Dietary Nitrate from Beetroot Juice for Hypertension: A Systematic Review
Abstract
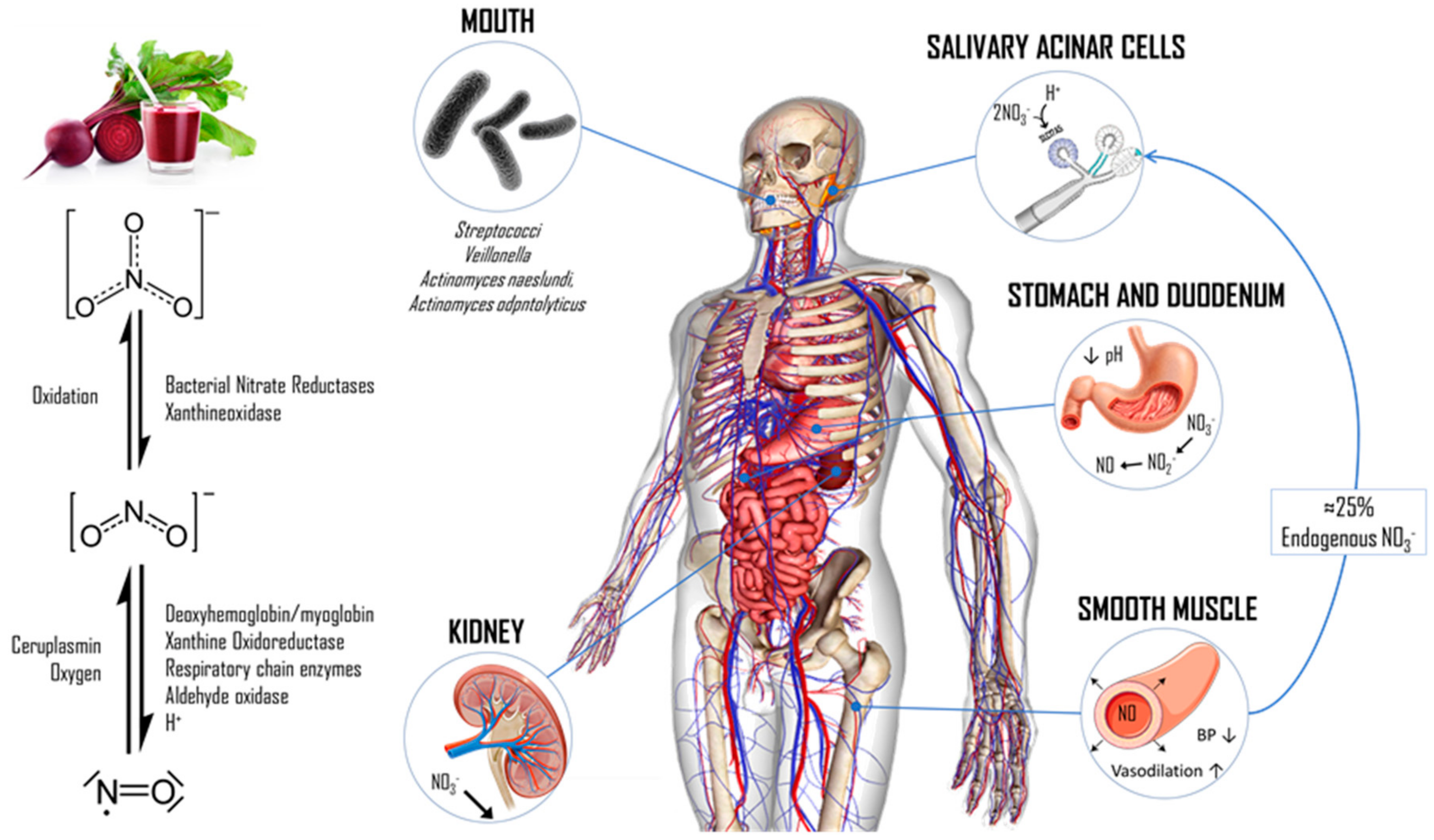
1. Introduction
2. Materials and Methods
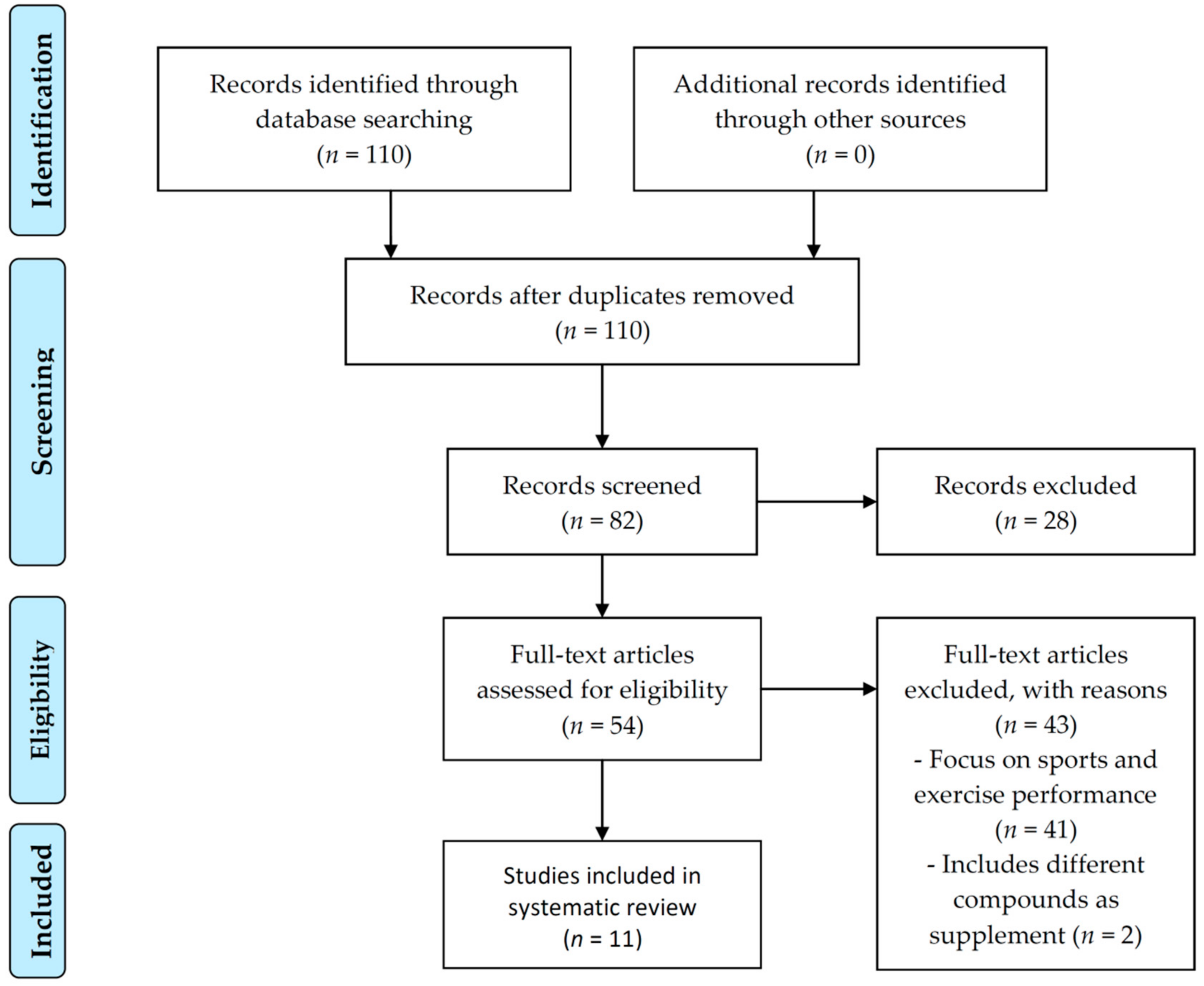
2.1. Search Strategy and Data Sources
2.2. Eligibility Criteria and Data Extraction
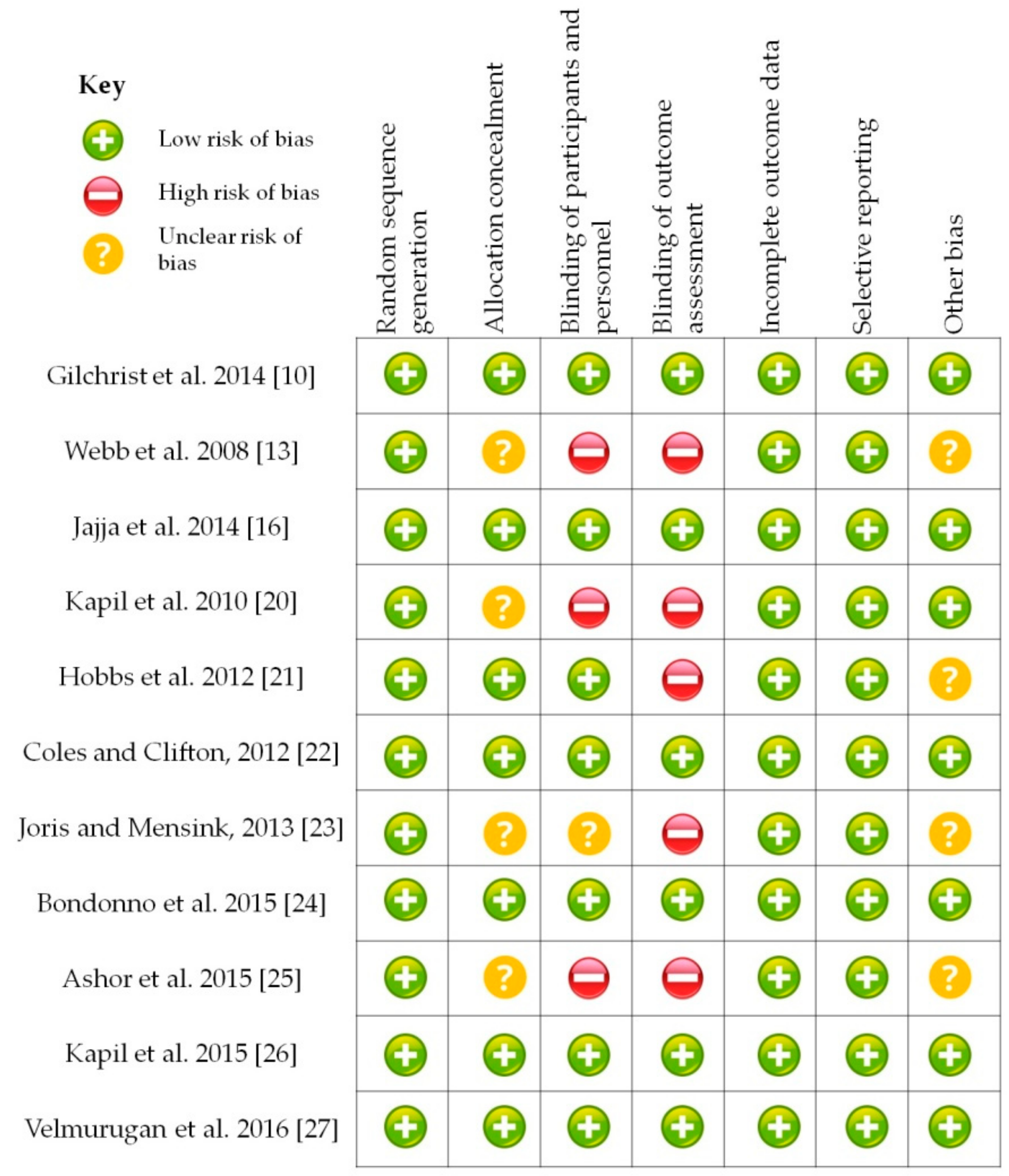
