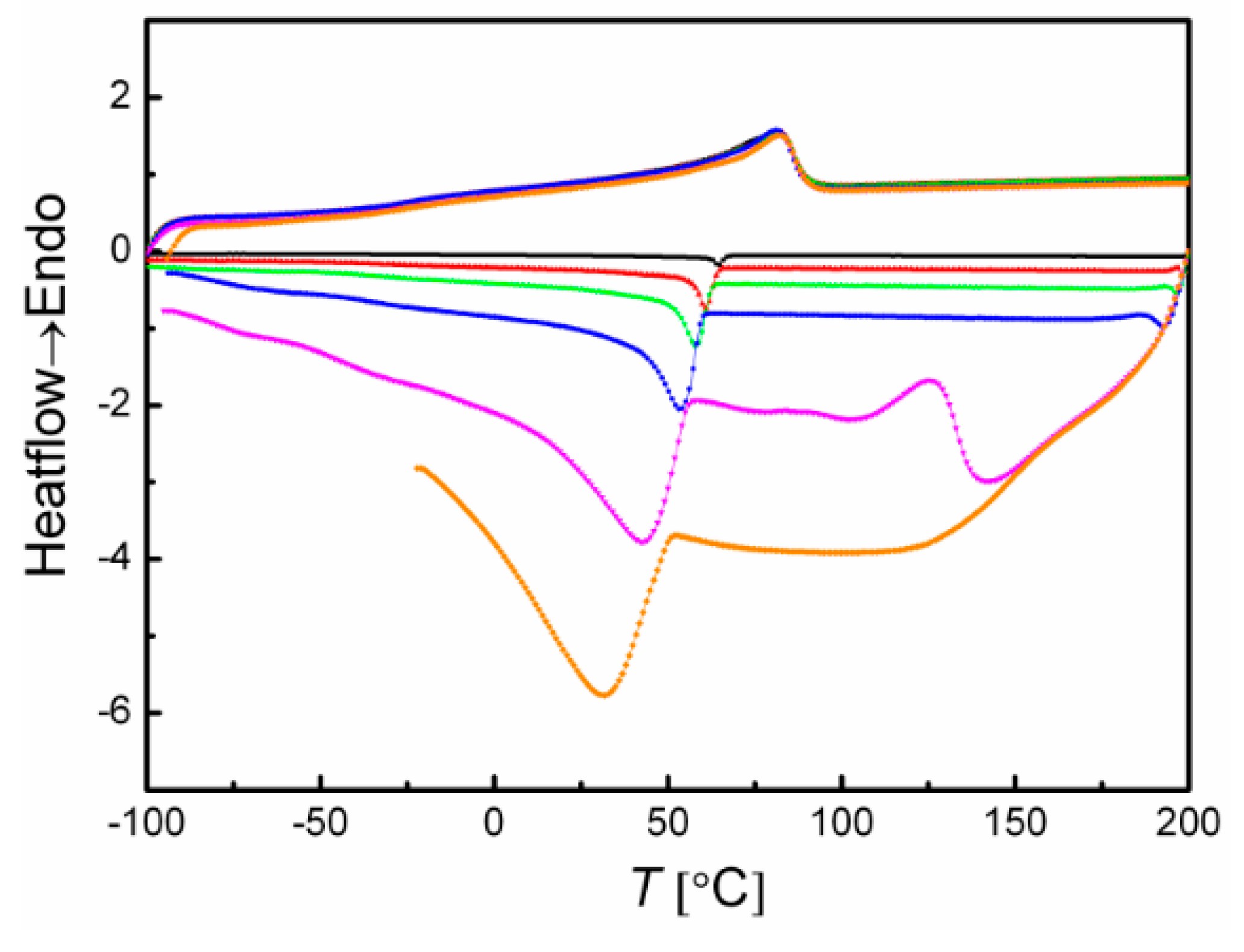
3.2.1. DSC Analysis
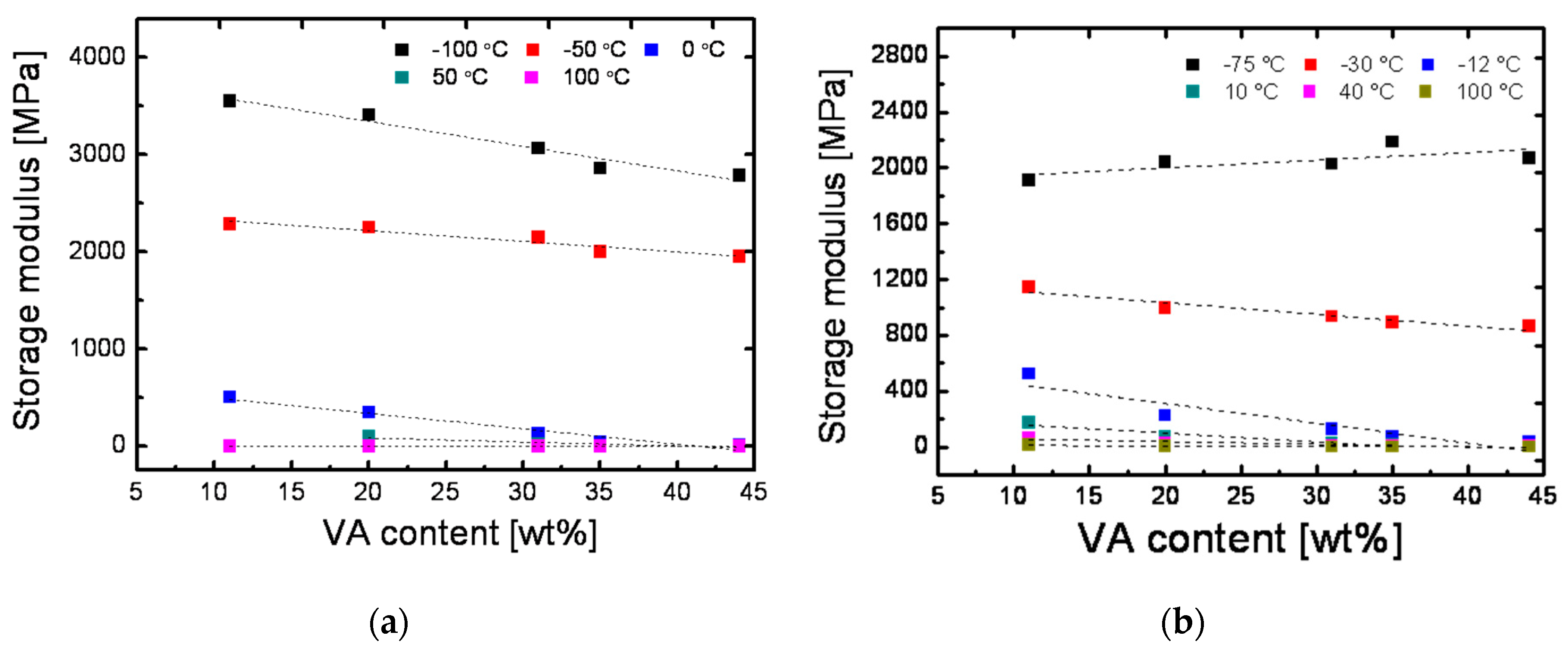
3.2.2. DMA Analysis
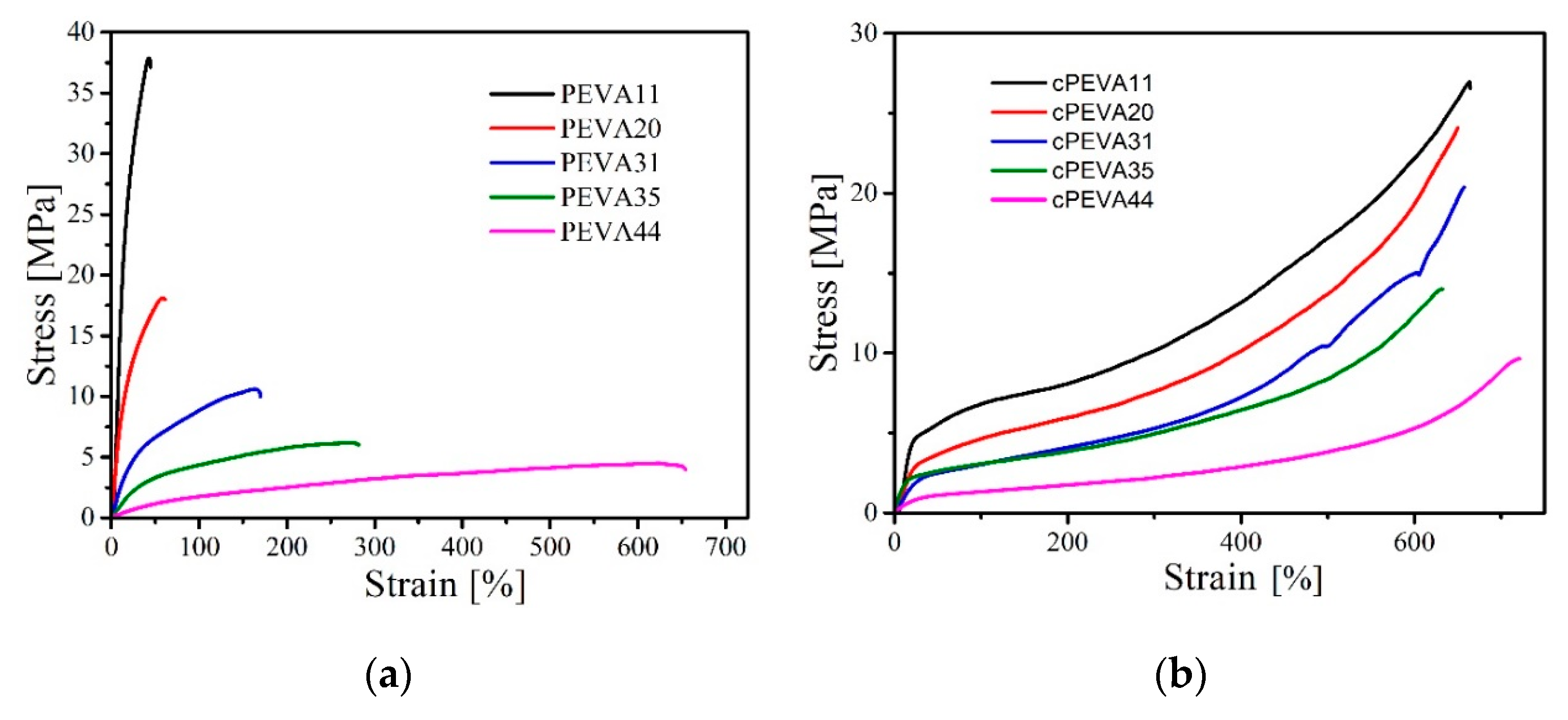
3.3. Mechanical Properties
4. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lendlein, A.; Kelch, S. Shape-memory polymers. Angew. Chem. Int. Ed. 2002, 41, 2034–2057. [Google Scholar] [CrossRef]
- Karl, K.; Madbouly, S.A.; Wolfgang, W.; Andreas, L. Temperature-Memory Polymer Networks with Crystallizable Controlling Units. Adv. Mater. 2011, 23, 4058–4062. [Google Scholar]
- Jiang, H.Y.; Kelch, S.; Lendlein, A. Polymers move in response to light. Adv. Mater. 2006, 18, 1471–1475. [Google Scholar] [CrossRef]
- Mohr, R.; Kratz, K.; Weigel, T.; Lucka-Gabor, M.; Moneke, M.; Lendlein, A. Initiation of shape-memory effect by inductive heating of magnetic nanoparticles in thermoplastic polymers. Proc. Natl. Acad. Sci. USA 2006, 103, 3540–3545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behl, M.; Ridder, U.; Feng, Y.; Kelch, S.; Lendlein, A. Shape-memory capability of binary multiblock copolymer blends with hard and switching domains provided by different components. Soft Matter 2009, 5, 676–684. [Google Scholar] [CrossRef]
- Miaudet, P.; Derre, A.; Maugey, M.; Zakri, C.; Piccione, P.M.; Inoubli, R.; Poulin, P. Shape and temperature memory of nanocomposites with broadened glass transition. Science 2007, 318, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Bertmer, M.; Buda, A.; Blomenkamp-Hofges, I.; Kelch, S.; Lendlein, A. Biodegradable shape-memory polymer networks: Characterization with solid-state NMR. Macromolecules 2005, 38, 3793–3799. [Google Scholar] [CrossRef]
- Choi, N.Y.; Kelch, S.; Lendlein, A. Synthesis, Shape-Memory Functionality and Hydrolytical Degradation Studies on Polymer Networks from Poly(rac-lactide)-b-poly(propylene oxide)-b-poly(rac-lactide) dimethacrylates. Adv. Eng. Mater. 2006, 8, 439–445. [Google Scholar] [CrossRef]
