Abstract
Atherosclerosis is the key factor responsible for cardiovascular events, which is a major cause of morbidities and mortalities worldwide. It is well known that high-density lipoprotein (HDL) oxidation and glycation increases the risk for atherosclerosis. Epimedium koreanum has been used as a traditional oriental medicine for treating erectile dysfunction, kidney diseases, osteoporosis, and breast cancer. However, no reports on the effects of E. koreanum on HDL modification exist. In this study, we investigated the inhibitory effects of E. koreanum extract and its eight flavonoids, which are: (1) anhydroicaritin 3-O-rhamnoside, (2) β-anhydroicaritin, (3–5) epimedins A-C, (6) epimedoside A, (7) icariin, and (8) des-O-methyl-β-anhydroicaritin, against HDL modification. HDLs obtained from pooled human plasma samples were incubated in vitro with E. koreanum extract or each compound in the presence of copper sulfate or fructose. The HDL modifications were evaluated by measuring generation of conjugated dienes, production of thiobarbituric acid reactive substances, change in electrophoretic mobility of apoA-I, advanced glycation end products formation, and apoA-I aggregation. Consequently, E. koreanum extract and compound 8 suppressed HDL modification through inhibition of lipid peroxidation, apoA-I aggregation, negative charge increase, and AGEs formation. In particular, compound 8 showed more potent inhibitory effect on HDL modification than the extracts, suggesting its protective role against atherosclerosis via inhibition of HDL oxidation and glycation.
1. Introduction
Atherosclerosis is a widespread and chronic progressive arterial disease that has been regarded as one of the major causes of morbidities and mortalities worldwide [1]. Numerous studies have demonstrated that high levels of low-density lipoprotein (LDL) and its modification are directly associated with the risk of developing atherosclerosis [2,3]. On the contrary, high level of HDL is well known for its protective role against atherosclerosis. The Framingham heart study, a long-term cardiovascular cohort study, revealed that elevated levels of high-density lipoprotein-cholesterol (HDL-C) were strongly and independently associated with a reduced risk of coronary heart disease (CHD) [4,5], which were also confirmed by further studies [5,6]. In addition, several previous studies have demonstrated that HDL plays a crucial role in the reduction of atherosclerosis risk through reverse cholesterol transport, anti-oxidative, anti-inflammatory, antithrombotic properties, anti-LDL oxidation, and endothelial cell maintenance functions [7,8,9,10]. However, HDL can easily be modified and impaired through a variety of mechanisms including oxidation and glycation and lead to loss of its anti-atherogenic activities [11]. Multiple studies have also reported that oxidized or glycated HDLs induce cytotoxicity and greater risk of cardiovascular and neurological diseases [12,13]. Furthermore, it has also been revealed that modified HDLs are recorded in patients with a variety of diseases, including rheumatoid arthritis, atrial fibrillation, and myocardial infarction [14,15,16]. Therefore, discovery of inhibitors of HDL modification could be a good strategy for the prevention of heart diseases.
Epimedium koreanum (E. koreanum) Nakai is a perennial and medicinal plant, which is widely distributed in China, Korea, and Japan [17]. In Korea, it has been traditionally used for enhancing erectile function, kidney tonifying, spermatorrhoea, and impotence [18]. Besides, it has also been used widely for the treatment of osteoporosis, immune suppression, cardiovascular diseases, and cancer [18,19,20,21]. E. koreanum has been reported to contain diverse constituents including lignans, polysaccharides, and flavonoids [17]. Among them, flavonoids are well-known to exhibit several biological and pharmacological activities including antioxidant, anti-tumor, osteoporosis inhibition, and antiviral effects [19,22,23,24]. In particular, icariin, a major flavonoid from E. koreanum, has been reported to have anti-atherogenic effects by modulating some cellular signaling pathways [25,26]. Although E. koreanum extract and its flavonoids have various pharmacological roles in the human body, its anti-atherosclerosis function via HDL oxidation and glycation has not been investigated so far. In this study, HDL was separated from plasma of young and healthy male volunteers. Herein, we report the inhibitory effects of ethanol extract of E. koreanum and its flavonoids on HDL oxidation and glycation.
2. Materials and Methods
2.1. Plant Materials and Compounds
The dried aerial parts of E. koreanum were purchased from the Herbmana (Gwangju, South Korea) in December 2018. This sample was botanically identified by the corresponding author (Sang Hee Shim). A voucher was deposited at the pharmacognosy laboratory of the College of Pharmacy, Duksung Women’s University (specimen no. NPC 12-6). The dried aerial parts of E. koreanum (25 g) were extracted thrice with 1 L of 70% aq ethanol (EtOH) for 1 h at 55 °C and the solvents were evaporated in vacuo at 40 °C, yielding the EtOH extract (4 g).
2.2. Standards and Reagents
The standard compounds, anhydroicaritin 3-O-rhamnoside (1), β-anhydroicaritin (2), epimedins A-C (3–5), epimedoside A (6), icariin (7), and des-O-methyl-β-anhydroicaritin (8), were isolated from the extract of E. koreanum by Emeritus professor Sam Sik Kang from Seoul National University. The compounds were identified by nuclear magnetic resonance (NMR) spectroscopy and their purities were assessed by NMR and thin layer chromatography (TLC) analysis before use for this study. Copper sulfate (CuSO4), dialysis tubing cellulose membrane, trichloroacetic acid (TCA), 1,1,3,3-Tetramethoxypropane, bovine serum albumin (BSA), and dichlorofluorescein diacetate (DCFH-DA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Coomassie Brilliant Blue R-250 was purchased from Tokyo Chemical Industry (Tokyo, Japan).
