The Effect of Probiotic Yogurt on Glycemic Control in Type 2 Diabetes or Obesity: A Meta-Analysis of Nine Randomized Controlled Trials
Abstract
:1. Introduction
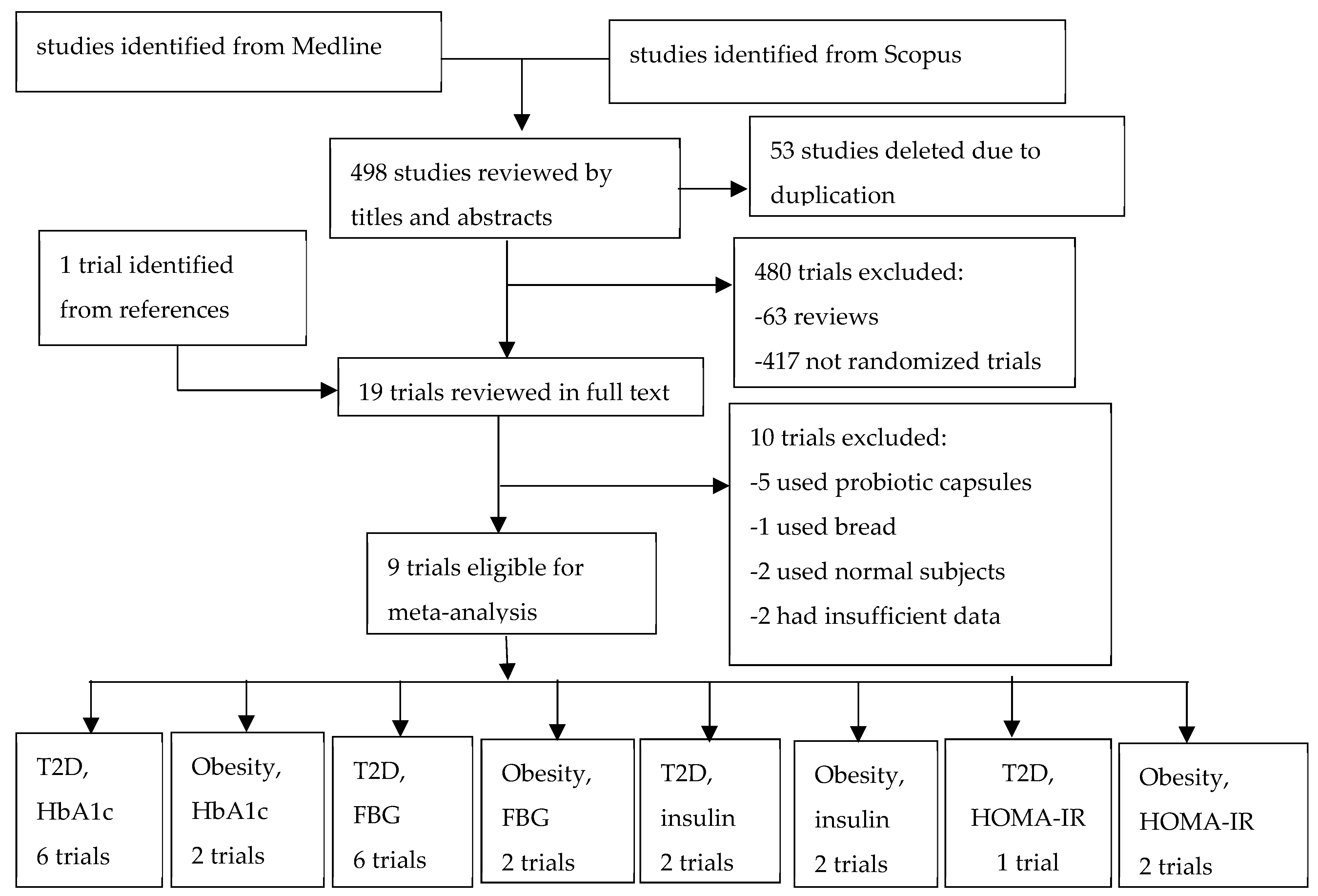
2. Methods
3. Results
3.1. Description of Included Trials
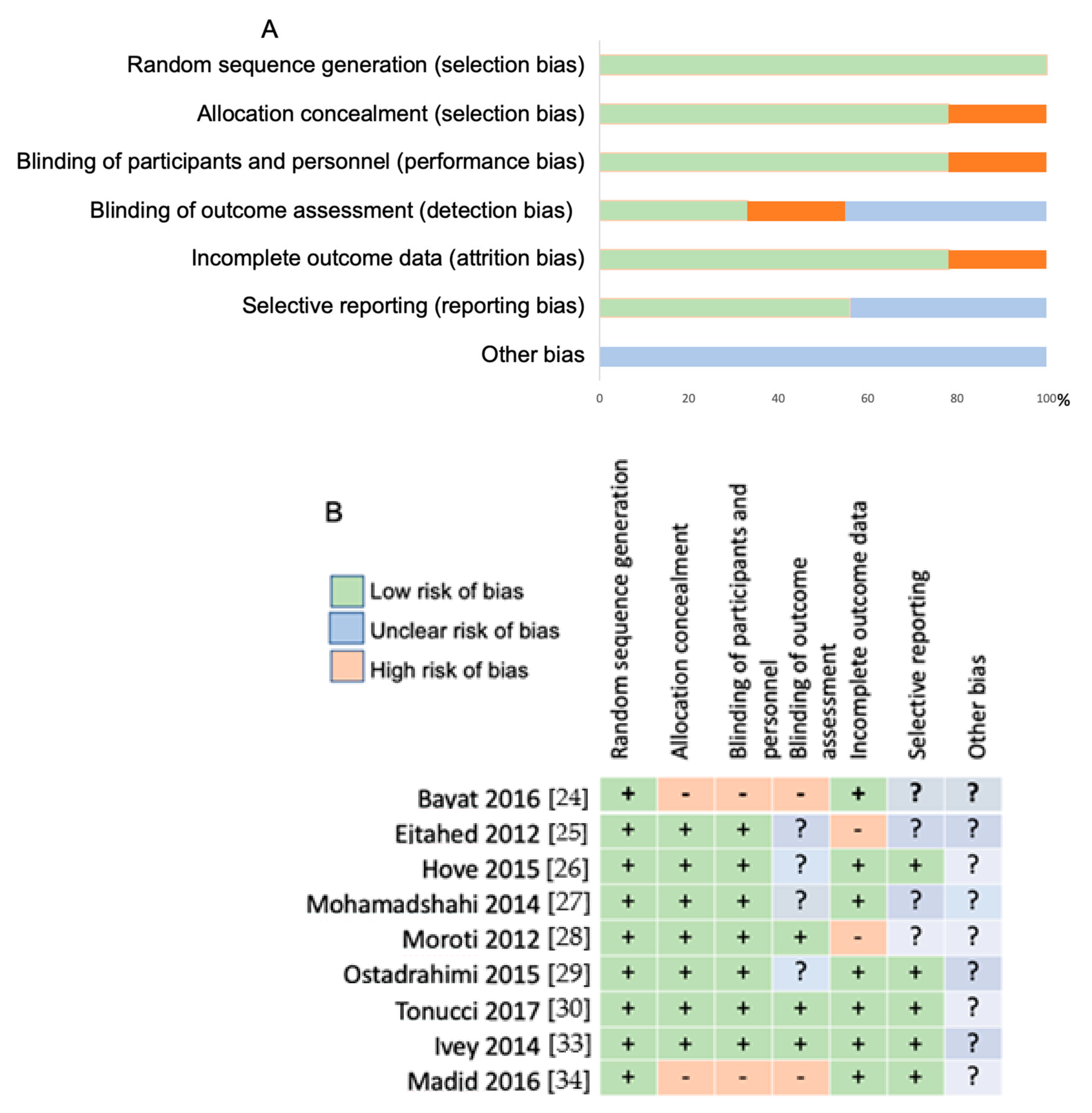