- Choi, N.Y.; Lendlein, A. Degradable shape-memory polymer networks from oligo [(l-lactide)-ran-glycolide] dimethacrylates. Soft Matter 2007, 3, 901–909. [Google Scholar] [CrossRef]
- Maitland, D.J.; Metzger, M.F.; Schumann, D.; Lee, A.; Wilson, T.S. Photothermal properties of shape memory polymer micro-actuators for treating stroke. Lasers Surg. Med. 2002, 30, 1–11. [Google Scholar] [CrossRef]
- Rickert, D.; Moses, M.A.; Lendlein, A.; Kelch, S.; Franke, R.P. The importance of angiogenesis in the interaction between polymeric biomaterials and surrounding tissue. Clin. Hemorheol. Microcirc. 2003, 28, 175–181. [Google Scholar] [PubMed]
- Rickert, D.; Lendlein, A.; Schmidt, A.M.; Kelch, S.; Roehlke, W.; Fuhrmann, R.; Franke, R.P. In vitro cytotoxicity testing of AB-polymer networks based on oligo(ε-caprolactone) segments after different sterilization techniques. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 67, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, J.; Lei, J.; Liu, D.; Xie, Z.; Qu, D.; Li, K.; Deng, T.; Tang, H. Advanced separators for lithium-ion and lithium–sulfur batteries: A review of recent progress. ChemSusChem 2016, 9, 3023–3039. [Google Scholar] [CrossRef] [PubMed]
- Lendlein, A.; Schmidt, A.M.; Langer, R. AB-polymer networks based on oligo (ϵ-caprolactone) segments showing shape-memory properties. Proc. Natl. Acad. Sci. USA 2001, 98, 842–847. [Google Scholar] [PubMed]
- Neuss, S.; Blomenkamp, I.; Stainforth, R.; Boltersdorf, D.; Jansen, M.; Butz, N.; Perez-Bouza, A.; Knüchel, R. The use of a shape-memory poly(ε-caprolactone) dimethacrylate network as a tissue engineering scaffold. Biomaterials 2009, 30, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Li, F.K.; Zhu, W.; Zhang, X.; Zhao, C.T.; Xu, M. Shape memory effect of ethylene–vinyl acetate copolymers. J. Appl. Polym. Sci. 1999, 71, 1063–1070. [Google Scholar] [CrossRef]
