Left Ventricular Systolic Function Assessed by Speckle Tracking Echocardiography in Athletes with and without Left Ventricle Hypertrophy
Abstract
1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Study Protocol
2.2.1. Echocardiography
2.2.2. Speckle Tracking Echocardiography
Observer Variability
2.3. Exercise Protocol
2.4. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Echocardiography
3.3. STE
4. Discussion
5. Conclusions
6. Limitations of the Study
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ryffel, C.P.; Eser, P.; Trachsel, L.D.; Brugger, N.; Wilhelm, M. Age at start of endurance training is associated with patterns of left ventricular hypertrophy in middle-aged runners. Int. J. Cardiol. 2018, 267, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Lo Iudice, F.; Petitto, M.; Ferrone, M.; Esposito, R.; Vaccaro, A.; Buonauro, A.; D’Andrea, A.; Trimarco, B.; Galderisi, M. Determinants of myocardial mechanics in top-level endurance athletes: Three-dimensional speckle tracking evaluation. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Prior, D.L.; La Gerche, A. The athlete’s heart. Heart 2012, 98, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Spence, A.; Halliwill, J.R.; Cable, N.T.; Thijssen, D.H. Exercise and vascular adaptation in asymptomatic humans. Exp. Physiol. 2011, 96, 57–70. [Google Scholar] [CrossRef]
- Wernstedt, P.; Sjostedt, C.; Ekman, I.; Du, H.; Thuomas, K.A.; Areskog, N.H.; Nylander, E. Adaptation of cardiac morphology and function to endurance and strength training. A comparative study using mr imaging and echocardiography in males and females. Scand. J. Med. Sci. Sports 2002, 12, 17–25. [Google Scholar]
- Cote, A.T.; Bredin, S.S.; Phillips, A.A.; Koehle, M.S.; Glier, M.B.; Devlin, A.M.; Warburton, D.E. Left ventricular mechanics and arterial-ventricular coupling following high-intensity interval exercise. J. Appl. Physiol. 2013, 115, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Fagard, R.H. Impact of different sports and training on cardiac structure and function. Cardiol. Clin. 1997, 15, 397–412. [Google Scholar] [CrossRef]