3.2. Risk of Bias Assessment
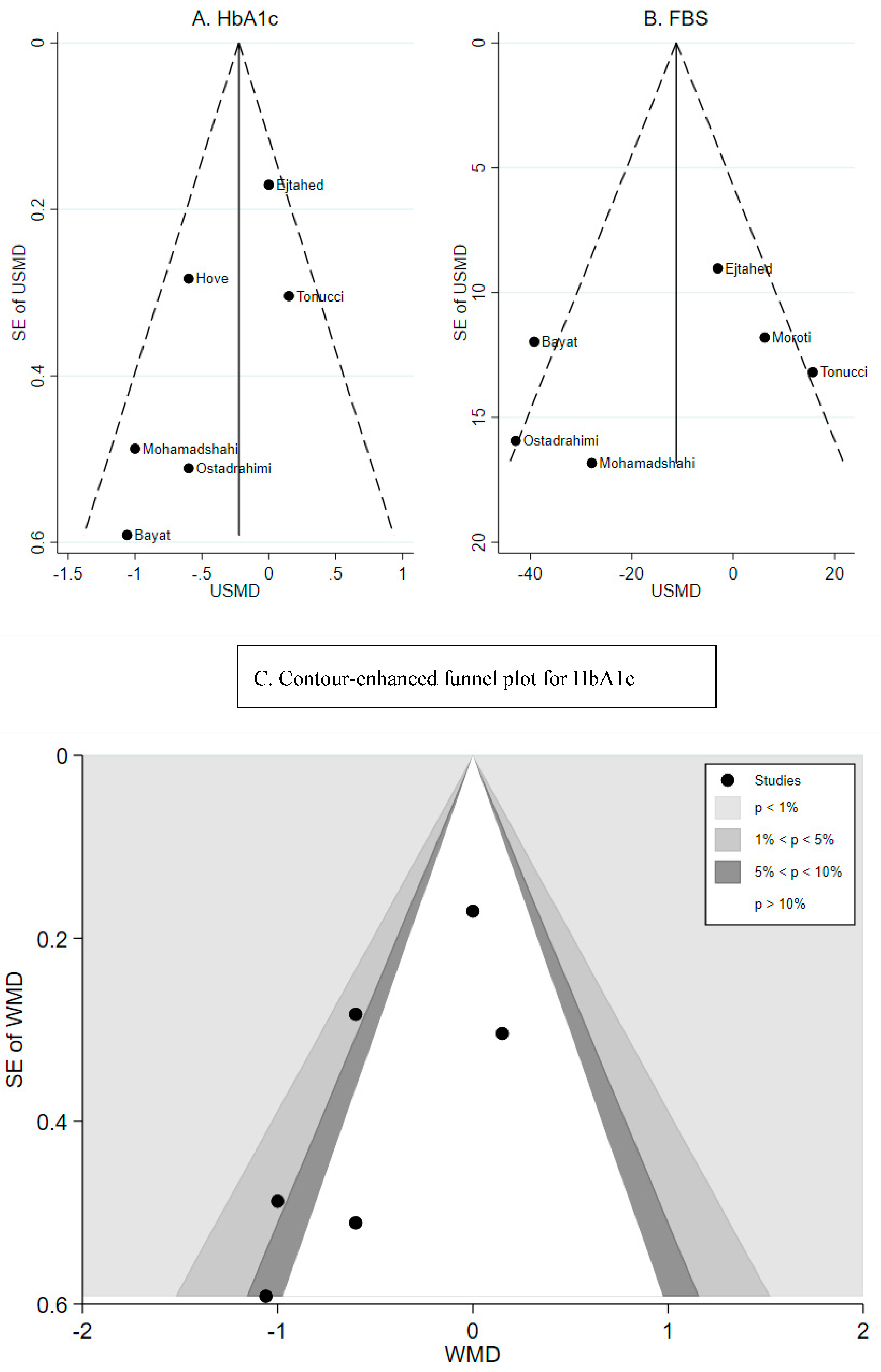
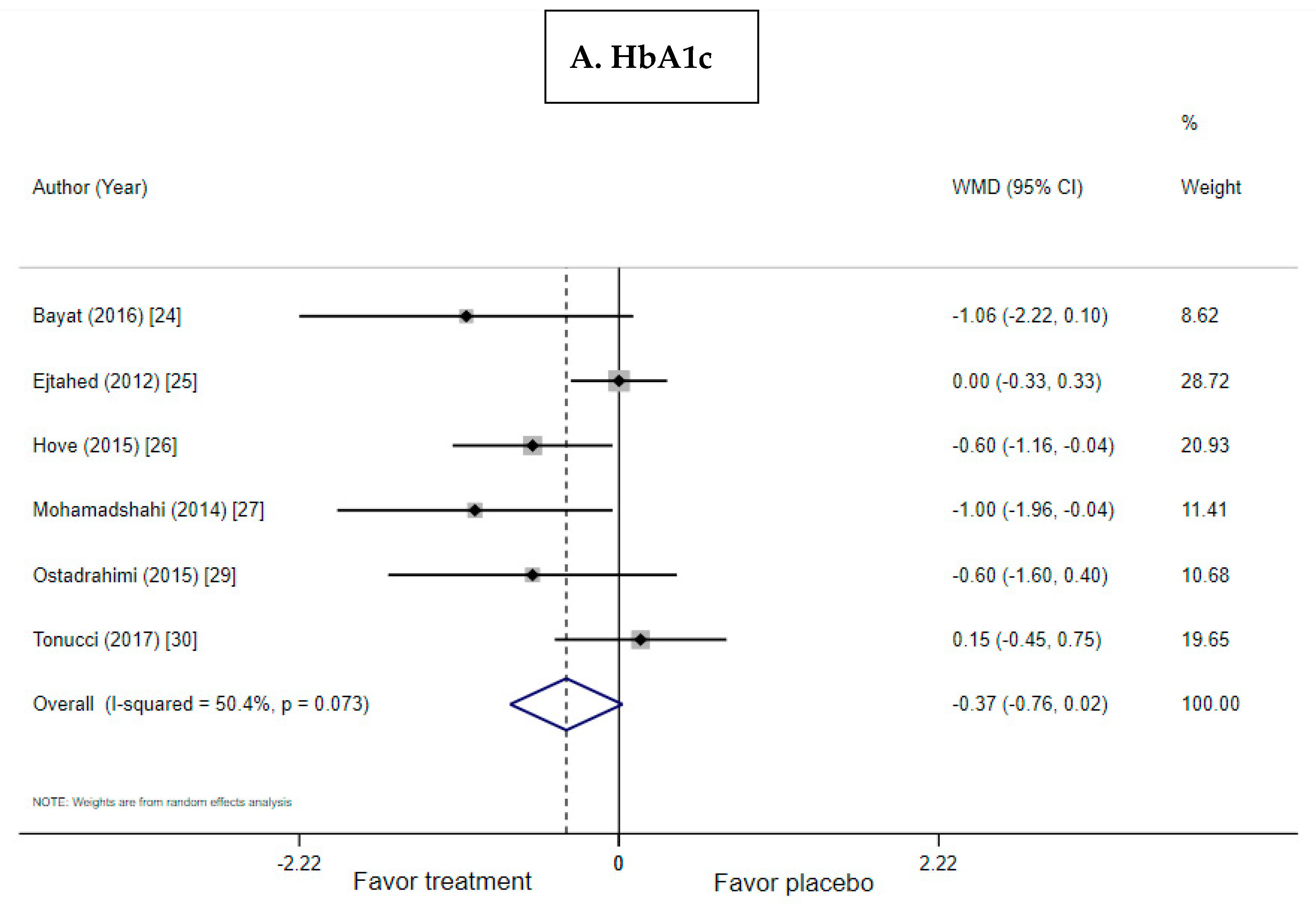
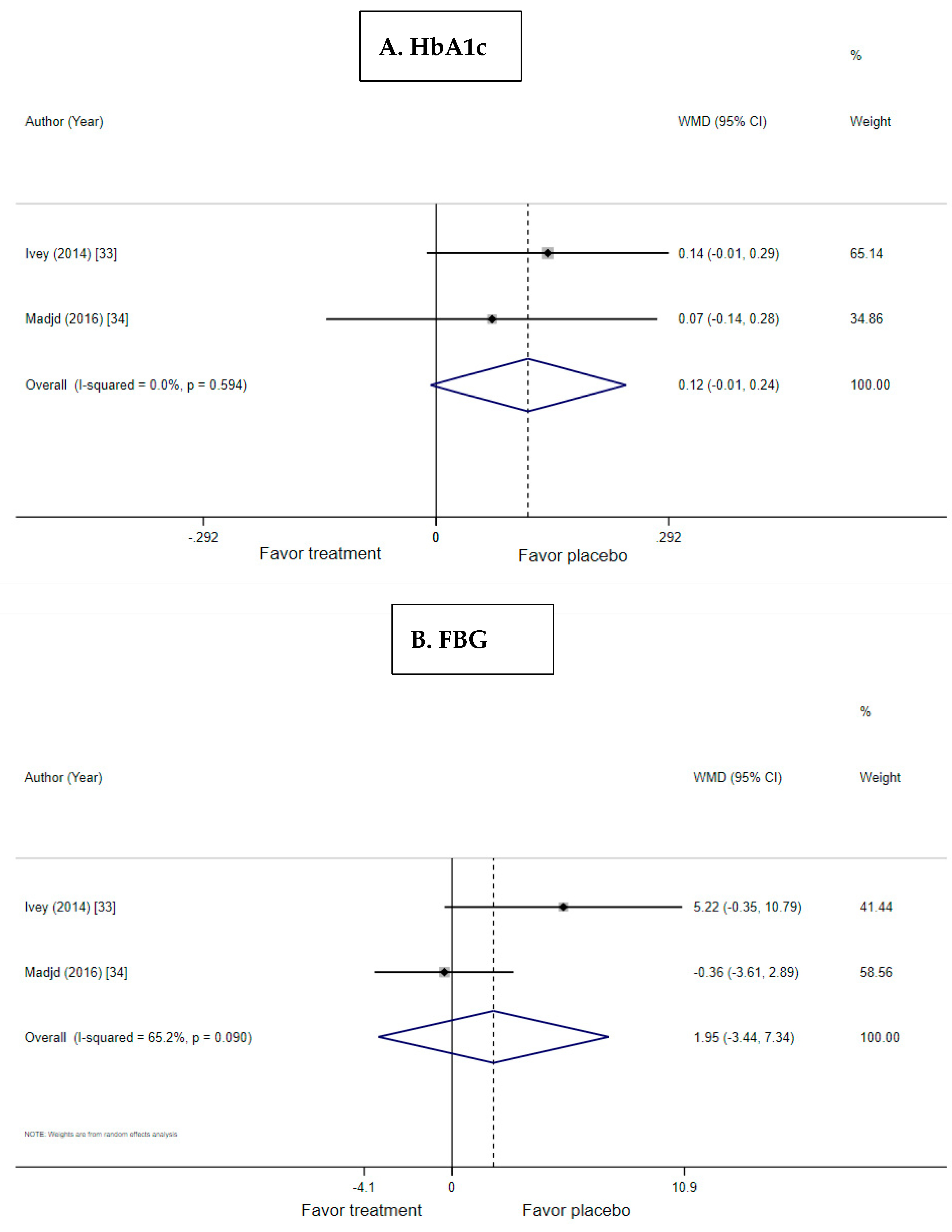
3.3. Effect of Probiotic Yogurt on HbA1c
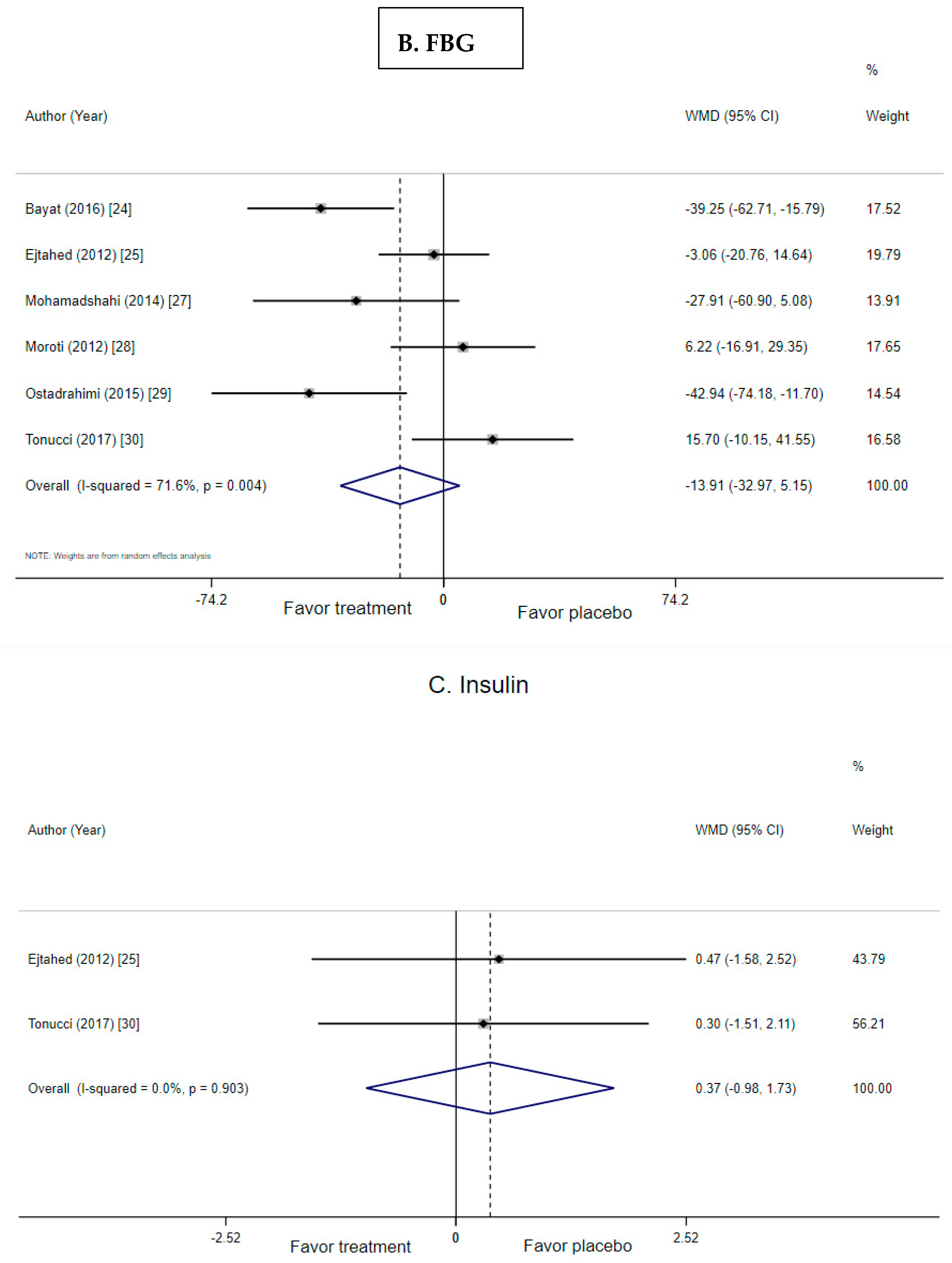
3.4. Effect of Probiotic Yogurt on Fasting Blood Glucose
