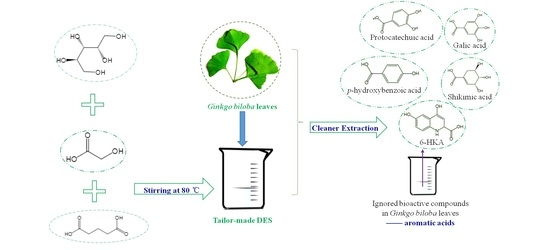
Tailor-Made Deep Eutectic Solvents for Simultaneous Extraction of Five Aromatic Acids from Ginkgo biloba Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
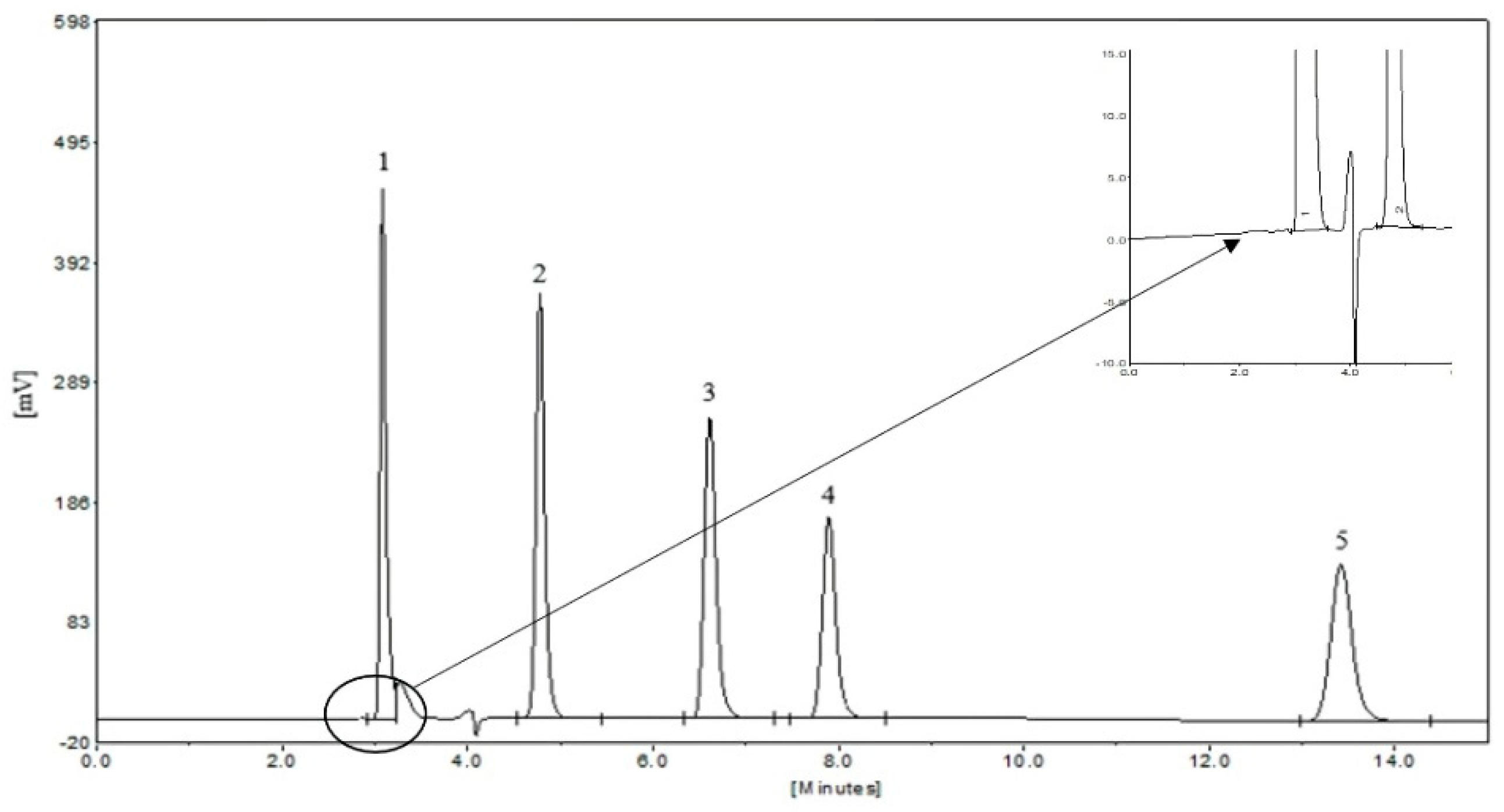
2.2. HPLC Analysis of Five Aromatic Acids
2.3. DESs Preparation
2.4. Extraction of Five Aromatic Acids from Ginkgo biloba Leaves Employing DESs
2.5. DESs Tailoring
2.6. Optimization of the Extraction Procedure for Five Aromatic Acids
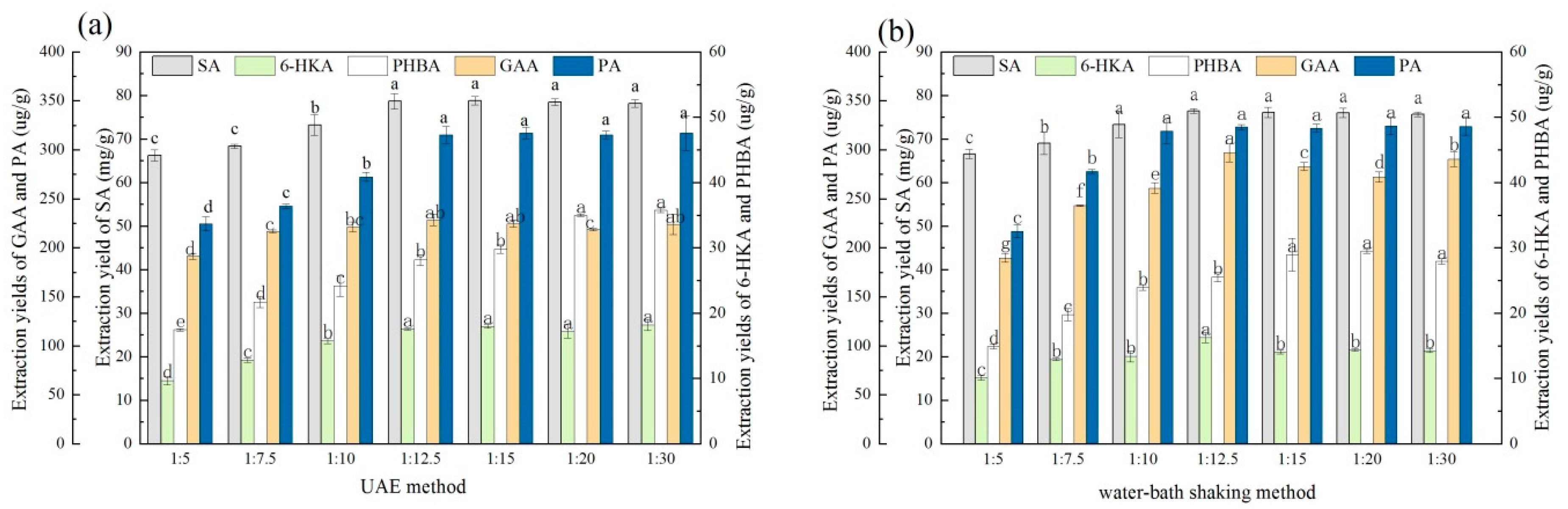
2.6.1. Effect of the Extraction Methods
2.6.2. Optimization of the Extraction Conditions
3. Results and Discussion
3.1. Various Types of DESs Preparation
3.2. Screening and Tailoring DESs for the Simultaneous Extraction of Five Aromatic Acids
3.2.1. Initial DESs Screening
3.2.2. Tailoring DESs by Changing the Molar Ratio between Component 1 and Component 2
3.2.3. Tailoring DESs by Forming the Ternary DESs
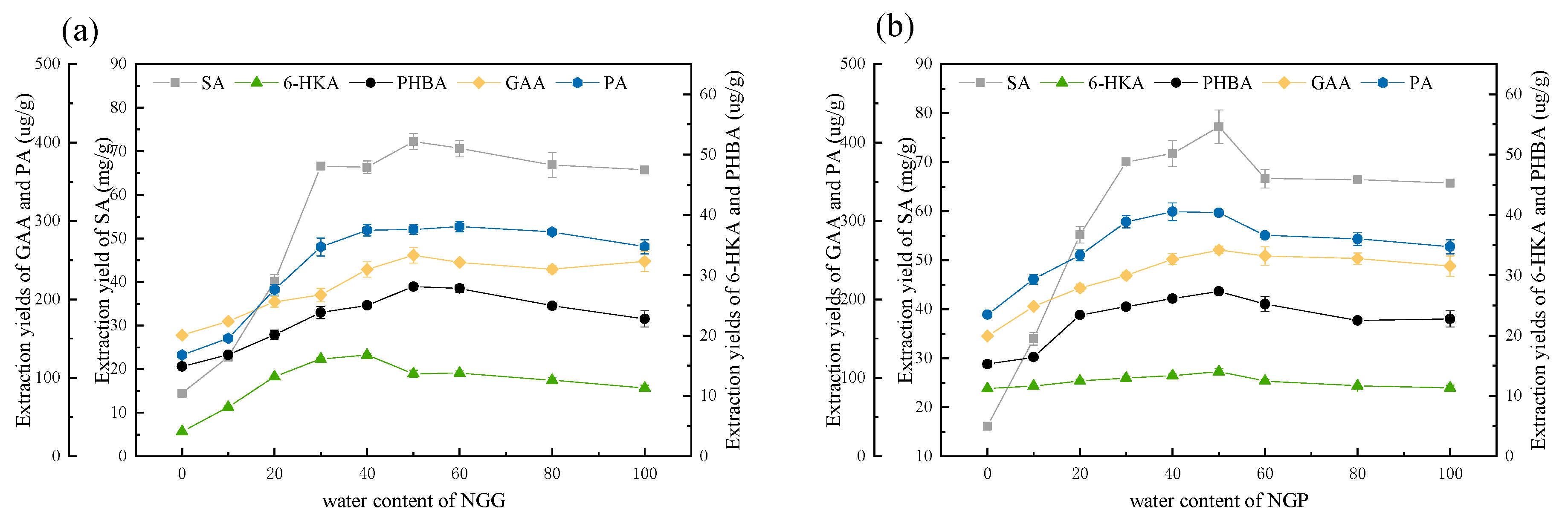
3.2.4. Tailoring DESs by Changing the Water Content
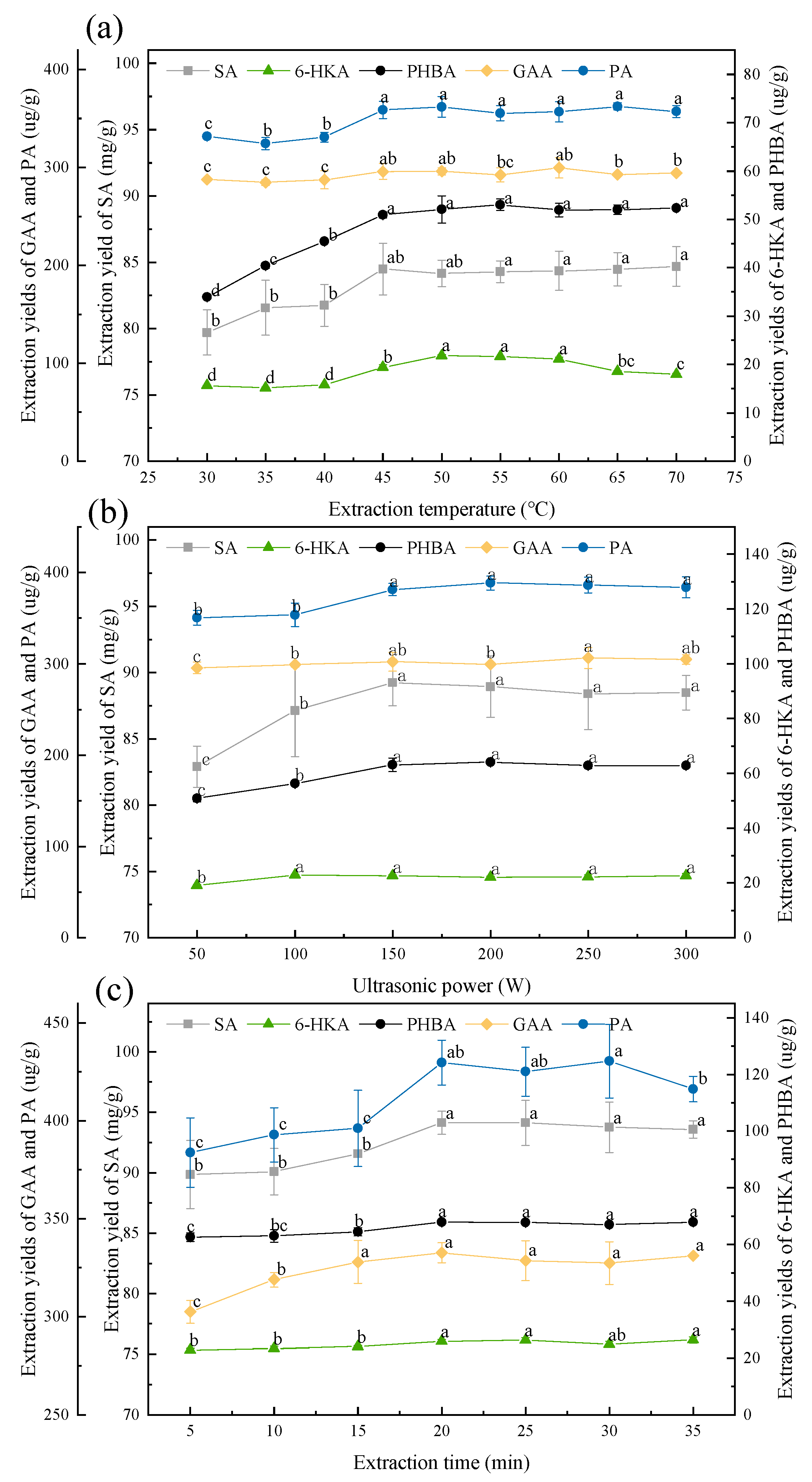
3.3. Optimization of the Extraction Conditions
3.3.1. Comparison of the Extraction Methods
3.3.2. Effect of the Solid to Solvent Ratio
3.3.3. Effect of the Extraction Temperature
3.3.4. Effect of the Ultrasonic Power
3.3.5. Effect of the Extraction Time
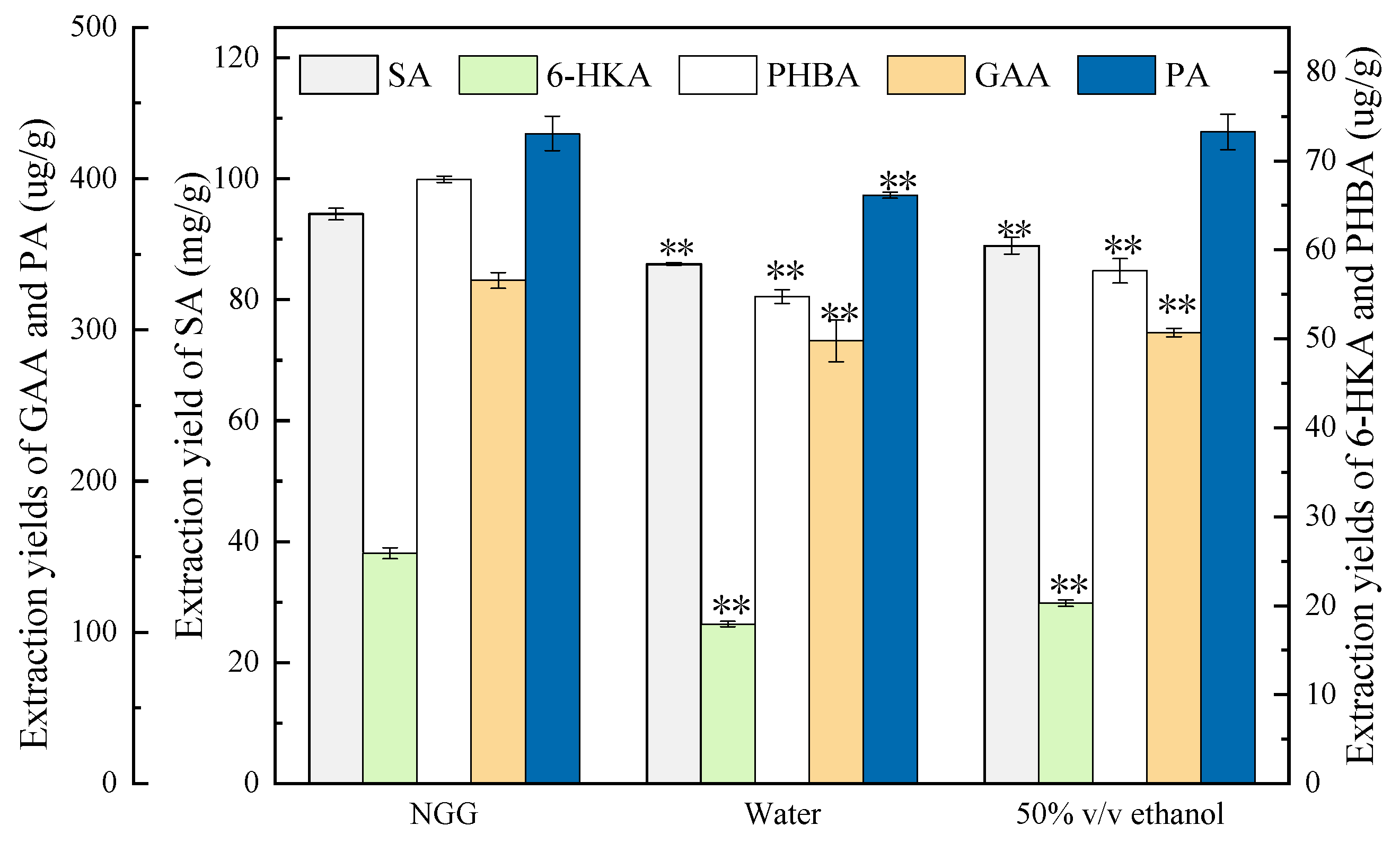
3.4. Evaluation of Extraction Efficiency of the NGG50 in Comparison to Conventional Solvents
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ude, C.; Schubert-Zsilavecz, M.; Wurglics, M. Ginkgo biloba extracts: A review of the pharmacokinetics of the active ingredients. Clin. Pharmacokinet. 2013, 52, 727–749. [Google Scholar] [CrossRef] [PubMed]
- Montes, P.; Ruiz-Sánchez, E.; Rojas, C.; Rojas, P. Ginkgo biloba extract 761: A review of basic studies and potential clinical use in psychiatric disorders. CNS Neurol. Disord-DR. 2015, 14, 132–149. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, P.; Singh, R.D.; Ahuja, P.S. Biology and chemistry of Ginkgo biloba. Fitoterapia 2008, 79, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Stanton, J.D.; Tolson, A.H.; Luo, Y.; Wang, H. Bioactive terpenoids and flavonoids from Ginkgo biloba extract induce the expression of hepatic drug-metabolizing enzymes through pregnane X receptor, constitutive androstane receptor, and aryl hydrocarbon receptor-mediated pathways. Pharm. Res. 2009, 26, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, T.A. Chemical analysis of Ginkgo biloba leaves and extracts. J. Chromatogrv. A. 2002, 967, 21–55. [Google Scholar] [CrossRef]
- Edmonds, M.; Payne, R. Isolation of shikimic acid from star aniseed. J. Chem. Educ. 2005, 82, 599–600. [Google Scholar] [CrossRef]
