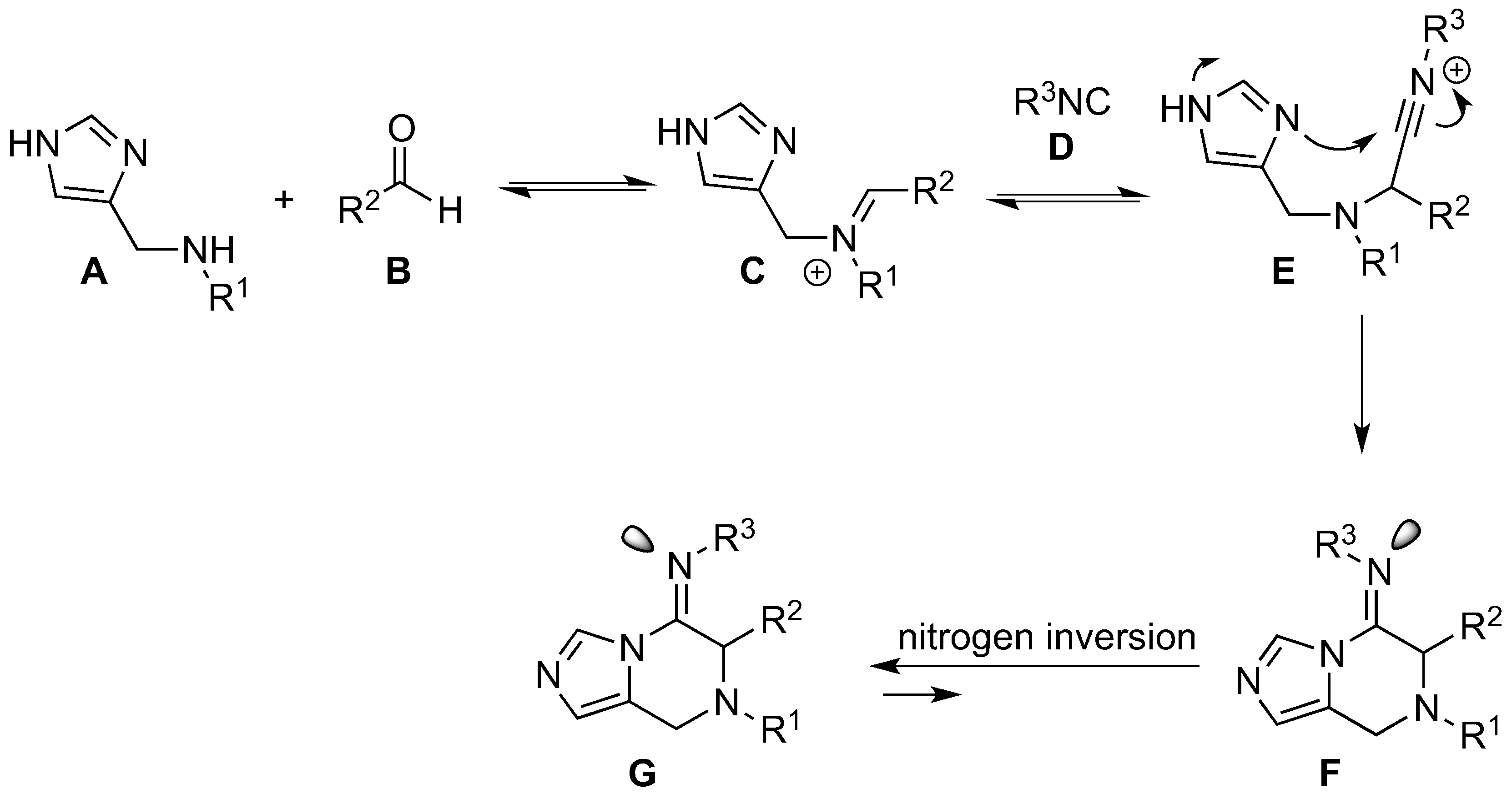
3.5. Synthesis of Substituted Imidazopyrazines and Benzoimidazopyrazines (45–80)
The secondary amine (0.5 mmol, 1 equiv.) was dissolved in methanol (2.0 mL). Aldehyde (0.5 mmol, 1 equiv.) and isocyanide (0.5 mmol, 1 equiv.) were added and the reaction was stirred at room temperature under nitrogen for 18 h. The reaction mixture was concentrated under reduced pressure and the crude material was purified by column chromatography.
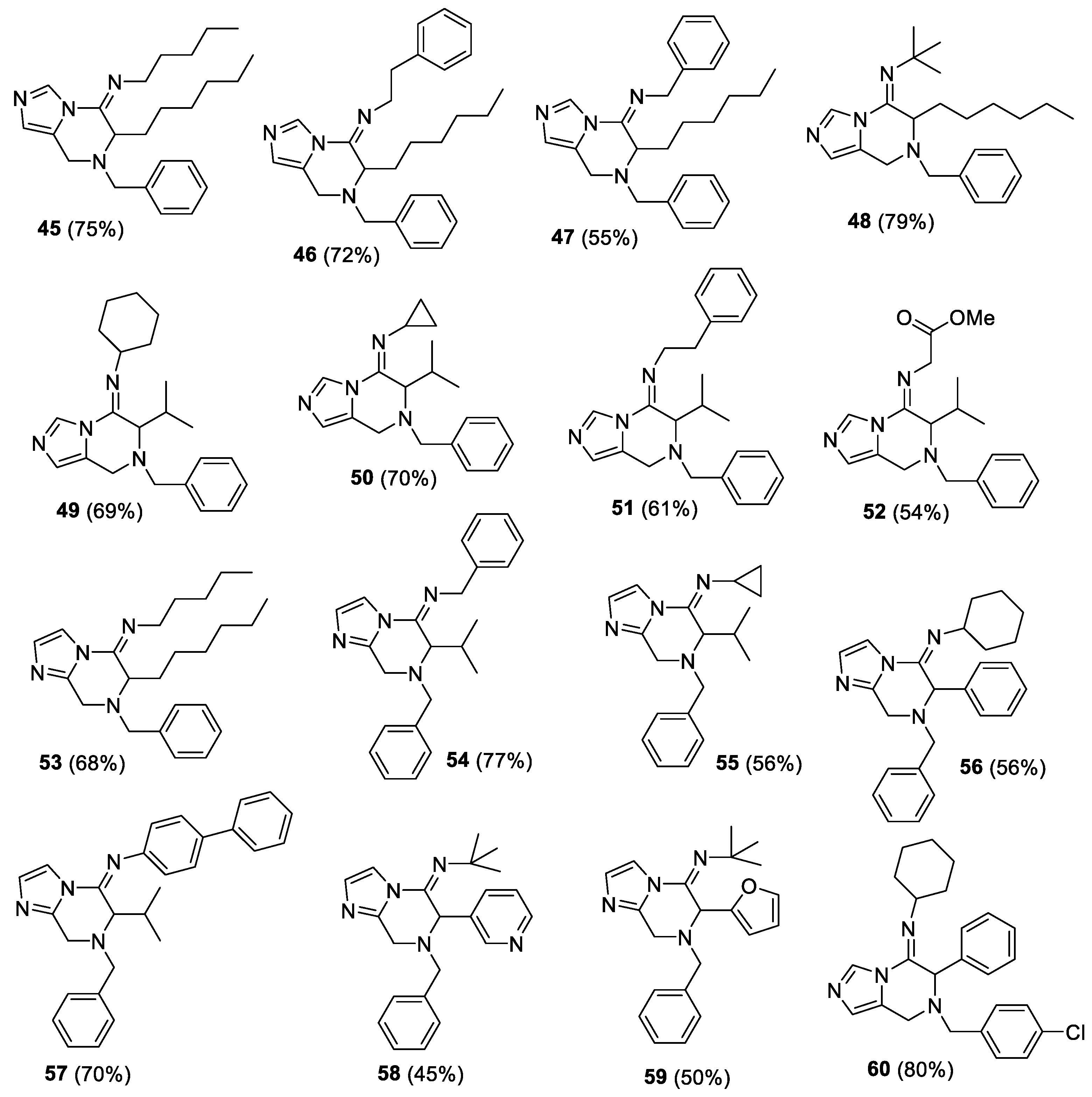
(E)-N-(7-benzyl-6-hexyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)pentan-1-amine (45). The crude material was purified by column chromatography (Pet/EtOAc 90:10 and Pet/EtOAc 70:30) to give the product as orange oil (153 mg, yield 75%). 1H-NMR (300 MHz, CDCl3) δ 8.32 (s, 1H), 7.29–7.27 (m, 5H), 6.78 (s, 1H), 4.13 (d, J = 17.1 Hz, AB system, 1H), 3.80–3.68 (m, 3H), 3.53 (d, J = 13.2 Hz, AB system, 1H), 3.24–3.22 (m, 2H), 1.78–1.16 (m, 16H), 1.06–0.88 (m, 6H); 13C-NMR (75 MHz, CDCl3) δ 150.0, 137.9, 132.8, 128.9, 128.5, 127.7, 124.9, 124.6, 58.9, 54.8, 48.3, 40.7, 31.8, 30.9, 29.7, 28.8, 25.9, 22.7, 22.6, 14.1. IR (neat) 2953, 2927, 2857, 1671, 1461, 1347, 1093, 923, 818, 699 νmax/cm−1; MS (ESI) m/z Calcd for C24H37N4+: 381.3013; Found: 381.3006 [M + H]+.