- Sung, Y.T.; Kum, C.K.; Lee, H.S.; Kim, J.S.; Yoon, H.G.; Kim, W.N. Effects of crystallinity and crosslinking on the thermal and rheological properties of ethylene vinyl acetate copolymer. Polymer 2005, 46, 11844–11848. [Google Scholar] [CrossRef]
- Yao, D.H.; Qu, B.J.; Wu, Q.H. Photoinitiated crosslinking of ethylene-vinyl acetate copolymers and characterization of related properties. Polym. Eng. Sci. 2007, 47, 1761–1767. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G.E. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef]
- Brogly, M.; Nardin, M.; Schultz, J. Effect of vinylacetate content on crystallinity and second-order transitions in ethylene—vinylacetate copolymers. J. Appl. Polym. Sci. 1997, 64, 1903–1912. [Google Scholar] [CrossRef]
- Arsac, A.; Carrot, C.; Guillet, J. Rheological characterization of ethylene vinyl acetate copolymers. J. Appl. Polym. Sci. 1999, 74, 2625–2630. [Google Scholar] [CrossRef]
- Bistac, S.; Kunemann, P.; Schultz, J. Crystalline modifications of ethylene-vinyl acetate copolymers induced by a tensile drawing: Effect of the molecular weight. Polymer 1998, 39, 4875–4881. [Google Scholar] [CrossRef]
- Gospodinova, N.; Zlatkov, T.; Terlemezyan, L. Relationship between microstructure and phaseand relaxation transitions in ethylene-(vinyl acetate) copolymers prepared by emulsion copolymerization. Polymer 1998, 39, 2583–2588. [Google Scholar] [CrossRef]
- Radhakrishnan, C.K.; Sujith, A.; Unnikrishnan, G.; Thomas, S. Effects of the blend ratio and crosslinking systems on the curing behavior, morphology, and mechanical properties of styrene–butadiene rubber/poly(ethylene-co-vinyl acetate) blends. J. Appl. Polym. Sci. 2004, 94, 827–837. [Google Scholar] [CrossRef]
- Varghese, H.; Johnson, T.; Bhagawan, S.S.; Joseph, S.; Thomas, S.; Groeninckx, G. Dynamic mechanical behavior of acrylonitrile butadiene rubber/poly(ethylene-co-vinyl acetate) blends. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1556–1570. [Google Scholar] [CrossRef]
