Elaboration and Characterization of Active Apple Starch Films Incorporated with Ellagic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Reagents
2.2. Preparation of Coating Solutions and Films
2.3. Films Characterization
2.3.1. Color
2.3.2. Optical Properties using Ultraviolet–visible (UV-vis) Spectroscopy
2.3.3. Scanning Electron Microscopy
2.3.4. Thickness Measurement
2.3.5. Moisture Content
2.3.6. Water Solubility
2.3.7. Water Vapor Permeability (WVP)
2.3.8. Mechanical Properties
2.3.9. X-ray Diffraction
2.3.10. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.11. Thermogravimetric Analysis
2.3.12. Antioxidant Capacity
2.4. Experimental Design
3. Results and Discussion
3.1. Physical Properties of Apple Starch Films Harvested at DAFB
3.2. Characterization of Films with Ellagic Acid (EA) in the FILM-70 Formulation
3.2.1. Scanning Electron Microscopy (SEM) Analysis
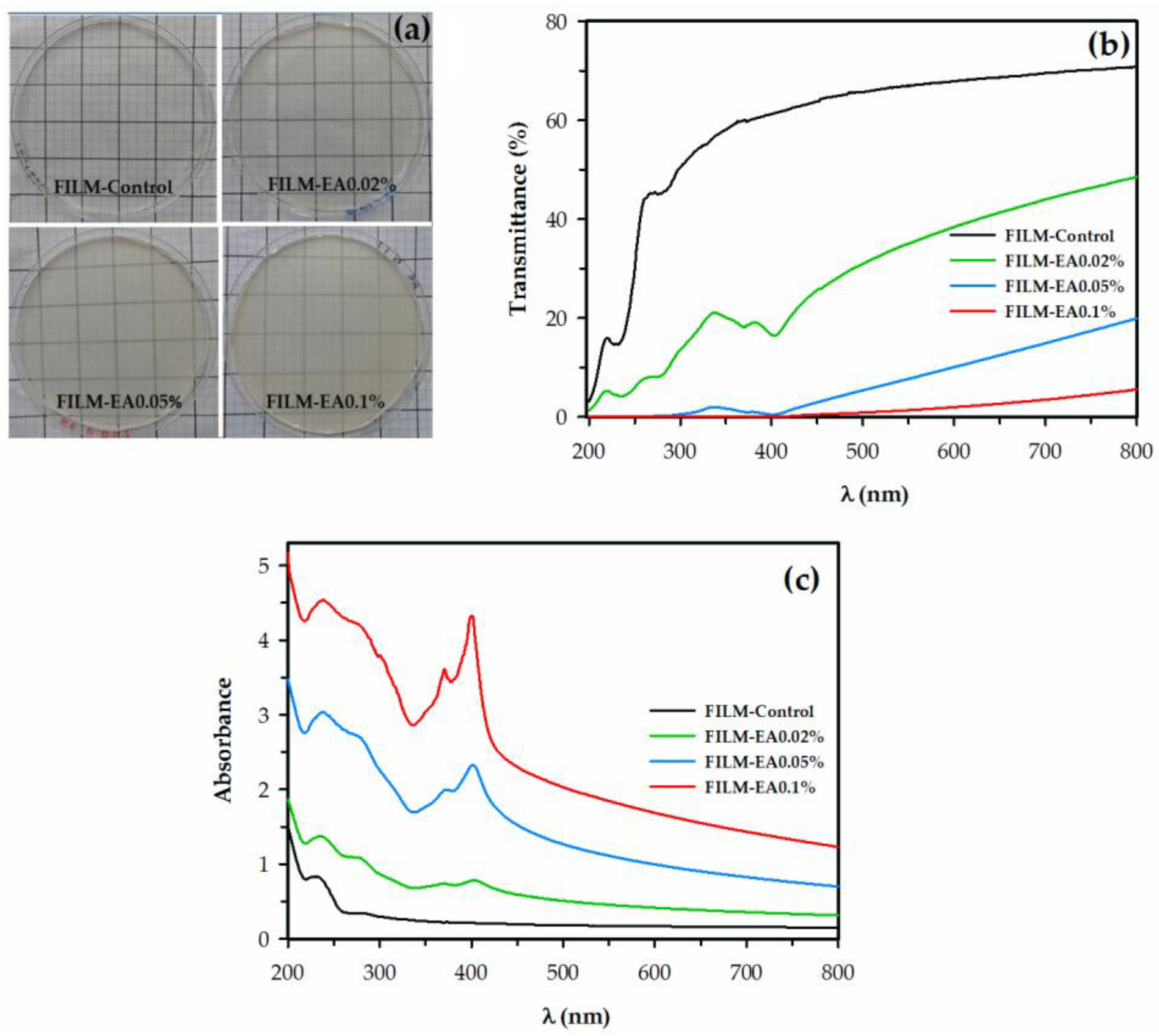
3.2.2. Color and UV-vis Spectroscopy
3.2.3. Moisture Content and Thickness of the Films