2.3. Data Synthesis
3. Results
4. Discussion
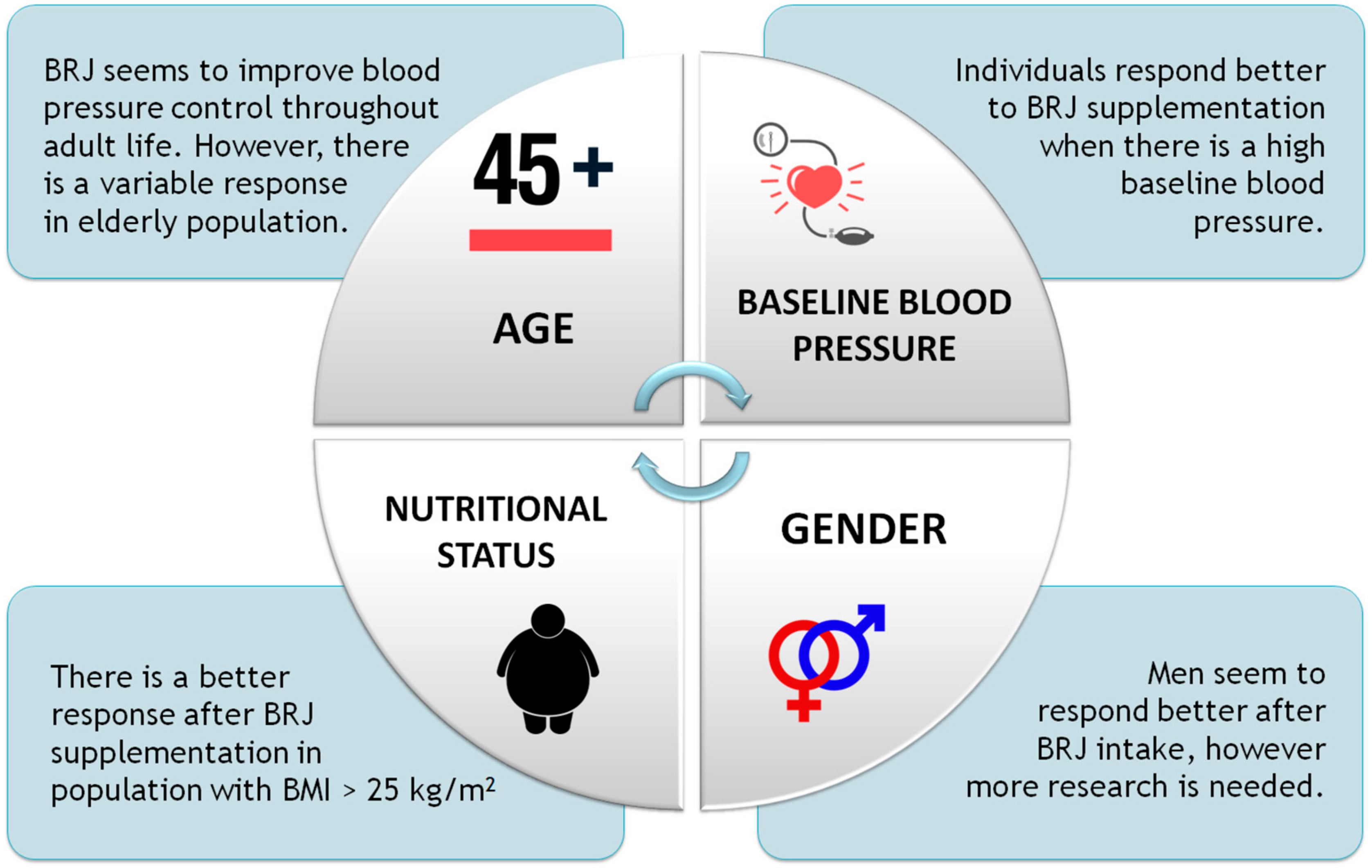
4.1. Effects on Intervention Measures
4.1.1. Age
4.1.2. Gender
4.1.3. Nutritional Status
4.1.4. Baseline Blood Pressure
4.2. Factors Related to Beetroot Juice Administration
4.2.1. Nitrate Concentration
4.2.2. Volume
4.2.3. Length
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Global Health Risks Global Health Risks. Mortality and Burden of Disease Attributable to Selected Major Risks. Available online: http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf (accessed on 19 August 2018).
- Zhou, B.; Bentham, J.; Di Cesare, M.; Bixby, H.; Danaei, G.; Cowan, M.J. Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef]
- World Health Organization. Información General Sobre la Hipertensión en el Mundo–OMS. 2013. Available online: http://apps.who.int/iris/bitstream/10665/87679/1/WHO_DCO_WHD_2013.2_spa.pdf (accessed on 29 June 2018).