(E)-N-(7-benzyl-6-hexyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)-2-phenylethanamine (46). The crude material was purified by column chromatography (Pet/EtOAc 90:10) to give the product as orange oil (160 mg, yield 72%). 1H-NMR (300 MHz, CDCl3) δ 8.38, (s, 1H), 7.31–7.17 (m, 10H), 6.79 (s, 1H), 4.07 (d, J = 16.8 HZ, AB system, 1H), 3.75–3.47 (m, 5H), 3.25 (d, J = 13.2 Hz, AB system, 1H), 3.04–2.84 (m, 2H), 1.68–1.48 (m, 2H), 1.28–1.14 (m, 8H), 0.89–0.83 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 150.7, 140.0, 138.0, 135.4, 129.2, 128.8, 128.6, 128.5, 127.6, 126.4, 125.1, 124.6, 58.7, 55.1, 50.3, 40.4, 37.8, 31.8, 28.8 (2C), 25.9, 22.7, 14.2. IR (neat) 2925, 2856, 1671, 1460, 1347, 1089, 923, 744, 698 νmax/cm−1; MS (ESI) m/z Calcd for C27H35N4+: 415.2857; found: 415.2853 [M + H]+.
(E)-N-(7-benzyl-6-hexyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)-1-phenylmethanamine (47). The crude material was purified by column chromatography (Pet/EtOAc 70:30) to give the product as orange oil (118 mg, yield 55%). 1H-NMR (300 MHz, CDCl3) δ 8.41, (s, 1H), 7.33–7.23 (m, 10H), 6.81 (s, 1H), 4.52 (s, 2H), 4.17 (d, J = 16.8 Hz, AB system, 1H), 3.91–3.71 (m, 3H), 3.54 (d, J = 12.8 Hz, AB system, 1H), 1.82–1.20 (m, 10H), 0.97–0.89 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 151.4, 139.5, 137.8, 133.0, 129.0, 128.6, 127.8. 127.5, 127.1, 125.2, 124.6, 59.0, 55.1, 51.8, 40.7, 31.8, 28.8 (2C), 26.0, 22.7, 14.2; IR (neat) 2926, 1671, 1460, 1346, 1213, 1086, 922, 734, 697 νmax/cm−1; MS (ESI) m/z Calcd for C26H33N4+: 401.2700; found: 401.2699 [M + H]+.
(E)-N-(7-benzyl-6-hexyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)-2-methylpropan-2-amine (48). The crude material was purified by column chromatography (Pet/EtOAc 95:5 and Pet/EtOAc 70:30) to give the product as brown amorphous solid (154 mg, yield 79%). 1H-NMR (300 MHz, CDCl3) δ 8.26 (s, 1H), 7.29–7.26 (m, 5H), 6.71 (s, 1H), 4.08 (d, J = 17.1 Hz, AB system, 1H), 3.93–3.89 (m, 1H), 3.70–3.65 (m, 3H), 1.84–1.21 (m, 19H), 1.10–0.90 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 146.2, 138.1, 133.1, 128.4 (2C), 127.4, 124.9, 124.5, 58.5 (2C), 54.5, 39.6, 31.7, 31.5, 29.7, 28.8, 26.0, 22.6, 14.0; IR (KBr) 2958, 2924, 2858, 1670, 1455, 1344, 1198, 1095, 738, 652 νmax/cm−1; MS (ESI) m/z Calcd for C23H35N4+: 367.2857; found: fragment ion (loss of 2-methyl-N-methylenepropan-2-amine moiety): 284.2121 [M + H]+.
(E)-N-(7-benzyl-6-isopropyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)cyclohexanamine (49). The crude material was purified by column chromatography (Pet/EtOAc 70:30) to give the product as yellow solid (121 mg, yield 69%). 1H-NMR (300 MHz, CDCl3) δ 8.26 (s, 1H), 7.30–7.25 (m, 5H), 6.72 (s, 1H), 4.11 (d, J = 17.4 Hz, AB system, 1H), 3.71–3.66 (m, 3H), 3.49 (d, J = 10.7 Hz, AB system, 1H), 3.29–3.30 (m, 1H), 1.95–1.92 (m, 1H), 1.79–1.45 (m, 6H), 1.24–1.20 (m, 7H), 0.93 (d, J = 6.7 Hz, 3H); 13C-NMR (75 MHz, CDCl3) δ 146.6, 138.2, 132.9, 128.5 (2C), 127.5, 125.3, 124.5, 62.5, 59.0, 57.4, 40.2, 34.7, 33.8, 27.9, 25.6, 24.4, 24.3, 20.2, 20.0; IR (KBr) 3033, 2922, 2850, 1672, 1618, 1455, 1340, 1245, 1214, 1090, 815 νmax/cm−1; m.p. 154–155 °C; MS (ESI) m/z Calcd for C22H31N4+: 351.2544; found: fragment ion (loss of N-methylenecyclohexanamine moiety): found: 242.1653 [M + H]+.
(E)-N-(7-benzyl-6-isopropyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)cyclopropanamine (50). The crude material was purified by column chromatography (Pet/EtOAc 70:30) to give the product as yellow solid (108 mg, yield 70%). 1H-NMR (300 MHz, CDCl3) δ 8.20 (s, 1H), 7.30–7.25 (m, 5H), 6.78 (s, 1H), 4.21 (d, J = 17.1 Hz, AB system, 1H), 3.81–3.47 (m, 4H), 2.70–2.63 (m, 1H), 2.56–2.42 (m, 1H), 2.06–1.95 (m, 1H), 1.24–0.81 (m, 9H); 13C-NMR (75 MHz, CDCl3) δ 148.6, 138.0, 132.5, 129.1, 128.4, 127.6, 124.8, 124.6, 61.0, 59.1, 41.4, 31.7, 28.3, 20.1 (2C), 9.6, 8.9; IR (KBr) 3002, 2960, 2925, 2870, 1668, 1454, 1359, 1344, 1246, 1090 νmax/cm−1; m.p. 107–108 °C; MS (ESI) m/z Calcd for C19H25N4+: 309.2074; found: 309.2072 [M + H]+.