2.3. Isolation of HDL from Human Plasma
Human whole blood samples were obtained from young and healthy male volunteers (IRB no. 2018-007-001). Blood was collected in the Vacutainer (BD sciences, Franklin Lakes, NJ, USA) containing ethylenediaminetetraacetic acid (EDTA, final concentration 1 mM) for each individual. Plasma was separated using high speed centrifugation for 10 min at 4 °C at 3000 rpm (5810R; Eppendorf, Hamburg, Germany). High-density lipoprotein (HDL; d = 1.125–1.225 g/mL) was isolated from the plasma via sequential ultracentrifugation in accordance with standard protocols [27]. Plasma was centrifuged for 22 h at 10 °C at 45,000 rpm using a LE-80 (Beckman, CA, USA) at the Instrumental Analysis Center of Duksung Women’s University. Isolated HDL was extensively dialyzed against Tris buffer containing 140 mM NaCl, 10 mM Tris–HCl, and 5 mM EDTA (pH 7.4) for 24 h at 4 °C. Protein quantification of HDL was determined according to the Lowry protein assay with slight modification [28].
2.4. Measurement of the Formed Conjugated Dienes (CD)
To measure the amounts of dienes formed by lipid peroxidation chain reaction, HDL (200 μg protein/mL) was incubated with CuSO4 (final concentration, 10 μM) under the presence of E. koreanum extract (final concentrations, 10 and 100 μg/mL, respectively), eight isolated compounds (final concentration, 40 μM), or compound 8 (final concentrations, 10 and 20 μM, respectively) in a medium containing 10 mM phosphate buffer (pH 7.4). During incubation, formation of conjugated dienes was continuously monitored by measuring the absorbance at 234 nm at 37 °C using a Spectra Max 190 spectrophotometer (Molecular Devices, CA, USA) [29].
2.5. Measurement of Thiobarbituric Acid Reactive Substances (TBARS)
Native HDL (500 μg protein/mL) with CuSO4 (final concentration, 10 μM) in the presence of E. koreanum extract (final concentration, 10 and 100 μg/mL, respectively), eight isolated compounds (final concentration, 40 μM), or compound 8 (final concentrations, 10 and 20 μM, respectively) were incubated for 4 h at 37 °C. After oxidation, 0.5 mM EDTA (pH 8.0) was added to terminate the reaction with nitrogen gas. Afterwards, 20% trichloroacetic acid (TCA) was added to HDL samples and reaction mixtures were incubated with thiobarbituric acid (TBA) solution (0.67% TBA in 0.05 N NaOH). The mixture was heated in a water bath at 90 °C for 20 min. The mixed samples were cooled at 4 °C and centrifuged at 3000 rpm for 15 min. After centrifugation, the absorbance of supernatant was measured at 532 nm using a UV–visible spectrophotometer Spectra Max 190 (Molecular Devices, CA, USA) [30].
2.6. Measurement of Advanced Glycation End Products (AGEs)
To measure the advanced glycation end products (AGEs) formation, HDL (500 μg protein/mL) was incubated with fructose (final concentration, 100 mM) and E. koreanum extract (final concentrations, 10 and 100 μg/mL, respectively), eight compounds (final concentration, 40 μM), or compound 8 (final concentrations, 10 and 20 μM, respectively) in 0.2 M sodium phosphate buffer, containing 0.02% sodium azide (pH 7.4) for 72 h at 37 °C. Afterwards, HDL samples were dialyzed for 24 h at 4 °C against 10 mM phosphate buffer (pH 7.4). AGEs of HDL samples were determined by measuring the fluorescence intensity (Ex = 360 nm, Em = 460 nm) using a Synergy 2 multi-mode microplate reader (BioTek, VT, USA) [31].
2.7. Measurement of Relative Electrophoretic Mobility (REM)
Electrophoretic mobilities of oxidized HDL by copper was examined using 0.5% agarose gel in TAE buffer (40 mM Tris-Acetate, 1 mM EDTA, pH 8.0) using electrophoretic system (100 V for 40 min). The gels were then fixed using a fixative solution (ethanol: acetic acid: distilled water, 60:10:30, v/v/v) for 30 min to 2 h. After being fixed, the gels were dried in an oven at 80 °C for 1 h, followed by staining with 0.15% Coomassie Brilliant Blue R-250 to visualize the HDL band [32].
2.8. Measurement of Apolipoprotein A-I (ApoA-I) Aggregation
After oxidation and glycation, each HDL sample was denatured with Laemmli sample buffer and 2-mercaptoethanol (15:1, v/v) at 90 °C for 5 min. 12% and 15% SDS-PAGE was conducted to detect apoA-I aggregation. Then, the gels were stained with 0.15% Coomassie Brilliant Blue R-250 to visualize apoA-I in HDL [33].
2.9. Measurement of Dichlorofluorescein (DCF) Fluorescence
Dichlorofluorescein diacetate (DCF-DA) dissolved in methanol (final concentration, 2.0 mg/mL) was incubated at room temperature in the dark for 30 min, which released DCFH. Interaction of DCFH with oxidized lipid generated DCF, which produced intense fluorescence. HDLs (500 μg protein/mL) were pre-treated with E. koreanum extract (final concentrations, 10 and 100 μg/mL, respectively), eight compounds (final concentration, 40 μM), compound 8 (final concentrations, 10 and 20 μM), or vitamin C (final concentration, 40 μM) for 4 h at 37 °C. Afterwards, HDL samples were dialyzed for 24 h at 4 °C against 10 mM phosphate buffer (pH 7.4). LDL (170 μg protein/mL) was oxidized by CuSO4 (final concentration, 0.5 μM) at 37 °C for 4 h in the absence or presence HDL (60 μg protein/mL). Subsequently, copper ions were removed through dialysis. 50 μL of protein mixture was mixed with 10 μL of DCFH and 460 μL of normal saline. Samples were incubated at 37 °C in the dark for 2 h. DCF fluorescence was determined by measuring the fluorescence intensity (Ex = 485 nm, Em = 538 nm) using a Synergy 2 multi-mode microplate reader (BioTek, VT, USA) [34,35].
