Bioactive Multilayer Polylactide Films with Controlled Release Capacity of Gallic Acid Accomplished by Incorporating Electrospun Nanostructured Coatings and Interlayers
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Extrusion
2.3. Solution Preparation
2.4. Solution Characterization
2.5. Electrospinning
2.6. Multilayer Preparation
2.7. Material Characterization
2.7.1. Morphology
2.7.2. Thermal Analysis
2.7.3. Release Measurements
2.7.4. Antioxidant Activity
2.7.5. Statistical Analysis
3. Results
3.1. Solution Properties
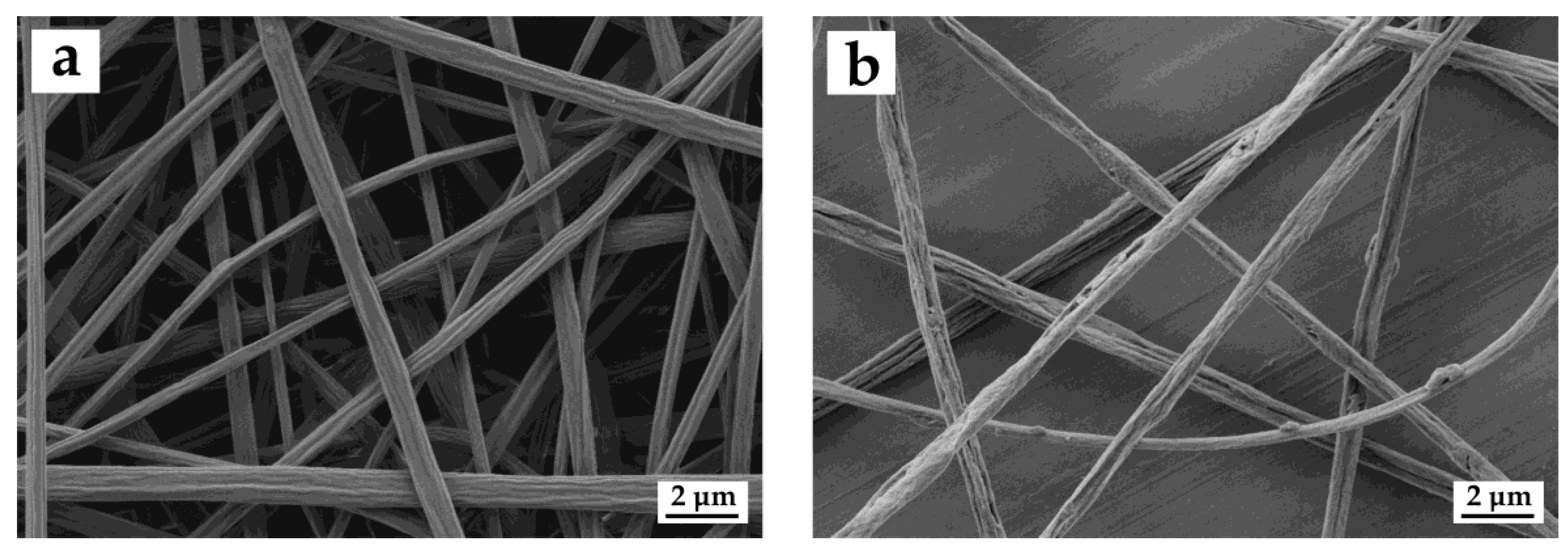
3.2. Morphology
3.3. Film Transparency
3.4. Thermal Stability
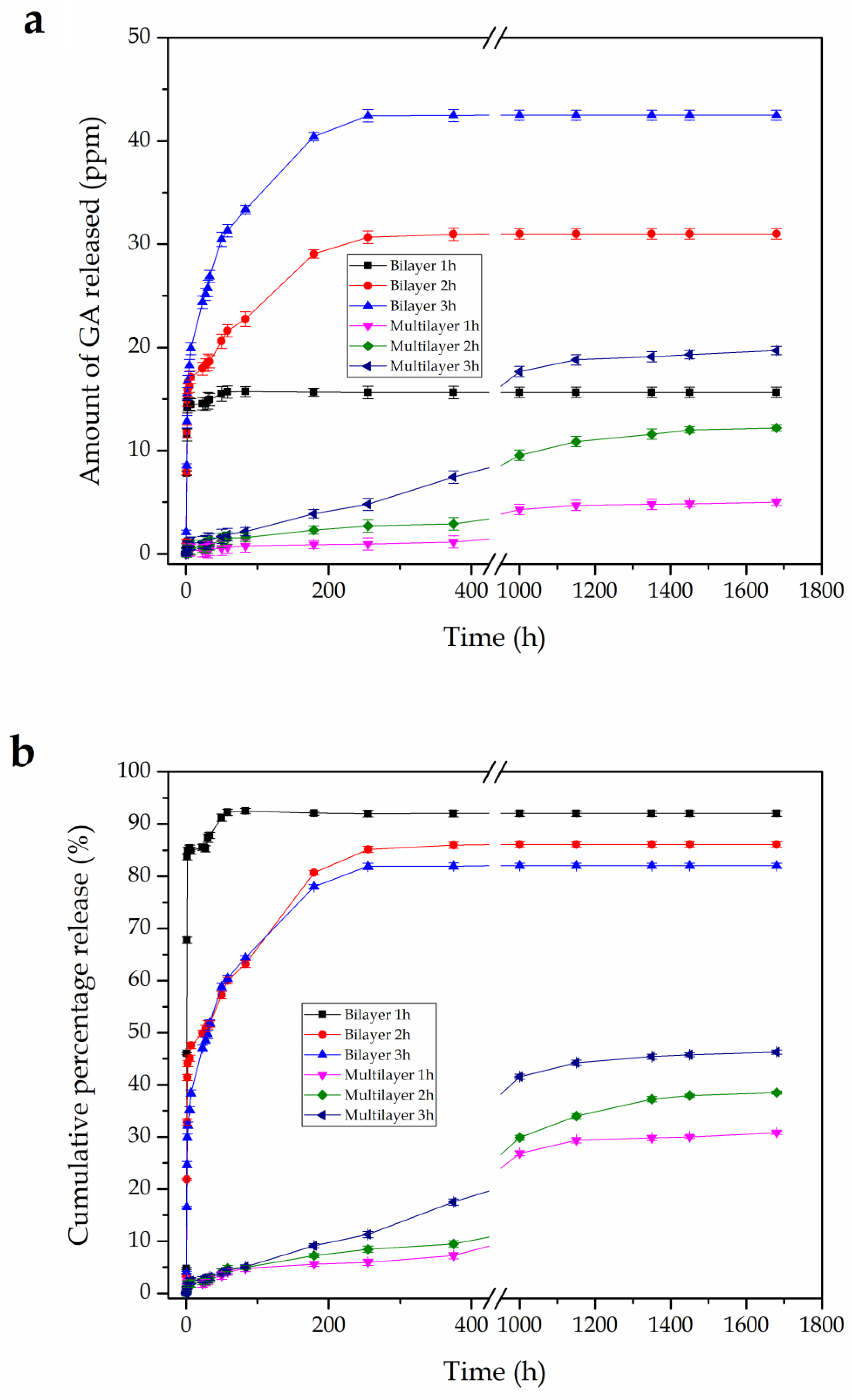
3.5. In vitro Release Studies
3.6. Antioxidant Activity
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Hasler, C.M. Functional foods: Benefits, concerns and challenges—A position paper from the american council on science and health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef] [PubMed]
- Fogliano, V.; Vitaglione, P. Functional foods: Planning and development. Mol. Nutr. Food Res. 2005, 49, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rubio, A.; Gavara, R.; Lagaron, J.M. Bioactive packaging: Turning foods into healthier foods through biomaterials. Trends Food Sci. Technol. 2006, 17, 567–575. [Google Scholar] [CrossRef]
- Mellinas, C.; Valdés, A.; Ramos, M.; Burgos, N.; Del Carmen Garrigós, M.; Jiménez, A. Active edible films: Current state and future trends. J. Appl. Polym. Sci. 2016, 133, 42631. [Google Scholar] [CrossRef]
- Lopez-Rubio, A. Bioactive food packaging strategies. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Lagaron, J.M., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2011; pp. 460–482. [Google Scholar]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly (lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, L.; Cascone, M.G.; Quiriconi, S.; Morabito, L.; Giusti, P. Biodegradable hollow microfibres to produce bioactive scaffolds. Polym. Int. 2005, 54, 101–107. [Google Scholar] [CrossRef]