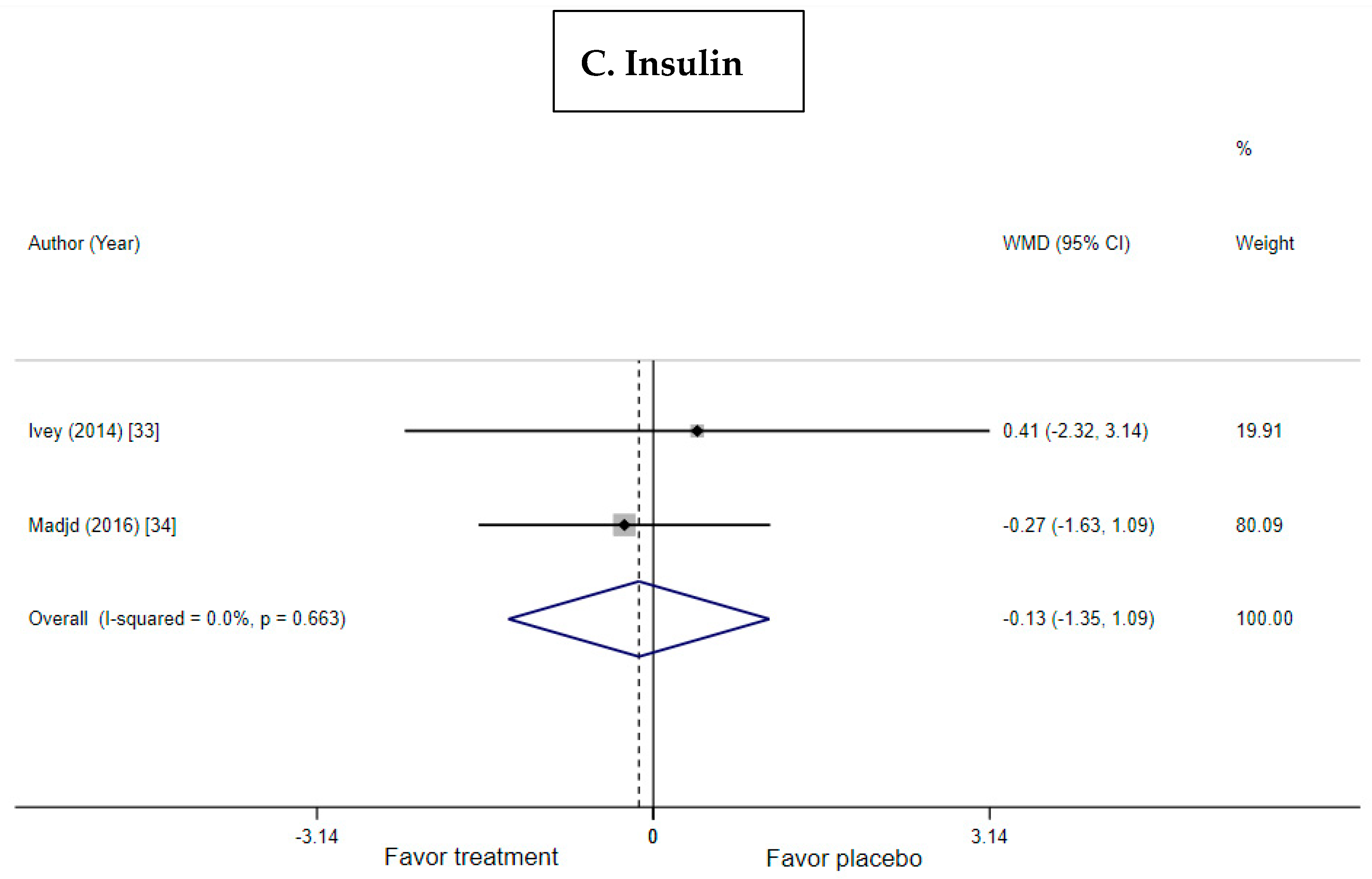
3.5. Effect of Probiotic Yogurt on Insulin and HOMA-IR
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- National Diabetes Statistics Report. U.S. Department of Health and Human Services; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017. [Google Scholar]
- Fryar, C.D.; Ogden, C.L. Prevalence of Overweight, Obesity, and Severe Obesity among Adults Aged 20 and Over: United States, 1960–1962 through 2015–2016. NCHS Health E-Stats. Available online: https://www.cdc.gov/nchs/data/hestat/obesity_adult_15_16/obesity_adult_15_16.htm (accessed on 12 March 2019).
- Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2019. Diabetes Care 2019, 42, S90–S102. [CrossRef] [PubMed]
- Obesity management for the treatment of type 2 diabetes: Standards of medical care in diabetes-2019. Diabetes Care 2019, 42, S81–S89. [CrossRef] [PubMed]
- Improving care and promoting health in populations: Standards of medical care in diabetes-2019. Diabetes Care 2019, 42, S7–S12. [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 aha/acc/tos guideline for the management of overweight and obesity in adults: A report of the american college of cardiology/american heart association task force on practice guidelines and the obesity society. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef] [PubMed]
- Lifestyle management: Standards of medical care in diabetes-2019. Diabetes Care 2019, 42, S46–S60. [CrossRef] [PubMed]
- Casazza, K.; Hanks, L.J.; Beasley, T.M.; Fernandez, J.R. Beyond thriftiness: Independent and interactive effects of genetic and dietary factors on variations in fat deposition and distribution across populations. Am. J. Phys. Anthropol. 2011, 145, 181–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ÉM, M. The Prolongation of Life: Optimistic Studies. Springer Classics in Longevity and Aging; Reprint of 1908 English Edition; Springer: New York, NY, USA, 2004. [Google Scholar]
- Barengolts, E. Gut microbiota, prebiotics, probiotics, and synbiotics in management of obesity and prediabetes: Review of randomized controlled trials. Endocr. Pract. 2016, 22, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Hu, Y.M.; Zhou, F.; Yuan, Y.; Xu, Y.C. Effects of probiotics supplement in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. Med. Clin. 2017, 148, 362–370. [Google Scholar] [CrossRef]
- Wang, X.; Juan, Q.F.; He, Y.W.; Zhuang, L.; Fang, Y.Y.; Wang, Y.H. Multiple effects of probiotics on different types of diabetes: A systematic review and meta-analysis of randomized, placebo-controlled trials. J. Pediatric Endocrinol. Metab. JPEM 2017, 30, 611–622. [Google Scholar] [CrossRef]
- Yao, K.; Zeng, L.; He, Q.; Wang, W.; Lei, J.; Zou, X. Effect of probiotics on glucose and lipid metabolism in type 2 diabetes mellitus: A meta-analysis of 12 randomized controlled trials. Med. Sci. Monit. 2017, 23, 3044–3053. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Martin, D.A.; DiMarco, D.M.; Bolling, B.W. Evidence for the effects of yogurt on gut health and obesity. Crit. Rev. Food Sci. Nutr. 2017, 57, 1569–1583. [Google Scholar] [CrossRef]
- Basic Report: 01116, Yogurt, Plain, Whole Milk United States Department of Agriculture Agricultural Research Service National Nutrient Database for Standard Reference. Available online: https://ndb.nal.usda.gov/ndb/foods/show/01116 (accessed on 12 March 2019).
- Goldbohm, R.A.; Chorus, A.M.; Galindo Garre, F.; Schouten, L.J.; van den Brandt, P.A. Dairy consumption and 10-y total and cardiovascular mortality: A prospective cohort study in the netherlands. Am. J. Clin. Nutr. 2011, 93, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Soedamah-Muthu, S.S.; Masset, G.; Verberne, L.; Geleijnse, J.M.; Brunner, E.J. Consumption of dairy products and associations with incident diabetes, chd and mortality in the whitehall ii study. Br. J. Nutr. 2013, 109, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Malekshah, A.F.; Pourshams, A.; Poustchi, H.; Sepanlou, S.G.; Sharafkhah, M.; Khoshnia, M.; Farvid, M.; Abnet, C.C.; Kamangar, F.; et al. Dairy food intake and all-cause, cardiovascular disease, and cancer mortality: The golestan cohort study. Am. J. Epidemiol. 2017, 185, 697–711. [Google Scholar] [CrossRef]
- Guo, J.; Astrup, A.; Lovegrove, J.A.; Gijsbers, L.; Givens, D.I.; Soedamah-Muthu, S.S. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: Dose-response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2017, 32, 269–287. [Google Scholar] [CrossRef]
- Tognon, G.; Nilsson, L.M.; Shungin, D.; Lissner, L.; Jansson, J.H.; Renstrom, F.; Wennberg, M.; Winkvist, A.; Johansson, I. Nonfermented milk and other dairy products: Associations with all-cause mortality. Am. J. Clin. Nutr. 2017, 105, 1502–1511. [Google Scholar] [CrossRef]
- Mazidi, M.; Mikhailidis, D.P.; Sattar, N.; Howard, G.; Graham, I.; Banach, M. Consumption of dairy product and its association with total and cause specific mortality—A population-based cohort study and meta-analysis. Clin. Nutr. (Edinb. Scotl.) 2018. [Google Scholar] [CrossRef]
- Bayat, A.; Azizi-Soleiman, F.; Heidari-Beni, M.; Feizi, A.; Iraj, B.; Ghiasvand, R.; Askari, G. Effect of cucurbita ficifolia and probiotic yogurt consumption on blood glucose, lipid profile, and inflammatory marker in type 2 diabetes. Int. J. Prev. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Hove, K.D.; Brøns, C.; Færch, K.; Lund, S.S.; Rossing, P.; Vaag, A. Effects of 12 weeks of treatment with fermented milk on blood pressure, glucose metabolism and markers of cardiovascular risk in patients with type 2 diabetes: A randomised double-blind placebo-controlled study. Eur. J. Endocrinol. 2015, 172, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Mohamadshahi, M.; Veissi, M.; Haidari, F.; Shahbazian, H.; Kaydani, G.A.; Mohammadi, F. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. BioImpacts 2014, 4, 83–88. [Google Scholar]
- Moroti, C.; Souza Magri, L.F.; de Rezende Costa, M.; Cavallini, D.C.; Sivieri, K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012, 11, 29. [Google Scholar] [CrossRef]
- Ostadrahimi, A.; Taghizadeh, A.; Mobasseri, M.; Farrin, N.; Payahoo, L.; Beyramalipoor Gheshlaghi, Z.; Vahedjabbari, M. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Iran J. Public Health 2015, 44, 228–237. [Google Scholar] [PubMed]
- Tonucci, L.B.; Olbrich Dos Santos, K.M.; Licursi de Oliveira, L.; Rocha Ribeiro, S.M.; Duarte Martino, H.S. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. (Edinb. Scotl.) 2017, 36, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. BMJ (Clin. Res. Ed.) 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. Ed.) 2011, 343, d5928. [Google Scholar] [CrossRef]