- Donal, E.; Rozoy, T.; Kervio, G.; Schnell, F.; Mabo, P.; Carre, F. Comparison of the heart function adaptation in trained and sedentary men after 50 and before 35 years of age. Am. J. Cardiol. 2011, 108, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Caselli, S.; Sharma, S.; Basso, C.; Bax, J.J.; Corrado, D.; D’Andrea, A.; D’Ascenzi, F.; Di Paolo, F.M.; Edvardsen, T.; et al. European association of preventive cardiology (eapc) and european association of cardiovascular imaging (eacvi) joint position statement: Recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete’s heart. Eur. Heart J. 2018, 39, 1949–1969. [Google Scholar] [PubMed]
- Pelliccia, A.; Thompson, P.D. The genetics of left ventricular remodeling in competitive athletes. J. Cardiovasc. Med. 2006, 7, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Cazorla, O.; Ait Mou, Y.; Goret, L.; Vassort, G.; Dauzat, M.; Lacampagne, A.; Tanguy, S.; Obert, P. Effects of high-altitude exercise training on contractile function of rat skinned cardiomyocyte. Cardiovasc. Res. 2006, 71, 652–660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zebrowska, A.; Waskiewicz, Z.; Zajac, A.; Gasior, Z.; Galbo, H.; Langfort, J. Igf-1 response to arm exercise with eccentric and concentric muscle contractions in resistance-trained athletes with left ventricular hypertrophy. Int. J. Sports Med. 2013, 34, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, A.; Grace, F.; Richards, J.; Hough, J.; Oxborough, D.; Sculthorpe, N. Left ventricular speckle tracking-derived cardiac strain and cardiac twist mechanics in athletes: A systematic review and meta-analysis of controlled studies. Sports Med. 2017, 47, 1145–1170. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, L.; George, K.; Oxborough, D. Speckle tracking echocardiography for the assessment of the athlete’s heart: Is it ready for daily practice? Curr. Treat. Options Cardiovasc. Med. 2018, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Geyer, H.; Caracciolo, G.; Abe, H.; Wilansky, S.; Carerj, S.; Gentile, F.; Nesser, H.J.; Khandheria, B.; Narula, J.; Sengupta, P.P. Assessment of myocardial mechanics using speckle tracking echocardiography: Fundamentals and clinical applications. J. Am. Soc. Echocardiogr. 2010, 23, 351–369, quiz 453-355. [Google Scholar] [CrossRef] [PubMed]