- Agroui, K.; Collins, G.; Farenc, J. Measurement of glass transition temperature of crosslinked EVA encapsulant by thermal analysis for photovoltaic application. Renew. Energy 2012, 43, 218–223. [Google Scholar] [CrossRef]
- Agroui, K.; Collins, G. Determination of thermal properties of crosslinked EVA encapsulant material in outdoor exposure by TSC and DSC methods. Renew. Energy 2014, 63, 741–746. [Google Scholar] [CrossRef]
- Jacobs, M.A.; Kemmere, M.F.; Keurentjes, J.T.F. Foam processing of poly(ethylene-co-vinyl acetate) rubber using supercritical carbon dioxide. Polymer 2004, 45, 7539–7547. [Google Scholar] [CrossRef]
- Mosnácek, J.; Basfar, A.A.; Shukri, T.M.; Bahattab, M.A. Poly(ethylene vinyl acetate) (EVA)/low density polyethylene (LDPE)/ammonium polyphosphate (APP) composites cross-linked by dicumyl peroxide for wire and cable applications. Polym. J. 2008, 40, 460–464. [Google Scholar] [CrossRef]
- Shi, X.; Jia, L.; Yan, M.; Li, C. Effects of fillers on the damping property of ethylene vinyl-acetate/polylactic acid blends. J. Mater. Sci. Chem. Eng. 2016, 4, 89–96. [Google Scholar] [CrossRef]
- Wang, L.; Hong, Y.; Li, J.X. Durability of running shoes with ethylene vinyl acetate or polyurethane midsoles. J. Sports Sci. 2012, 30, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Narkis, M.; Miltz, J. Nanostructured thermoplastic vulcanizates by selectively cross-linking a thermoplastic blend with similar chemical structures. J. Appl. Polym. Sci. 2010, 21, 703–709. [Google Scholar] [CrossRef]
- Ganesh, B.; Unnikrishnan, G. Cure characteristics, morphology, mechanical properties, and aging characteristics of silicone rubber/ethylene vinyl acetate blends. J. Appl. Polym. Sci. 2010, 99, 1069–1082. [Google Scholar] [CrossRef]
- Bianchi, O.; Oliveira, R.; Fiorio, R. Assessment of Avrami, Ozawa and Avrami–Ozawa equations for determination of EVA crosslinking kinetics from DSC measurements. Polym. Test. 2008, 27, 722–729. [Google Scholar] [CrossRef]