- Ivey, K.L.; Hodgson, J.M.; Kerr, D.A.; Lewis, J.R.; Thompson, P.L.; Prince, R.L. The effects of probiotic bacteria on glycaemic control in overweight men and women: A randomised controlled trial. Eur. J. Clin. Nutr. 2014, 68, 447–452. [Google Scholar] [CrossRef]
- Madjd, A.; Taylor, M.A.; Mousavi, N.; Delavari, A.; Malekzadeh, R.; Macdonald, I.A.; Farshchi, H.R. Comparison of the effect of daily consumption of probiotic compared with low-fat conventional yogurt on weight loss in healthy obese women following an energy-restricted diet: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 323–329. [Google Scholar] [CrossRef]
- Kasinska, M.A.; Drzewoski, J. Effectiveness of probiotics in type 2 diabetes: A meta-analysis. Pol. Arch. Med. Wewn. 2015, 125, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Buys, N.J. Glucose- and glycaemic factor-lowering effects of probiotics on diabetes: A meta-analysis of randomised placebo-controlled trials. Br. J. Nutr. 2016, 115, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, Y.; Fei, X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina (Kaunas Lith.) 2016, 52, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, A.S.; Larsen, N.; Pedersen-Skovsgaard, T.; Berg, R.M.; Moller, K.; Svendsen, K.D.; Jakobsen, M.; Pedersen, B.K. Effects of lactobacillus acidophilus ncfm on insulin sensitivity and the systemic inflammatory response in human subjects. Br. J. Nutr. 2010, 104, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Mazloom, Z.; Yousefinejad, A.; Dabbaghmanesh, M.H. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: A clinical trial. Iran. J. Med. Sci. 2013, 38, 38–43. [Google Scholar] [PubMed]
- Mahboobi, S.; Iraj, B.; Maghsoudi, Z.; Feizi, A.; Ghiasvand, R.; Askari, G.; Maayeshi, N. The effects of probiotic supplementation on markers of blood lipids, and blood pressure in patients with prediabetes: A randomized clinical trial. Int. J. Prev. Med. 2014, 5, 1239–1246. [Google Scholar]
- Asemi, Z.; Khorrami-Rad, A.; Alizadeh, S.A.; Shakeri, H.; Esmaillzadeh, A. Effects of synbiotic food consumption on metabolic status of diabetic patients: A double-blind randomized cross-over controlled clinical trial. Clin. Nutr. (Edinb. Scotl.) 2014, 33, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Tajabadi-Ebrahimi, M.; Sharifi, N.; Farrokhian, A.; Raygan, F.; Karamali, F.; Razzaghi, R.; Taheri, S.; Asemi, Z. A randomized controlled clinical trial investigating the effect of synbiotic administration on markers of insulin metabolism and lipid profiles in overweight type 2 diabetic patients with coronary heart disease. Exp. Clin. Endocrinol. Diabetes 2017, 125, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Mobini, R.; Tremaroli, V.; Stahlman, M.; Karlsson, F.; Levin, M.; Ljungberg, M.; Sohlin, M.; Berteus Forslund, H.; Perkins, R.; Backhed, F.; et al. Metabolic effects of lactobacillus reuteri dsm 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 2017, 19, 579–589. [Google Scholar] [CrossRef]
- Firouzi, S.; Majid, H.A.; Ismail, A.; Kamaruddin, N.A.; Barakatun-Nisak, M.Y. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: A randomized controlled trial. Eur. J. Nutr. 2017, 56, 1535–1550. [Google Scholar] [CrossRef]
- Shakeri, H.; Hadaegh, H.; Abedi, F.; Tajabadi-Ebrahimi, M.; Mazroii, N.; Ghandi, Y.; Asemi, Z. Consumption of synbiotic bread decreases triacylglycerol and vldl levels while increasing hdl levels in serum from patients with type-2 diabetes. Lipids 2014, 49, 695–701. [Google Scholar] [CrossRef]
- Electronic Code of Federal Regulations (e-CFR). Title 21, sec. 131.200 Yogurt. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=c5dc0f4a8e02731cc415cc69fd722812& mc=true&tpl=/ecfrbrowse/Title21/ 21cfrv2_02.tpl#0 (accessed on 12 March 2019).
- O’Connor, L.M.; Lentjes, M.A.; Luben, R.N.; Khaw, K.T.; Wareham, N.J.; Forouhi, N.G. Dietary dairy product intake and incident type 2 diabetes: A prospective study using dietary data from a 7-day food diary. Diabetologia 2014, 57, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Sun, Q.; Giovannucci, E.; Mozaffarian, D.; Manson, J.E.; Willett, W.C.; Hu, F.B. Dairy consumption and risk of type 2 diabetes: 3 cohorts of us adults and an updated meta-analysis. BMC Med. 2014, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Lopez, A.; Bullo, M.; Martinez-Gonzalez, M.A.; Corella, D.; Estruch, R.; Fito, M.; Gomez-Gracia, E.; Fiol, M.; Garcia de la Corte, F.J.; Ros, E.; et al. Dairy product consumption and risk of type 2 diabetes in an elderly spanish mediterranean population at high cardiovascular risk. Eur. J. Nutr. 2016, 55, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Eussen, S.J.; van Dongen, M.C.; Wijckmans, N.; den Biggelaar, L.; Oude Elferink, S.J.; Singh-Povel, C.M.; Schram, M.T.; Sep, S.J.; van der Kallen, C.J.; Koster, A.; et al. Consumption of dairy foods in relation to impaired glucose metabolism and type 2 diabetes mellitus: The maastricht study. Br. J. Nutr. 2016, 115, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferre, M.; Becerra-Tomas, N.; Ruiz-Canela, M.; Corella, D.; Schroder, H.; Estruch, R.; Ros, E.; Aros, F.; Gomez-Gracia, E.; Fiol, M.; et al. Total and subtypes of dietary fat intake and risk of type 2 diabetes mellitus in the prevencion con dieta mediterranea (predimed) study. Am. J. Clin. Nutr. 2017, 105, 723–735. [Google Scholar] [CrossRef]
- Panahi, S.; Doyon, C.Y.; Despres, J.P.; Perusse, L.; Vohl, M.C.; Drapeau, V.; Tremblay, A. Yogurt consumption, body composition, and metabolic health in the quebec family study. Eur. J. Nutr. 2018, 57, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Ning, N.; Wang, C.; Wang, Y.; Li, Q.; Meng, Z.; Liu, Y.; Li, Q. Dairy products consumption and risk of type 2 diabetes: Systematic review and dose-response meta-analysis. PLoS ONE 2013, 8, e73965. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Dairy products and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Am. J. Clin. Nutr. 2013, 98, 1066–1083. [Google Scholar] [CrossRef]