(E)-N-(7-benzyl-6-isopropyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)-2-phenylethanamine (51). The crude material was purified by column chromatography (Pet/EtOAc 90:10 and Pet/EtOAc 70:30) to give the product as brown oil (113.5 mg, yield 61%). 1H-NMR (300 MHz, CDCl3) δ 8.34 (s, 1H), 7.29–7.16 (m, 10H), 6.76 (s, 1H), 4.12 (d, J = 17.1 Hz, AB system, 1H), 3.69 (d, J = 17.1 Hz, AB system, 1H), 3.54–3.47 (m, 3H), 3.34–3.20 (m, 2H), 3.05–2.84 (m, 2H), 2.05–1.90 (m, 1H), 1.14–1.11 (m, 3H), 0.86–0.83 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 149.6, 140.0, 138.1, 132.8, 129.2, 128.6 (2C), 128.5, 127.6, 126.4, 125.2, 124.8, 61.3, 58.6, 51.5, 40.8, 37.8, 28.4, 20.1, 19.9; IR (neat) 2960, 1672, 1494, 1456, 1353, 1218, 1103, 743, 698 νmax/cm−1; MS (ESI) m/z Calcd for C24H29N4+: 373.2387; found: fragment ion (loss of N-methylene-2-phenylethanamine moiety): found: 242.1654 [M + H]+.
(E)-methyl2-((7-benzyl-6-isopropyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)amino)-acetate (52). The crude material was purified by column chromatography (Pet/EtOAc 70:30) to give the product as brown oil (92 mg, yield 54%). 1H-NMR (300 MHz, CDCl3) δ 8.33 (s, 1H), 7.28–7.25 (m, 5H), 6.77 (s, 1H), 4.21 (d, J = 17.4 Hz, AB system, 1H), 4.05 (br s, 2H), 3.81–3.76 (m, 5H), 3.60 (d, J = 13.2 Hz, AB system, 1H), 3.27 (d, J = 10.7 Hz, 1H), 2.02–1.93 (m, 1H) 1.13 (d, J = 6.4 Hz, 3H), 0.86 (d, J = 7.8 Hz, 3H); 13C-NMR (75 MHz, CDCl3) δ 170.2, 152.9, 137.7, 133.2, 128.8, 128.6, 127.8, 125.0 (2C), 61.0, 59.0, 52.3, 51.2, 41.2, 28.3, 20.0, 19.8; IR (neat) 2961, 1743, 1678, 1465, 1358, 1198, 1178, 741 νmax/cm−1; MS (ESI) m/z Calcd for C19H25N4O2+: 341.1973; found: 341.1971 [M + H]+.
(E)-N-(7-benzyl-6-hexyl-7,8-dihydroimidazo[1,2-a]pyrazin-5(6H)-ylidene)pentan-1-amine (53). The crude material was purified by column chromatography (Pet/EtOAc 80:20) to give the product as yellow oil (129 mg, yield 68%). 1H-NMR (300 MHz, CDCl3) δ 7.62 (s, 1H), 7.31–7.29 (m, 5H), 7.00 (s, 1H), 4.25 (d, J = 17.8 Hz, AB system, 1H), 3.93 (d, J = 17.8 Hz, AB system, 1H), 3.89–3.76 (m, 3H), 3.58 (d, J = 13.2 Hz, AB system, 1H), 3.23 (t, J = 6.7 Hz, 2H) 1.75–1.27 (m, 15H), 0.94–0.87 (m, 6H); 13C-NMR (75 MHz, CDCl3) δ 150.9, 143.6, 137.7, 129.0, 128.6, 128.4, 127.8, 113.5, 59.4, 54.4, 48.7, 44.5, 31.8, 31.0, 29.7, 28.8 (2C), 26.0, 22.7, 22.6, 14.2 (2C); IR (neat) 2927, 2857, 1670, 1491, 1455, 1413, 1289, 1093, 889, 743, 698 νmax/cm−1; MS (ESI) m/z Calcd for C24H37N4+: 381.3013; found: 381.3006 [M + H]+.
(E)-N-(7-benzyl-6-isopropyl-7,8-dihydroimidazo[1,2-a]pyrazin-5(6H)-ylidene)-1-phenylmethanamine (54). The crude material was purified by column chromatography (Pet/EtOAc 80:20) to give the product as yellow oil (147 mg, yield 77%). 1H-NMR (300 MHz, CDCl3) δ 7.69 (s, 1H), 7.36–7.23 (m, 10H), 7.03 (s, 1H), 4.54–4.33 (m, 3H), 3.96–3.80 (m, 2H), 3.61–3.51 (m, 2H), 2.06–1.96 (m, 1H), 1.19 (d, J = 6.4 Hz, 3H), 0.93 (d, J = 6.4 Hz, 3H); 13C-NMR (75 MHz, CDCl3) δ 150.8, 143.9, 139.6, 137.7, 128.9, 128.6, 128.5 (2C), 127.8, 127.5, 127.0, 114.0, 60.7, 59.6, 53.1, 45.0, 28.6, 20.2, 20.0; IR (neat) 2961, 1671, 1491, 1410, 1289, 1258, 1100, 737, 697 νmax/cm−1; MS (ESI) m/z Calcd for C23H27N4+: 359.2231; found: 359.2229 [M + H]+.
(E)-N-(7-benzyl-6-isopropyl-7,8-dihydroimidazo[1,2-a]pyrazin-5(6H)-ylidene)cyclopropanamine (55). The crude material was purified by column chromatography (Pet/EtOAc 70:30) to give the product as yellow oil (91 mg, yield 56%). 1H-NMR (300 MHz, CDCl3) δ 7.45 (br d, 1H), 7.29–7.24 (m, 5H), 6.96 (br d, 1H), 4.30 (d, J = 18.0 Hz, AB system, 1H), 3.92–3.78 (m, 2H), 3.61–3.47 (m, 2H), 2.65–2.61 (m, 1H), 2.02–1.86 (m, 1H), 1.19–0.78 (m, 10H); 13C-NMR (75 MHz, CDCl3) δ 149.2, 143.3, 137.7, 129.4, 129.1, 128.4, 128.2, 127.6, 113.6, 60.5, 59.6, 45.1, 31.9, 28.3, 20.1, 20.0, 9.6, 8.9; IR (neat) 2962, 1665, 1530, 1488, 1415, 1367, 1289, 1261, 1087, 743, 698 νmax/cm−1; MS (ESI) m/z Calcd for C19H25N4+: 309.2074; found: 309.2072 [M + H]+.
(E)-N-(7-benzyl-6-phenyl-7,8-dihydroimidazo[1,2-a]pyrazin-5(6H)-ylidene)cyclohexanamine (56). The crude material was purified by column chromatography (Pet/EtOAc 90:10 and Pet/EtOAc 70:30) to give the product as yellow oil (114 mg, yield 56%). 1H-NMR (300 MHz, CDCl3) δ 7.78 (br d, 1H), 7.41–7.23 (m, 10H), 7.02 (br d, 1H), 5.04 (s, 1H), 3.91–3.67 (m, 4H), 3.09–2.99 (m, 1H), 1.73–1.00 (m, 10H); 13C-NMR (75 MHz, CDCl3) δ 146.3, 144.3, 137.5, 134.7, 129.0, 128.9, 128.7, 128.6, 128.5, 128.1, 127.9, 113.7, 58.9, 58.7, 57.2, 44.9, 34.3, 33.6, 25.5, 24.2, 24.1; IR (neat) 2928, 2853, 1668, 1536, 1492, 1450, 1412, 1289, 1090, 740, 698 νmax/cm−1; MS (ESI) m/z Calcd for C25H29N4+: 385.2387; found: 385.2387 [M + H]+.