2.10. Statistical Analyses
All data were presented as the mean ± standard deviation (SD), and statistical comparisons between groups were made using paired t-tests with the Sigma plot 12.0 statistical software (Systat Software Inc, San Jose, CA, USA). All data were representative of at least 3 independent experiments. P-values of less than 0.05 were regarded as significant
3. Results
3.1. Inhibitory Activities of E. koreanum Extract and Its Flavonoids on HDL Oxidation
The inhibitory effects of the E. koreanum extract and its eight flavonoids (Figure 1) on HDL oxidation were evaluated by measuring the amounts of CD and TBARS formed, which are the indicators of lipid peroxidation. The extract (at 10 and 100 μg/mL) and its eight flavonoids (at 40 μM) significantly reduced maximum CD formation and further extended the lag time by copper-induced HDL oxidation (Figure 2A and Figure 3A). In addition, 100 μg/mL of E. koreanum extract and 40 μM of compounds (1–2, and 8) significantly inhibited the formation of TBARS (Figure 2B and Figure 3B). Despite copper-induced oxidation, HDLs treated with 100 μg/mL of E. koreanum extract and 40 μM of compound 8 showed similar or even lower levels of CD and TBARS compared with native HDL (Figure 2A,B and Figure 3A,B). In particular, compound 8 showed greater reduction of CD and TBARS formation than other compound (Figure 3A,B). As compound 8 showed strong activities, concentrations lower than 40 μM were used for treatment of the HDLs (at 10 and 20 μM). Low concentrations of the samples also strongly inhibited the formation of CD and TBARS, exhibiting similar levels of CD and TBARS formation to that of native HDL (Figure 4A,B). Vitamin C used as a positive control did not inhibit HDL oxidation at 40 μM and the results were similar to those treated with 10 μM of compound 8.
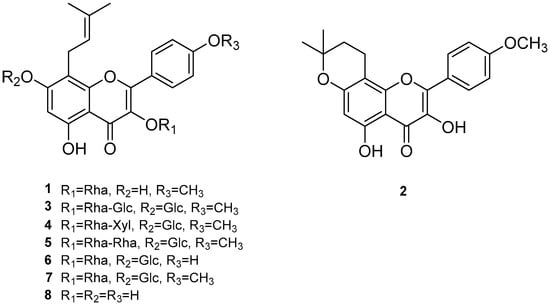
Figure 1.
Chemical structures of compounds 1–8; (1) anhydroicaritin 3-O-rhamnoside, (2) β-anhydroicaritin, (3) epimedin A, (4) epimedin B, (5) epimedin C, (6) epimedoside A, (7) icariin, and (8) des-O-methyl-β-anhydroicaritin.
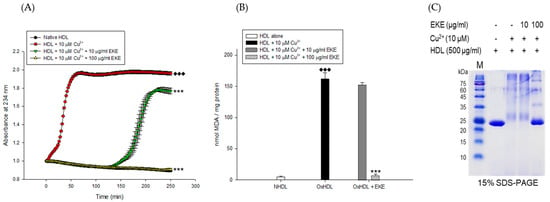
Figure 2.
Effects of E. koreanum extract (EKE) on Cu2+ induced HDL oxidation. (A) Continuous monitoring of conjugated diene levels by absorbance at 234 nm (A234) during 10 μM Cu2+- mediated HDL oxidation in the presence of E. koreanum extract (final concentrations, 10 and 100 μg/mL). (B) Effects of E. koreanum extract (final concentrations, 10 and 100 μg/mL) on TBARS production during HDL oxidation induced by CuSO4 for 4 h at 37 °C. (C) SDS-PAGE of HDL modified by 10 μM CuSO4 for 4 h in the absence or presence of E. koreanum extract (final concentration, 10 and 100 μg/mL). These data are expressed as mean ± SD of three independent experiments. ♦♦♦, p < 0.001 vs. NHDL (native HDL); ***, p < 0.001 vs. OxHDL (oxidized HDL).
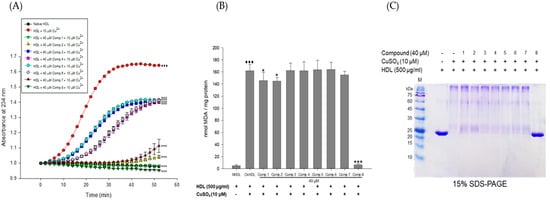
Figure 3.
Effects of eight flavonoids from E. koreanum on Cu2+-induced HDL oxidation. (A) Continuous monitoring of conjugated diene levels by absorbance at 234 nm wavelength (A234) during 10 μM copper ion mediated HDL oxidation in the presence of the eight compounds (final concentration, 40 μM). (B) Effect of the eight flavonoids (final concentration, 40 μM) in TBARS production during HDL oxidation induced by CuSO4 for 4 h at 37 °C. (C) SDS-PAGE showing HDL modified by 10 μM CuSO4 for 4 h in the absence or presence of the eight flavonoids (final concentration, 40 μM). These data are expressed as mean ± SD of three independent experiments. ♦♦♦, p < 0.001 vs. NHDL (native HDL); *, p < 0.01; ***, p < 0.001 vs. OxHDL (oxidized HDL).