- Gijsbers, L.; Ding, E.L.; Malik, V.S.; de Goede, J.; Geleijnse, J.M.; Soedamah-Muthu, S.S. Consumption of dairy foods and diabetes incidence: A dose-response meta-analysis of observational studies. Am. J. Clin. Nutr. 2016, 103, 1111–1124. [Google Scholar] [CrossRef]
- Bourrie, B.C.; Willing, B.P.; Cotter, P.D. The microbiota and health promoting characteristics of the fermented beverage kefir. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.L.; Hendrich, S. Glycemic index, insulinemic index, and satiety index of kefir. J. Am. Coll. Nutr. 2012, 31, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Fathi, Y.; Faghih, S.; Zibaeenezhad, M.J.; Tabatabaei, S.H. Kefir drink leads to a similar weight loss, compared with milk, in a dairy-rich non-energy-restricted diet in overweight or obese premenopausal women: A randomized controlled trial. Eur. J. Nutr. 2016, 55, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Fathi, Y.; Ghodrati, N.; Zibaeenezhad, M.J.; Faghih, S. Kefir drink causes a significant yet similar improvement in serum lipid profile, compared with low-fat milk, in a dairy-rich diet in overweight or obese premenopausal women: A randomized controlled trial. J. Clin. Lipidol. 2017, 11, 136–146. [Google Scholar] [CrossRef]
- Yadav, H.; Jain, S.; Sinha, P.R. Oral administration of dahi containing probiotic lactobacillus acidophilus and lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J. Dairy Res. 2008, 75, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Jung, S.R.; Lee, S.Y.; Lee, N.K.; Paik, H.D.; Lim, S.I. Lactobacillus plantarum strain ln4 attenuates diet-induced obesity, insulin resistance, and changes in hepatic mrna levels associated with glucose and lipid metabolism. Nutrients 2018, 10, 643. [Google Scholar] [CrossRef]
- Gillespie, A.L.; Calderwood, D.; Hobson, L.; Green, B.D. Whey proteins have beneficial effects on intestinal enteroendocrine cells stimulating cell growth and increasing the production and secretion of incretin hormones. Food Chem. 2015, 189, 120–128. [Google Scholar] [CrossRef]
- Sari, E.K.; Bakir, B.; Aydin, B.D.; Sozmen, M. The effects of kefir, koumiss, yogurt and commercial probiotic formulations on pparalpha and ppar-beta/delta expressions in mouse kidney. Biotech. Histochem. 2014, 89, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Turek, K.; Domagala, J.; Wszolek, M. Fatty acid profile and oxidation tests of fat extracted from yogurt using rose hip seed oil. Acta Sci. Pol. Technol. Aliment. 2018, 17, 51–58. [Google Scholar] [Green Version]
- Pei, R.; DiMarco, D.M.; Putt, K.K.; Martin, D.A.; Chitchumroonchokchai, C.; Bruno, R.S.; Bolling, B.W. Premeal low-fat yogurt consumption reduces postprandial inflammation and markers of endotoxin exposure in healthy premenopausal women in a randomized controlled trial. J. Nutr. 2018, 148, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Chung, W.C.; Chun, S.H.; Han, J.H.; Song, M.J.; Lee, K.W. Enhancing the natural killer cell activity and anti-influenza effect of heat-treated lactobacillus plantarum nf1-fortified yogurt in mice. J. Dairy Sci. 2018, 101, 10675–10684. [Google Scholar] [CrossRef]
- Hursel, R.; van der Zee, L.; Westerterp-Plantenga, M.S. Effects of a breakfast yoghurt, with additional total whey protein or caseinomacropeptide-depleted alpha-lactalbumin-enriched whey protein, on diet-induced thermogenesis and appetite suppression. Br. J. Nutr. 2010, 103, 775–780. [Google Scholar] [CrossRef]
- Rosa, D.D.; Dias, M.M.S.; Grzeskowiak, L.M.; Reis, S.A.; Conceicao, L.L.; Peluzio, M. Milk kefir: Nutritional, microbiological and health benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef]
- Zubiria, M.G.; Gambaro, S.E.; Rey, M.A.; Carasi, P.; Serradell, M.L.A.; Giovambattista, A. Deleterious metabolic effects of high fructose intake: The preventive effect of lactobacillus kefiri administration. Nutrients 2017, 9, E470. [Google Scholar] [CrossRef]
- Teruya, K.; Yamashita, M.; Tominaga, R.; Nagira, T.; Shim, S.Y.; Katakura, Y.; Tokumaru, S.; Tokumaru, K.; Barnes, D.; Shirahata, S. Fermented milk, kefram-kefir enhances glucose uptake into insulin-responsive muscle cells. Cytotechnology 2002, 40, 107–116. [Google Scholar] [CrossRef]
- Punaro, G.R.; Maciel, F.R.; Rodrigues, A.M.; Rogero, M.M.; Bogsan, C.S.; Oliveira, M.N.; Ihara, S.S.; Araujo, S.R.; Sanches, T.R.; Andrade, L.C.; et al. Kefir administration reduced progression of renal injury in stz-diabetic rats by lowering oxidative stress. Nitric Oxide 2014, 37, 53–60. [Google Scholar] [CrossRef]
- Mert, H.; Yilmaz, H.; Irak, K.; Yildirim, S.; Mert, N. Investigation of the protective effect of kefir against isoproterenol induced myocardial infarction in rats. Korean J. Food Sci. Anim. Resour. 2018, 38, 259–272. [Google Scholar]
- Hsu, Y.J.; Huang, W.C.; Lin, J.S.; Chen, Y.M.; Ho, S.T.; Huang, C.C.; Tung, Y.T. Kefir supplementation modifies gut microbiota composition, reduces physical fatigue, and improves exercise performance in mice. Nutrients 2018, 10, E862. [Google Scholar] [CrossRef]
- Gangoiti, M.V.; Puertas, A.I.; Hamet, M.F.; Peruzzo, P.J.; Llamas, M.G.; Medrano, M.; Prieto, A.; Duenas, M.T.; Abraham, A.G. Lactobacillus plantarum cidca 8327: An alpha-glucan producing-strain isolated from kefir grains. Carbohydr. Polym. 2017, 170, 52–59. [Google Scholar] [CrossRef]
- Fels, L.; Jakob, F.; Vogel, R.F.; Wefers, D. Structural characterization of the exopolysaccharides from water kefir. Carbohydr. Polym. 2018, 189, 296–303. [Google Scholar] [CrossRef]