- Usuki, T.; Yasuda, N.; Yoshizawa-Fujita, M.; Rikukawa, M. Extraction and isolation of shikimic acid from Ginkgo biloba leaves utilizing an ionic liquid that dissolves cellulose. Chem. Commun. 2011, 47, 10560–10562. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Dietrich, D.; Gräsel, I.; Reuter, G.; Seifert, G.; Steinhäuser, C. 6-Hydroxykynurenic acid and kynurenic acid differently antagonise AMPA and NMDA receptors in hippocampal neurones. J. Neurochem. 2001, 77, 1108–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuchta, K.; Yao, J.B.; Du, X.; Jin, H.H.; Fang, L.; Min, H.; Wang, R.W. Seasonal variations of the contents of genistein and 6-HKA in Ginkgo biloba leaves from different regions of China. Planta. Med. 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Guan, S.; Bao, Y.M.; Jiang, B.; An, L.J. Protective effect of protocatechuic acid from Alpinia oxyphylla on hydrogen peroxide-induced oxidative PC12 cell death. Eur. J. Pharmacol. 2006, 538, 73–79. [Google Scholar] [CrossRef]
- Yip, E.C.H.; Chan, A.S.L.; Pang, H.; Tam, Y.K.; Wong, Y.H. Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c-Jun N-terminal kinase-dependent mechanism. Cell Biol. Toxicol. 2006, 22, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents–solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low transition temperature mixtures (LTTMs): A new generation of designer solvents. Angew. Chem. Int. Edit. 2013, 52, 3074–3085. [Google Scholar] [CrossRef] [PubMed]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Natesan, J.S. New Horizons in the extraction of bioactive compounds using deep eutectic solvents: a review. Aanl. Chim. Acta. 2017, 979, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yang, M.; Cao, F.L.; Wang, J.H.; Su, E.Z. Tailor-made hydrophobic deep eutectic solvents for cleaner extraction of polyprenyl acetates from Ginkgo biloba leaves. J. Clean. Prod. 2017, 152, 399–405. [Google Scholar] [CrossRef]
- Su, E.Z.; Yang, M.; Cao, J.; Lu, C.; Wang, J.H.; Cao, F.L. Deep eutectic solvents as green media for efficient extraction of terpene trilactones from Ginkgo biloba leaves. J. Liq. Chromatogr. R. T. 2017, 40, 1–7. [Google Scholar] [CrossRef]
- Xue, M.; Wang, Y.; Meng, Z.; Zhang, W.; Wu, Y.; Jiang, S. Extraction of shikimic acid from Chinese star anise using flash column chromatography on a molecularly-imprinted ploymer column. J. Liq. Chromatogr. R. T. 2013, 36, 2677–2686. [Google Scholar]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Xiao, Y.; Wang, Y.M.; Kong, J.; Hou, Y.C.; Wu, W.Z. Effect of water on the separation of phenol from model oil with choline chloride via forming deep eutectic solvent. Fuel Process Technol. 2015, 137, 104–108. [Google Scholar] [CrossRef]
- Xie, J.Y.; Chen, X.P.; Chen, F. Study on ultrasonic extraction technology of shikimic acid from Pinu elliottii Engelm. Food Sci. Tech. 2010, 8, 258–2662. [Google Scholar]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 627–6278. [Google Scholar] [CrossRef] [PubMed]
- Row, K.H. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Figueiredo, M.; Gomes, C.; Costa, R.; Martins, A.; Pereira, C.M.; Silva, F. Differential capacity of a deep eutectic solvent based on choline chloride and glycerol on solid electrodes. Electrochim. Aata. 2009, 54, 2630–2634. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Srček, V.G.; Bubalo, M.C.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT-Food Sci. Technol. 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Lu, C.; Cao, J.; Wang, N.; Su, E.Z. Significantly improving the solubility of non-steroidal anti-inflammatory drugs in deep eutectic solvents for potential non-aqueous liquid administration. Med. Chem. Comm. 2016, 7, 955–959. [Google Scholar] [CrossRef]
