A Simple Method to Synthesize Lignin Nanoparticles
Abstract
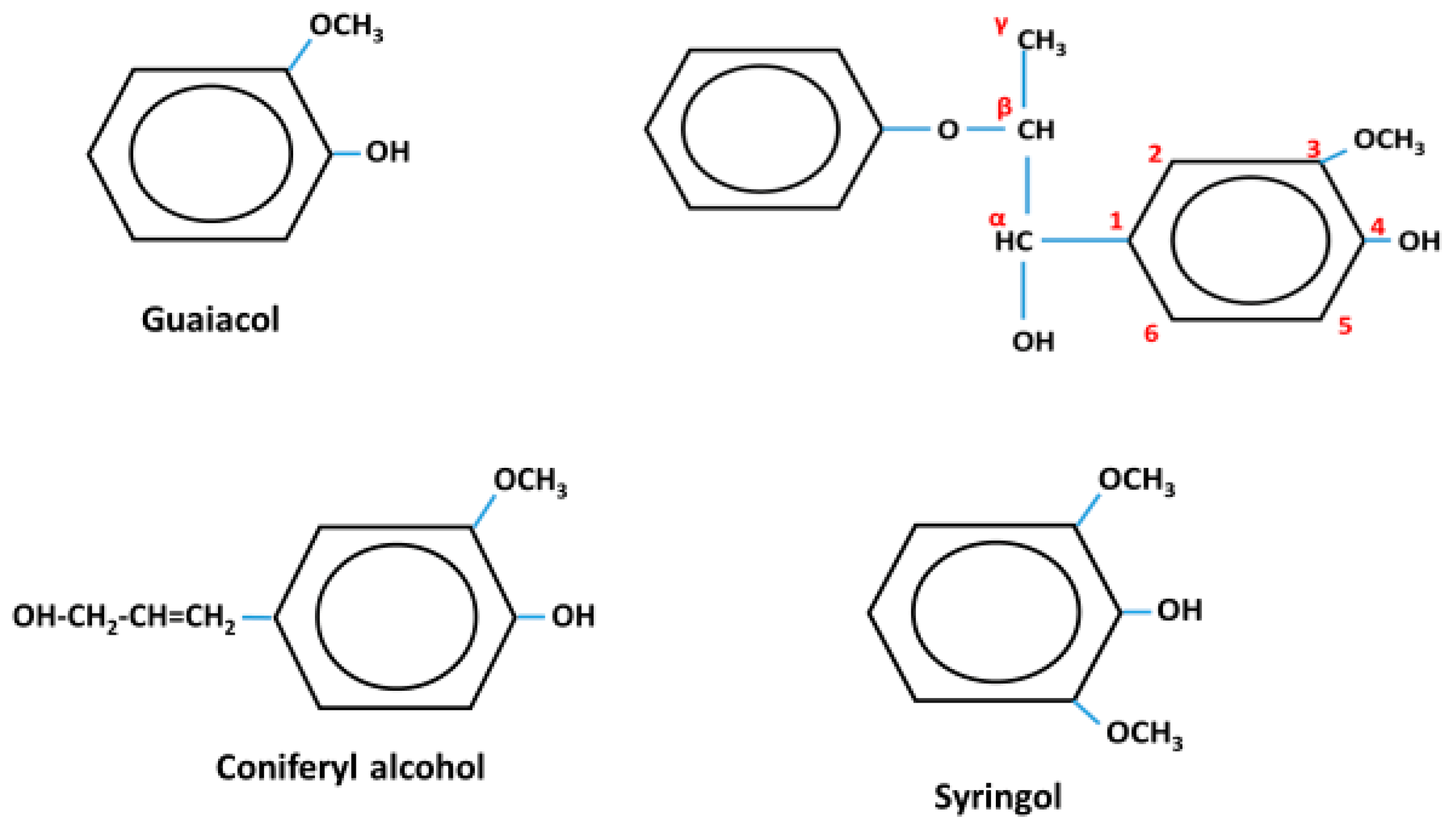
1. Introduction
2. Materials and Methods
2.1. Materials
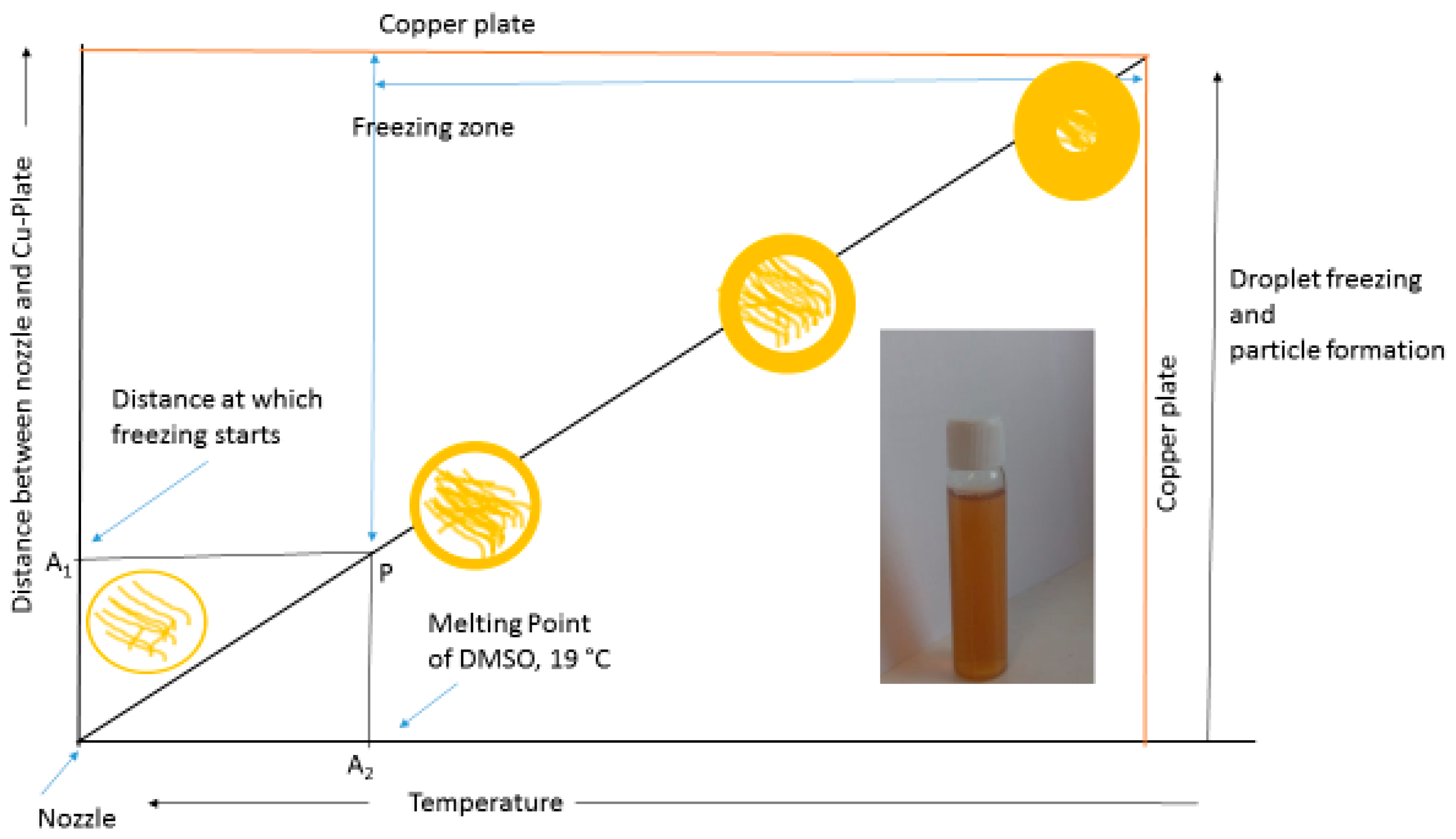
2.2. Synthesis of Lignin Nanoparticles
2.3. Particle Size Determination
2.4. Scanning Electron Microscopy (SEM)
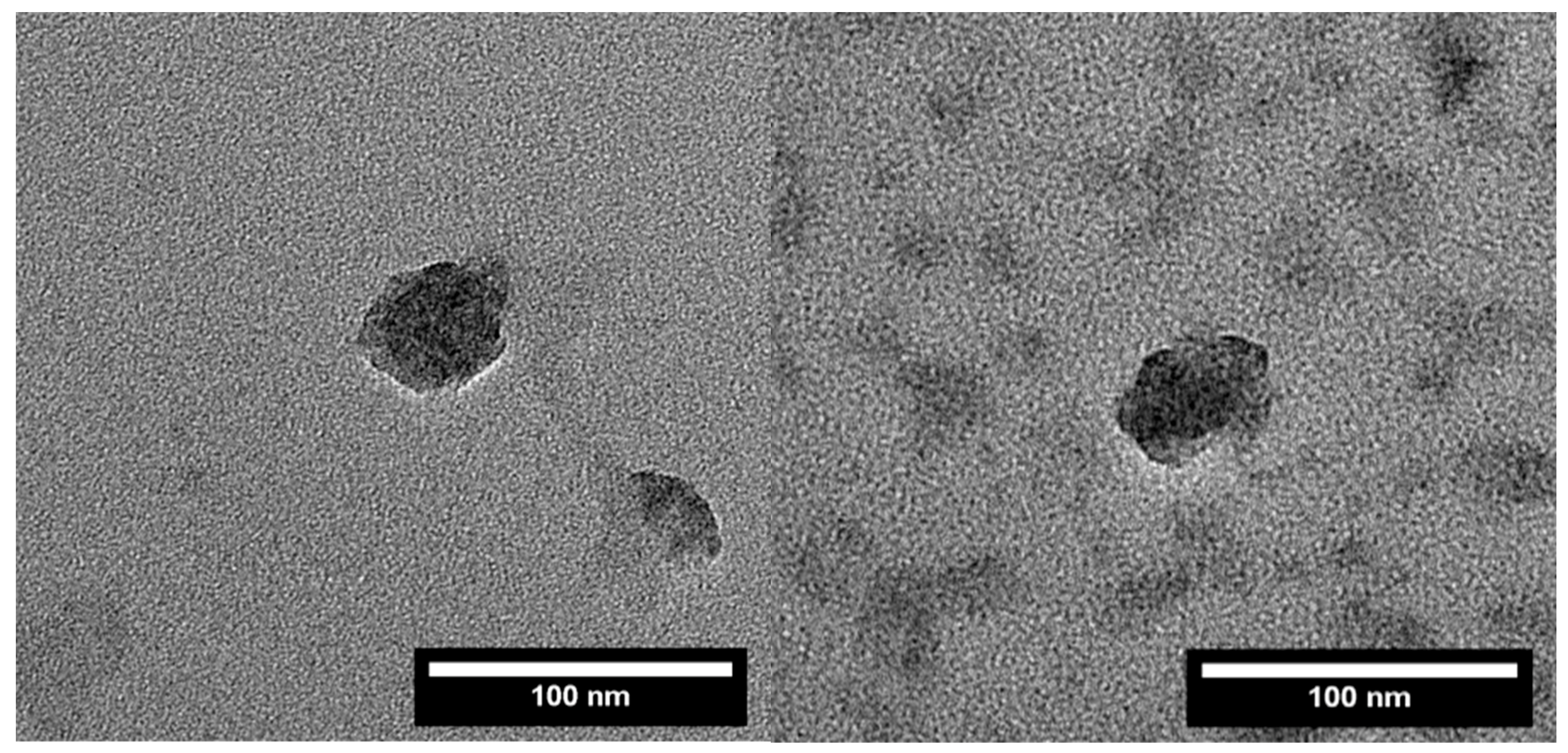
2.5. Transmission Electron Microscopy (TEM)
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siddiqui, L.; Mishra, H.; Mishra, P.K.; Iqbal, Z.; Talegaonkar, S. Novel 4-in-1 strategy to combat colon cancer, drug resistance and cancer relapse utilizing functionalized bioinspiring lignin nanoparticle. Med. Hypotheses 2018, 121, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Král, P.; Klímek, P.; Mishra, P.K.; Rademacher, P.; Wimmer, R. Preparation and Characterization of Cork Layered Composite Plywood Boards. BioResources 2014, 9, 1977–1985. [Google Scholar] [CrossRef]
- Mishra, P.K.; Giagli, K.; Tsalagkas, D.; Mishra, H.; Talegaonkar, S.; Gryc, V.; Wimmer, R. Changing Face of Wood Science in Modern Era: Contribution of Nanotechnology. Recent Pat. Nanotechnol. 2018, 12, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Jansson, R.; Widhe, M.; Benselfelt, T.; Håkansson, K.M.O.; Lundell, F.; Hedhammar, M.; Söderberg, L.D. Ultrastrong and Bioactive Nanostructured Bio-Based Composites. ACS Nano 2017, 11, 5148–5159. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Ansari, F.; Gowda, V.K.; Brouzet, C.; Chen, P.; Larsson, P.T.; Roth, S.V.; Lundell, F.; Wågberg, L.; Kotov, N.A.; et al. Multiscale Control of Nanocellulose Assembly: Transferring Remarkable Nanoscale Fibril Mechanics to Macroscale Fibers. ACS Nano 2018, 12, 6378–6388. [Google Scholar] [CrossRef] [PubMed]
- Constant, S.; Wienk, H.L.; Frissen, A.E.; de Peinder, P.; Boelens, R.; Van Es, D.S.; Grisel, R.J.; Weckhuysen, B.M.; Huijgen, W.J.; Gosselink, R.J. New insights into the structure and composition of technical lignins: a comparative characterisation study. Green Chem. 2016, 18, 2651–2665. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Frangville, C.; Rutkevicius, M.; Richter, A.P.; Velev, O.D.; Stoyanov, S.D.; Paunov, V.N. Fabrication of Environmentally Biodegradable Lignin Nanoparticles. Chemphyschem 2012, 13, 4235–4243. [Google Scholar] [CrossRef] [PubMed]
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro- and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- ur Rahman, O.; Shi, S.; Ding, J.; Wang, D.; Ahmad, S.; Yu, H. Lignin nanoparticles: synthesis, characterization and corrosion protection performance. New J. Chem. 2018, 42, 3415–3425. [Google Scholar] [CrossRef]