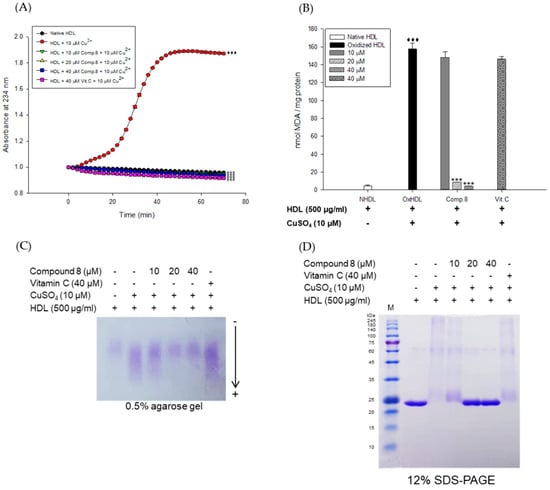
Figure 4.
Effect of low concentrations of compound 8 (des-O-methyl-β-anhydroicaritin) from E. koreanum on Cu2+-induced HDL oxidation. (A) Continuous monitoring of the conjugated diene levels by absorbance at 234 nm (A234) during 10 μM copper-ion mediated HDL oxidation in the presence of compound 8 (final concentrations, 10, 20, and 40 μM). (B) Effects of compound 8 (final concentrations, 10, 20, and 40 μM) in TBARS production during HDL oxidation induced by CuSO4 for 4 h at 37 °C. (C) Electrophoretic mobility profile of HDL, which was treated with compound 8 (final concentrations, 10, 20, and 40 μM) in the presence 10 μM copper ion. Oxidized HDL diffused more than native HDL. (D) SDS-PAGE of HDL modified by 10 μM CuSO4 for 4 h in the absence or presence of compound 8 (final concentrations, 10, 20, and 40 μM). These data are expressed as mean ± SD of three independent experiments. ♦♦♦, p < 0.001 vs. NHDL (native HDL); ***, p < 0.001 vs. OxHDL (oxidized HDL). Vitamin C was used as a positive control.
The inhibitory effects of E. koreanum extract and eight flavonoids on apoA-I aggregation were examined using SDS-PAGE (Sodium dodecyl sulfate-Polyacrylamide gel electrophoresis). The oxidized HDL showed a multimeric pattern of apoA-I, which meant that apoA-I was aggregated by copper ions (Figure 2C). However, HDL treatment with E. koreanum extract (at 100 μg/mL) and compound 8 (at 40 μM) inhibited apoA-I aggregation (Figure 2C and Figure 3C). In particular, the apoA-I band of HDL treated with compound 8 (at 40 μM) showed a similar pattern to that of native HDL (Figure 3C). Besides, the low concentration of compound 8 (at 20 μM) also showed similar activity (Figure 4D) to that HDL treated with 40 μM of compound 8. In an agarose gel electrophoresis, oxidized HDL band was more smeared than that seen in case of native HDL, while the presence of compound 8 (at 20 and 40 μM) recovered copper-mediated changes of positive charge on oxidized HDL (Figure 4C).
3.2. Inhibitory Activities of E. koreanum Extract and Its Flavonoids on HDL Glycation
The inhibitory activities of E. koreanum extract and its eight flavonoids on HDL glycation were evaluated by measuring advanced glycation end products (AGEs) and apoA-I aggregation. Glycated HDL induced by fructose showed much higher amount of AGEs than that in native HDL (Figure 5A). However, HDL treated with 100 μg/mL of E. koreanum extract and 40 μM of compounds (1, 2, 4, and 6–8) significantly inhibited the formation of AGEs compared to glycated HDL and HDL treated with aminoguanidine (AG) used as a positive control (Figure 5A and Figure 6A). Furthermore, low concentration of compound 8 (10 and 20 μM) also significantly lessened the formation of AGEs (Figure 7A). In the SDS-PAGE analysis, apoA-I in glycated HDL showed multimeric band because of the aggregation caused by fructose, while distinct apoA-I band was observed in case of native HDL (Figure 5B). Despite fructose-induced glycation, HDL treated with 100 μg/mL of E. koreanum extract and 40 μM of compounds (1, 2, 4, and 6–8) reduced apoA-I aggregation (Figure 5B and Figure 6B). Surprisingly, 40 μM of compounds (1, 2, 4, and 6–8) showed a pattern similar to that of native HDL (Figure 6B). The low concentrations of compound 8 (at 10 μM and 20 μM) also remarkably reduced multimeric apoA-I (Figure 7B).
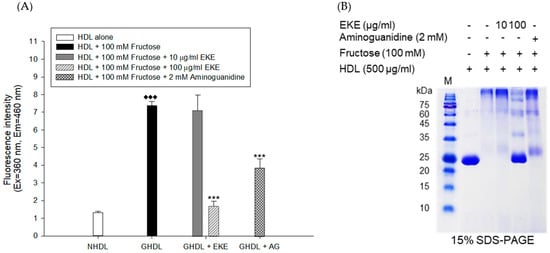
Figure 5.
Effect of E. koreanum extract (EKE) on fructose induced HDL glycation. (A) The formation of AGEs was determined by measuring the fluorescence intensity (Ex = 360 nm, Em = 460 nm). (B) SDS-PAGE of HDL modified by 100 mM fructose for 72 h in the absence or presence of E. koreanum extract (final concentrations, 10 and 100 μg/mL). These data are expressed as mean ± SD of three independent experiments. ♦♦♦, p < 0.001 vs. NHDL (native HDL); ***, p < 0.001 vs. GHDL (glycated HDL).