- Ma, J.; Luo, X.D.; Protiva, P.; Yang, H.; Ma, C.; Basile, M.J.; Weinstein, I.B.; Kennelly, E.J. Bioactive novel polyphenols from the fruit of manilkara zapota (sapodilla). J. Nat. Prod. 2003, 66, 983–986. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Sitthimonchai, S.; Worasuttayangkurn, L.; Mahidol, C.; Ruchirawat, M.; Satayavivad, J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem. Toxicol. 2007, 45, 328–336. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, N.J.; Lee, B.K.; Lee, K.W.; Lee, H.J. Gallic acid, a metabolite of the antioxidant propyl gallate, inhibits gap junctional intercellular communication via phosphorylation of connexin 43 and extracellular-signal-regulated kinase1/2 in rat liver epithelial cells. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2008, 638, 175–183. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, C.G.; Borges, C.D.; Zambiazi, R.C.; Nunes, M.R.; Benvenutti, E.V.; da Luz, S.R.; D’Avila, R.F.; Rutz, J.K. Microencapsulation of gallic acid in chitosan, β-cyclodextrin and xanthan. Ind. Crops Prod. 2013, 46, 138–146. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ray, S.; Jin, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Liu, D.; Quek, S.Y. Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein–gallic acid system. Food Chem. 2013, 136, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G.H.; Jin Park, H. Recent developments in microencapsulation of food ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Pérez-Masiá, R.; Lagaron, J.M. A review on electrospun polymer nanostructures as advanced bioactive platforms. Polym. Eng. Sci. 2016, 56, 500–527. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Busolo, M.A.; Torres-Giner, S.; Prieto, C.; Lagaron, J.M. Electrospraying assisted by pressurized gas as an innovative high-throughput process for the microencapsulation and stabilization of docosahexaenoic acid-enriched fish oil in zein prolamine. Innov. Food Sci. Emerg. Technol. 2018. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Wilkanowicz, S.; Melendez-Rodriguez, B.; Lagaron, J.M. Nanoencapsulation of aloe vera in synthetic and naturally occurring polymers by electrohydrodynamic processing of interest in food technology and bioactive packaging. J. Agric. Food Chem. 2017, 65, 4439–4448. [Google Scholar] [CrossRef]