- Mishra, S.B.; Luyt, A.S. Effect of organic peroxides on the morphological, thermal and tensile properties of EVA-organoclay nanocomposites. Express Polym. Lett. 2008, 2, 256–264. [Google Scholar] [CrossRef]
- Lendlein, A.; Schmidt, A.M.; Schroeter, M.; Langer, R. Shape-memory polymer networks from oligo(ϵ-caprolactone) dimethacrylates. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1369–1381. [Google Scholar] [CrossRef]
- Blundell, D.J.; Beckett, D.R.; Willcocks, P.H. Routine crystallinity measurements of polymers by DSC. Polymer 1981, 22, 704–707. [Google Scholar] [CrossRef]
- Höhne, G.W.H.; Hemminger, W.F.; Flammersheim, H.-J. Differential Scanning Calorimetry; Springer: Berlin, Germany, 2003. [Google Scholar]
- Mirabella, F.M.; Bafna, A. Determination of the crystallinity of polyethylene/α-olefin copolymers by thermal analysis: Relationship of the heat of fusion of 100% polyethylene crystal and the density. J. Polym. Sci. B: Polym. Phys. 2002, 40, 1637–1643. [Google Scholar] [CrossRef]
- Wunderlich, B.; Cormier, C.M. Heat of fusion of polyethylene. J. Polym. Sci. Part A Polym. Phys. 1967, 5, 987–988. [Google Scholar] [CrossRef]
- Wunderlich, B. The ATHAS database on heat capacities of polymers. Pure Appl. Chem. 1995, 67, 1019–1026. [Google Scholar] [CrossRef]
- McGrattan, B.J. Decomposition of Ethylene-Vinyl Acetate Copolymers Examined by Combined Thermogravimetry, Gas-Chromatography, and Infrared-Spectroscopy. In Proceedings of the Symposium on Hyphenated Techniques in Polymer Characterization—Thermal-Spectroscopic and Other Methods, at the 206th National Meeting of the American-Chemical-Society, America Chemical Society, Chicago, IL, USA, 22–27 August 1993; pp. 103–115. [Google Scholar]
- Lendlein, A.; Kelch, S. Shape-memory polymers as stimuli-sensitive implant materials. Clin. Hemorheol. Microcirc. 2005, 32, 105–116. [Google Scholar] [PubMed]
- Kim, S.J.; Shin, B.S.; Hong, J.L.; Cho, W.J.; Ha, C.S. Reactive compatibilization of the PBT/EVA blend by maleic anhydride. Polymer 2001, 42, 4073–4080. [Google Scholar] [CrossRef]