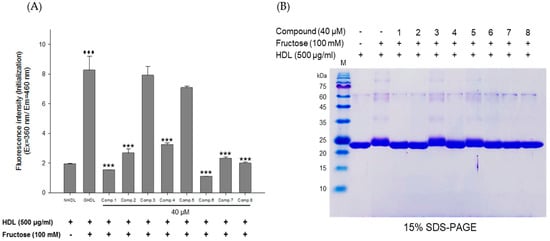
Figure 6.
Effects of eight flavonoids from E. koreanum on fructose induced HDL glycation. (A) The formation of AGEs was determined by measuring the fluorescence intensity (Ex = 360 nm, Em = 460 nm). (B) SDS-PAGE of HDL modified by 100 mM fructose for 72 h in the absence or presence of the eight flavonoids (final concentration, 40 μM). These data are expressed as mean ± SD of three independent experiments. ♦♦♦, p < 0.001 vs. NHDL (native HDL); ***, p < 0.001 vs. GHDL (glycated HDL).
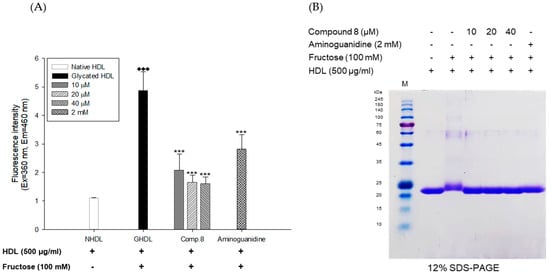
Figure 7.
Dose dependent effect of compound 8 (des-O-methyl-β-anhydroicaritin) on fructose-induced HDL glycation. (A) formation of AGEs was determined by measuring the fluorescence intensity (Ex = 360 nm, Em = 460 nm). (B) SDS-PAGE of HDL modified by 100 mM fructose for 72 h in the absence or presence of compound 8 (final concentrations, 10, 20, and 40 μM). These data are expressed as mean ± SD of three independent experiments. ♦♦♦, p < 0.001 vs. NHDL (native HDL); ***, p < 0.001 vs. GHDL (glycated HDL).
3.3. Effect of E. koreanum Extract and Its Eight Flavonoids on HDL Function
To evaluate the ability of HDL to inhibit LDL oxidation, a cell-free assay was employed by measuring DCF fluorescence [34]. Oxidized LDL induced by copper-ion significantly increased DCF fluorescence compared to native LDL (Figure 8A). However, it was remarkably reduced by addition of HDL, indicating that HDL inhibited the oxidation of LDL (Figure 8A,B). HDLs treated with E. koreanum extract (final concentration, 10 and 100 μg/mL), eight compounds (final concentration, 40 μM), and vit.C (final concentration, 40 μM) also inhibited the LDL oxidation in a similary way to the native HDL (control). In particular, HDLs treated with 100 μg/mL of E. koreanum extract and 40 μM of compounds (1–4, and 8) showed stronger anti-LDL oxidation activity than the control (Figure 8A,B).
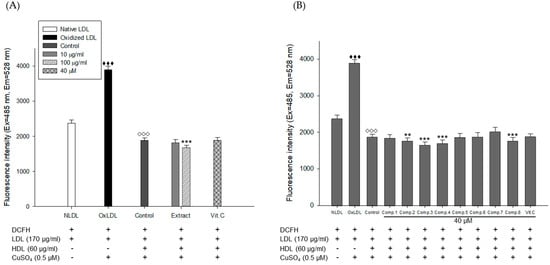
Figure 8.
HDL treated with E. koreanum extract or its eight flavonoids inhibited ROS production from Cu2+-induced LDL oxidation. (A) Inhibitory effect of E. koreanum extract treated-HDL on LDL oxidation. (B) Inhibitory effect of eight flavonoids treated-HDL on LDL oxidation. The formation of DCF was determined by measuring the fluorescence intensity (Ex = 485 nm, Em = 538 nm). These data are expressed as mean ± SD of three independent experiments. ♦♦♦, p < 0.001 vs. NLDL (native LDL); ◊◊◊, p < 0.001 vs. OxLDL (oxidized LDL); **, p < 0.01, ***, p < 0.001 vs. Control.
4. Discussion
E. koreanum Nakai, mainly comprising flavonoids, has been traditionally used for the treatment of many diseases including cardiovascular diseases [36]. Even though there are some reports that icariin, a major flavonoid from E. koreanum, is effective for hyperlipidemia, it remains uncertain whether E. koreanum extract and its flavonoids could hinder the oxidation and glycation of HDL or not. In this study, we evaluated the inhibitory effects of E. koreanum extract and its flavonoids on the oxidation and glycation of the human HDL. The inhibitory activities against HDL oxidation and glycation were evaluated by measuring the amounts of CDs, MDA, multimeric apoA-I, and AGEs generated from modification of HDL.
CDs are formed by rearrangement of double bonds in polyunsaturated fatty acids (PUFAs) and primary lipid oxidation products of PUFAs in HDL. The contents of CDs can be quantitatively determined by UV spectroscopy at 234 nm, which have been used to assess the extent of lipid oxidation. Cleavage of these products is known to generate malondialdehyde (MDA). Measuring the formation of CD and MDA is the most commonly employed method to evaluate lipid peroxidation in vitro [31]. CD and MDA have been reportedly generated in substantially large amounts in copper-mediated oxidization of HDL [12,37]. In our results, E. koreanum extract and its flavonoids significantly reduced CD formation (Figure 2A and Figure 3A) and inhibited MDA production (Figure 3B). Even low concentrations of compound 8 strongly inhibited the formation of CD and MDA, which were similar to those from HDL treated with the extract and native HDL (Figure 4A,B). These results indicate that E. koreanum extract and compound 8 could inhibit lipid peroxidation by reducing CD and MDA formation on copper-mediated oxidized HDL.