- Horuz, T.İ.; Belibağlı, K.B. Nanoencapsulation of carotenoids extracted from tomato peels into zein fibers by electrospinning. J. Sci. Food Agric. 2019, 99, 759–766. [Google Scholar] [CrossRef]
- Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. Use of the electrohydrodynamic process to develop active/bioactive bilayer films for food packaging applications. Food Hydrocoll. 2016, 55, 11–18. [Google Scholar] [CrossRef]
- Torres-Giner, S. Electrospun nanofibers for food packaging applications. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Lagaron, J.M., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2011; pp. 108–125. [Google Scholar]
- Torres-Giner, S.; Busolo, M.; Cherpinski, A.; Lagaron, J.M. Electrospinning in the packaging industry. In Electrospinning: From Basic Research to Commercialization; Kny, E., Ghosal, K., Thomas, S., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2018; pp. 238–260. [Google Scholar]
- Torres-Giner, S.; Martinez-Abad, A.; Lagaron, J.M. Zein-based ultrathin fibers containing ceramic nanofillers obtained by electrospinning. II. Mechanical properties, gas barrier, and sustained release capacity of biocide thymol in multilayer polylactide films. J. Appl. Polym. Sci. 2014, 131, 9270–9276. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Nahvi, Z.; Zandi, M. Antioxidant peptide-loaded electrospun chitosan/poly(vinyl alcohol) nanofibrous mat intended for food biopackaging purposes. Food Hydrocoll. 2019, 89, 637–648. [Google Scholar] [CrossRef]
- Alehosseini, A.; Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; López-Rubio, A. Electrospun curcumin-loaded protein nanofiber mats as active/bioactive coatings for food packaging applications. Food Hydrocoll. 2019, 87, 758–771. [Google Scholar] [CrossRef]