- Yusof, R.; Abdulmalek, E.; Sirat, K.; Rahman, M.B.A. Tetrabutylammonium bromide (TBABr)-based deep eutectic solvents (DESs) and their physical properties. Molecules 2014, 19, 8011–8026. [Google Scholar] [CrossRef]
- Tang, B.; Row, K.H. Recent developments in deep eutectic solvents in chemical sciences. Monatsh Chem. 2013, 144, 1427–1454. [Google Scholar] [CrossRef]
- Shah, D.; Mjalli, F.S. Effect of water on the thermos-physical properties of Reline: An experimental and molecular simulation based approach. Phys. Chem. Chem. Phys. 2014, 16, 23900–23907. [Google Scholar] [CrossRef]
- Passos, H.; Tavares, D.J.; Ferreira, A.M.; Freire, M.G.; Coutinho, J.A. Are aqueous biphasic systems composed of deep eutectic solvents ternary or quaternary systems? ACS Sustain. Chem. Eng. 2016, 4, 2881–2886. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.; Wu, W.; Liu, D.; Ji, Y.; Ren, S. Roles of a hydrogen bond donor and a hydrogen bond acceptor in the extraction of toluene from n-heptane using deep eutectic solvents. Green Chem. 2016, 18, 3089–3097. [Google Scholar] [CrossRef]
- Ghosh, S.; Chisti, Y.; Banerjee, U.C. Production of shikimic acid. Biotechnol. Adv. 2012, 30, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Ohira, H.; Torii, N.; Aida, T.M.; Watanabe, M.; Smith, R.L. Rapid separation of shikimic acid from Chinese star anise (Illicium verum Hook. f.) with hot water extraction. Sep. Purif. Technol. 2009, 69, 102–108. [Google Scholar] [CrossRef]
Sample Availability: Samples of the shikimic acid, gallic acid, 6-hydroxykynurenic acid, protocatechuic acid, and p-hydroxybenzoic acid are available from the authors. |

| DESs | SA (mg/g) | GAA (µg/g) | 6-HKA (µg/g) | PA (µg/g) | PHBA (µg/g) |
|---|---|---|---|---|---|
| ChCl-G | 23.78 ± 0.60 | 224.02 ± 9.11 | 10.18 ± 0.21 | 41.08 ± 0.85 | 26.04 ± 0.24 |
| ChCl-EG | 25.59 ± 0.73 | 222.32 ± 7.21 | 23.73 ± 0.64 | 200.93 ± 2.51 | 17.50 ± 0.24 |
| ChCl-P | 20.45 ± 0.83 | 148.04 ± 4.27 | 21.55 ± 0.91 | 167.36 ± 4.84 | 14.17 ± 0.56 |
| ChCl-B | 16.23 ± 0.40 | 113.77 ± 2.38 | 17.83 ± 0.33 | 136.07 ± 3.02 | 19.68 ± 0.73 |
| ChCl-DS | 18.47 ± 0.81 | 174.81 ± 4.81 | 17.96 ± 0.25 | 162.38 ± 0.54 | 15.70 ± 0.03 |
| ChCl-DG | 15.77 ± 0.17 | 144.73 ± 2.57 | 6.58 ± 0.24 | 121.91 ± 0.23 | 13.42 ± 0.51 |
| ChCl-PA | 23.78 ± 0.45 | 411.37 ± 13.99 | 30.59 ± 1.08 | 207.94 ± 7.08 | 16.78 ± 0.72 |
| ChCl-GA | 25.00 ± 0.95 | 217.48 ± 0.54 | 34.39 ± 1.21 | 237.66 ± 4.42 | 16.40 ± 0.16 |
| ChCl-MA1 | 25.01 ± 0.31 | 200.25 ± 7.76 | 31.90 ± 0.08 | 210.50 ± 4.74 | 16.37 ± 0.23 |
| ChCl-MA2 | 42.91 ± 1.26 | 164.95 ± 4.92 | 20.71 ± 0.60 | 237.95 ± 7.79 | 16.25 ± 0.63 |
| ChCl-LA1 | 23.24 ± 1.01 | 200.19 ± 4.82 | 26.65 ± 0.51 | 186.13 ± 3.68 | 40.84 ± 0.14 |
| ChCl-LA2 | 20.79 ± 0.13 | 183.95 ± 4.25 | 52.64 ± 1.26 | 189.13 ± 6.16 | 16.52 ± 0.44 |
| ChCl-CA | 44.09 ± 0.23 | 264.49 ± 4.9 | 24.68 ± 0.10 | 187.63 ± 4.72 | 19.86 ± 0.21 |
| ChCl-TA | 24.26 ± 0.98 | 200.37 ± 0.59 | 27.72 ± 0.39 | 189.86 ± 6.34 | 16.14 ± 0.05 |
| ChCl-U | 26.59 ± 0.93 | 168.25 ± 4.41 | 30.74 ± 1.03 | 194.21 ± 8.41 | 20.19 ± 0.81 |