ApoA-I, a major constituent protein of HDLs, is responsible for mediating several beneficial effects in HDL. Modification of apoA-I is directly related to the production of dysfunctional HDL, which has pro-atherosclerotic and pro-inflammatory properties. In a previous study, oxidized HDL induced by CuSO4 increased apoA-I denaturation, along with elevating the negative charge on HDL and lipid peroxides in comparison to the native HDL [38]. In the present evaluation, both oxidized HDL and glycated HDL increased formation of multimeric apoA-I (Figure 4D and Figure 5B). In our agarose gel electrophoresis experiment, the band corresponding to the oxidized HDL diffused more than the native HDL, indicating increase of negative charge on the oxidized HDL (Figure 4C). However, HDL treated with E. koreanum extract and compound 8 restored the change of charge and inhibited apoA-I aggregation induced by cupric-ion (Figure 2C, Figure 3C, and Figure 4C,D). In glycated HDL, apoA-I aggregation was significantly reduced by treatment of E. koreanum extract and compounds (1, 2, 4, and 6–8) (Figure 5B and Figure 6B). In particular, band for apoA-I of HDL treated with compound 8 showed a pattern similar to that of native HDL, even at low concentrations (Figure 7B). These results suggest that E. koreanum extract and compound 8 remarkably inhibit the aggregation of apoA-I and can aid to prevent dysfunctional HDL formation.
AGEs are formed by non-enzymatic reactions between reducing sugars and proteins or lipids. High levels of AGEs have been linked with the development of many diseases such as diabetes, kidney failure, cataracts, Alzheimer’s, and atherosclerosis [38]. Glycated HDL has been reported to significantly increase AGEs formation and reduce activities of paraoxonase [39]. Paraoxonase, an enzyme physically associated with HDL, has an antioxidant activity and prevents oxidation of LDL [40,41]. Recent clinical studies have demonstrated that the glycated HDL-C/HDL-C ratios in patients with metabolic syndrome are higher than that found in controls, suggesting that glycated HDL could be a risk factor to the development of atherosclerosis and diabetes [42]. Our present findings showed that E. koreanum extract and its compounds (1, 2, 4, and 6–8) significantly reduced AGEs formation in fructose-induced glycated HDL (Figure 5A and Figure 6A). In particular, low concentrations of compound 8 (at 10 and 20 μM) reduced AGE formation similar to E. koreanum extract (at 100 μg/mL) and compound 8 (at 40 μM). Furthermore, E. koreanum extract (at 100 μg/mL) and compound 8 (at 10, 20, and 40 μM) showed a higher potent activity than aminoguanidine (positive control, final concentration, 2 mM) (Figure 7A). These results lead us to believe that E. koreanum extract and compound 8 could be considered as an active compound against HDL glycation.
To evaluate the function of HDL to protect LDL against oxidation, a cell-free assay was conducted. The normal HDL significantly decreased the fluorescent signal indicating that the normal HDL strongly inhibited the LDL oxidation. Treatment of E. koreanum extract and some flavonoids (1–4, and 8) to the HDL also decreased the fluorescent signal even though they did not show dramatic changes compared with the HDL without the extract or compounds. Therefore, this plant and compounds showed additive effects on HDL function against LDL oxidation.
In this study, E. koreanum extract and its flavonoids exhibited potent inhibitory activities against oxidation and glycation of HDL. There are some previous reports that polyphenol-rich dark chocolates and red wine suppressed HDL and LDL modification by lowering oxidative stress levels [43,44]. For a single compound level, genistein, daidzein, luteolin, isohramnetin, quercetin, glabridin, and some anthocyanins increased activities of paraoxonase 1, an enzyme which protects HDL from oxidation, and eventually improved the cholesterol efflux capacity of HDL [45,46,47]. In addition, flavonoid-rich fractions of Camellia nitidissima flowers and Retama sphaerocarpa fruits showed anti-glycation effects in the glycated-bovine serum albumin (BSA) [48,49]. In our results, the non-glycosylated prenylflavonoid, des-O-methyl-β-anhydroicaritin (8), was found to show much stronger activities against HDL modifications than the glycosylated prenylflavonoids (1 and 3–7). In a literature study to compare our results with previously reported ones, limonin, a natural limonoid-type triterpenoid, showed a stronger inhibitory activity on LDL oxidation than its glycoside, limonin 17-β-D-glucopyranoside [50]. Regarding the flavonoid-type compounds, naringenin was also reported to show stronger inhibitory effects on LDL oxidation and glycation than its glycoside, naringin [51], which were quite coherent with our current findings.
5. Conclusions
To the best of our knowledge, this is the first study on inhibitory effects of E. koreanum and its flavonoids on HDL oxidation and glycation. Our findings suggest that extract of E. koreanum and compound 8 (des-O-methyl-β-anhydroicaritin) might harbor potential to act as good supplements for the prevention of atherosclerosis via inhibition of HDL oxidation and glycation. Further studies dealing with the mechanistic and in vivo efficacies for compound 8 will be required.
Author Contributions
J.-Y.K. conducted experiments and prepared the manuscript; S.H.S. designed and supervised the study.