- Aydogdu, A.; Sumnu, G.; Sahin, S. Fabrication of gallic acid loaded hydroxypropyl methylcellulose nanofibers by electrospinning technique as active packaging material. Carbohydr. Polym. 2019, 208, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Cherpinski, A.; Torres-Giner, S.; Cabedo, L.; Lagaron, J.M. Post-processing optimization of electrospun submicron poly(3-hydroxybutyrate) fibers to obtain continuous films of interest in food packaging applications. Food Addit. Contam. Part A 2017, 34, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Rodriguez, B.; Castro-Mayorga, J.L.; Reis, M.A.M.; Sammon, C.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Preparation and characterization of electrospun food biopackaging films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) derived from fruit pulp biowaste. Front. Sustain. Food Syst. 2018, 2, 38. [Google Scholar] [CrossRef]
- Cherpinski, A.; Kossel, C.; Torres-Giner, S.; Lagaron, J.M. Electrospun Hydrophilic/Hydrophobic Double Coating for Property Enhanced Fiber Based Biopackaging Applications; TAPPI Press: London, UK, 2018; pp. 145–150. [Google Scholar]
- Torres-Giner, S.; Montanes, N.; Fenollar, O.; García-Sanoguera, D.; Balart, R. Development and optimization of renewable vinyl plastisol/wood flour composites exposed to ultraviolet radiation. Mater. Des. 2016, 108, 648–658. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Chimnoi, N.; Techasakul, S.; Supaphol, P. Gallic acid-loaded electrospun poly (l-lactic acid) fiber mats and their release characteristic. Macromol. Chem. Phys. 2009, 210, 814–822. [Google Scholar] [CrossRef]
- Casasola, R.; Thomas, N.L.; Trybala, A.; Georgiadou, S. Electrospun poly lactic acid (pla) fibres: Effect of different solvent systems on fibre morphology and diameter. Polymer 2014, 55, 4728–4737. [Google Scholar] [CrossRef]
- Aytac, Z.; Kusku, S.I.; Durgun, E.; Uyar, T. Encapsulation of gallic acid/cyclodextrin inclusion complex in electrospun polylactic acid nanofibers: Release behavior and antioxidant activity of gallic acid. Mater. Sci. Eng. C 2016, 63, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishna, S.; Fujihara, K.; Teo, W.E.; Lim, T.C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2005. [Google Scholar]