| BE-G | 17.82 ± 0.22 | 121.98 ± 3.42 | 19.51 ± 0.51 | 129.97 ± 6.05 | 13.22 ± 0.45 |
| BE-EG | 15.49 ± 0.03 | 150.57 ± 6.54 | 24.33 ± 1.06 | 167.68 ± 6.70 | 13.97 ± 0.39 |
| BE-P | 16.07 ± 0.59 | 502.83 ± 10.71 | 12.32 ± 0.55 | 140.56 ± 6.18 | 42.62 ± 0.26 |
| BE-B | 15.66 ± 0.45 | 120.00 ± 4.52 | 12.94 ± 0.46 | 126.19 ± 0.92 | 23.33 ± 1.01 |
| BE-X | 3.70 ± 0.16 | 209.73 ± 4.38 | 13.28 ± 0.28 | 249.72 ± 6.13 | 12.03 ± 0.58 |
| BE-DS | 10.55 ± 0.28 | 124.30 ± 3.82 | 17.80 ± 0.37 | 122.84 ± 0.62 | 23.86 ± 0.07 |
| BE-GA | 25.20 ± 0.45 | 162.56 ± 3.31 | 20.57 ± 0.67 | 156.26 ± 2.89 | 18.82 ± 0.53 |
| BE-MA1 | 27.15 ± 1.32 | 248.94 ± 9.41 | 37.10 ± 0.84 | 184.09 ± 2.94 | 18.69 ± 0.69 |
| BE-MA2 | 23.02 ± 0.80 | 237.65 ± 4.65 | 11.26 ± 0.50 | 183.55 ± 2.79 | 20.72 ± 0.72 |
| BE-LA1 | 19.96 ± 0.53 | 156.53 ± 3.81 | 47.38 ± 1.84 | 184.09 ± 2.94 | 38.61 ± 1.32 |
| BE-LA2 | 7.61 ± 0.09 | 141.97 ± 0.54 | 51.69 ± 2.35 | 166.92 ± 5.46 | 14.21 ± 0.19 |
| BE-CA | 39.04 ± 0.37 | 233.34 ± 5.22 | 19.75 ± 0.44 | 203.24 ± 3.74 | 16.39 ± 0.16 |
| B-LA2 | 28.87 ± 0.51 | 188.89 ± 7.26 | 72.39 ± 0.90 | 211.55 ± 8.03 | 14.97 ± 0.32 |
| B-LA1 | 27.81 ± 0.39 | 257.62 ± 2.23 | 23.61 ± 0.56 | 208.20 ± 10.33 | 29.59 ± 0.75 |
| B-MA2 | 41.58 ± 0.55 | 233.31 ± 11.17 | 43.83 ± 2.03 | 221.45 ± 6.36 | 15.56 ± 0.30 |
| B-CA | 31.07 ± 0.16 | 183.33 ± 6.57 | 29.49 ± 1.09 | 272.37 ± 7.44 | 47.07 ± 1.23 |
| B-GA | 34.60 ± 1.38 | 236.58 ± 9.90 | 29.59 ± 1.13 | 218.16 ± 2.58 | 15.14 ± 0.42 |
| B-PA | 23.63 ± 0.78 | 155.78 ± 5.15 | 22.34 ± 0.59 | 200.25 ± 4.94 | 12.91 ± 0.50 |
| P-LA2 | 29.64 ± 0.35 | 226.56 ± 2.22 | 65.38 ± 1.53 | 237.44 ± 5.73 | 13.61 ± 0.65 |
| P-LA1 | 25.87 ± 0.77 | 177.59 ± 8.57 | 22.19 ± 0.95 | 209.62 ± 7.15 | 34.27 ± 0.90 |
| P-MA2 | 37.32 ± 1.41 | 230.85 ± 8.40 | 20.24 ± 0.62 | 254.80 ± 12.37 | 15.09 ± 0.09 |
| P-CA | 18.85 ± 0.50 | 166.23 ± 7.15 | 27.63 ± 0.16 | 192.01 ± 8.48 | 12.80 ± 0.44 |
| P-GA | 35.00 ± 1.14 | 133.55 ± 2.26 | 30.83 ± 0.10 | 253.69 ± 11.14 | 13.07 ± 0.48 |
| P-PA | 24.12 ± 0.17 | 132.11 ± 5.57 | 23.45 ± 0.92 | 204.87 ± 2.72 | 12.17 ± 0.29 |
| X-LA2 | 33.12 ± 0.82 | 242.25 ± 6.62 | 45.31 ± 1.72 | 192.03 ± 3.36 | 21.70 ± 0.70 |
| X-LA1 | 32.21 ± 1.17 | 433.09 ± 2.49 | 24.44 ± 0.38 | 204.64 ± 8.49 | 22.33 ± 0.90 |
| X-MA2 | 32.13 ± 0.93 | 276.84 ± 13.31 | 16.86 ± 0.22 | 170.61 ± 2.04 | 20.98 ± 0.62 |
| X-CA | 26.83 ± 0.99 | 123.49 ± 3.10 | 20.62 ± 0.46 | 161.19 ± 1.70 | 19.54 ± 0.62 |
| X-GA | 47.98 ± 0.45 | 305.42 ± 0.04 | 19.00 ± 0.56 | 143.14 ± 7.11 | 12.57 ± 0.51 |
| X-PA | 28.42 ± 0.53 | 169.53 ± 5.65 | 19.46 ± 0.90 | 177.33 ± 4.10 | 14.07 ± 0.58 |
| DESs | Molar Ratio | SA (mg/g) | GAA (μg/g) | 6-HKA (μg/g) | PA (μg/g) | PHBA (μg/g) |
|---|---|---|---|---|---|---|
| X-GA | 3:1 | 25.23 ± 0.46 | 201.56 ± 1.65 | 3.69 ± 0.08 | 57.81 ± 2.05 | 13.34 ± 0.53 |
| 2:1 | 28.61 ± 1.37 | 204.45 ± 7.94 | 9.38 ± 0.31 | 107.74 ± 3.15 | 11.38 ± 0.32 | |
| 1:1 | 47.98 ± 0.01 | 305.42 ± 0.04 | 19.00 ± 0.56 | 143.14 ± 7.11 | 12.57 ± 0.51 | |
| 1:2 | 50.96 ± 0.94 | 248.04 ± 9.30 | 17.74 ± 0.64 | 233.07 ± 5.00 | 12.62 ± 0.15 | |
| 1:3 | 58.35 ± 1.50 | 281.54 ± 8.75 | 23.10 ± 0.48 | 274.20 ± 8.43 | 13.68 ± 0.43 | |