- Nöchel, U.; Reddy, C.S.; Ke, W.; Jing, C.; Zizak, I.; Behl, M.; Kratz, K.; Lendlein, A. Nanostructural changes in crystallizable controlling units determine the temperature-memory of polymers. J. Mater. Chem. A 2015, 3, 8284–8293. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, T.; Seguchi, T. ESR study on chemical crosslinking reaction mechanisms of polyethylene using a chemical agent. J. Polym. Sci. Part A Polym. Chem. 1996, 35, 279–284. [Google Scholar] [CrossRef]
- Horkay, F.; Burchard, W.; Hecht, A.M.; Geissler, E. Scattering properties of poly(vinyl acetate) gels in different solvents. Macromolecules 1993, 26, 4203–4207. [Google Scholar] [CrossRef]
- Reding, F.P.; Whitman, R.D.; Faucher, J.A. Glass transitions in ethylene copolymers and vinyl homopolymers and copolymers. J. Polym. Sci. 1962, 57, 483–498. [Google Scholar] [CrossRef]
- Lam, R.; Geil, P.H. The Tg of amorphous linear polyethylene: A torsion braid analysis. Polym. Bull. 1978, 1, 127–131. [Google Scholar] [CrossRef]
- Mihaylova, M.; Kresteva, M. Dynamic mechanical properties of polymer blends of polypropylene and poly(ethylene-co-vinyl acetate) irradiated with fast electrons. Bulg. J. Phys. 2001, 28, 85–94. [Google Scholar]
- Mandelkern, L.; Martin, G.M.; Quinn, F.A. Glassy state transitions of poly-(chlorotrifluoroethylene), poly-(vinylidenefluoride), and their copolymers. J. Res. Natl. Bur. Stand. 1957, 58, 137–143. [Google Scholar] [CrossRef]
- Stark, W.; Jaunich, M. Investigation of Ethylene/Vinyl Acetate Copolymer (EVA) by thermal analysis DSC and DMA. Polym. Test. 2011, 30, 236–242. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, W.N. Glass transition temperatures and rigid amorphous fraction of poly(ether ether ketone) and poly(ether imide) blends. Polymer 1997, 38, 2657–2663. [Google Scholar]
- Kim, S.L.; Skibo, M.D.; Manson, J.A.; Hertzberg, R.W.; Janiszewski, J. Tensile, impact and fatigue behavior of an amine-cured epoxy resin. Polym. Eng. Sci. 1978, 18, 1093–1100. [Google Scholar] [CrossRef]