- Cherpinski, A.; Torres-Giner, S.; Cabedo, L.; Méndez, J.A.; Lagaron, J.M. Multilayer structures based on annealed electrospun biopolymer coatings of interest in water and aroma barrier fiber-based food packaging applications. J. Appl. Polym. Sci. 2018, 135, 45501. [Google Scholar] [CrossRef]
- Cherpinski, A.; Gozutok, M.; Sasmazel, H.; Torres-Giner, S.; Lagaron, J. Electrospun oxygen scavenging films of poly(3-hydroxybutyrate) containing palladium nanoparticles for active packaging applications. Nanomaterials 2018, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Lasprilla-Botero, J.; Torres-Giner, S.; Pardo-Figuerez, M.; Álvarez-Láinez, M.; Lagaron, J.M. Superhydrophobic bilayer coating based on annealed electrospun ultrathin poly(ε-caprolactone) fibers and electrosprayed nanostructured silica microparticles for easy emptying packaging applications. Coatings 2018, 8, 173. [Google Scholar] [CrossRef]
- Santos, N.A.; Cordeiro, A.M.T.M.; Damasceno, S.S.; Aguiar, R.T.; Rosenhaim, R.; Carvalho Filho, J.R.; Santos, I.M.G.; Maia, A.S.; Souza, A.G. Commercial antioxidants and thermal stability evaluations. Fuel 2012, 97, 638–643. [Google Scholar] [CrossRef]
- Garro Galvez, J.M.; Fechtal, M.; Riedl, B. Gallic acid a model of tannins in condensation with formaldehyde. Thermochim. Acta 1996, 274, 149–163. [Google Scholar] [CrossRef]
- Luzi, F.; Puglia, D.; Dominici, F.; Fortunati, E.; Giovanale, G.; Balestra, G.M.; Torre, L. Effect of gallic acid and umbelliferone on thermal, mechanical, antioxidant and antimicrobial properties of poly (vinyl alcohol-co-ethylene) films. Polym. Degrad. Stabil. 2018, 152, 162–176. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Lagaron, J.M.; Balart, R.; Torres-Giner, S. In situ compatibilization of biopolymer ternary blends by reactive extrusion with low-functionality epoxy-based styrene–acrylic oligomer. J. Polym. Environ. 2019, 27, 84–96. [Google Scholar] [CrossRef]
- Wu, J.; Chen, S.; Ge, S.; Miao, J.; Li, J.; Zhang, Q. Preparation, properties and antioxidant activity of an active film from silver carp (hypophthalmichthys molitrix) skin gelatin incorporated with green tea extract. Food Hydrocoll. 2013, 32, 42–51. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Gil, L.; Pascual-Ramírez, L.; Garde-Belza, J.A. Packaging: Food waste reduction. In Encyclopedia of Polymer Applications; Mishra, M., Ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2018; pp. 1990–2009. [Google Scholar]