- Van Dalen, B.M.; Soliman, O.I.; Vletter, W.B.; Kauer, F.; van der Zwaan, H.B.; ten Cate, F.J.; Geleijnse, M.L. Feasibility and reproducibility of left ventricular rotation parameters measured by speckle tracking echocardiography. Eur. J. Echocardiogr. 2009, 10, 669–676. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Blessberger, H.; Binder, T. Non-invasive imaging: Two dimensional speckle tracking echocardiography: Basic principles. Heart 2010, 96, 716–722. [Google Scholar] [CrossRef]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Vitarelli, A.; Capotosto, L.; Placanica, G.; Caranci, F.; Pergolini, M.; Zardo, F.; Martino, F.; De Chiara, S.; Vitarelli, M. Comprehensive assessment of biventricular function and aortic stiffness in athletes with different forms of training by three-dimensional echocardiography and strain imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Von Lueder, T.G.; Hodt, A.; Gjerdalen, G.F.; Steine, K. Left ventricular biomechanics in professional football players. Scand. J. Med. Sci. Sports 2018, 28, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; Reichek, N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977, 55, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Savage, D.D.; Garrison, R.J.; Anderson, K.M.; Kannel, W.B.; Castelli, W.P. Echocardiographic criteria for left ventricular hypertrophy: The framingham heart study. Am. J. Cardiol. 1987, 59, 956–960. [Google Scholar] [CrossRef]
- Laaksonen, M.S.; Kalliokoski, K.K.; Luotolahti, M.; Kemppainen, J.; Teras, M.; Kyrolainen, H.; Nuutila, P.; Knuuti, J. Myocardial perfusion during exercise in endurance-trained and untrained humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R837–R843. [Google Scholar] [CrossRef] [PubMed]
- Samesina, N.; Azevedo, L.F.; DE Matos, L.D.N.J.; Echenique, L.S.; Negrao, C.E.; Pastore, C.A. Comparison of electrocardiographic criteria for identifying left ventricular hypertrophy in athletes from different sports. Clinics 2017, 72, 343–350. [Google Scholar] [CrossRef]