| 1:4 | 58.80 ± 0.68 | 363.69 ± 4.14 | 47.04 ± 1.54 | 301.51 ± 4.40 | 15.26 ± 0.20 | |
| 1:5 | 51.76 ± 0.68 | 300.39 ± 10.22 | 32.00 ± 0.35 | 280.86 ± 3.13 | 14.81 ± 0.20 | |
| ChCl-MA2 | 1:1 | 42.91 ± 1.26 | 164.95 ± 4.92 | 20.71 ± 0.60 | 237.95 ± 7.79 | 16.25 ± 0.63 |
| 1:2 | 39.64 ± 1.27 | 154.64 ± 4.33 | 12.86 ± 0.46 | 222.09 ± 10.05 | 18.66 ± 0.16 | |
| 1:3 | 37.70 ± 0.49 | 166.37 ± 5.76 | 18.99 ± 0.21 | 167.86 ± 0.08 | 22.29 ± 0.92 | |
| 1:4 | 37.09 ± 0.22 | 107.98 ± 3.73 | 18.91 ± 0.19 | 216.10 ± 1.79 | 18.94 ± 0.85 | |
| 1:5 | 28.71 ± 0.69 | 80.70 ± 1.44 | 18.38 ± 0.69 | 214.53 ± 7.14 | 18.94 ± 0.19 | |
| ChCl-CA | 1:1 | 44.09 ± 0.23 | 264.49 ± 4.90 | 24.68 ± 0.1 | 187.63 ± 4.72 | 19.86 ± 0.21 |
| 1:2 | 30.10 ± 1.48 | 326.94 ± 4.88 | 14.07 ± 0.33 | 131.62 ± 1.90 | 14.43 ± 0.43 | |
| 1:3 | 26.14 ± 0.13 | 159.49 ± 4.35 | 13.35 ± 0.43 | 119.76 ± 1.31 | 14.02 ± 0.19 | |
| 1:4 a | - | - | - | - | - | |
| 1:5 b | - | - | - | - | - | |
| B-MA2 | 3:1 | 28.15 ± 0.47 | 50.60 ± 2.02 | 14.44 ± 0.31 | 122.86 ± 2.12 | 18.40 ± 0.44 |
| 2:1 | 32.16 ± 1.53 | 153.18 ± 3.33 | 13.87 ± 0.53 | 133.69 ± 6.42 | 18.93 ± 0.89 | |
| 1:1 | 41.58 ± 0.55 | 233.31 ± 11.17 | 43.83 ± 2.03 | 221.45 ± 6.36 | 15.56 ± 0.30 | |
| 1:2 | 40.84 ± 1.71 | 321.20 ± 2.45 | 16.81 ± 0.21 | 161.22 ± 0.38 | 21.68 ± 0.86 | |
| 1:3 | 40.67 ± 0.42 | 431.65 ± 8.36 | 35.72 ± 0.02 | 177.93 ± 5.26 | 22.42 ± 0.81 | |
| 1:4 | 34.07 ± 0.55 | 520.44 ± 4.63 | 17.88 ± 0.77 | 168.12 ± 4.82 | 18.33 ± 0.64 | |
| 1:5 | 30.61 ± 0.89 | 273.85 ± 2.80 | 12.68 ± 1.20 | 168.28 ± 1.76 | 18.45 ± 0.37 |
| DESs | SA (mg/g) | GAA (μg/g) | 6-HKA (μg/g) | PA (μg/g) | PHBA (μg/g) |
|---|---|---|---|---|---|
| 1-1 | 37.14 ± 1.66 | 149.36 ± 2.29 | 11.51 ± 1.10 | 256.7 ± 12.5 | 24.82 ± 0.30 |
| 1-2 | 44.24 ± 0.24 | 87.63 ± 2.08 | 12.71 ± 1.02 | 282.47 ± 4.60 | 28.28 ± 0.40 |
| 1-3 | 49.64 ± 1.83 | 94.01 ± 0.93 | 11.50 ± 0.45 | 265.13 ± 11.3 | 17.38 ± 0.01 |
| 1-4 | 48.93 ± 1.83 | 182.82 ± 0.42 | 18.94 ± 0.78 | 301.24 ± 8.19 | 14.14 ± 0.27 |
| 1-5 | 58.80 ± 0.67 | 163.69 ± 4.14 | 17.04 ± 1.54 | 301.51 ± 4.40 | 15.26 ± 0.20 |
| 2-1 a | - | - | - | - | - |
| 2-2 | 35.59 ± 1.01 | 193.61 ± 3.14 | 17.76 ± 0.07 | 204.18 ± 7.23 | 13.63 ± 0.56 |
| 2-3 | 50.05 ± 0.15 | 123.51 ± 1.15 | 14.72 ± 0.90 | 260.18 ± 0.43 | 12.87 ± 0.17 |
| 2-4 | 52.56 ± 1.62 | 185.54 ± 3.31 | 12.01 ± 0.17 | 287.08 ± 12.6 | 14.55 ± 0.48 |
| 2-5 | 58.80 ± 0.67 | 163.69 ± 4.14 | 17.04 ± 1.54 | 301.51 ± 4.40 | 15.26 ± 0.20 |
| 3-1 | 25.11 ± 0.86 | 137.59 ± 11.4 | 13.05 ± 0.47 | 191.55 ± 7.01 | 15.16 ± 0.36 |
| 3-2 | 52.23 ± 1.49 | 143.98 ± 8.22 | 18.01 ± 0.64 | 251.09 ± 5.08 | 17.07 ± 0.47 |
| 3-3 | 57.59 ± 0.90 | 158.40 ± 1.48 | 14.01 ± 0.01 | 266.67 ± 12.2 | 17.04 ± 0.40 |
| 3-4 | 61.67 ± 2.31 | 157.06 ± 0.68 | 15.31 ± 0.90 | 282.55 ± 4.66 | 15.26 ± 0.42 |
| 3-5 | 58.80 ± 0.67 | 133.69 ± 4.14 | 17.04 ± 1.54 | 301.51 ± 4.40 | 15.26 ± 0.20 |
| 4-1 | 40.13 ± 1.82 | 169.95 ± 7.12 | 17.18 ± 1.29 | 280.20 ± 6.56 | 14.69 ± 0.70 |
| 4-2 | 47.88 ± 0.98 | 123.16 ± 14.5 | 10.37 ± 1.48 | 285.30 ± 4.24 | 14.89 ± 0.51 |
| 4-3 | 51.33 ± 1.46 | 168.29 ± 10.9 | 17.26 ± 0.95 | 272.23 ± 8.87 | 15.11 ± 0.36 |
| 4-4 | 50.05 ± 0.54 | 143.76 ± 1.02 | 16.77 ± 0.58 | 254.08 ± 7.88 | 15.05 ± 0.21 |