- Gupta, V.; Drzal, L.; Lee, C.C.; Rich, M. The temperature-dependence of some mechanical properties of a cured epoxy resin system. Polym. Eng. Sci. 1985, 25, 812–823. [Google Scholar] [CrossRef]
- Safranski, D.L.; Gall, K. Effect of chemical structure and crosslinking density on the thermo-mechanical properties and toughness of (meth)acrylate shape memory polymer networks. Polymer 2008, 49, 4446–4455. [Google Scholar] [CrossRef] [Green Version]
- Voit, W.; Ware, T.; Dasari, R.R.; Smith, P.; Danz, L.; Simon, D.; Barlow, S.; Marder, S.R.; Gall, K. High-strain shape-memory polymers. Adv. Funct. Mater. 2010, 20, 162–171. [Google Scholar] [CrossRef]
- Li, K.; Zheng, J. Study on the Correlation of Crosslink Network Structure and Tensile Properties of HTPB Binder. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; p. 012091. [Google Scholar]




| Sample ID a | Product Name b | VA-Content b [wt%] | VA-Content c [wt%] | VA-Content d [wt%] | Ge [%] | Qf [%] | DOC g [%] | χch [%] |
|---|---|---|---|---|---|---|---|---|
| PEVA | ||||||||
| PEVA11 | Greenflex ML30 | 9 | 11 | - | - | - | 45.7 ± 0.7 | 33.7% |
| PEVA20 | Elvax460 | 18 | 20 | - | - | - | 36.7 ± 0.8 | 24.8% |
| PEVA31 | Elvax3175LG | 28 | 31 | - | - | - | 27.6 ± 0.7 | 14.3% |
| PEVA35 | Elvax150 | 32 | 35 | - | - | - | 13.1 ± 0.7 | 6.0% |
| PEVA44 | Elvax40w | 40 | 44 | - | - | - | 8.4 ± 0.8 | 3.7% |
| cPEVA | ||||||||
| cPEVA11 | 13 | 10 | n.d * | n.d * | 35.9 ± 0.1 | 30.0% | ||
| cPEVA20 | 21 | 18 | 96 ± 1 | 625 ± 15 | 27.6 ± 0.2 | 21.3% | ||
| cPEVA31 | 33 | 29 | 96 ± 1 | 695 ± 7 | 15.2 ± 0.1 | 16.4% | ||
| cPEVA35 | 37 | 33 | 94 ± 1 | 645 ± 8 | 7.8 ± 0.3 | 11.3% | ||
| cPEVA44 | 44 | 41 | 95 ± 1 | 615 ± 4 | 5.6 ± 0.4 | 5.2% | ||
| Sample ID | Tca [°C] | Tmb [°C] | ΔHm c [J·g−1] | ΔT d [°C] | Tge [°C] | Tg, PEf [°C] | Tδ,maxg [°C] | Tmh [°C] | Ei [MPa] | σbj [MPa] | εbk [%] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PEVA11 | 73 | 102 | 96.9 | 108 | −25 | −84.5 | −3 | - | 118.0 ± 70 | 18.9 ± 8 | 47 ± 6 |