(E)-N-(7-benzyl-6-isopropyl-7,8-dihydroimidazo[1,2-a]pyrazin-5(6H)-ylidene)-[1,1′-biphenyl]-4-amine (57). The crude material was purified by column chromatography (Pet/EtOAc 90:10 and Pet/EtOAc 70:30) to give the product as yellow oil (158 mg, yield 70%). 1H-NMR (300 MHz, CDCl3) δ 7.77 (br d, 1H), 7.77–7.29 (m, 12H), 7.11 (br d, 1H), 6.86 (br d, 2H), 4.32–4.14 (m, 1H), 4.02–3.84 (m, 3H), 3.70–3.63 (m, 1H), 2.07–1.97 (m, 1H), 1.11 (br d, 3H), 0.82 (br s 3H); 13C-NMR (75 MHz, CDCl3) δ 150.9, 146.0, 144.7, 140.6, 137.8, 137.0, 129.3, 128.9, 128.7, 128.6, 127.8, 127.7, 127.2, 126.8, 120.8, 114.1, 62.8, 59.7, 44.1, 28.9, 20.4 (2C); IR (neat) 2963, 1667, 1535, 1484, 1407, 1259, 1220, 908, 848, 730, 697 νmax/cm−1; MS (ESI) m/z Calcd for C28H29N4+: 421.2387; found: 421.2386 [M + H]+.
(E)-N-(7-benzyl-6-(pyridin-3-yl)-7,8-dihydroimidazo[1,2-a]pyrazin-5(6H)-ylidene)-2-methylpropan-2-amine (58). The crude material was purified by column chromatography (Pet/EtOAc 50:50 and EtOAc) to give the product as yellow oil (86 mg, yield 45%). 1H-NMR (300 MHz, CDCl3) δ 8.56 (br d, 2H), 7.76 (s, 1H), 7.46–7.22 (m, 7H), 7.00 (s, 1H), 5.19 (s, 1H), 3.91–3.81 (m, 3H), 3.64 (d, J = 18.3 Hz, AB system, 1H), 1.12 (s, 9H); 13C-NMR (75 MHz, CDCl3) δ 157.8, 150.3, 149.8, 143.8, 142.5, 137.2, 135.7, 131.4, 128.8, 128.7, 128.0, 123.5, 114.1, 59.5, 58.5, 55.0, 44.7, 31.3; IR (neat) 2970, 1674, 1454, 1418, 1360, 1294, 1206, 1092, 911, 729, 700 νmax/cm−1; MS (ESI) m/z Calcd for C22H26N5+: 360.2183; found: 360.2181 [M + H]+.
(E)-N-(7-benzyl-6-(furan-2-yl)-7,8-dihydroimidazo[1,2-a]pyrazin-5(6H)-ylidene)-2-methylpropan-2-amine (59). The crude material was purified by column chromatography (Pet/EtOAc 90:10 and Pet/EtOAc 70:30) to give the product as yellow oil (93 mg, yield 50%). 1H-NMR (300 MHz, CDCl3) δ 7.63 (br d, 1H), 7.45–7.26 (m, 6H), 6.98 (br d, 1H), 6.31 (br d, 1H), 5.94–5.93 (m, 1H), 5.19 (m, 1H), 3.99–3.57 (m, 4H), 1.06 (s, 9H); 13C-NMR (75 MHz, CDCl3) δ 148.0, 144.7, 143.1, 142.7, 137.2, 129.3, 128.7, 128.2, 127.9, 114.2, 110.6, 110.5, 58.4, 54.9, 54.5, 46.7, 30.9; IR (neat) 2970, 1684, 1542, 1493, 1418, 1364, 1208, 1087, 1014, 742, 699 νmax/cm−1; MS (ESI) m/z Calcd for C21H25N4O+: 349.2023; found: 349.2022 [M + H]+.
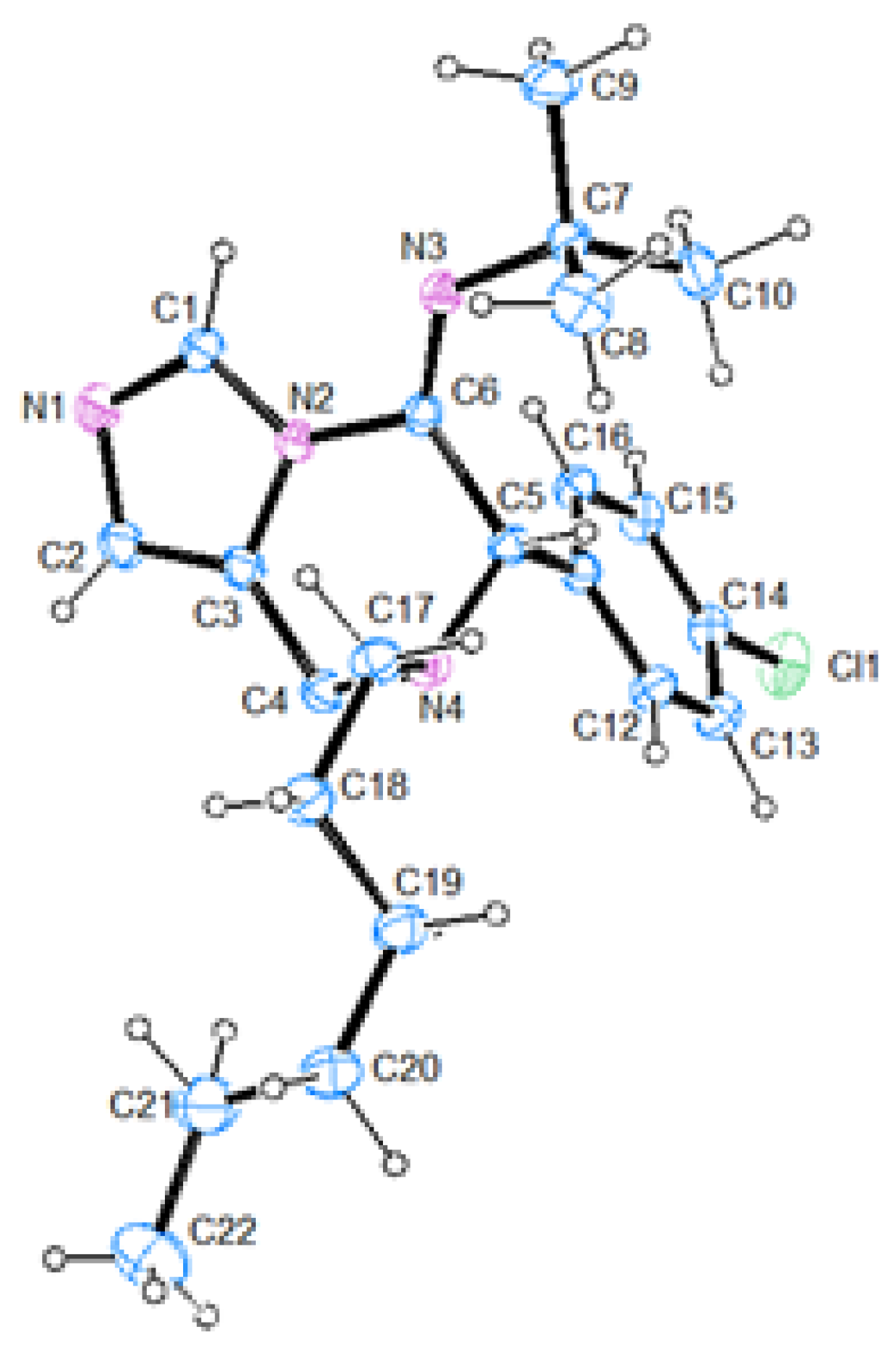
(E)-N-(7-(4-chlorobenzyl)-6-phenyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)cyclohexanamine (60). The crude material was purified by column chromatography (Pet/EtOAc 70:30) to give the product as orange oil (167 mg, yield 80%). 1H-NMR (300 MHz, CDCl3) δ 8.48 (s, 1H), 7.41–7.26 (m, 9H), 6.74 (s, 1H), 4.99 (s, 1H), 3.81–3.63 (m, 4H), 3.12–3.08 (m, 1H), 1.74–0.88 (m, 10H); 13C-NMR (75 MHz, CDCl3) δ 145.3, 136.3, 134.7, 133.6, 133.0, 130.2, 128.9 (2C), 128.6, 128.2, 125.1, 125.0, 59.8, 57.6, 56.9, 41.1, 34.3, 33.5, 25.5, 24.2, 24.1; IR (neat) 2928, 2854, 1669, 1488, 1462, 1347, 1191, 1088, 803, 733, 699, 653 νmax/cm−1; MS (ESI) m/z Calcd for C25H28ClN4+: 419.1997; found: 419.1998 [M + H]+.