- Akagawa, E.; Murata, K.; Tanaka, N.; Yamada, H.; Miura, T.; Kunichika, H.; Wada, Y.; Hadano, Y.; Tanaka, T.; Nose, Y.; et al. Augmentation of left ventricular apical endocardial rotation with inotropic stimulation contributes to increased left ventricular torsion and radial strain in normal subjects: Quantitative assessment utilizing a novel automated tissue tracking technique. Circ. J. 2007, 71, 661–668. [Google Scholar] [CrossRef]
- Forsythe, L.; MacIver, D.H.; Johnson, C.; George, K.; Somauroo, J.; Papadakis, M.; Brown, B.; Qasem, M.; Oxborough, D. The relationship between left ventricular structure and function in the elite rugby football league athlete as determined by conventional echocardiography and myocardial strain imaging. Int. J. Cardiol. 2018, 261, 211–217. [Google Scholar] [CrossRef]
- Uznanska, B.; Chrzanowski, L.; Plewka, M.; Lipiec, P.; Krzeminska-Pakula, M.; Kasprzak, J.D. The relationship between left ventricular late-systolic rotation and twist, and classic parameters of ventricular function and geometry. Kardiol. Pol. 2008, 66, 740–747, discussion 748–749. [Google Scholar]
- George, K.; Shave, R.; Oxborough, D.; Cable, T.; Dawson, E.; Artis, N.; Gaze, D.; Hew-Butler, T.; Sharwood, K.; Noakes, T. Left ventricular wall segment motion after ultra-endurance exercise in humans assessed by myocardial speckle tracking. Eur. J. Echocardiogr. 2009, 10, 238–243. [Google Scholar] [CrossRef]
- Nottin, S.; Doucende, G.; Schuster-Beck, I.; Dauzat, M.; Obert, P. Alteration in left ventricular normal and shear strains evaluated by 2d-strain echocardiography in the athlete’s heart. J. Physiol. 2008, 586, 4721–4733. [Google Scholar] [CrossRef] [PubMed]
- Lumens, J.; Delhaas, T.; Arts, T.; Cowan, B.R.; Young, A.A. Impaired subendocardial contractile myofiber function in asymptomatic aged humans, as detected using mri. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1573–H1579. [Google Scholar] [CrossRef] [PubMed]
- Aksakal, E.; Kurt, M.; Ozturk, M.E.; Tanboğa, I.H.; Kaya, A.; Nacar, T.; Sevimli, S.; Gurlertop, Y. The effect of incremental endurance exercise training on left ventricular mechanics: A prospective observational deformation imaging study. Anadolu Kardiyol Derg 2013, 13, 432–438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eun, L.Y.; Chae, H.W. Assessment of myocardial function in elite athlete’s heart at rest—2D speckle tracking echocardiography in Korean elite soccer players. Sci. Rep. 2016, 6, 39772. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Solari, M.; Mazzolai, M.; Cameli, M.; Lisi, M.; Andrei, V.; Focardi, M.; Bonifazi, M.; Mondillo, S. Two-dimensional and three-dimensional left ventricular deformation analysis: A study in competitive athletes. Int. J. Cardiovasc. Imaging 2016, 32, 1697–1705. [Google Scholar] [CrossRef]
- Carasso, S.; Yang, H.; Woo, A.; Vannan, M.A.; Jamorski, M.; Wigle, E.D.; Rakowski, H. Systolic myocardial mechanics in hypertrophic cardiomyopathy: Novel concepts and implications for clinical status. J. Am. Soc. Echocardiogr. 2008, 21, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Van Dalen, B.M.; Kauer, F.; Soliman, O.I.; Vletter, W.B.; Michels, M.; ten Cate, F.J.; Geleijnse, M.L. Influence of the pattern of hypertrophy on left ventricular twist in hypertrophic cardiomyopathy. Heart 2009, 95, 657–661. [Google Scholar] [CrossRef]
- Schnell, F.; Matelot, D.; Daudin, M.; Kervio, G.; Mabo, P.; Carre, F.; Donal, E. Mechanical dispersion by strain echocardiography: A novel tool to diagnose hypertrophic cardiomyopathy in athletes. J. Am. Soc. Echocardiogr. 2017, 30, 251–261. [Google Scholar] [CrossRef]
- Diffee, G.M.; Nagle, D.F. Regional differences in effects of exercise training on contractile and biochemical properties of rat cardiac myocytes. J. Appl. Physiol. 2003, 95, 35–42. [Google Scholar] [CrossRef][Green Version]
- Notomi, Y.; Lysyansky, P.; Setser, R.M.; Shiota, T.; Popovic, Z.B.; Martin-Miklovic, M.G.; Weaver, J.A.; Oryszak, S.J.; Greenberg, N.L.; White, R.D.; et al. Measurement of ventricular torsion by two-dimensional ultrasound speckle tracking imaging. J. Am. Coll. Cardiol. 2005, 45, 2034–2041. [Google Scholar] [CrossRef]
- Neri Serneri, G.G.; Boddi, M.; Modesti, P.A.; Cecioni, I.; Coppo, M.; Padeletti, L.; Michelucci, A.; Colella, A.; Galanti, G. Increased cardiac sympathetic activity and insulin-like growth factor-i formation are associated with physiological hypertrophy in athletes. Circ. Res. 2001, 89, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Modesti, P.A.; Vanni, S.; Bertolozzi, I.; Cecioni, I.; Lumachi, C.; Perna, A.M.; Boddi, M.; Gensini, G.F. Different growth factor activation in the