| PEVA20 | 65 | 91 | 71.2 | 104 | −29 | −65.4 | −9 | 100 | 90.9 ± 17 | 13.2 ± 2.8 | 65 ± 4 |
| PEVA31 | 45 | 73 | 41.0 | 91 | −30 | −92.4 | −13 | 84 | 25.8 ± 5 | 8.5 ± 6 | 190 ± 8 |
| PEVA35 | 36 | 63 | 17.2 | 65 | −23 | −87.8 | −19 | 66 | 9.7 ± 1.2 | 4.1 ± 1 | 280 ± 30 |
| PEVA44 | 24 | 45 | 10.6 | 62 | −20 | −84.5 | −32 | 55 | 3.4 ± 0.2 | 3.2 ± 0.1 | 650 ± 36 |
| cPEVA11 | 68 | 94 | 86.1 | 92 | −26 | n.o. l | −5 | - | 33.7 ± 1.5 | 26.9 ± 5.3 | 664 ± 58 |
| cPEVA20 | 55 | 81 | 61.1 | 79 | −27 | n.o. | −15 | - | 21.0 ± 5 | 24.1 ± 10 | 650 ± 30 |
| cPEVA31 | 37 | 65 | 47.0 | 85 | −30 | n.o. | −16 | - | 10.4 ± 1.2 | 20.6 ± 3.4 | 657 ± 37 |
| cPEVA35 | 29 | 56 | 32.6 | 73 | −28 | n.o. | −18 | - | 7.8 ± 0.7 | 14.0 ± 1.1 | 632 ± 18 |
| cPEVA44 | 14 | 41 | 14.9 | 60 | −26 | n.o. | −18 | - | 2.9 ± 0.4 | 9.6 ± 0.7 | 721 ± 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Deng, Q. The Thermal and Mechanical Properties of Poly(ethylene-co-vinyl acetate) Random Copolymers (PEVA) and its Covalently Crosslinked Analogues (cPEVA). Polymers 2019, 11, 1055. https://doi.org/10.3390/polym11061055
Wang K, Deng Q. The Thermal and Mechanical Properties of Poly(ethylene-co-vinyl acetate) Random Copolymers (PEVA) and its Covalently Crosslinked Analogues (cPEVA). Polymers. 2019; 11(6):1055. https://doi.org/10.3390/polym11061055
Chicago/Turabian StyleWang, Ke, and Qibo Deng. 2019. "The Thermal and Mechanical Properties of Poly(ethylene-co-vinyl acetate) Random Copolymers (PEVA) and its Covalently Crosslinked Analogues (cPEVA)" Polymers 11, no. 6: 1055. https://doi.org/10.3390/polym11061055
APA StyleWang, K., & Deng, Q. (2019). The Thermal and Mechanical Properties of Poly(ethylene-co-vinyl acetate) Random Copolymers (PEVA) and its Covalently Crosslinked Analogues (cPEVA). Polymers, 11(6), 1055. https://doi.org/10.3390/polym11061055