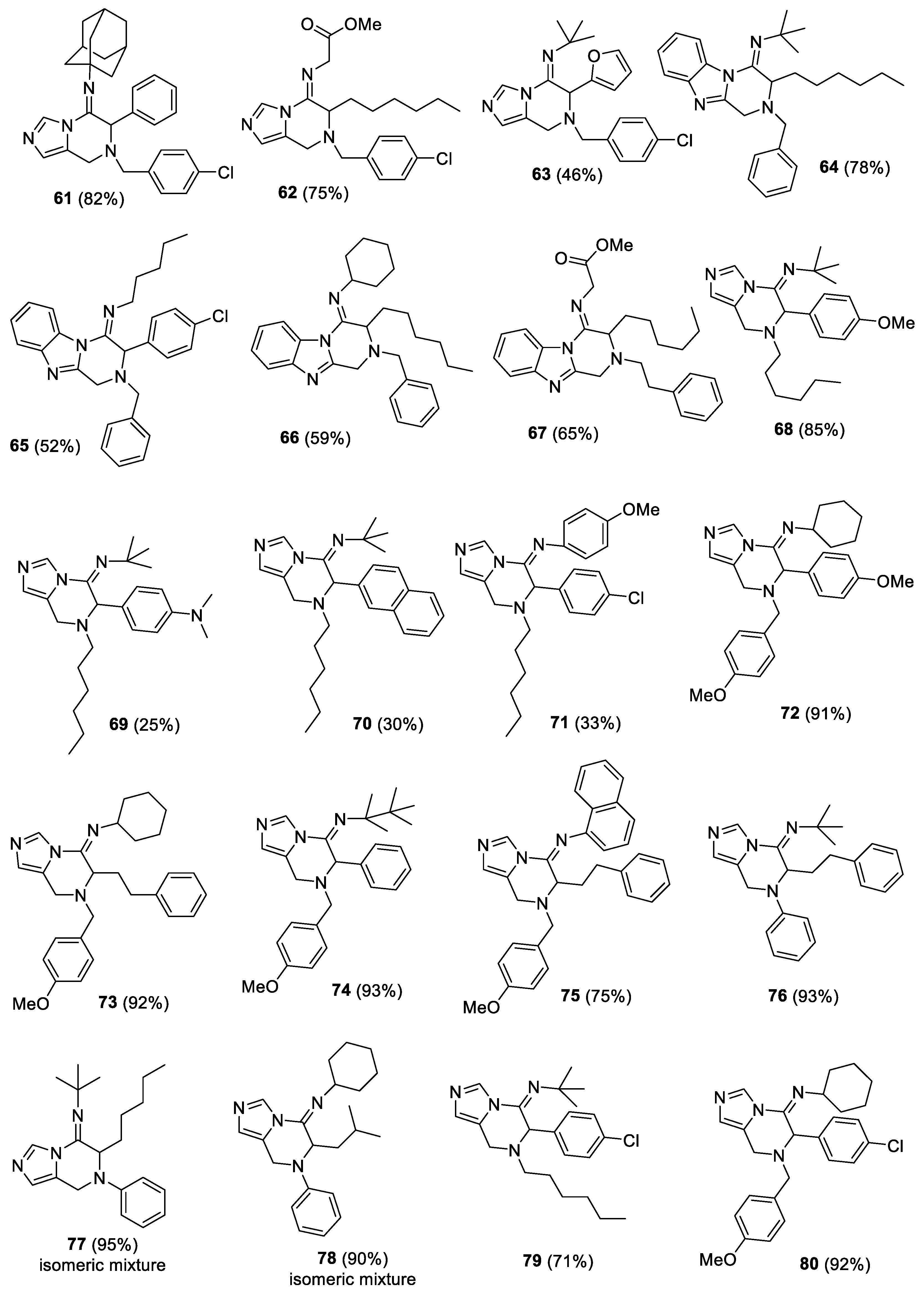
(E)-1-((3r,5r,7r)-adamantan-1-yl)-N-(7-(4-chlorobenzyl)-6-phenyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)methanamine (61). The crude material was purified by column chromatography (Pet/EtOAc 90:10 and Pet/EtOAc 70:30) to give the product as yellow oil (193 mg, yield 82%). 1H-NMR (300 MHz, CDCl3) δ 8.49 (s, 1H), 7.36–7.26 (m, 9H), 6.71 (s, 1H), 5.18 (s, 1H), 3.81 (s, 2H), 3.59 (s, 2H), 1.96 (s, 3H), 1.70–1.50 (m, 12H); 13C-NMR (75 MHz, CDCl3) δ 142.4, 136.5, 135.9, 133.6, 133.3, 130.2, 128.9, 128.8, 128.6, 128.4, 125.6, 124.9, 63.0, 57.5, 56.1, 44.0, 41.0, 36.3, 29.7; IR (neat) 2906, 2850, 1675, 1491, 1459, 1340, 1190, 1088, 909, 804, 729, 698 νmax/cm−1; MS (ESI) m/z Calcd for C29H32ClN4+: 471.2310; found: 471.2320 [M + H]+.
(E)-methyl2-((7-(4-chlorobenzyl)-6-hexyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)amino)acetate (62). The crude material was purified by column chromatography (Pet/EtOAc 80:20 and Pet/EtOAc 60:40) to give the product as yellow oil (156 mg, yield 75%). 1H-NMR (300 MHz, CDCl3) δ 8.35 (s, 1H), 7.297.18 (m, 4H), 6.78 (s, 1H), 4.16–4.12 (m, 3H), 3.78–3.52 (m, 7H), 1.76–1.16 (m, 10H), 0.87–0.85 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 170.0, 154.0, 136.3, 133.6, 133.4, 130.2, 128.8, 125.4, 124.3, 58.2, 55.5, 52.3, 50.2, 40.5, 31.7, 29.0, 28.8, 26.0, 22.6, 14.1; IR (neat) 2927, 2856, 1746, 1673, 1464, 1351, 1197, 1176, 1087, 1014, 807, 652 νmax/cm−1; MS (ESI) m/z Calcd for C22H30ClN4O2+: 417.2052; found: 417.2051[M + H]+.
(E)-N-(7-(4-chlorobenzyl)-6-(furan-2-yl)-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)-2-methylpropan-2-amine (63). The crude material was purified by column chromatography (Pet/EtOAc 90:10 and Pet/EtOAc 70:30) to give the product as yellow oil (88 mg, yield 46%). 1H-NMR (300 MHz, CDCl3) δ 8.33 (s, 1H), 7.43–7.34 (m, 5H), 6.77 (br d, 1H), 6.33–6.32 (m, 1H), 6.01 (br d, 1H), 5.13 (s, 1H), 3.83–3.54 (m, 4H) 1.10 (s, 9H); 13C-NMR (75 MHz, CDCl3) δ 147.8, 141.7, 135.8, 133.7, 133.1, 130.4, 128.8, 126.0, 124.0, 110.7, 110.5, 57.6, 55.6, 54.6, 43.0, 30.8; IR (neat) 2969, 1685, 1490, 1461, 1346, 1201, 1089, 1014, 809, 740, 654 νmax/cm−1; MS (ESI) m/z Calcd for C21H24ClN4O+: 383.1634; found: 383.1633 [M + H]+.