- Phiriyawirut, M.; Phaechamud, T. Gallic acid-loaded cellulose acetate electrospun nanofibers: Thermal properties, mechanical properties, and drug release behavior. Open J. Polym. Chem. 2012, 2, 17550. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Garcia-Garcia, D.; Carbonell-Verdu, A.; Balart, R.; Torres-Giner, S. Effect of different compatibilizers on injection-molded green composite pieces based on polylactide filled with almond shell flour. Compos. Part B Eng. 2018, 147, 76–85. [Google Scholar] [CrossRef]
- Lu, Y.; Yeap Foo, L. Antioxidant activities of polyphenols from sage (salvia officinalis). Food Chem. 2001, 75, 197–202. [Google Scholar] [CrossRef]
- Ghitescu, R.-E.; Popa, A.-M.; Popa, V.I.; Rossi, R.M.; Fortunato, G. Encapsulation of polyphenols into phema e-spun fibers and determination of their antioxidant activities. Int. J. Pharm. 2015, 494, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Chen, T.-X.; Branford-White, C.J.; Zhu, L.-M. Electrospun shikonin-loaded pcl/ptmc composite fiber mats with potential biomedical applications. Int. J. Pharm. 2009, 382, 215–221. [Google Scholar] [CrossRef] [PubMed]
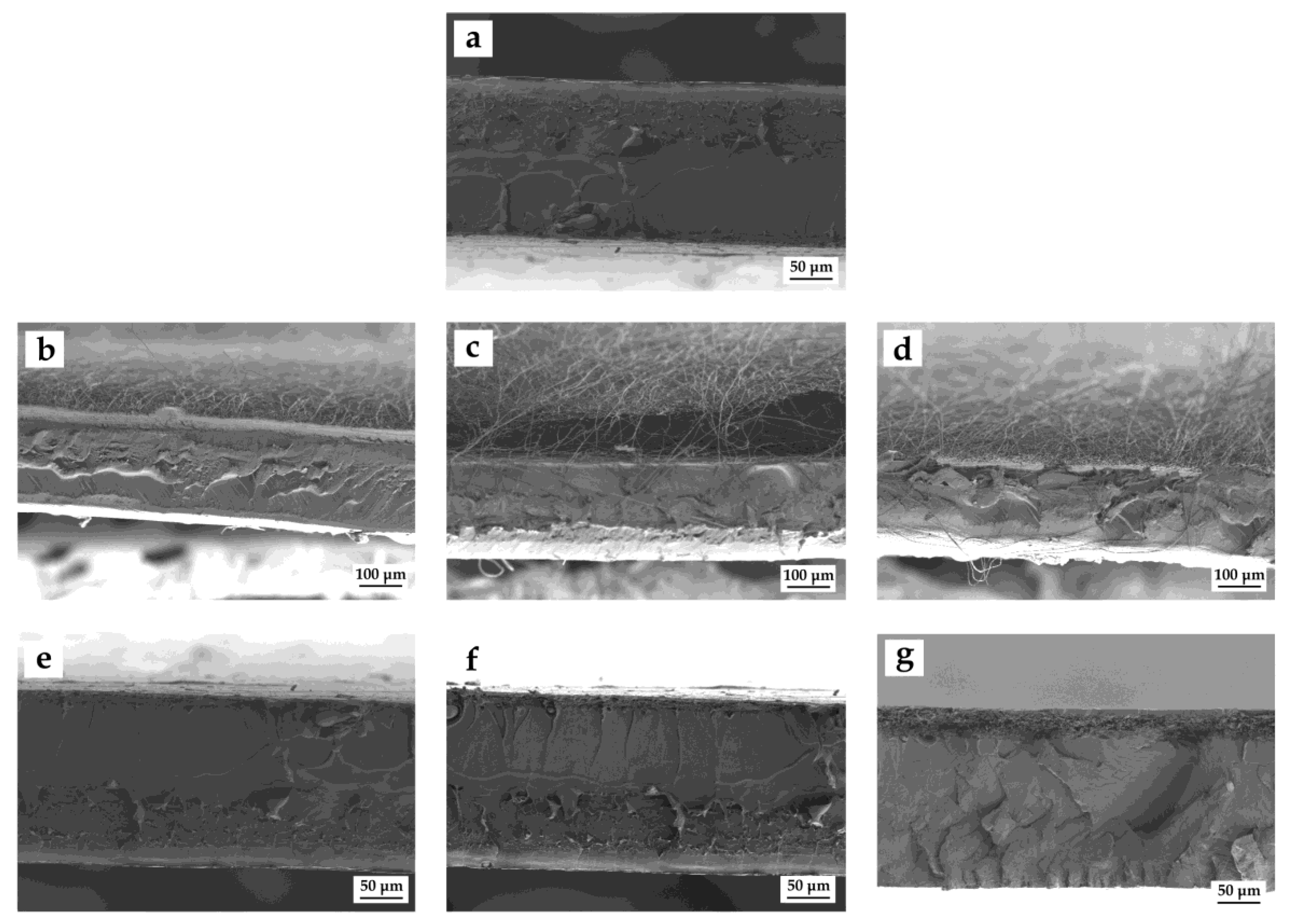


| Film | Structure | Time (h) | Thickness (µm) |
|---|---|---|---|
| Monolayer | 200-µm PLA | 0 | 200.1 ± 0.9 |
| Bilayer 1h | 200-µm PLA/Electrospun PLA+GA fibers | 1 | 210.2 ± 2.8 |
| Bilayer 2h | 200-µm PLA/Electrospun PLA+GA fibers | 2 | 215.0 ± 3.1 |
| Bilayer 3h | 200-µm PLA/Electrospun PLA+GA fibers | 3 | 225.2 ± 2.0 |
| Multilayer 1h | 200-µm PLA/Electrospun PLA+GA film/10-µm PLA | 1 | 213.3 ± 1.8 |
| Multilayer 2h | 200-µm PLA/Electrospun PLA+GA film/10-µm PLA | 2 | 214.8 ± 1.9 |
| Multilayer 3h | 200-µm PLA/Electrospun PLA+GA film/10-µm PLA | 3 | 220.1 ± 2.1 |
| Solution | Surface Tension (mN/m) | Conductivity (µS/cm) | Viscosity (cP) |
|---|---|---|---|