right and left ventricles in experimental volume overload. Hypertension 2004, 43, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Laviades, C.; Mayor, G.; Diez, J. Elevated circulating levels of insulin-like growth factor i in essential hypertensive patients with left ventricular hypertrophy. Archives des Maladies du Coeur et des Vaisseaux 1991, 84, 1039–1041. [Google Scholar] [PubMed]
- Sharma, S. Athlete’s heart—Effect of age, sex, ethnicity and sporting discipline. Exp. Physiol. 2003, 88, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Kayancicek, H. Elevated LV mass and LV mass index sign on the athlete’s ECG: Athletes hearts are prone to ventricular arrythmia. J. Clin. Med. 2018, 7, 122. [Google Scholar] [CrossRef]
- Brownlee, K.K.; Moore, A.W.; Hackney, A.C. Relationship between circulating cortisol and testosterone: Influence of physical exercise. J. Sports Sci. Med. 2005, 4, 76–83. [Google Scholar] [PubMed]
- Parr, M.K.; Flenker, U.; Schanzer, W. Sports-related issues and biochemistry of natural and synthetic anabolic substances. Endocrinol. Metab. Clin. N. Am. 2010, 39, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Bleumink, G.S.; Schut, A.F.; Sturkenboom, M.C.; Janssen, J.A.; Witteman, J.C.; van Duijn, C.M.; Hofman, A.; Stricker, B.H. A promoter polymorphism of the insulin-like growth factor-i gene is associated with left ventricular hypertrophy. Heart 2005, 91, 239–240. [Google Scholar] [CrossRef][Green Version]
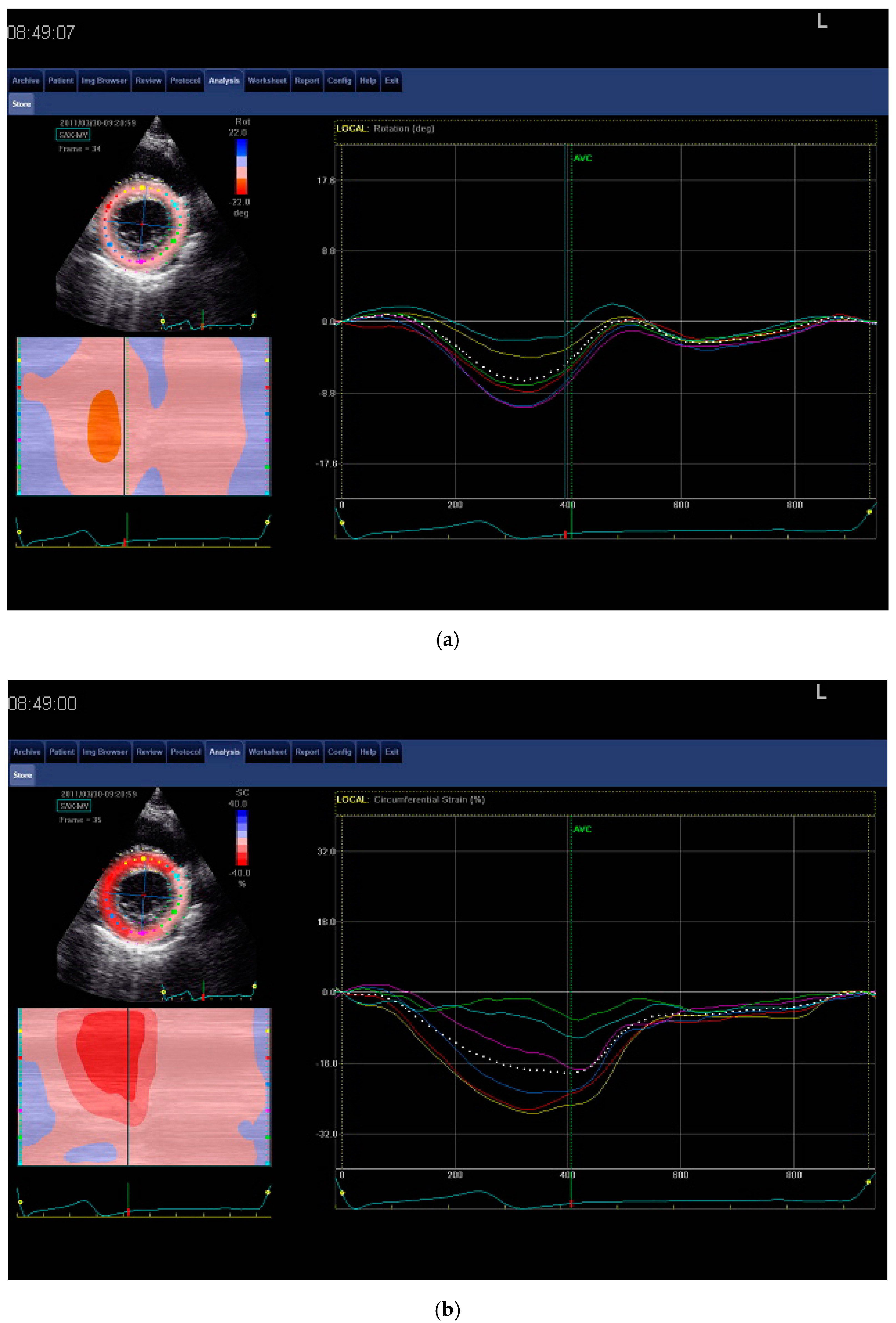
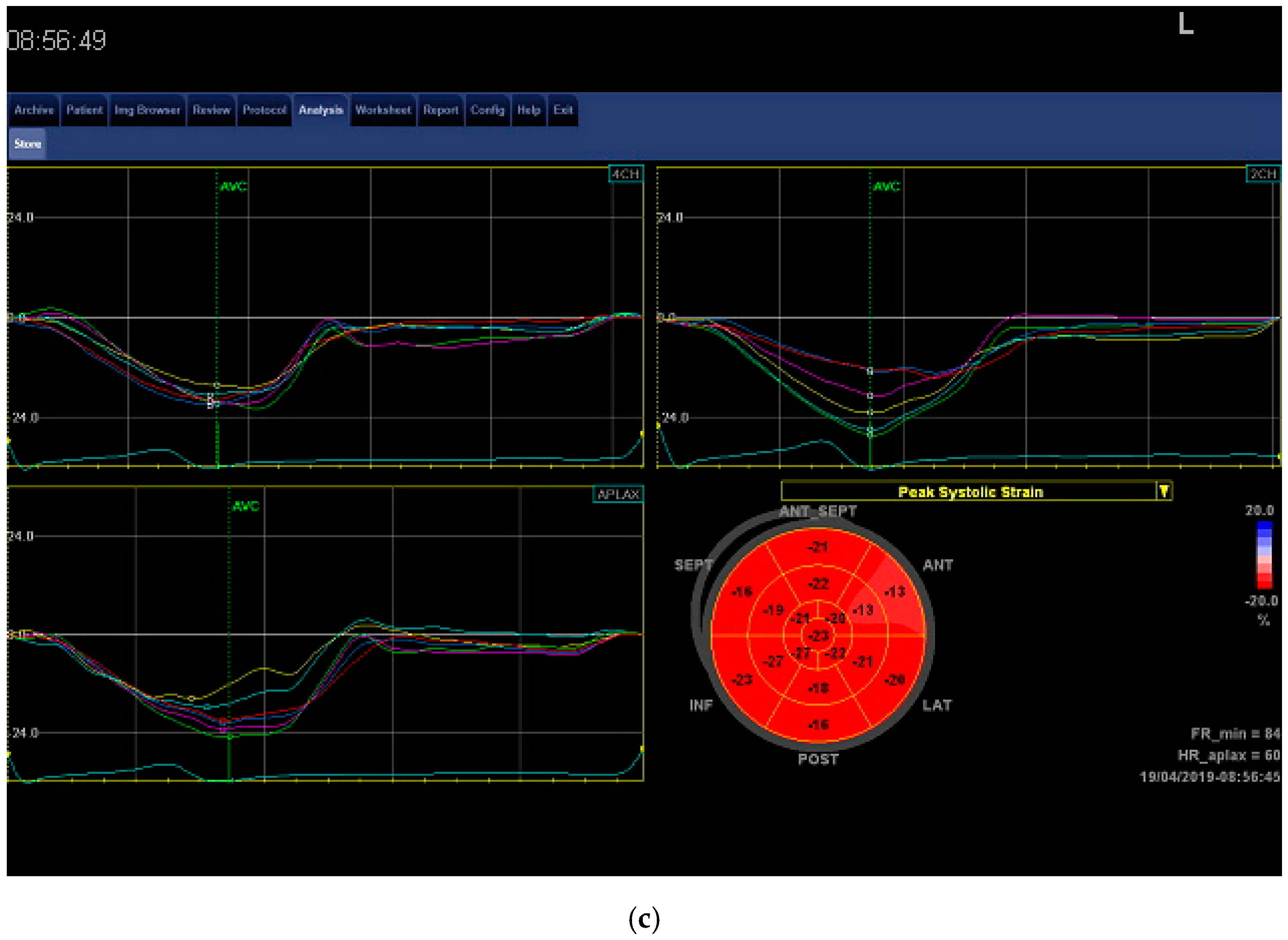
| Variable | CG (n = 14) | Non-LVH (n = 14) | LVH (n = 14) |
|---|---|---|---|
| Age (years) | 20.13 (0.4) | 27.21 (12.4) | 24.43 (3.5) |
| Body height (cm) | 178.38 (4.9) | 177.43 (3.6) | 179.39 (7.3) |
| Body mass (kg) | 75.87 (7.7) | 70.70 (5.0) | 71.96 (7.3) |
| BSA (m2) | 1.94 (0.1) | 1.88 (0.1) | 1.90 (0.1) |
| BMI (kg/m2) | 23.85 (2.4) | 22.38 (1.7) | 22.37 (2.0) |
| FM (kg) | 11.72 (2.9) | 8.50 (3.2) * | 8.58 (2.7) ** |
| FFM (kg) | 62.77 (4.6) | 61.46 (4.6) | 65.96 (6.3) |
| TBW (kg) | 45.85 (3.4) | 46.78 (4.3) | 48.35 (4.6) |
| VO2max (mL/kg/min) | 50.33 (4.8) | 62.18 (7.8) * | 62.00 (7.5) ** |
| Training status (years) | 0 | 9.10 (4.3) | 9.90 (5.1) |