(E)-N-(2-benzyl-3-hexyl-2,3-dihydrobenzo[4,5]imidazo[1,2-a]pyrazin-4(1H)-ylidene)-2-methylpropan-2-amine (64). The crude material was purified by column chromatography Pet/EtOAc 95:5 and Pet/EtOAc 80:20) to give the product as yellow oil (208 mg, yield 78%). 1H-NMR (300 MHz, CDCl3) δ 8.50–8.47 (m, 1H), 7.72–7.69 (m, 1H), 7.33–7.26 (m, 7H), 4.40 (d, J = 18.3 Hz, AB system, 1H), 4.09–4.00 (m, 2H), 3.85 (br s, 2H), 1.91–1.71 (m, 4H), 1.58–1.35 (m, 15H), 0.92–0.94 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 149.9, 149.3, 143.2, 137.9, 132.6, 128.6 (2C), 127.7, 123.5 (2C), 118.9, 116.6, 59.3, 59.2, 55.0, 44.1, 31.8, 30.2, 28.9 (2C), 26.2, 22.7, 14.1; IR (neat) 2961, 2927, 1670, 1539, 1448, 1361, 1343, 1164, 746 νmax/cm−1; MS (ESI) m/z Calcd for C27H37N4+: 417.3013; found: 417.3010 [M + H]+.
(E)-N-(2-benzyl-3-(4-chlorophenyl)-2,3-dihydrobenzo[4,5]imidazo[1,2-a]pyrazin-4(1H)-ylidene)pentan-1-amine (65). The crude material was purified by column chromatography (Pet/EtOAc 95:5 and Pet/EtOAc 90:10) to give the product as yellow oil (119 mg, yield 52%). 1H-NMR (300 MHz, CDCl3) δ 8.64–8.62 (m, 1H), 7.75–7.73 (m, 1H), 7.40–7.20 (m, 11H), 5.10 (s, 1H), 4.02–3.95 (m, 3H), 3.77 (d, J = 12.8 Hz, AB system, 1H), 3.32–3.11 (m, 2H), 1.71–1.67 (m, 2H), 1.41–1.30 (m, 4H), 0.92 (t, J = 6.9 Hz, 3H); 13C-NMR (75 MHz, CDCl3) δ 150.0, 149.5, 143.1, 137.2, 134.6, 132.9, 132.2, 129.6, 129.2, 129.1, 128.9, 128.1, 124.1 (2C), 119.3, 116.7, 58.9, 58.8, 49.0, 45.5, 30.9, 29.7, 22.5, 14.1; IR (neat) 2928, 2855, 1665, 1650, 1489, 1449, 1357, 1167, 1090, 746, 698 νmax/cm−1; MS (ESI) m/z Calcd for C28H30ClN4+: 457.2154; found: 457.2157 [M + H]+.
(E)-N-(2-benzyl-3-hexyl-2,3-dihydrobenzo[4,5]imidazo[1,2-a]pyrazin-4(1H)-ylidene)cyclohexanamine (66). The crude material was purified by column chromatography (Pet/EtOAc 95:5 and Pet/EtOAc 90:10) to give the product as yellow oil (130 mg, yield 59%). 1H-NMR (300 MHz, CDCl3) δ 8.55–8.52 (m, 1H), 7.73–7.70 (m, 1H), 7.35–7.26 (m, 7H), 4.43 (d, J = 18.1 Hz, AB system, 1H), 4.11 (d, J = 18.1 Hz, AB system, 1H), 3.98 (dd, J1 = 10.7 Hz, J2 = 4.3 Hz, 1H), 3.83 (d, J = 13.1 Hz, AB system, 1H), 3.70 (d, J = 13.1 Hz, AB system, 1H), 3.38–3.30 (m, 1H), 1.93–1.29 (m, 20H), 0.89 (t, J = 6.4 Hz, 3H); 13C-NMR (75 MHz, CDCl3) δ 150.8, 149.5, 143.1, 137.7, 132.4, 128.9, 128.5, 127.8, 123.7 (2C), 119.0, 116.8, 59.5, 56.9, 55.1, 44.8, 34.9, 34.7, 31.7, 29.8, 28.7, 25.8 (2C), 24.3, 24.2, 22.6, 14.1; IR (neat) 2926, 2853, 1666, 1540, 1449, 1346, 1173, 744, 698 νmax/cm−1; MS (ESI) m/z Calcd for C29H39N4+: 443.3170; found: 443.3170 [M + H]+.
(E)-methyl2-((3-hexyl-2-phenethyl-2,3-dihydrobenzo[4,5]imidazo[1,2-a]pyrazin-4(1H)-ylidene)amino)acetate (67). The crude material was purified by column chromatography Pet/EtOAc 95:5 and Pet/EtOAc 90:10) to give the product as yellow oil (145 mg, yield 65%). 1H-NMR (300 MHz, CDCl3) δ 8.53–8.50 (m, 1H), 7.70–7.67 (m, 1H), 7.34–7.15 (m, 7H), 4.38 (d, J = 18.3 Hz, AB system, 1H), 4.33 (br d, 1H), 4.15 (d, J = 18.0 Hz, AB system, 1H), 3.90–3.77 (m, 5H), 2.93–2.78 (m, 4H), 1.84–1.51 (m, 3H), 1.37–1.23 (m, 7H), 0.91–0.87 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 170.5, 156.7, 149.3, 142.9, 139.6, 132.2, 128.9, 128.6, 126.5, 124.5, 124.3, 119.1, 116.9, 57.5, 57.1, 52.4, 50.8, 44.6, 35.2, 31.8, 29.4, 29.0, 26.2, 22.7, 14.1; IR (neat) 2928, 2885, 1746, 1670, 1451, 1375, 1200, 1174, 746, 699 νmax/cm−1; MS (ESI) m/z Calcd for C27H35N4O2+: 447.2755; found: 447.2757 [M + H]+.
(E)-N-(7-hexyl-6-(4-methoxyphenyl)-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)-2-methylpropan-2-amine (68). The crude material was purified by column chromatography (n-hexane/ EtOAc 97:3) to give the product as brownish solid (162 mg, 85% yield). 1H-NMR (400 MHz, CDCl3) δ 8.34 (s, 1H), 7.08 (d, J = 8.4 Hz, 2H), 6.77 (d, J = 8.4 Hz, 2H), 6.65 (s, 1H), 5.03 (s, 1H), 3.71 (s, 3H), 3.52 (dd, Jab = 16.4 Hz, 2H), 2.62–2.48 (m, 2H), 1.56–1.49 (m, 2H), 1.35–1.26 (m, 6H), 1.20 (s, 9H), 0.86–0.83 (m, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.2, 144.2, 132.9, 129.7, 127.5, 125.8, 124.5, 113.8, 62.7, 55.1, 54.5, 53.6, 40.5, 31.6, 31.3, 28.1, 26.8, 22.5, 14.0. IR (neat) 2965, 2929, 2861, 2833, 1668, 1511, 1460, 1338, 1246, 1037, 819 νmax/cm−1; Mp 78–79 °C; MS (ESI) m/z Calcd for C23H35N4O+: 383.2806; found: fragment ion (loss of 2-methyl-N-methylenepropan-2-amine moiety): 300.2072 [M + H]+.
(E)-4-(5-(tert-butylimino)-7-hexyl-5,6,7,8-tetrahydroimidazo[1,5-a]pyrazin-6-yl)-N,N-dimethylaniline (69). The crude material was purified by column chromatography (n-hexane/ EtOAc 85:15) to give the product as yellowish solid (49 mg, 25% yield). 1H-NMR (400 MHz, CDCl3) δ 8.36 (s, 1H), 7.01 (d, J = 8.0 Hz, 2H), 6.68 (s, 1H), 6.60 (d, J = 8.0 Hz, 2H), 5.03 (s, 1H), 3.57 (dd, Jab = 15.2 Hz, 2H), 2.91 (s, 6H), 2.62–2.49 (m, 2H), 1.58–1.52 (m, 2H), 1.39–1.30 (m, 6H), 1.24 (s, 9H), 0.90–0.87 (m, 3H); 13C-NMR (100 MHz, CDCl3) δ 150.0, 144.7, 132.9, 129.4, 126.1, 124.4, 122.5, 112.0, 62.8, 54.4, 53.6, 40.7, 40.3, 31.7, 31.3, 29.7, 28.2, 26.8, 22.6, 14.0. IR (neat) 3115, 2952, 2926, 2857, 1669, 1529, 1466, 1349, 1206, 1192, 811 νmax/cm−1; Mp 104–105 °C; MS (ESI) m/z Calcd for C24H38N5+: 396.3122; found: fragment ion (loss of 2-methyl-N-methylenepropan-2-amine moiety): 313.2388 [M + H]+.