| PLA | 30.6 ± 0.1 a | 1.52 ± 0.08 b | 204.4 ± 0.4 c |
| PLA + GA | 30.7 ± 0.1 a | 5.83 ± 0.11 b | 195.9 ± 0.7 c |
| Film | T5% (°C) | Tdeg (°C) | Residual Mass (%) |
|---|---|---|---|
| GA | 261.5 ± 1.1 | 290.6 ± 1.1 / 337.5 ± 1.2 | 5.32 ± 0.12 |
| Monolayer | 338.1 ± 1.3 | 375.1 ± 1.4 | 0.12 ± 0.04 |
| Bilayer 1h | 348.2 ± 1.2 | 375.2 ± 1.1 | 0.11 ± 0.05 |
| Bilayer 2h | 348.9 ± 1.0 | 378.7 ± 1.2 | 0.12 ± 0.03 |
| Bilayer 3h | 349.1 ± 1.4 | 379.5 ± 1.3 | 0.19 ± 0.04 |
| Multilayer 1h | 345.3 ± 0.9 | 375.1 ± 1.1 | 0.14 ± 0.03 |
| Multilayer 2h | 346.4 ± 1.2 | 377.4 ± 1.0 | 0.20 ± 0.05 |
| Multilayer 3h | 347.3 ± 1.4 | 377.5 ± 1.2 | 0.18 ± 0.04 |
| Film | Inhibition (%) | ||
|---|---|---|---|
| 2 Weeks | 6 Weeks | 10 Weeks | |
| Bilayer 1h | 42.4 ± 0.7 a | 38.4 ± 0.9 a | 25.6 ± 1.2 a |
| Bilayer 2h | 65.7 ± 0.9 a | 54.9 ± 1.2 a | 47.7 ± 0.9 a |
| Bilayer 3h | 96.0 ± 2.4 a | 78.9 ± 1.3 a | 54.7 ± 1.3 a |
| Multilayer 1h | 5.9 ± 0.8 b | 29.2 ± 0.7 b | 38.1 ± 0.7 b |
| Multilayer 2h | 8.1 ± 0.8 b | 37.4 ± 1.0 b | 52.4 ± 1.2 b |
| Multilayer 3h | 12.3 ± 1.2 b | 48.7 ± 0.9 b | 71.4 ± 0.8 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiles-Carrillo, L.; Montanes, N.; Lagaron, J.M.; Balart, R.; Torres-Giner, S. Bioactive Multilayer Polylactide Films with Controlled Release Capacity of Gallic Acid Accomplished by Incorporating Electrospun Nanostructured Coatings and Interlayers. Appl. Sci. 2019, 9, 533. https://doi.org/10.3390/app9030533
Quiles-Carrillo L, Montanes N, Lagaron JM, Balart R, Torres-Giner S. Bioactive Multilayer Polylactide Films with Controlled Release Capacity of Gallic Acid Accomplished by Incorporating Electrospun Nanostructured Coatings and Interlayers. Applied Sciences. 2019; 9(3):533. https://doi.org/10.3390/app9030533
Chicago/Turabian StyleQuiles-Carrillo, Luis, Nestor Montanes, José M. Lagaron, Rafael Balart, and Sergio Torres-Giner. 2019. "Bioactive Multilayer Polylactide Films with Controlled Release Capacity of Gallic Acid Accomplished by Incorporating Electrospun Nanostructured Coatings and Interlayers" Applied Sciences 9, no. 3: 533. https://doi.org/10.3390/app9030533