| Powermax (Watt) | 260 (40.0) | 368.89 (36.67) * | 400 (34,11) **# |
| Variable | CG (n = 14) | Non-LVH (n = 14) | LVH (n = 14) |
|---|---|---|---|
| LVM (g) | 159.04 (21.2) | 196.20 (33.8) | 301.29 (58.4) ***### |
| LVMI (g/m2) | 82.07 (10.5) | 104.54 (17.2) | 158.04 (25.6) ***### |
| IVS (mm) | 8.69 (1.0) | 10.18 (2.0) | 12.14 (0.7) ***## |
| PWT (mm) | 8.5 (1.2) | 9.18 (1.6) | 11 (1.1) ***# |
| RWT (%) | 36.00 (6.0) | 38.00 (10) | 41.00 (5.0) * |
| LVEDd (mm) | 47.88 (2.1) | 49.07 (3.9) | 53.93 (4.2) *# |
| LVESd (mm) | 28.63 (2.1) | 30.71 (2.9) | 31.00 (3.5) |
| LVEF% | 64.50 (5.3) | 60.36 (5.4) | 60.57 (4.2) |
| SV (mL) | 77.00 (7.1) | 86.00 (12.2) | 94.36 (10.1) * |
| HRRest (b/min) | 73.00 (7.5) | 63.00 (2.5) | 60.00 (3.0) |
| HR Peak (b/min) | 184.00 (7.1) | 185.00 (8.0) | 192.00 (3.0) |
| SBPRest (mmHg) | 126.86 (10.7) | 118.57 (13.9) | 124.64 (14.1) |
| SBP Post-Ex (mmHg) | 182.90 (8.1) | 185.7 (13.6) | 196.2 (7.4) |
| DBPRest (mmHg) | 81.25 (11.3) | 78.57 (12.2) | 77.50 (11.2) |
| DBP Post-Ex (mmHg) | 64.50 (10.0) | 60.00 (6.7) | 57.10 (11.1) |
| E/A | 1.98 (0.6) | 1.80 (0.5) | 1.91 (0.3) |
| E (m/s) | 0.91 (0.1) | 0.90 (0.1) | 0.90 (0.2) |
| A (m/s) | 0.48 (0.1) | 0.52 (0.1) | 0.47 (0.05) |
| Indicator | CG | Non-LVH | LVH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rest | Post-Ex | Δ | Rest | Post-Ex | Δ | Rest | Post-Ex | Δ | |
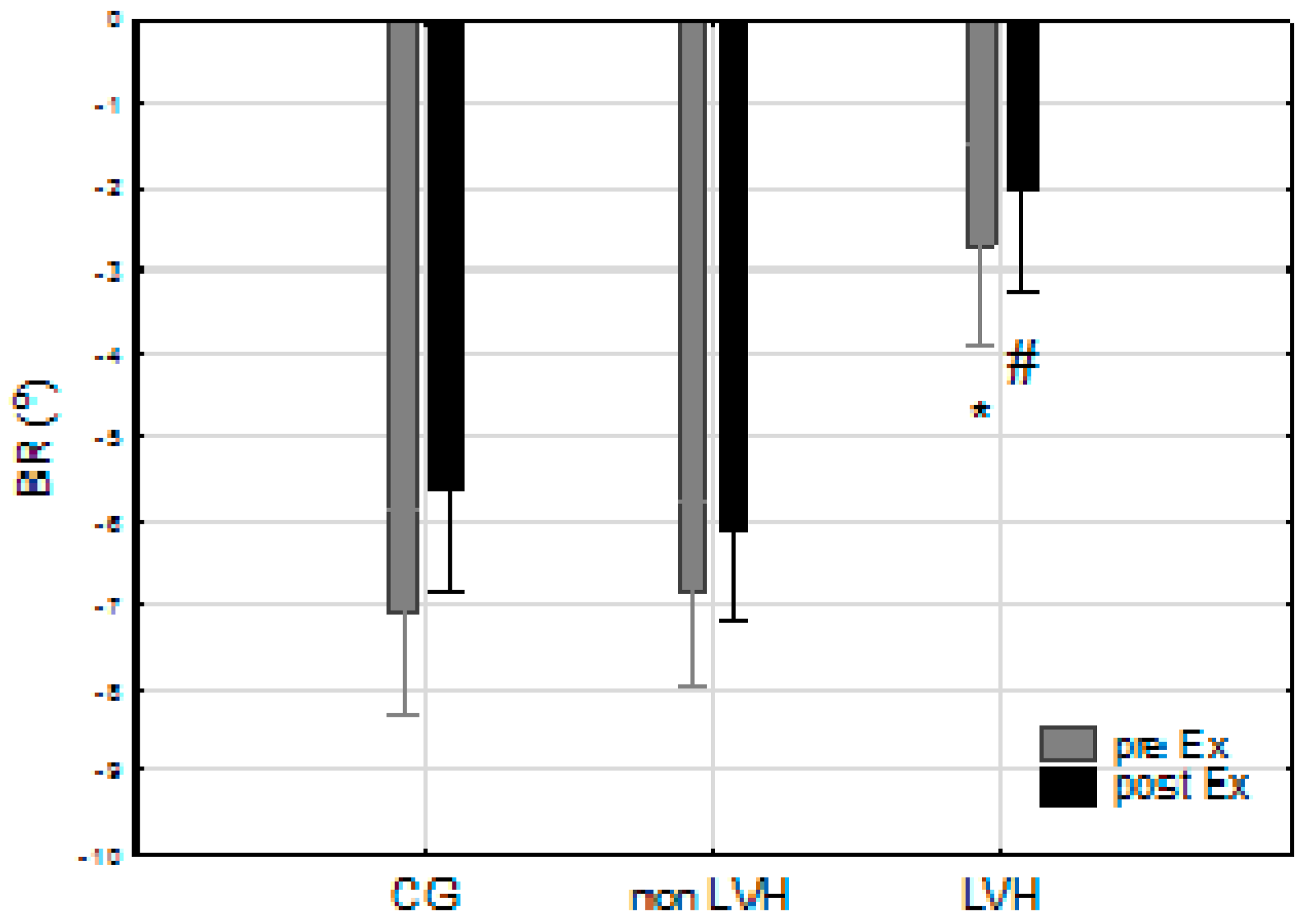
| Basal rotation (BR) (°) | −7.09 (2.86) | −5.63 (2.30) | 1.47 (3.96) | −6.84 (2.12) | −6.09 (3.18) | 0.76 (1.69) | −2.11 (0.60) *# | −2.02 (0.51) *# | 0.69 (2.36) |
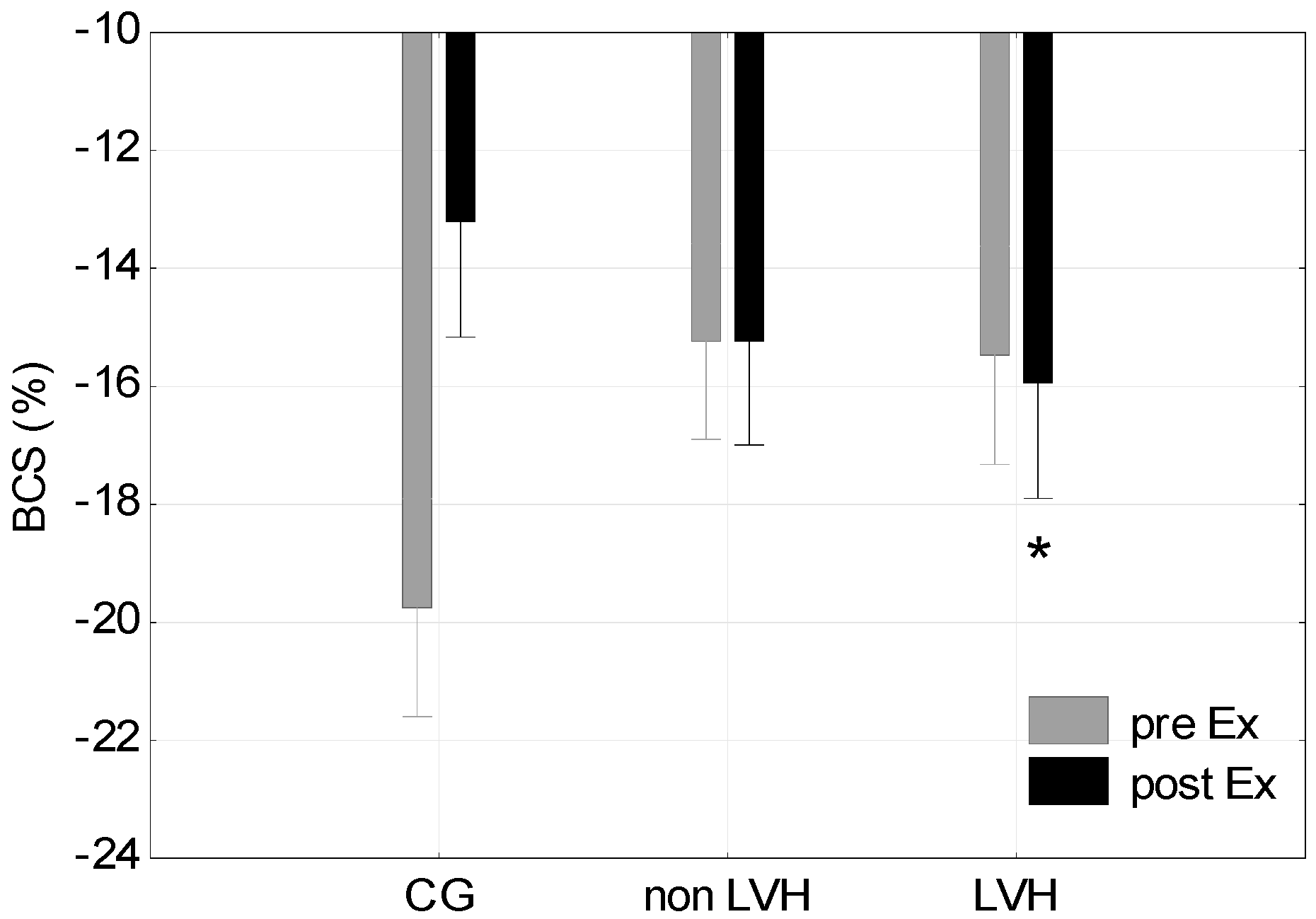
| Basal circumferential strain (BCS) (%) | −19.75 (4.28) | −13.21 (2.82) | 6.55 (3.59) | −15.91 (1.96) | −15.24 (5.28) | 0.67 (4.47) | −15.47 (3.52) | −15.93 (1.94) * | 0.46 (4.60) |
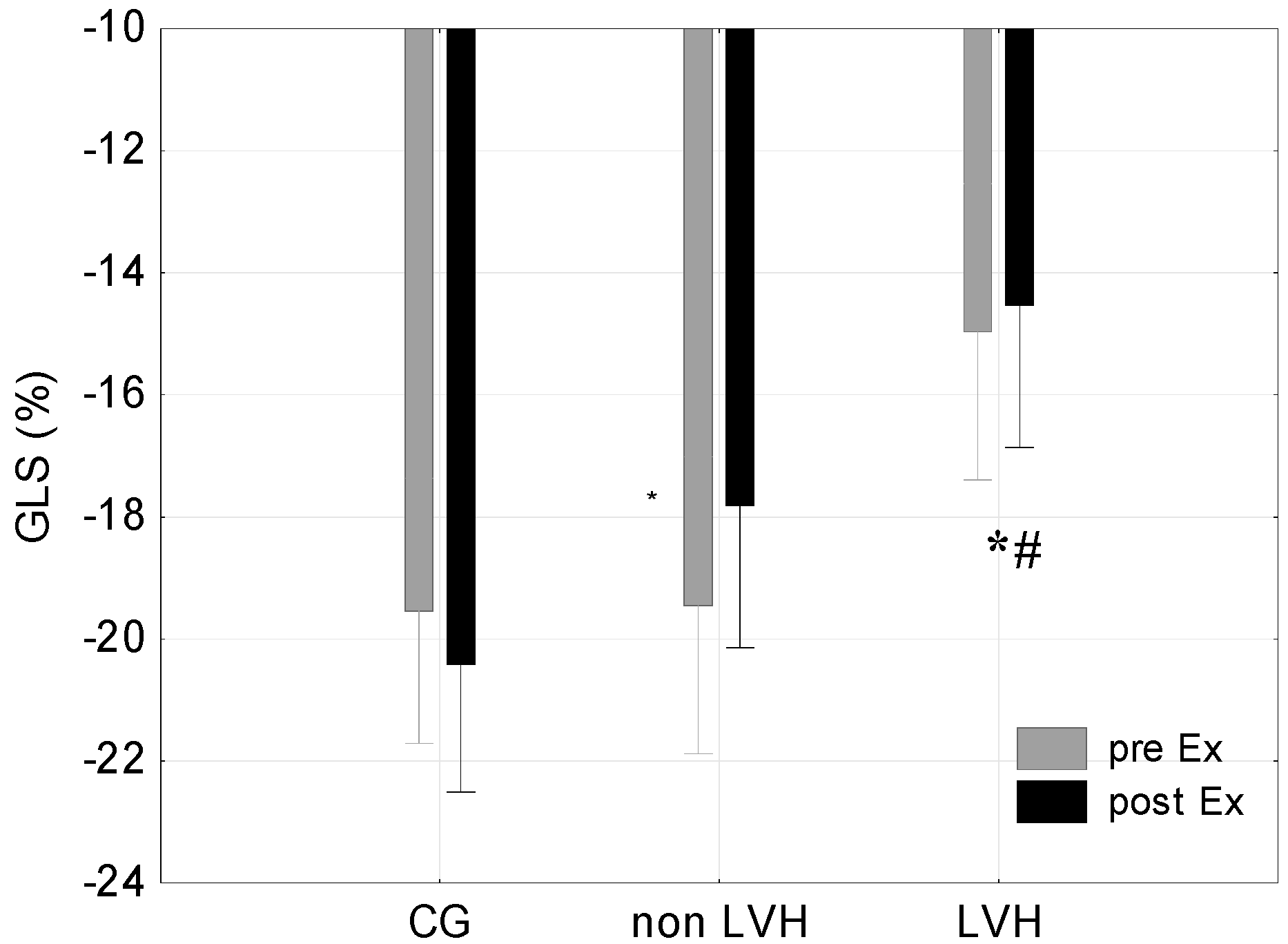
| Global longitudinal strain (GLS) (%) | −19.54 (5.88) | −20.42 (0.96) | −0.88 (1.72) | −19.45 (2.73) | −17.82 (2.75) | 1.64 (0.29) | −14.97 (4.75) | −14.54 (2.64) *# | 0.43 (1.57) |
| Variables | BR Rest | BR Post-Ex | BCS Rest | BCS Post-Ex | GLS Rest | GLS Post-Ex |
|---|---|---|---|---|---|---|
| LVM (g) | r = 0.64 p < 0.05 | r = 0.65 p > 0.05 | r = 0.36 p > 0.05 | r = −0.01 p > 0.05 | r = 0.08 p > 0.05 | r = 0.30 p > 0.05 |