| 4-5 | 58.80 ± 0.67 | 163.69 ± 4.14 | 17.04 ± 1.54 | 301.51 ± 4.40 | 15.26 ± 0.20 |
| 5-1 | 27.37 ± 0.73 | 147.22 ± 5.76 | 19.19 ± 0.01 | 175.62 ± 7.75 | 13.61 ± 0.30 |
| 5-2 | 35.22 ± 1.75 | 187.14 ± 6.95 | 11.10 ± 0.57 | 204.07 ± 1.90 | 13.96 ± 0.22 |
| 5-3 | 41.10 ± 1.73 | 183.06 ± 6.53 | 19.64 ± 0.57 | 227.41 ± 10.1 | 12.59 ± 0.52 |
| 5-4 | 70.02 ± 0.23 | 162.84 ± 10.6 | 17.57 ± 0.27 | 336.88 ± 13.2 | 15.68 ± 0.51 |
| 5-5 | 58.80 ± 0.67 | 163.69 ± 4.14 | 17.04 ± 1.54 | 301.51 ± 4.40 | 15.26 ± 0.20 |
| 6-1 a | - | - | - | - | - |
| 6-2 a | - | - | - | - | - |
| 6-3 a | - | - | - | - | - |
| 6-4 | 66.56 ± 0.81 | 123.63 ± 7.68 | 16.69 ± 0.44 | 294.11 ± 9.39 | 22.82 ± 0.02 |
| 6-5 | 58.80 ± 0.67 | 163.69 ± 4.14 | 17.04 ± 1.54 | 301.51 ± 4.40 | 15.26 ± 0.20 |
| 7-1 a | - | - | - | - | - |
| 7-2 a | - | - | - | - | - |
| 7-3 | 56.03 ± 2.45 | 106.88 ± 3.52 | 14.41 ± 0.6 | 303.46 ± 35.2 | 20.84 ± 0.89 |
| 7-4 | 56.50 ± 2.38 | 131.67 ± 14.1 | 14.50 ± 1.90 | 315.80 ± 3.80 | 16.60 ± 0.30 |
| 7-5 | 58.80 ± 0.67 | 163.69 ± 4.14 | 17.04 ± 1.54 | 301.51 ± 4.40 | 15.26 ± 0.20 |
| DESs | SA (mg/g) | GAA (μg/g) | 6-HKA (μg/g) | PA (μg/g) | PHBA (μg/g) |
|---|---|---|---|---|---|
| 8-1 | 31.18 ± 0.20 | 164.49 ± 4.90 | 14.68 ± 0.10 | 187.63 ± 4.72 | 19.86 ± 0.21 |
| 8-2 | 36.67 ± 1.17 | 184.26 ± 3.19 | 13.25 ± 0.22 | 151.47 ± 3.81 | 16.67 ± 0.38 |
| 8-3 | 46.98 ± 0.05 | 128.85 ± 2.37 | 10.93 ± 0.20 | 194.70 ± 3.24 | 21.94 ± 0.68 |
| 8-4 | 42.52 ± 0.08 | 137.57 ± 5.43 | 10.84 ± 0.21 | 212.63 ± 4.87 | 19.90 ± 0.14 |
| 8-5 | 42.91 ± 1.26 | 164.95 ± 4.92 | 10.71 ± 0.60 | 237.95 ± 7.79 | 16.25 ± 0.63 |
| 9-1 | 23.24 ± 1.01 | 100.19 ± 4.82 | 16.65 ± 0.51 | 186.13 ± 3.68 | 40.84 ± 0.14 |
| 9-2 | 35.03 ± 0.89 | 126.47 ± 4.60 | 13.36 ± 0.09 | 151.12 ± 5.97 | 11.02 ± 0.31 |
| 9-3 | 37.68 ± 0.06 | 155.88 ± 6.06 | 15.53 ± 0.46 | 162.01 ± 4.74 | 10.96 ± 0.09 |
| 9-4 | 40.01 ± 0.30 | 162.88 ± 4.37 | 18.74 ± 0.74 | 139.58 ± 0.82 | 11.03 ± 0.34 |
| 9-5 | 42.91 ± 1.26 | 164.95 ± 4.92 | 11.59 ± 0.50 | 237.95 ± 7.79 | 16.25 ± 0.63 |
| 10-1 | 20.79 ± 0.13 | 183.95 ± 4.25 | 12.64 ± 0.84 | 189.13 ± 6.16 | 16.52 ± 0.44 |
| 10-2 | 35.53 ± 0.49 | 124.28 ± 2.19 | 12.86 ± 0.48 | 175.35 ± 7.10 | 11.43 ± 0.16 |
| 10-3 | 36.49 ± 0.61 | 138.05 ± 6.57 | 19.34 ± 0.14 | 154.73 ± 1.90 | 16.07 ± 0.75 |
| 10-4 | 36.72 ± 0.65 | 102.49 ± 4.53 | 13.87 ± 0.27 | 153.54 ± 1.38 | 11.11 ± 0.23 |
| 10-5 | 42.91 ± 1.26 | 164.95 ± 4.92 | 20.71 ± 0.60 | 237.95 ± 7.79 | 16.25 ± 0.63 |
| 11-1 a | - | - | - | - | - |
| 11-2 | 18.29 ± 0.59 | 97.71 ± 2.19 | 7.64 ± 0.04 | 118.15 ± 4.03 | 11.32 ± 0.41 |
| 11-3 | 22.94 ± 0.80 | 139.39 ± 2.68 | 8.52 ± 0.19 | 158.79 ± 3.84 | 11.72 ± 0.40 |
| 11-4 | 27.34 ± 0.51 | 144.88 ± 5.80 | 9.62 ± 0.16 | 173.17 ± 4.44 | 11.07 ± 0.31 |
| 11-5 | 42.91 ± 1.26 | 164.95 ± 4.92 | 10.71 ± 0.60 | 237.95 ± 7.79 | 16.25 ± 0.63 |
| 12-1 | 23.78 ± 0.45 | 111.37 ± 14.0 | 10.59 ± 1.08 | 207.94 ± 7.08 | 16.78 ± 0.72 |
| 12-2 | 27.96 ± 0.93 | 185.20 ± 8.79 | 10.00 ± 0.09 | 157.90 ± 4.15 | 10.39 ± 0.40 |
| 12-3 | 34.47 ± 1.13 | 185.72 ± 8.75 | 13.29 ± 0.25 | 176.67 ± 5.07 | 12.98 ± 0.23 |
| 12-4 | 35.70 ± 0.77 | 146.81 ± 0.98 | 13.00 ± 0.35 | 185.11 ± 5.66 | 11.96 ± 0.14 |