Funding
This study was supported by the National Research Foundation (NRF) of Korea (grant no. NRF-2016R1A6A1A03007648, NRF-2017R1A6A3A11033480, NRF-2018R1A2B6001733, and NRF-2016R1D1A1B03930222).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mozaffarian, D.; Benjaminm, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [PubMed]
- Maiolino, G.; Rossitto, G.; Caielli, P.; Bisogni, V.; Rossi, G.P.; Calò, L.A. The role of oxidized low-density lipoproteins in atherosclerosis: The myths and the facts. Mediat. Inflamm. 2013, 2013, 714653. [Google Scholar] [CrossRef]
- Soran, H.; Durrington, P.N. Susceptibility of LDL and its subfractions to glycation. Curr. Opin. Lipidol. 2011, 22, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; Packard, C.J.; et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009, 302, 1993–2000. [Google Scholar] [PubMed]
- Rye, K.A.; Barter, P.J. Cardioprotective functions of HDLs. J. Lipid Res. 2014, 55, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; Abbott, R.D.; Castelli, W.P. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis 1988, 8, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Oram, J.F.; Yokoyama, S. Apolipoprotein-mediated removal of cellular cholesterol and phospholipids. J. Lipid Res. 1996, 37, 2473–2491. [Google Scholar] [PubMed]
- Navab, M.; Hama, S.Y.; Anantharamaiah, G.M.; Hassan, K.; Hough, G.P.; Watson, A.D.; Reddy, S.T.; Sevanian, A.; Fonarow, G.C.; Fogelman, A.M. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: Steps 2 and 3. J. Lipid Res. 2000, 41, 1495–1508. [Google Scholar]
- Barter, P.J.; Nicholls, S.; Rye, K.A.; Anantharamaiah, G.M.; Navab, M.; Fogelman, A.M. Anti-inflammatory properties of HDL. Circ. Res. 2004, 95, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Namiri-Kalantari, R.; Gao, F.; Chattopadhyay, A.; Wheeler, A.A.; Navab, K.D.; Farias Eisner, R.; Reddy, S.T. The dual nature of HDL: Anti-inflammatory and pro-inflammatory. Biofactors 2015, 41, 153–159. [Google Scholar] [CrossRef]
- Feng, H.; Li, X.A. Dysfunctional high-density lipoprotein. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 156–162. [Google Scholar] [CrossRef]
- Hurtado, I.; Fiol, C.; Gracia, V.; Caldú, P. In vitro oxidised HDL exerts a cytotoxic effect on macrophages. Atherosclerosis 1996, 125, 39–46. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Marchionni, C.; Dousset, N. Effect of non-enzymatic glycation on aluminium-induced lipid peroxidation of human high density lipoproteins (HDL). Nutr. Metab. Cardiovasc. Dis. 2004, 14, 358–365. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, E.Y.; Park, J.K.; Song, Y.W.; Kim, J.R.; Cho, K.H. Patients with rheumatoid arthritis show altered lipoprotein profiles with dysfunctional high-density lipoproteins that can exacerbate inflammatory and atherogenic process. PLoS ONE 2016, 11, e0164564. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kim, J.M.; Shin, D.G.; Kim, J.R.; Cho, K.H. Relation of atrial fibrillation (AF) and change of lipoproteins: Male patients with AF exhibited severe pro-inflammatory and pro-atherogenic properties in lipoproteins. Clin. Biochem. 2014, 47, 869–875. [Google Scholar] [CrossRef]
- Cho, K.H.; Shin, D.G.; Baek, S.H.; Kim, J.R. Myocardial infarction patients show altered lipoprotein properties and functions when compared with stable angina pectoris patients. Exp. Mol. Med. 2009, 41, 67–76. [Google Scholar] [CrossRef][Green Version]
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541. [Google Scholar] [CrossRef]
- Liu, C.R.; Xu, L.X. Analysis of active ingredients of traditional Chinese herbal drug. Assay of icariin in Epidemium. Chin. J. Pharm. Anal. 1984, 4, 81–84. [Google Scholar]
- Xu, F.; Ding, Y.; Guo, Y.; Liu, B.; Kou, Z.; Xiao, W.; Zhu, J. Anti-osteoporosis effect of Epimedium via an estrogen-like mechanism based on a system-level approach. J. Ethnopharmacol. 2016, 177, 148–160. [Google Scholar] [CrossRef]
- Fan, Y.; Ren, M.; Hou, W.; Guo, C.; Tong, D.; Ma, L.; Zhang, W.; He, M.; Song, X. The activation of Epimedium polysaccharide-propolis flavone liposome on Kupffer cells. Carbohydr. Polym. 2015, 133, 613–623. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Yang, X.; Wang, K.; Ni, J.; Qu, X. Inhibitory effect of Epimedium extract on S-adenosyl-L-homocysteine hydrolase and biomethylation. Life Sci. 2005, 78, 180–186. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zhang, X.; Yang, X.H.; Qiu, N.X.; Wang, Y.; Wang, Z.Z. Microwave assisted extraction of flavonoids from cultivated Epimedium sagittatum: Extraction yield and mechanism, antioxidant activity and chemical composition. Ind. Crops Prod. 2013, 50, 857–865. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Meng, J.; Wang, Z.Y. An anticancer agent icaritin induces sustained activation of the extracellular signal-regulated kinase (ERK) pathway and inhibits growth of breast cancer cells. Eur. J. Pharmacol. 2011, 658, 114–122. [Google Scholar] [CrossRef]