| LVMI (g/m2) | r = 0.64 p < 0.05 | r = 0.66 p > 0.05 | r = 0.48 p > 0.05 | r = 0.01 p > 0.05 | r = 0.02 p > 0.05 | r = 0.17 p > 0.05 |
| LVEDd (mm) | r = 0.61 p < 0.05 | r = 0.73 p < 0.05 | r = 0.64 p < 0.05 | r = 0.39 p > 0.05 | r = 0.11 p > 0.05 | r = 0.27 p > 0.05 |
| LVESd (mm) | r = 0.48 p > 0.05 | r = 0.41 p < 0.05 | r = 0.41 p > 0.05 | r = 0.26 p > 0.05 | r = 0.09 p > 0.05 | r = 0.19 p > 0.05 |
| SV (mL) | r = 0.20 p > 0.05 | r = 0.34 p > 0.05 | r = 0.64 p < 0.05 | r = 0.12 p > 0.05 | r = −0.23 p > 0.05 | r = −0.24 p > 0.05 |
| LVEF (%) | r = 0.49 p > 0.05 | r = 0.36 p > 0.05 | r = −0.01 p > 0.05 | r = −0.20 p > 0.05 | r = 0.40 p > 0.05 | r = 0.49 p > 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żebrowska, A.; Mikołajczyk, R.; Waśkiewicz, Z.; Gąsior, Z.; Mizia-Stec, K.; Kawecki, D.; Rosemann, T.; Nikolaidis, P.T.; Knechtle, B. Left Ventricular Systolic Function Assessed by Speckle Tracking Echocardiography in Athletes with and without Left Ventricle Hypertrophy. J. Clin. Med. 2019, 8, 687. https://doi.org/10.3390/jcm8050687
Żebrowska A, Mikołajczyk R, Waśkiewicz Z, Gąsior Z, Mizia-Stec K, Kawecki D, Rosemann T, Nikolaidis PT, Knechtle B. Left Ventricular Systolic Function Assessed by Speckle Tracking Echocardiography in Athletes with and without Left Ventricle Hypertrophy. Journal of Clinical Medicine. 2019; 8(5):687. https://doi.org/10.3390/jcm8050687
Chicago/Turabian StyleŻebrowska, Aleksandra, Rafał Mikołajczyk, Zbigniew Waśkiewicz, Zbigniew Gąsior, Katarzyna Mizia-Stec, Damian Kawecki, Thomas Rosemann, Pantelis T. Nikolaidis, and Beat Knechtle. 2019. "Left Ventricular Systolic Function Assessed by Speckle Tracking Echocardiography in Athletes with and without Left Ventricle Hypertrophy" Journal of Clinical Medicine 8, no. 5: 687. https://doi.org/10.3390/jcm8050687
APA StyleŻebrowska, A., Mikołajczyk, R., Waśkiewicz, Z., Gąsior, Z., Mizia-Stec, K., Kawecki, D., Rosemann, T., Nikolaidis, P. T., & Knechtle, B. (2019). Left Ventricular Systolic Function Assessed by Speckle Tracking Echocardiography in Athletes with and without Left Ventricle Hypertrophy. Journal of Clinical Medicine, 8(5), 687. https://doi.org/10.3390/jcm8050687