| 12-5 | 42.91 ± 1.26 | 164.95 ± 4.92 | 10.71 ± 0.60 | 237.95 ± 7.79 | 16.25 ± 0.63 |
| 13-1 | 25.01 ± 0.31 | 100.25 ± 7.76 | 11.90 ± 0.08 | 210.50 ± 4.74 | 16.37 ± 0.23 |
| 13-2 | 31.42 ± 0.54 | 119.96 ± 4.20 | 12.37 ± 0.07 | 206.66 ± 4.88 | 14.85 ± 0.14 |
| 13-3 | 38.97 ± 1.36 | 167.08 ± 4.03 | 15.88 ± 0.07 | 243.57 ± 10.9 | 12.19 ± 0.40 |
| 13-4 | 39.40 ± 1.78 | 180.78 ± 6.68 | 14.05 ± 0.18 | 210.78 ± 4.70 | 11.64 ± 0.34 |
| 13-5 | 42.91 ± 1.26 | 164.95 ± 4.92 | 20.71 ± 0.60 | 237.95 ± 7.79 | 16.25 ± 0.63 |
| DESs | SA (mg/g) | GAA (μg/g) | 6-HKA (μg/g) | PA (μg/g) | PHBA (μg/g) |
|---|---|---|---|---|---|
| 14-1 | 23.24 ± 1.01 | 200.19 ± 4.82 | 16.65 ± 0.51 | 186.13 ± 3.68 | 40.84 ± 0.14 |
| 14-2 | 28.37 ± 1.29 | 180.57 ± 1.99 | 10.62 ± 0.31 | 153.67 ± 5.90 | 21.23 ± 0.22 |
| 14-3 | 30.84 ± 0.65 | 165.97 ± 5.58 | 10.95 ± 0.13 | 204.52 ± 8.48 | 11.93 ± 0.23 |
| 14-4 | 29.78 ± 1.04 | 180.00 ± 2.80 | 10.78 ± 0.19 | 198.21 ± 7.99 | 11.27 ± 0.30 |
| 14-5 | 44.09 ± 0.23 | 264.49 ± 4.90 | 14.68 ± 0.10 | 187.63 ± 4.72 | 19.86 ± 0.21 |
| 15-1 | 20.79 ± 0.13 | 183.95 ± 4.25 | 12.64 ± 0.84 | 189.13 ± 6.16 | 16.52 ± 0.44 |
| 15-2 | 25.76 ± 1.07 | 174.46 ± 0.81 | 14.31 ± 0.13 | 178.25 ± 6.95 | 20.49 ± 0.78 |
| 15-3 | 25.70 ± 0.86 | 241.97 ± 10.6 | 16.49 ± 0.28 | 202.97 ± 2.51 | 18.78 ± 0.84 |
| 15-4 | 37.19 ± 0.91 | 211.99 ± 0.55 | 19.75 ± 0.05 | 242.74 ± 4.86 | 21.41 ± 0.40 |
| 15-5 | 44.09 ± 0.23 | 264.49 ± 4.90 | 14.68 ± 0.10 | 187.63 ± 4.72 | 19.86 ± 0.21 |
| 16-1 a | - | - | - | - | - |
| 16-2 | 20.87 ± 0.44 | 219.76 ± 11.0 | 16.01 ± 0.27 | 158.10 ± 4.98 | 17.84 ± 0.73 |
| 16-3 | 29.58 ± 0.33 | 260.91 ± 8.78 | 13.85 ± 0.42 | 174.52 ± 4.90 | 19.38 ± 0.84 |
| 16-4 | 39.25 ± 1.04 | 280.17 ± 2.16 | 11.28 ± 0.05 | 184.27 ± 5.79 | 21.04 ± 0.66 |
| 16-5 | 44.09 ± 0.23 | 264.49 ± 4.90 | 14.68 ± 0.10 | 187.63 ± 4.72 | 19.86 ± 0.21 |
| 17-1 | 35.67 ± 0.67 | 111.37 ± 14.0 | 19.73 ± 0.35 | 207.94 ± 7.08 | 16.78 ± 0.72 |
| 17-2 | 49.11 ± 1.57 | 264.16 ± 3.18 | 12.93 ± 1.03 | 297.12 ± 8.03 | 18.57 ± 0.34 |
| 17-3 | 41.32 ± 1.18 | 213.04 ± 4.50 | 19.7 ± 0.91 | 268.72 ± 5.99 | 19.42 ± 0.74 |
| 17-4 | 40.74 ± 2.00 | 209.41 ± 4.71 | 12.91 ± 0.60 | 216.47 ± 4.44 | 16.93 ± 0.65 |
| 17-5 | 44.09 ± 0.23 | 264.49 ± 4.90 | 14.68 ± 0.10 | 187.63 ± 4.72 | 19.86 ± 0.21 |
| 18-1 | 37.51 ± 0.47 | 200.25 ± 7.76 | 18.21 ± 1.07 | 210.5 ± 4.74 | 16.37 ± 0.23 |
| 18-2 | 42.21 ± 0.30 | 251.42 ± 4.20 | 15.74 ± 1.19 | 222.09 ± 3.90 | 18.04 ± 0.80 |
| 18-3 | 42.79 ± 0.55 | 242.45 ± 0.64 | 13.34 ± 0.40 | 272.46 ± 1.11 | 20.79 ± 0.90 |
| 18-4 | 45.49 ± 0.18 | 201.05 ± 3.66 | 10.39 ± 0.49 | 254.53 ± 5.15 | 18.15 ± 0.38 |
| 18-5 | 44.09 ± 0.23 | 264.49 ± 4.90 | 14.68 ± 0.10 | 187.63 ± 4.72 | 19.86 ± 0.21 |
| DESs | SA (mg/g) | GAA (μg/g) | 6-HKA (μg/g) | PA (μg/g) | PHBA (μg/g) |
|---|---|---|---|---|---|
| 19-1 | 31.07 ± 0.16 | 166.23 ± 7.15 | 9.49 ± 0.73 | 272.37 ± 7.44 | 47.07 ± 1.23 |
| 19-2 | 27.79 ± 0.40 | 214.74 ± 10.4 | 18.87 ± 0.40 | 241.08 ± 6.21 | 16.07 ± 0.40 |
| 19-3 | 25.61 ± 0.25 | 208.26 ± 3.68 | 12.02 ± 0.23 | 301.09 ± 2.60 | 14.18 ± 0.54 |
| 19-4 | 31.17 ± 0.15 | 229.53 ± 9.45 | 16.61 ± 0.19 | 272.19 ± 4.35 | 15.11 ± 0.30 |