- McCartney, D.M.A.; Byrne, D.G.; Turner, M.J. Dietary contributors to hypertension in adults reviewed. Ir. J. Med. Sci. 2015, 184, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; De Jesus, J.M.; Houston Miller, N.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2014, 63, 2960–2984. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Morris, P.B.; Ballantyne, C.M.; Birtcher, K.K.; Daly, D.D.; DePalma, S.M.; Minissian, M.B.; Orringer, C.E.; Smith, S.C., Jr. 2017 Focused Update of the 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J. Am. Coll. Cardiol. 2017, 70, 1785–1822. [Google Scholar] [CrossRef] [PubMed]
- Dharmashankar, K.; Widlansky, M.E. Vascular endothelial function and hypertension: Insights and directions. Curr. Hypertens. Rep. 2010, 12, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Lara, J.; Ashor, A.W.; Oggioni, C.; Ahluwalia, A.; Mathers, J.C.; Siervo, M. Effects of inorganic nitrate and beetroot supplementation on endothelial function: A systematic review and meta-analysis. Eur. J. Nutr. 2016, 55, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, M.; Winyard, P.G.; Fulford, J.; Anning, C.; Shore, A.C.; Benjamin, N. Dietary nitrate supplementation improves reaction time in type 2 diabetes: Development and application of a novel nitrate-depleted beetroot juice placebo. Nitric Oxide Biol. Chem. 2014, 40, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Stanaway, L.; Rutherfurd-Markwick, K.; Page, R.; Ali, A. Performance and health benefits of dietary nitrate supplementation in older adults: A systematic review. Nutrients 2017, 9, 1171. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.K.; Hirai, D.M.; Copp, S.W.; Holdsworth, C.T.; Allen, J.D.; Jones, A.M.; Musch, T.I.; Poole, D.C. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J. Physiol. 2013, 591, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–90. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.M.; Wu, Z.F.; Pang, B.X.; Jin, L.Y.; Qin, L.Z.; Wang, S.L. From nitrate to nitric oxide: The role of salivary glands and oral bacteria. J. Dent. Res. 2016, 95, 1452–1456. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, S.; Kapil, V.; Ghosh, S.M.; Davies, S.; McKnight, A.; Aboud, Z.; Khambata, R.S.; Webb, A.J.; Poole, A.; Ahluwalia, A.; et al. Antiplatelet effects of dietary nitrate in healthy volunteers: Involvement of cGMP and influence of sex. Free Radic. Biol. Med. 2013, 65, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Jajja, A.; Sutyarjoko, A.; Lara, J.; Rennie, K.; Brandt, K.; Qadir, O.; Siervo, M. Beetroot supplementation lowers daily systolic blood pressure in older, overweight subjects. Nutr. Res. 2014, 34, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011, 343, D5928. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Milsom, A.B.; Okorie, M.; Maleki-Toyserkani, S.; Akram, F.; Rehman, F.; Arghandawi, S.; Pearl, V.; Benjamin, N.; Loukogeorgakis, S.; et al. Inorganic nitrate supplementation lowers blood pressure in humans: Role for nitrite-derived NO. Hypertension 2010, 56, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, D.A.; Kaffa, N.; George, T.W.; Methven, L.; Lovegrove, J.A. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br. J. Nutr. 2012, 108, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Coles, L.T.; Clifton, P.M. Effect of beetroot juice on lowering blood pressure in free-living, disease-free adults: A randomized, placebo-controlled trial. Nutr. J. 2012, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.J.; Mensink, R.P. Beetroot juice improves in overweight and slightly obese men postprandial endothelial function after consumption of a mixed meal. Atherosclerosis 2013, 231, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Liu, A.H.; Croft, K.D.; Ward, N.C.; Shinde, S.; Moodley, Y.; Lundberg, J.O.; Puddey, I.B.; Woodman, R.J.; Hodgson, J.M.; et al. Absence of an effect of high nitrate intake from beetroot juice on blood pressure in treated hypertensive individuals: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Jajja, A.; Sutyarjoko, A.; Brandt, K.; Qadir, O.; Lara, J. Effects of beetroot juice supplementation on microvascular blood flow in older overweight and obese subjects: A pilot randomised controlled study. J. Hum. Hypertens. 2015, 29, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double-blind, placebo-controlled study. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, S.; Gan, J.M.; Rathod, K.S.; Khambata, R.S.; Ghosh, S.M.; Hartley, A. Dietary nitrate improves vascular function in patients with hypercholesterolemia: A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2016, 103, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; He, J.; Appel, L.J.; Cutler, J.A.; Havas, S.; Kotchen, T.A.; Roccella, E.J.; Stout, R.; Vallbona, C.; Winston, M.C.; et al. Primary prevention of hypertension: Clinical and public health advisory from The National High Blood Pressure Education Program. JAMA 2002, 288, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Kabir, A.; Azizi, F.; Ghasemi, A. The nitrate-independent blood pressure–lowering effect of beetroot juice: A systematic review and meta-analysis. Adv. Nutr. Int. Rev. J. 2017, 8, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Lara, J.; Siervo, M. Medium-term effects of dietary nitrate supplementation on systolic and diastolic blood pressure in adults: A systematic review and meta-analysis. J. Hypertens. 2017, 35, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Hajifathalian, K.; Ezzati, M.; Woodward, M.; Rimm, E.B.; Danaei, G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: A pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet 2014, 383, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Speijers, G.; Brandt, P.A.V.D. Nitrate Food Additives Series; Food additives Series, 50; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Siervo, M.; Lara, J.; Ogbonmwan, I.; Mathers, J.C. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: A systematic review and meta-analysis. J. Nutr. 2013, 143, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef]
- Ashworth, A.; Mitchell, K.; Blackwell, J.R.; Vanhatalo, A.; Jones, A.M. High-nitrate vegetable diet increases plasma nitrate and nitrite concentrations and reduces blood pressure in healthy women. Public Health Nutr. 2015, 18, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
| Reference | Sample Population and Gender | Age and BMI | Baseline Blood Pressure (SBP; DBP) | Supplementation Duration | BRJ Dosage | NO3− Concentration | NO3—Depleted as Placebo? | Effect on SBP | Effect on DBP |
|---|---|---|---|---|---|---|---|---|---|
| Gilchrist et al. 2014 [10] | 27 both | 67.2 ± 4.9 years | 142.9 ± 13.9; | 14 days | 250 mL | 30.7 mM; | Yes | NS | NS |
| (18 M; 9 F) | 30.8 ± 3.2 kg/m2 | 81.1 ± 9.2 | 7.6 mmol | ||||||