(E)-N-(7-hexyl-6-(naphthalen-2-yl)-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)-2-methylpropan-2-amine (70). The crude material was purified by column chromatography (n-hexane/ EtOAc 90:10) to give the product as yellowish solid (32 mg, 16% yield). 1H-NMR (400 MHz, CDCl3) δ 8.48 (s, 1H), 7.83–7.72 (m, 3H), 7.54 (s, 1H), 7.50–7.44 (m, 3H), 6.70 (s, 1H), 5.27 (s, 1H), 3.58 (dd, Jab = 16.4 Hz, 2H), 2.72–2.61 (m, 2H), 1.66–1.59 (m, 2H), 1.42–1.29 (m, 6H), 1.26 (s, 9H), 0.93–0.90 (m, 3H); 13C-NMR (100 MHz, CDCl3) δ 143.9, 140.4, 133.6, 133.0, 132.9, 128.5, 128.1, 127.5, 127.2, 126.5, 126.4, 126.3, 125.7, 124.7, 63.5, 54.7, 53.9, 40.8, 31.7, 31.3, 28.3, 26.8, 22.6, 14.1. IR (neat) 3120, 3055, 2964, 2930, 2857, 1669, 1464, 1347, 1362, 1203, 795 νmax/cm−1; Mp 108–109 °C; MS (ESI) m/z Calcd for C26H35N4+: 403.2857; found: fragment ion (loss of 2-methyl-N-methylenepropan-2-amine moiety): 320.2123 [M + H]+.
(E)-N-(6-(4-chlorophenyl)-7-hexyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)-4-methoxyaniline (71). The crude material was purified by column chromatography (n-hexane/ EtOAc 85:15) to give the product as colorless to yellowish oil (72 mg, 33% yield). 1H-NMR (400 MHz, CDCl3) δ 8.49 (s, 1H), 7.30 (d, J = 8.4 Hz, 2H), 7.13 (d, J = 8.4 Hz, 2H), 6.82 (s, 1H), 6.77–6.74 (m, 2H), 6.60–6.58 (m, 2H), 4.77 (s, 1H), 3.76 (s, 3H), 3.66 (dd, Jab = 16.8 Hz, 2H), 2.62–2.42 (m, 2H), 1.46–1.39 (m, 2H), 1.32–1.21 (m, 6H), 0.88–0.85 (m, 3H); 13C-NMR (100 MHz, CDCl3) δ 156.7, 148.6, 139.1, 134.3, 134.0, 133.1, 129.6, 129.0, 125.6, 125.4, 121.1, 114.3, 60.7, 55.4, 53.7, 41.2, 31.6, 27.7, 26.6, 22.5, 14.0. IR (neat) 2927, 2855, 1666, 1505, 1462, 1395, 1351, 1242, 1210, 1090, 833 νmax/cm−1; MS (ESI) m/z Calcd for C25H30ClN4O+: 437.2103; found: 437.2107 [M + H]+.
(E)-N-(7-(4-methoxybenzyl)-6-(4-methoxyphenyl)-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)cyclohexanamine (72). The crude material was purified by column chromatography (n-hexane/ EtOAc 70:30) to give the product as yellowish oil (202 mg, 91% yield). 1H-NMR (400 MHz, CDCl3) δ 8.46 (s, 1H), 7.29 (d, J = 8.4 Hz, 2H), 7.15 (d, J = 8.4 Hz, 2H), 6.89 (d, J = 8.4 Hz, 2H), 6.84 (d, J = 8.4 Hz, 2H), 6.74 (s, 1H), 4.95 (s, 1H), 3.82 (s, 3H), 3.79 (s, 3H), 3.77–3.73 (m, 1H), 3.65–3.57 (m, 3H), 3.13–3.07 (m, 1H), 1.74–1.01 (m, 10H); 13C-NMR (100 MHz, CDCl3) δ 159.5, 159.1, 145.8, 132.8, 130.0, 129.6, 129.3, 126.8, 125.4, 124.8, 114.0, 113.9, 58.7, 57.4, 56.7, 55.2 (2C), 40.8, 34.1, 33.4, 25.4, 24.1, 24.0. IR (neat) 2928, 1676, 1598, 1509, 1459, 1345, 1249, 1201, 1033, 732, 698 νmax/cm−1; MS (ESI) m/z Calcd for C27H33N4O2+: 445.2599; found: 445.2600 [M + H]+.
(E)-N-(7-(4-methoxybenzyl)-6-phenethyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)cyclohexanamine (73). The crude material was purified by column chromatography (n-hexane/ EtOAc 70:30) to give the product as off-white solid (216 mg, 98% yield). 1H-NMR (400 MHz, CDCl3) δ 8.31 (s, 1H), 7.27–7.10 (m, 7H), 6.84 (d, J = 8.4 Hz, 2H), 6.76 (s, 1H), 4.14–4.10 (m, 1H), 3.80 (s, 3H), 3.81–3.72 (m, 2H), 3.59–3.43 (m, 2H), 2.95–2.70 (m, 3H), 2.17–2.13 (m, 1H), 1.73–1.00 (m, 11H); 13C-NMR (100 MHz, CDCl3) δ 159.2, 147.8, 140.9, 130.2, 129.8, 128.5 (4C), 126.2, 125.0, 124.4, 113.8, 58.2, 56.3, 55.3, 54.3, 39.7, 34.3, 34.0, 31.6, 31.4, 25.4, 24.2. IR (neat) 3022, 2933, 2858, 1661, 1513, 1467, 1349, 1252, 1220, 1037, 815 νmax/cm−1; Mp 119–120 °C; MS (ESI) m/z Calcd for C28H35N4O+: 443.2806; found: 443.2805 [M + H]+.
(E)-N-(7-(4-methoxybenzyl)-6-phenyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)-2,4,4-trimethylpentan-2-amine (74). The crude material was purified by column chromatography (n-hexane/ EtOAc 80:20) to give the product as colorless to yellowish oil (206 mg, 93% yield). 1H-NMR (400 MHz, CDCl3) δ 8.43 (s, 1H), 7.33–7.19 (m, 7H), 6.90 (d, J = 8.0 Hz, 2H), 6.73 (s, 1H), 5.14 (s, 1H), 3.82 (s, 3H), 3.73 (s, 2H), 3.57 (dd, Jab = 16.4 Hz, 2H), 1.53 (dd, Jab = 14.4 Hz, 2H), 1.17 (s, 3H), 1.03 (s, 3H), 0.95 (s, 9H); 13C-NMR (100 MHz, CDCl3) δ 159.1, 142.0, 135.6, 129.8, 129.7, 128.7, 128.6, 128.2, 125.7, 124.7, 113.9, 62.3, 58.7, 58.4, 57.5, 55.3, 40.7, 31.9, 31.8, 30.7. IR (neat) 2953, 1674, 1511, 1459, 1338, 1246, 1207, 1191, 699, 654 νmax/cm−1; MS (ESI) m/z Calcd for C28H37N4O+: 445.2962; found: fragment ion (loss of 2,4,4-trimethyl-N-methylenepentan-2-amine moiety): 306.1602 [M + H]+.