- Cho, E.J.; Hwang, H.J.; Kim, S.W.; Oh, J.Y.; Baek, Y.M.; Choi, J.W.; Bae, S.H.; Yun, J.W. Hypoglycemic effects of exopolysaccharides produced by mycelial cultures of two different mushrooms tremella fuciformis and phellinus baumii in ob/ob mice. Appl. Microbiol. Biotechnol. 2007, 75, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Saxelin, M. Probiotic formulations and applications, the current probiotics market, and changes in the marketplace: A european perspective. Clin. Infect. Dis. 2008, 46 (Suppl. 2), S76–S79, discussion S144–S151. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Kort, R.; Alvarez, S.; Bourdet-Sicard, R.; Benoit, V.; Cunningham, M.; Saulnier, D.M.; van Hylckama Vlieg, J.E.T.; Verstraelen, H.; Sybesma, W. Expanding the reach of probiotics through social enterprises. Benef. Microbes 2018, 9, 707–715. [Google Scholar] [CrossRef] [PubMed]
| Author Country Year | Design Duration | Subject Number T/C, Male/Female | Age BMI T/C | Treatment, Dose | Control, Dose | Probiotic Strains and Strength (CFU/mL) | Glycemic Out-Comes |
|---|---|---|---|---|---|---|---|
| T2D | |||||||
| Bayat, Iran, 2016 [24] | Open-label, RP 8 wks | 20/20 12/28 | 54/47 29/30 | PY 150 g/d | Dietary advice | Cultures not provided | A1c, FBG |
| Ejtahed, Iran, 2012 [25] | DB, PC, RP 6 wks | 30/30 23/37 | 51/51 29/29 | PY 300 g/d | CY 300 g/d | L. acidophilus La5 and B. lactis Bb12 (1.8 × 106 both) | A1c, FBG, insulin |
| Hove, Denmark, 2015 [26] | DB, PC, RP 12 wks | 23/18 41/0 | 59/61 29/28 | PY (low fat) (Cardi04® yogurt) 300 mL/d | Milk (low fat) (Artificially acidified milk) 300 mL/d | L. helveticus (Cardi04) (CFU not provided) | A1c, FBG, insulin, HOMA-IR, C-peptide, proinsulin |
| Mohamadshahi, Iran, 2014 [27] | DB, PC, RP 8 wks | 21/21 30/32 | 53/49 28/29 | PY 300 g/day | CY 300 g/day | L. acidophilus La-5 and B. lactis BB-12 (106 both) | A1c, FBG |
| Moroti, Brazil, 2012 [28] | DB, PC, RP 30 days | 9/9 0/18 | 56/57 28/28 | PY (Symbiotic shake) 100 mL BID | CY (shake without active ingredients) 100 mL BID | L. acidophilus, B. bifidum (4 × 108 both) and 1 g oligofructose | FBG |
| Ostadrahimi, Iran, 2015 [29] | DB, PC, RP 8 wks | 30/30 34/26 | 35-65 29/27 | PY (kefir, low fat) 600 mL BID | CY (dough, low fat) 600 mL BID | L. casei, L. acidophilus, B. lactis (106 both) | A1c, FBG |
| Tonucci, Brazil, 2017 [30] | DB, PC RP 6 wks | 23/22 26/19 | 52/51 28/28 | PY (goat milk) 120 g/d | Milk (goat milk) 120 g/d | L. acidophilus La-5 (6 × 107), B. lactis BB-12 (2 × 107) | A1c, FBG, insulin, HOMA-IR, fructosamine |
| OW/OB | |||||||
| Ivey, Australia, 2014 [33] a | DB, PC, RP 6 wks | 37/40 48/29 | 68/65 30/31 | PY 200 mL/d | Milk 200 mL/d | L. acidophilus La-5, B. lactis Bb-12 (3 × 109) | A1c, FBG, insulin, HOMA-IR |
| Madjd, Iran, 2016 [34] | SB, PC, RP 12 wks | 44/45 0/89 | 32/32 32/32 | PY (low fat) 200 g BID | CY (low fat) 200 g BID | L. acidophilus LA-5, B. lactis BB12 (107 both) | A1c, FBG, 2-h PG, insulin, HOMA-IR |
| Glycemic Outcome | Baseline Data | Final Data | p-Value a | ||
|---|---|---|---|---|---|
| Control | Treatment | Control | Treatment | ||
| HbA1c, % | |||||
| T2D subjects | |||||
| Bayat | 7.54 [2.03] | 7.06 [1.58] | 7.55 [1.87] | 6.49 [1.33] | <0.01 c |
| Ejtahed | 6.87 [0.81] | 7.29 [1.21] | 7.17 [0.66] | 7.17 [1.24] | <0.05 d |
| Hove | 7.3 [0.6] | 6.8 [0.5] | 7.7 [0.9] | 7.1 [0.6] | NS |
| Mohamadshahi | 8.33 [1.46] | 8.24 [1.68] | 8.09 [1.58] | 7.09 [1.23] | <0.05 c,d |
| Ostadrahimi | 6.98 [1.63] | 7.61 [1.22] | 7.0 [1.98] | 6.40 [1.91] | 0.02 d |
| Tonucci | 5.82 [1.04] | 6.38 [1.04] e | 5.94 [1.02] | 6.09 [1.17] | 0.02 b |
| Obese subjects | |||||
| Ivey | 5.56 [0.36] | 5.86 [0.65] | 5.60 [0.34] | 5.74 [3.19] | NS |
| Madjd | 5.01 [0.57] | 5.11 [0.48] | 4.74 [0.50] | 4.81 [0.47] | NS |
| FBG, mg/dL | |||||
| T2D subjects | |||||
| Bayat | 145.2 [41.9] | 148.95 [47.26] | 165.5 [41.34] | 126.25 [34.01] | <0.01 c |
| Ejtahed | 132.3 [23.04] | 145.08 [44.82] | 135.54 [23.76] | 132.48 [43.38] | <0.05 d |
| Mohamadshahi | 187.42 [55.13] | 175.24 [46.63 | 185.19 [63.60] | 157.28 [43.63] | NS |
| Moroti | 136.78 [19.47] | 191.11 [18.31] | 110.56 [29.90] | 116.78 [18.96] | <0.05 b |
| Ostadrahimi | 183.42 [74.76] | 161.63 [57.71] | 182.16 [73.78] | 139.22 [182.16] | 0.02 d |
| Tonucci | 132.8 [42.7] | 142.0 [41.0] | 135.7 [45.0] | 151.4 [43.4] | NS |
| Obese subjects | |||||
| Ivey | 96.48 [9.9] | 101.52 [18.18] | 93.24 [11.7] | 98.46 [13.14] | NS |
| Madjd | 90.72 [8.28] | 91.44 [8.64] | 86.40 [7.74] | 86.04 [7.92] | 0.059 d |
| Insulin, μU/mL | |||||
| T2D subjects | |||||
| Ejtahed | 6.31 [3.72] | 7.47 [4.89] | 6.50 [3.57] | 6.97 [4.49] | NS |
| Tonucci | 7.90 [2.51] | 8.53 [3.74] | 7.84 [2.99] | 8.14 [3.21] | NS |
| Obese subjects | |||||
| Ivey | 9.99 [4.49] | 9.63 [4.82] | 10.18 [5.36] | 10.59 [6.74] | NS |
| Madjd | 12.69 [3.63] | 12.85 [3.98] | 11.36 [3.25] | 11.09 [3.31] | <0.01 d |
| HOMA-IR | |||||
| T2D subjects | |||||
| Tonucci | 2.67 [1.17] | 3.01 [1.41] | 2.80 [1.38] | 3.04 [1.31] | NS |
| Obese subjects | |||||
| Madjd | 2.86 [0.92] | 2.93 [1.03] | 2.43 [0.77] | 2.38 [0.80] | <0.01 d |
| Ivey | 2.44 [1.29] | 2.48 [1.45] | 2.38 [1.39] | 2.63 [1.91] | NS |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barengolts, E.; Smith, E.D.; Reutrakul, S.; Tonucci, L.; Anothaisintawee, T. The Effect of Probiotic Yogurt on Glycemic Control in Type 2 Diabetes or Obesity: A Meta-Analysis of Nine Randomized Controlled Trials. Nutrients 2019, 11, 671. https://doi.org/10.3390/nu11030671
Barengolts E, Smith ED, Reutrakul S, Tonucci L, Anothaisintawee T. The Effect of Probiotic Yogurt on Glycemic Control in Type 2 Diabetes or Obesity: A Meta-Analysis of Nine Randomized Controlled Trials. Nutrients. 2019; 11(3):671. https://doi.org/10.3390/nu11030671
Chicago/Turabian StyleBarengolts, Elena, Emily Daviau Smith, Sirimon Reutrakul, Livia Tonucci, and Thunyarat Anothaisintawee. 2019. "The Effect of Probiotic Yogurt on Glycemic Control in Type 2 Diabetes or Obesity: A Meta-Analysis of Nine Randomized Controlled Trials" Nutrients 11, no. 3: 671. https://doi.org/10.3390/nu11030671
APA StyleBarengolts, E., Smith, E. D., Reutrakul, S., Tonucci, L., & Anothaisintawee, T. (2019). The Effect of Probiotic Yogurt on Glycemic Control in Type 2 Diabetes or Obesity: A Meta-Analysis of Nine Randomized Controlled Trials. Nutrients, 11(3), 671. https://doi.org/10.3390/nu11030671