| Webb et al. 2008 [13] | 14 both | 25.5 ± 4.5 years | 108.0 ± 1.3; | Acute | 500 mL | 45.0 ± 2.6 mM; | No | ↓ 10.4 ± 3.0 mmHg after 2.5 h | ↓ 8.1± 2.1 mmHg after 3 h |
| 22.5 mmol; | |||||||||
| (9 M; 5 F) | 22.54 kg/m2 | 70.3 ± 1.0 | 2.79 g/L | ||||||
| Jajja et al. 2014 [16] | 21 both | 62.0 ± 1.4 years | 129.8 ± 19.1; | 21 days | 70 mL | ≈69.1–92.1 mM; | Yes | ↓ 7.3 ± 5.9 mmHg during final week | NS |
| (12 M; 9 F) | 30.1 ± 1.2 kg/m2 | 77.1 ± 15.4 | ≈4.8–6.4 mmol; | ||||||
| 300–400 mg | |||||||||
| Kapil et al. 2010 [19] | 9 both | 18–45 years | 120.6 ± 4.1; | Acute | 250 mL | 22.4 ± 3.8 mM; | No | ↓5.4 ± 1.5 mmHg after 3 h | NS |
| 18–40 kg/m2 | 70.9 ± 2.5 | 5.6 mmol | |||||||
| Hobbs et al. 2012 [20] | 18 M | 31.4 ± 3.0 years | 130.6 ± 3.2; | Acute with different dosages | 500 mL | 4.6, 11.4, and 22.8 mM; 2.3, 5.7, and 11.4 mmol | No | ↓ 13.1, 20.5, and 22.2 mmHg according to [NO3−] after 2–3 h | ↓ 16.6, 14.6 y 18.3 mmHg according to [NO3−] after 2–3 h |
| 24,4 ± 3.0 kg/m2 | 82.1 ± 5.6 | ||||||||
| Coles and Clifton, 2012 [21] | 30 both | 42.5 ± 3.4 years | 132.4 ± 1.6; | Acute | 500 g | 15 mM; | No | ↓ 4–5 mmHg after 6 h only in men | NS |
| (15 M; 15 F) | 28.2 ± 1.3 kg/m2 | 81.1 ± 1.2 | 7.5 mmol | ||||||
| Joris and Mensink, 2013 [22] | 20 M | 61 ± 7 years | 135.2 ± 18.2; | Acute | 140 mL | 57.59 mM; 8.06 mmol; 500 mg | Yes | NS | ↓ 3–6 mmHg after 1–4 h |
| 30.1 ± 1.9 kg/m2 | 93.2 ± 12.0 | ||||||||
| Bondonno et al. 2015 [23] | 27 both | 63.2 ± 4.4 years | 132.9 ± 11.8; | 7 days | 140 mL | 49.99 mM; 6.99 mmol; 3.1 g/L | Yes | NS | NS |
| (10 M; 17 F) | 26.9 ± 3.2 kg/m2 | 76.2 ± 10.4 | |||||||
| Ashor et al. 2015 [24] | 21 both | 62.0 ± 4.5 years | 135.1 ± 14.9; | 21 days | 70 mL | ≈69.1–92.1 mM; | Yes | ↓ 10 mmHg after 3 weeks | ↓ 3 mmHg after 3 weeks |
| (12 M; 9 F) | 29.9 ± 4.2 kg/m2 | 77.5 ± 9.6 | ≈4.8–6.4 mmol; | ||||||
| 300–400 mg | |||||||||
| Kapil et al., 2015 [25] | 32 both | 56.3 ± 16.4 years | 138.4 ± 17.1; | 4 weeks | 250 mL | 25.7 ± 5.3 mM; 6.4 mmol | Yes | ↓ 7.7 mmHg after 24 h and 4 weeks | ↓ 5.2 and 2.4 mmHg after 24 h and 4 weeks |
| (16 M; 16 F) | 26.5 ± 4.0 kg/m2 | 82.8 ± 11.2 | |||||||
| Velmurugan et al., 2016 [26] | 33 both | 53.3 ± 10.1 years | 125.2 ± 15.1; | 6 weeks | 250 mL | 24.2 ± 7.7 mM; 6.05 mmol | Yes | ↓ 4.1 mmHg after 6 weeks | ↓ 1.5 mmHg after 6 weeks |
| (12 M; 21 F) | 26.8 ± 4.9 kg/m2 | 76.3 ± 8.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonilla Ocampo, D.A.; Paipilla, A.F.; Marín, E.; Vargas-Molina, S.; Petro, J.L.; Pérez-Idárraga, A. Dietary Nitrate from Beetroot Juice for Hypertension: A Systematic Review. Biomolecules 2018, 8, 134. https://doi.org/10.3390/biom8040134
Bonilla Ocampo DA, Paipilla AF, Marín E, Vargas-Molina S, Petro JL, Pérez-Idárraga A. Dietary Nitrate from Beetroot Juice for Hypertension: A Systematic Review. Biomolecules. 2018; 8(4):134. https://doi.org/10.3390/biom8040134
Chicago/Turabian StyleBonilla Ocampo, Diego A., Andrés F. Paipilla, Estevan Marín, Salvador Vargas-Molina, Jorge L. Petro, and Alexandra Pérez-Idárraga. 2018. "Dietary Nitrate from Beetroot Juice for Hypertension: A Systematic Review" Biomolecules 8, no. 4: 134. https://doi.org/10.3390/biom8040134
APA StyleBonilla Ocampo, D. A., Paipilla, A. F., Marín, E., Vargas-Molina, S., Petro, J. L., & Pérez-Idárraga, A. (2018). Dietary Nitrate from Beetroot Juice for Hypertension: A Systematic Review. Biomolecules, 8(4), 134. https://doi.org/10.3390/biom8040134