| 19-5 | 41.58 ± 0.55 | 233.31 ± 11.2 | 13.83 ± 1.35 | 221.45 ± 6.36 | 15.56 ± 0.30 |
| 20-1 | 27.81 ± 0.39 | 257.62 ± 2.23 | 13.61 ± 0.56 | 208.20 ± 10.3 | 29.59 ± 0.75 |
| 20-2 | 27.06 ± 0.67 | 88.82 ± 3.04 | 17.92 ± 0.58 | 172.45 ± 6.10 | 11.02 ± 0.37 |
| 20-3 | 28.20 ± 0.45 | 169.89 ± 6.22 | 18.14 ± 0.18 | 172.04 ± 3.41 | 12.10 ± 0.03 |
| 20-4 | 34.87 ± 0.67 | 271.81 ± 6.28 | 16.95 ± 0.09 | 217.78 ± 3.02 | 11.12 ± 0.54 |
| 20-5 | 41.58 ± 0.55 | 233.31 ± 11.2 | 13.83 ± 1.35 | 221.45 ± 6.36 | 15.56 ± 0.30 |
| 21-1 | 28.87 ± 0.51 | 188.89 ± 7.26 | 12.39 ± 0.90 | 211.55 ± 8.03 | 14.97 ± 0.32 |
| 21-2 | 26.52 ± 1.15 | 80.68 ± 2.57 | 18.77 ± 0.82 | 208.79 ± 4.40 | 12.32 ± 0.10 |
| 21-3 | 33.70 ± 0.24 | 100.43 ± 3.82 | 14.87 ± 0.76 | 197.62 ± 1.71 | 11.01 ± 0.19 |
| 21-4 | 35.75 ± 0.32 | 155.04 ± 5.62 | 10.56 ± 1.25 | 229.50 ± 10.4 | 11.59 ± 0.31 |
| 21-5 | 41.58 ± 0.55 | 233.31 ± 11.2 | 13.83 ± 1.35 | 221.45 ± 6.36 | 15.56 ± 0.30 |
| 22-1 a | - | - | - | - | - |
| 22-2 | 22.64 ± 0.81 | 112.94 ± 1.11 | 10.69 ± 0.25 | 160.06 ± 6.84 | 11.76 ± 0.24 |
| 22-3 | 26.93 ± 0.37 | 178.24 ± 4.13 | 11.38 ± 0.87 | 185.78 ± 0.72 | 11.22 ± 0.10 |
| 22-4 | 28.97 ± 1.04 | 94.25 ± 1.87 | 15.32 ± 0.88 | 217.78 ± 8.11 | 14.08 ± 0.25 |
| 22-5 | 41.58 ± 0.55 | 233.31 ± 11.2 | 13.83 ± 1.35 | 221.45 ± 6.36 | 15.56 ± 0.30 |
| 23-1 a | - | - | - | - | - |
| 23-2 | 51.56 ± 0.34 | 141.68 ± 2.55 | 15.98 ± 1.06 | 275.93 ± 2.72 | 15.50 ± 0.15 |
| 23-3 | 54.25 ± 1.26 | 259.33 ± 8.62 | 10.87 ± 0.60 | 286.31 ± 4.27 | 16.50 ± 0.33 |
| 23-4 | 47.55 ± 1.78 | 242.84 ± 9.34 | 16.35 ± 1.14 | 204.45 ± 2.18 | 15.12 ± 0.49 |
| 23-5 | 41.58 ± 0.55 | 233.31 ± 11.2 | 13.83 ± 1.35 | 221.45 ± 6.36 | 15.56 ± 0.30 |
| 24-1 | 41.73 ± 0.37 | 190.98 ± 4.33 | 16.70 ± 0.85 | 269.59 ± 10.1 | 14.60 ± 0.40 |
| 24-2 | 40.38 ± 0.82 | 186.48 ± 7.46 | 10.00 ± 0.56 | 299.50 ± 1.04 | 15.80 ± 0.37 |
| 24-3 | 31.72 ± 0.20 | 216.30 ± 3.62 | 12.88 ± 1.21 | 330.73 ± 15.7 | 16.73 ± 0.54 |
| 24-4 | 34.02 ± 0.46 | 232.19 ± 3.71 | 10.62 ± 0.89 | 273.51 ± 7.66 | 15.49 ± 0.24 |
| 24-5 | 41.58 ± 0.55 | 233.31 ± 11.2 | 13.83 ± 1.35 | 221.45 ± 6.36 | 15.56 ± 0.30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Wang, H.; Zhang, W.; Cao, F.; Ma, G.; Su, E. Tailor-Made Deep Eutectic Solvents for Simultaneous Extraction of Five Aromatic Acids from Ginkgo biloba Leaves. Molecules 2018, 23, 3214. https://doi.org/10.3390/molecules23123214
Cao J, Wang H, Zhang W, Cao F, Ma G, Su E. Tailor-Made Deep Eutectic Solvents for Simultaneous Extraction of Five Aromatic Acids from Ginkgo biloba Leaves. Molecules. 2018; 23(12):3214. https://doi.org/10.3390/molecules23123214
Chicago/Turabian StyleCao, Jun, Huimin Wang, Wei Zhang, Fuliang Cao, Geli Ma, and Erzheng Su. 2018. "Tailor-Made Deep Eutectic Solvents for Simultaneous Extraction of Five Aromatic Acids from Ginkgo biloba Leaves" Molecules 23, no. 12: 3214. https://doi.org/10.3390/molecules23123214
APA StyleCao, J., Wang, H., Zhang, W., Cao, F., Ma, G., & Su, E. (2018). Tailor-Made Deep Eutectic Solvents for Simultaneous Extraction of Five Aromatic Acids from Ginkgo biloba Leaves. Molecules, 23(12), 3214. https://doi.org/10.3390/molecules23123214