(E)-N-(7-(4-methoxybenzyl)-6-phenethyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)naphthalen-1-amine (75). The crude material was purified by column chromatography (n-hexane/ EtOAc 70:30) to give the product as yellowish solid (182 mg, 75% yield). 1H-NMR (400 MHz, CDCl3) δ 8.47 (s, 1H), 7.81 (d, J = 8.0 Hz, 1H), 7.72 (d, J = 8.0 Hz, 1H), 7.60 (d, J = 7.6 Hz, 1H), 7.48–7.42 (m, 2H), 7.26 (s, 1H), 7.14 (d, J = 8.0 Hz, 2H), 6.96–6.59 (m, 9H), 3.99 (dd, Jab = 16.8 Hz, 2H), 3.81 (s, 3H), 3.76–3.72 (m, 1H), 3.61 (dd, Jab = 13.2 Hz, 2H), 2.68–2.48 (m, 2H), 2.11–2.04 (m, 1H), 1.85–1.76 (m, 1H); 13C-NMR (100 MHz, CDCl3) δ 159.1, 151.9, 144.1, 139.8, 134.0, 133.4, 130.7, 129.9, 129.3 (2C), 128.0, 127.9, 127.7, 127.3, 126.4, 125.9, 125.7, 124.9, 124.8, 120.6, 115.6, 113.9, 58.1, 55.3, 55.0, 39.7, 31.1, 29.8. IR (neat) 3052, 2954, 2937, 2846, 1667, 1510, 1356, 1246, 1208, 1031, 862 νmax/cm−1; Mp 136–137 °C; MS (ESI) m/z Calcd for C32H31N4O+: 487.2493; found: 487.2498 [M + H]+.
(E)-2-methyl-N-(6-phenethyl-7-phenyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)propan-2-amine (76). The crude material was purified by column chromatography (n-hexane/ EtOAc 70:30) to give the product as colorless to brownish oil (173 mg, 93% yield). 1H-NMR (400 MHz, CDCl3) δ 8.18 (s, 1H), 7.31–7.15 (m, 7H), 6.93–6.87 (m, 4H), 4.72–4.69 (m, 1H), 4.58 (s, 2H), 2.97–2.70 (m, 2H), 2.31–1.97 (m, 2H), 1.15 (s, 9H); 13C-NMR (100 MHz, CDCl3) δ 150.2, 144.8, 140.2, 129.4, 128.7, 128.5, 126.4, 125.2, 123.9, 121.6, 118.9, 58.2, 54.4, 38.9, 32.1, 31.4, 31.3. IR (neat) 2967, 2924, 1678, 1598, 1494, 1458, 1344, 1201, 924, 754, 697 νmax/cm−1; MS (ESI) m/z Calcd for C24H29N4+: 373.2387; found: fragment ion (loss of 2-methyl-N-methylenepropan-2-amine): 290.1654 [M + H]+.
2-methyl-N-(6-pentyl-7-phenyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)propan-2-amine (77). The crude material was purified by column chromatography (n-hexane/ EtOAc 90:10) to give the product as yellowish oil (160.5 mg, 95% yield). 1H-NMR (400 MHz, CDCl3) main isomer δ 8.16 (s, 1H), 7.23–7.19 (m, 2H), 6.91–6.86 (m, 4H), 4.84–4.80 (m, 1H), 4.48 (dd, Jab = 16.8 Hz, 2H), 1.96–1.87 (m, 1H), 1.65–1.52 (m, 3H), 1.37–1.17 (m, 4H), 1.27 (s, 9H), 0.86–0.83 (m, 3H); 13C-NMR (100 MHz, CDCl3) main isomer δ 156.4, 145.4, 140.6, 129.4, 127.1, 123.6, 121.3, 118.5, 59.1, 55.6, 38.7, 31.4, 31.3, 28.6, 25.9, 22.5, 13.9. IR (neat) 2959, 2930, 2870, 1672, 1493, 1456, 1361, 1201, 1103, 750, 695νmax/cm−1; MS (ESI) m/z Calcd for C21H31N4+: 339.2544; found: fragment ion (loss of 2-methyl-N-methylenepropan-2-amine): 256.1810 [M + H]+.
N-(6-isobutyl-7-phenyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)cyclohexanamine (78). The crude material was purified by column chromatography (n-hexane/ EtOAc 90:10) to give the product as yellowish oil (157.5 mg, 90% yield). 1H-NMR (400 MHz, CDCl3) main isomer δ 8.22 (s, 1H), 7.22–7.18 (m, 2H), 6.89–6.84 (m, 4H), 4.91–4.87 (m, 1H), 4.51 (dd, JAB = 16.8 Hz, 2H), 3.47–3.42 (m, 1H), 1.97–1.11 (m, 13H), 0.98–0.96 (m, 6H); 13C-NMR (100 MHz, CDCl3) main isomer δ 174.4, 149.9, 133.0, 129.4, 124.9, 123.9, 121.1, 117.7, 70.07, 53.6, 43.7, 39.3, 34.1, 33.1, 25.5, 24.5, 24.4, 23.6. IR (neat) 2928, 2855, 1671, 1598, 1497, 1463, 1354, 1216, 924, 754, 693 νmax/cm−1; MS (ESI) m/z Calcd for C22H31N4+: 351.2544; found: 351.2543 [M + H]+.
(E)-N-(6-(4-chlorophenyl)-7-hexyl-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)-2-methylpropan-2-amine (79). The crude material was purified by column chromatography (n-hexane/ EtOAc 97:3) to give the product as an off-white solid (137 mg, 71% yield). 1H-NMR (400 MHz, CDCl3) δ 8.35 (s, 1H), 7.24 (d, J = 8.4 Hz, 2H), 7.15 (d, J = 8.4 Hz, 2H), 6.67 (s, 1H), 5.05 (s, 1H), 3.53 (dd, Jab = 16.8 Hz, 2H), 2.64–2.51 (m, 2H), 1.57–1.50 (m, 2H), 1.34–1.24 (m, 6H), 1.21 (s, 9H), 0.88–0.85 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 143.4, 134.5, 134.0, 133.0, 129.9, 128.7, 125.4, 124.8, 62.7, 54.6, 53.8, 40.5, 31.6, 31.3, 28.1, 26.7, 22.6, 14.0. IR (neat) 2949, 2933, 2865, 2815, 1665, 1489, 1458, 1340, 1202, 1088, 1013 νmax/cm−1; Mp 108–109 °C; MS (ESI) m/z Calcd for C22H32ClN4+: 387.2310; found: fragment ion (loss of 2-methyl-N-methylenepropan-2-amine): 304.1577 [M + H]+.
(E)-N-(6-(4-chlorophenyl)-7-(4-methoxybenzyl)-7,8-dihydroimidazo[1,5-a]pyrazin-5(6H)-ylidene)cyclohexanamine (80). The crude material was purified by column chromatography (n-hexane/EtOAc 70:30) to give the product as amorphous solid (205 mg, 91.5% yield). 1H-NMR (400 MHz, CDCl3) δ 8.46 (s, 1H), 7.31–7.18 (m, 6H), 6.89 (dd, J = 8.4 Hz, 2H), 6.75 (s, 1H), 4.93 (s, 1H), 3.82 (s, 3H), 3.77–3.59 (m, 4H), 3.08–3.02 (m, 1H), 1.75–1.01 (m, 10H); 13C NMR (100 MHz, CDCl3) δ 159.2, 144.9, 134.2, 133.7, 130.0, 129.5, 129.3, 128.9, 125.1, 125.0 (2C), 114.0, 58.4, 57.6, 56.8, 55.3, 40.9 (2C), 34.1, 33.4, 25.4, 24.1, 24.0. IR (neat) 2929, 2854, 1669, 1511, 1463, 1348, 1247, 1190, 1090, 815, 731 νmax/cm−1; MS (ESI) m/z Calcd for C26H30ClN4O+: 449.2103; found: 449.2106 [M + H]+.