- Cho, W.K.; Weeratunga, P.; Lee, B.H.; Park, J.S.; Kim, C.J.; Ma, J.Y.; Lee, J.S. Epimedium koreanum Nakai displays broad spectrum of antiviral activity in vitro and in vivo by inducing cellular antiviral state. Viruses 2015, 7, 352–377. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, K.; Yan, M.; Zhang, Y.; Wang, Y.; Ren, L. Icariin inhibits oxidized low-density lipoprotein-induced proliferation of vascular smooth muscle cells by suppressing activation of extracellular signal-regulated kinase 1/2 and expression of proliferating cell nuclear antigen. Mol. Med. Rep. 2016, 13, 2899–2903. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.B.; Sui, G.G.; Lu, X.Y. Icariin improves eNOS/NO pathway to prohibit the atherogenesis of apolipoprotein E-null mice. Can. J. Physiol. Pharmacol. 2017, 95, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Markwell, M.A.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Esterbauer, H.; Striegl, G.; Puhl, H.; Rotheneder, M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic. Res. Commun. 1989, 6, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Sabokdast, M.; Habibi-Rezaei, M.; Moosavi-Movahedi, A.A.; Ferdousi, M.; Azimzadeh-Irani, E.; Poursasan, N. Protection by beta-hydroxybutyric acid against insulin glycation, lipid peroxidation and microglial cell apoptosis. Daru 2015, 23, 42. [Google Scholar] [CrossRef]
- Sparks, D.L.; Phillips, M.C. Quantitative measurement of lipoprotein surface charge by agarose gel electrophoresis. J. Lipid Res. 1992, 33, 123–130. [Google Scholar]
- Cameron, S.J.; Morrell, C.N.; Bao, C.; Swaim, A.F.; Rodriguez, A.; Lowenstein, C.J. A novel anti-Inflammatory effect for high density lipoprotein. PLoS ONE 2015, 10, e0144372. [Google Scholar] [CrossRef]
- Hillstrom, R.J.; Yacapin-Ammons, A.K.; Lynch, S.M. Vitamin C inhibits lipid oxidation in human HDL. J. Nutr. 2013, 133, 3047–3051. [Google Scholar] [CrossRef]
- Navab, M.; Hama, S.Y.; Hough, G.P.; Subbanagounder, G.; Reddy, S.T.; Fogelman, A.M. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipid. J. Lipid Res. 2001, 42, 1308–1317. [Google Scholar]
- Zhao, Y.; Chen, S.; Wang, Y.; Lv, C.; Wang, J.; Lu, J. Effect of drying processes on prenylflavonoid content and antioxidant activity of Epimedium koreanum Nakai. J. Food Drug Anal. 2018, 26, 796–806. [Google Scholar] [CrossRef]
- Nagano, Y.; Arai, H.; Kita, T. High density lipoprotein loses its effect to stimulate efflux of cholesterol from foam cells after oxidative modification. Proc. Natl. Acad. Sci. USA 1991, 88, 6457–6461. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, C.C.; Thorpe, S.R.; Fu, M.X.; Harper, C.M.; Yoo, J.; Kim, S.M.; Wong, H.; Peters, A.L. Glycation impairs high-density lipoprotein function. Diabetologia 2000, 43, 312–320. [Google Scholar] [CrossRef]
- Mackness, B.; Durrington, P.N.; Mackness, M.I. Human serum paraoxonase. Gen. Pharmacol. 1998, 31, 329–336. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M.; Bisgaier, C.L.; Newton, R.S.; Primo-Parmo, S.L.; La Du, B.N. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J. Clin. Investig. 1998, 101, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Al Saudi, R.M.; Kasabri, V.; Naffa, R.; Bulatova, N.; Bustanji, Y. Glycated LDL-C and glycated HDL-C in association with adiposity, blood and atherogenicity indices in metabolic syndrome patients with and without prediabetes. Ther. Adv. Endocrinol. Metab. 2018, 9, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Nanetti, L.; Vignini, A.; Gregori, A.; Raffaelli, F.; Moroni, C.; Bertoli, E.; Faloia, E.; Mazzanti, L. Effect of consumption of dark chocolate on lipoproteins and serum lipids. Mediterr. J. Nutr. Metab. 2008, 12, 1–6. [Google Scholar]
- Berrougui, H.; Grenier, G.; Loued, S.; Drouin, G.; Khalil, A. A new insight into resveratrol as an atheroprotective compound: Inhibition of lipid peroxidation and enhancement of cholesterol efflux. Atherosclerosis 2009, 207, 420–427. [Google Scholar] [CrossRef]
- Atrahimovich, D.; Vaya, J.; Tavori, H.; Khatib, S. Glabridin protects paraoxonase 1 from linoleic acid hydroperoxide inhibition via specific interaction: A fluorescence-quenching study. J. Agric. Food Chem. 2012, 60, 3679–3685. [Google Scholar] [CrossRef] [PubMed]
- Schrader, C.; Ernst, I.M.; Sinnecker, H.; Soukup, S.T.; Kulling, S.E.; Rimbach, G. Genistein as a potential inducer of the anti-atherogenic enzyme paraoxonase-1: Studies in cultured hepatocytes in vitro and in rat liver in vivo. J. Cell Mol. Med. 2012, 16, 2331–2341. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, X.; Zhang, Y.; Wang, Y.; Liu, Y.; Sun, R. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J. Clin. Endocrinol. Metab. 2014, 99, 561–569. [Google Scholar] [CrossRef]
- Yang, R.; Wang, W.X.; Chen, H.J.; He, Z.C.; Jia, A.Q. The inhibition of advanced glycation end-products by five fractions and three main flavonoids from Camellia nitidissima Chi flowers. J. Food Drug Anal. 2018, 26, 252–259. [Google Scholar] [CrossRef]
- Boussahel, S.; Cacciola, F.; Dahamna, S.; Mondello, L.; Saija, A.; Cimino, F.; Speciale, A.; Cristani, M. Flavonoid profile, antioxidant and antiglycation properties of Retama sphaerocarpa fruits extracts. Nat. Prod. Res. 2018, 32, 1911–1919. [Google Scholar] [CrossRef]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Wu, C.H.; Lin, J.A.; Hsieh, W.C.; Yen, G.C. Low-density-lipoprotein (LDL)-bound flavonoids increase the resistance of LDL to oxidation and glycation under pathophysiological concentrations of glucose in vitro. J. Agric. Food Chem. 2009, 57, 5058–5064. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).