- Beisl, S.; Miltner, A.; Friedl, A. Lignin from Micro-to Nanosize: Production Methods. Int. J. Mol. Sci. 2017, 18, 1244. [Google Scholar] [CrossRef] [PubMed]
- Ago, M.; Tardy, B.L.; Wang, L.; Guo, J.; Khakalo, A.; Rojas, O.J. Supramolecular assemblies of lignin into nano-and microparticles. MRS Bull. 2017, 42, 371–378. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ekielski, A. The Self-Assembly of Lignin and Its Application in Nanoparticle Synthesis: A Short Review. Nanomaterials 2019, 9, 243. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, W.; Singh, S.; Simmons, B.; Cheng, G. On the solution structure of kraft lignin in ethylene glycol and its implication for nanoparticle preparation. Nanoscale Adv. 2018. [Google Scholar] [CrossRef]
- Mishra, P.K.; Wimmer, R. Aerosol assisted self-assembly as a route to synthesize solid and hollow spherical lignin colloids and its utilization in layer by layer deposition. Ultrason. Sonochem. 2017, 35, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Salentinig, S.; Schubert, M. Softwood Lignin Self-Assembly for Nanomaterial Design. Biomacromolecules 2017. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Feng, X.; Yang, D.; Yi, C.; Qiu, X. Pi-Pi STACKING OF THE AROMATIC GROUPS IN LIGNOSULFONATES. BioResources 2012, 7, 1145–1156. [Google Scholar]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and Formation Mechanism of Renewable Lignin Hollow Nanospheres with a Single Hole by Self-Assembly. ACS Sustain. Chem. Eng. 2017, 5, 2273–2281. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, W.; Yao, H.; Fan, P.; Yang, J.; Fei, Z.; Zhong, M. Self-assembly of NiO nanoparticles in lignin-derived mesoporous carbons for supercapacitor applications. Green Chem. 2013, 15, 3057–3063. [Google Scholar] [CrossRef]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.-L.; Hult, E.-L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A simple process for lignin nanoparticle preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, P.K.; Ekielski, A. A Simple Method to Synthesize Lignin Nanoparticles. Colloids Interfaces 2019, 3, 52. https://doi.org/10.3390/colloids3020052
Mishra PK, Ekielski A. A Simple Method to Synthesize Lignin Nanoparticles. Colloids and Interfaces. 2019; 3(2):52. https://doi.org/10.3390/colloids3020052
Chicago/Turabian StyleMishra, Pawan Kumar, and Adam Ekielski. 2019. "A Simple Method to Synthesize Lignin Nanoparticles" Colloids and Interfaces 3, no. 2: 52. https://doi.org/10.3390/colloids3020052
APA StyleMishra, P. K., & Ekielski, A. (2019). A Simple Method to Synthesize Lignin Nanoparticles. Colloids and Interfaces, 3(2), 52. https://doi.org/10.3390/colloids3020052