3.2.4. Solubility and Water Vapor Permeability (WVP)
3.2.5. Mechanical Properties
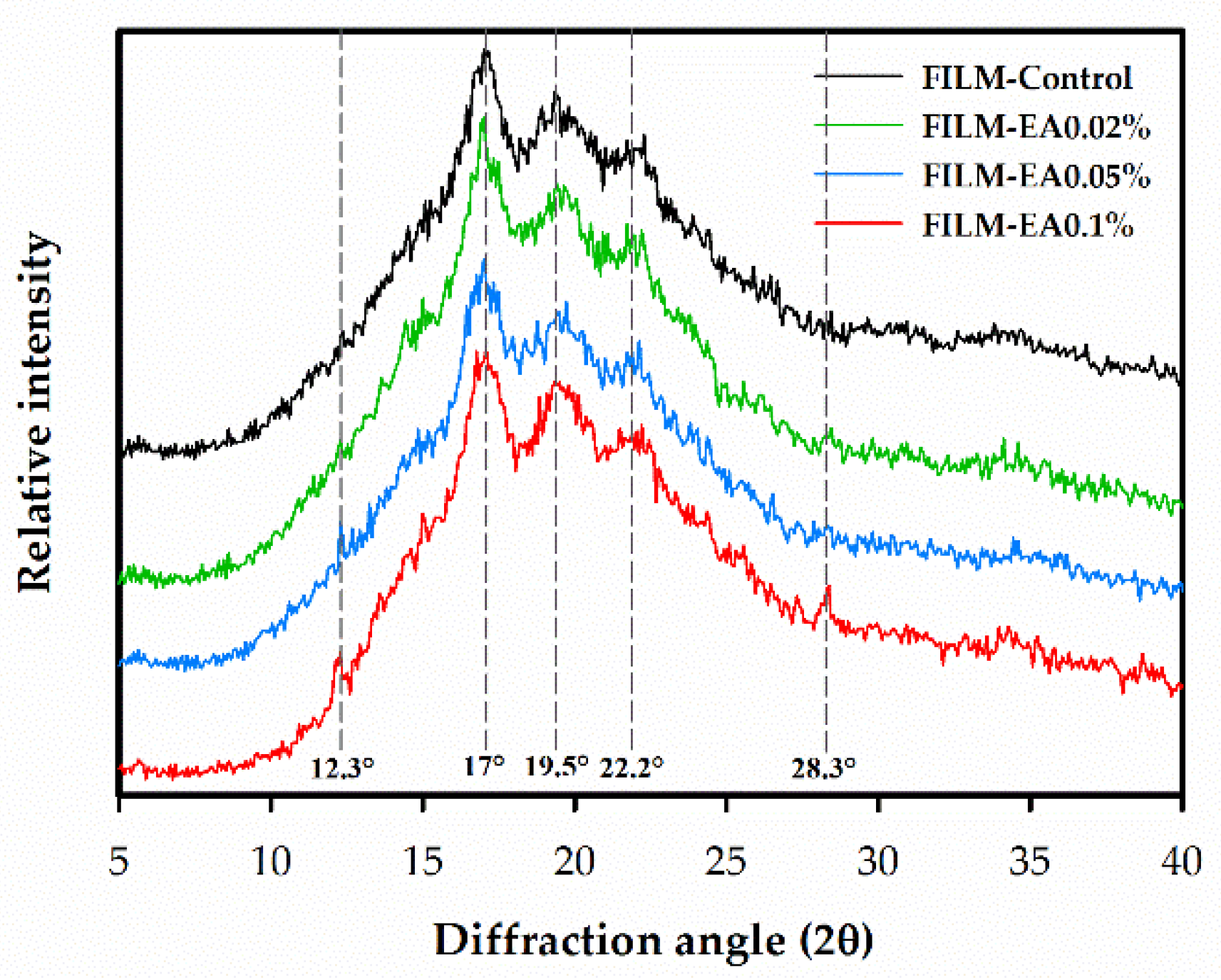
3.2.6. X-ray Diffraction and Crystallinity
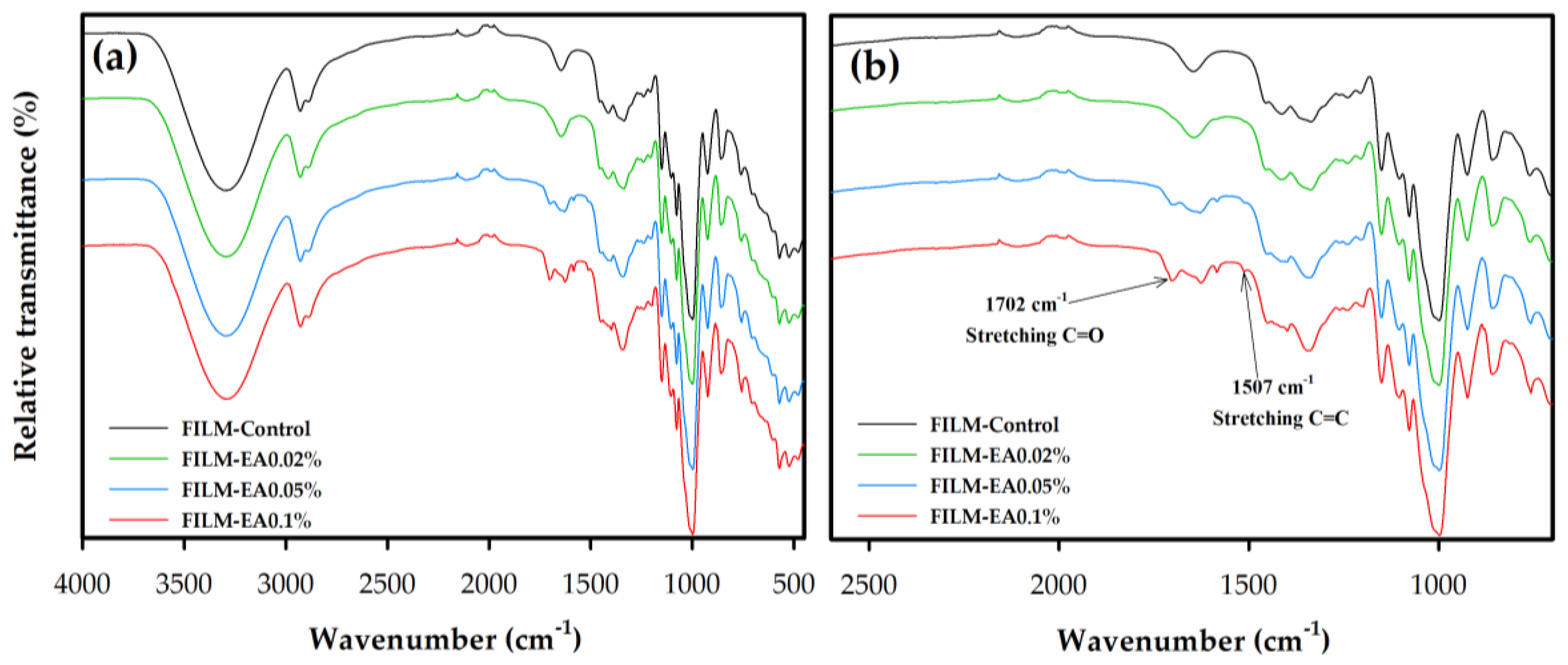
3.2.7. Fourier Transform Infrared Spectroscopy
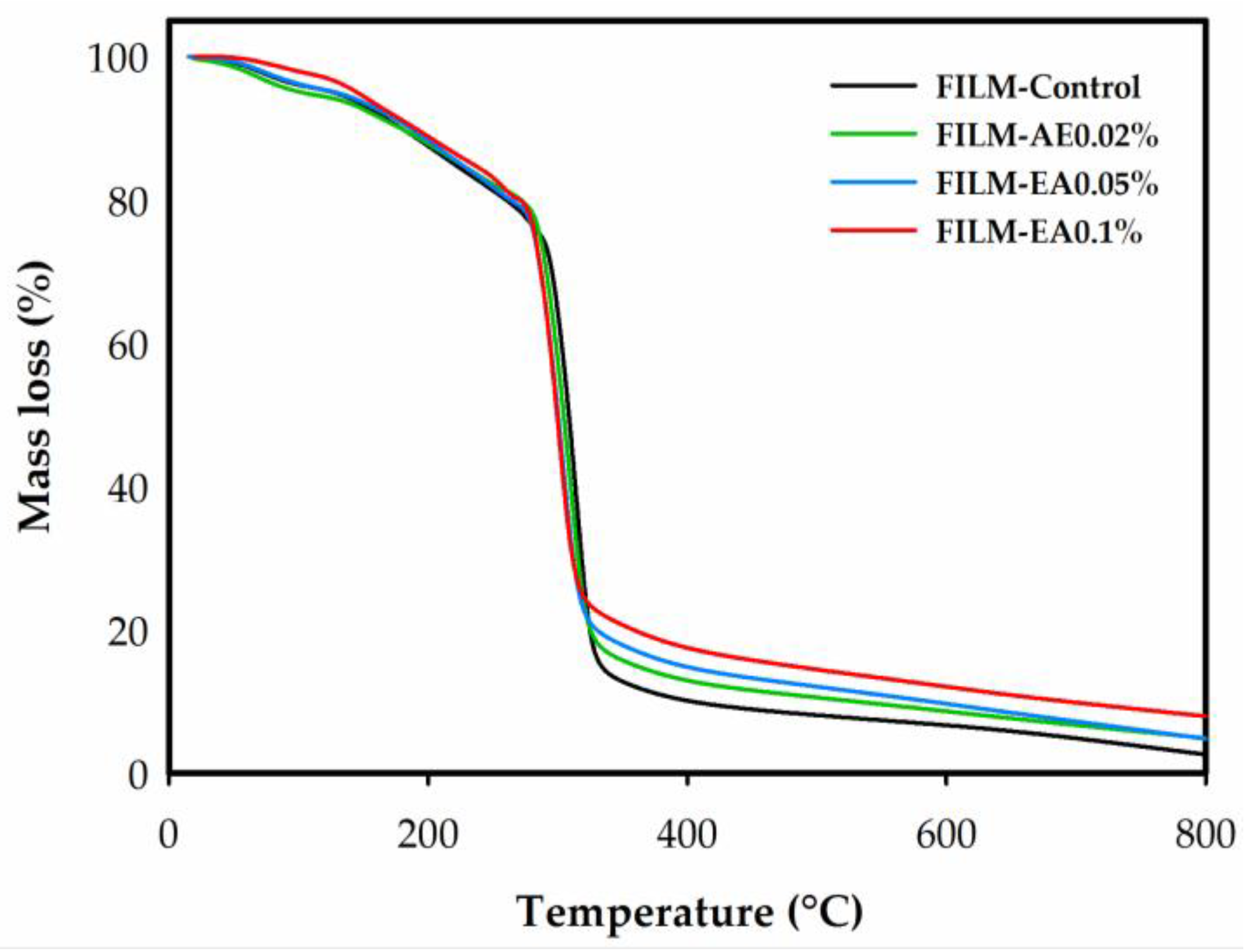
3.2.8. Thermogravimetric Analysis
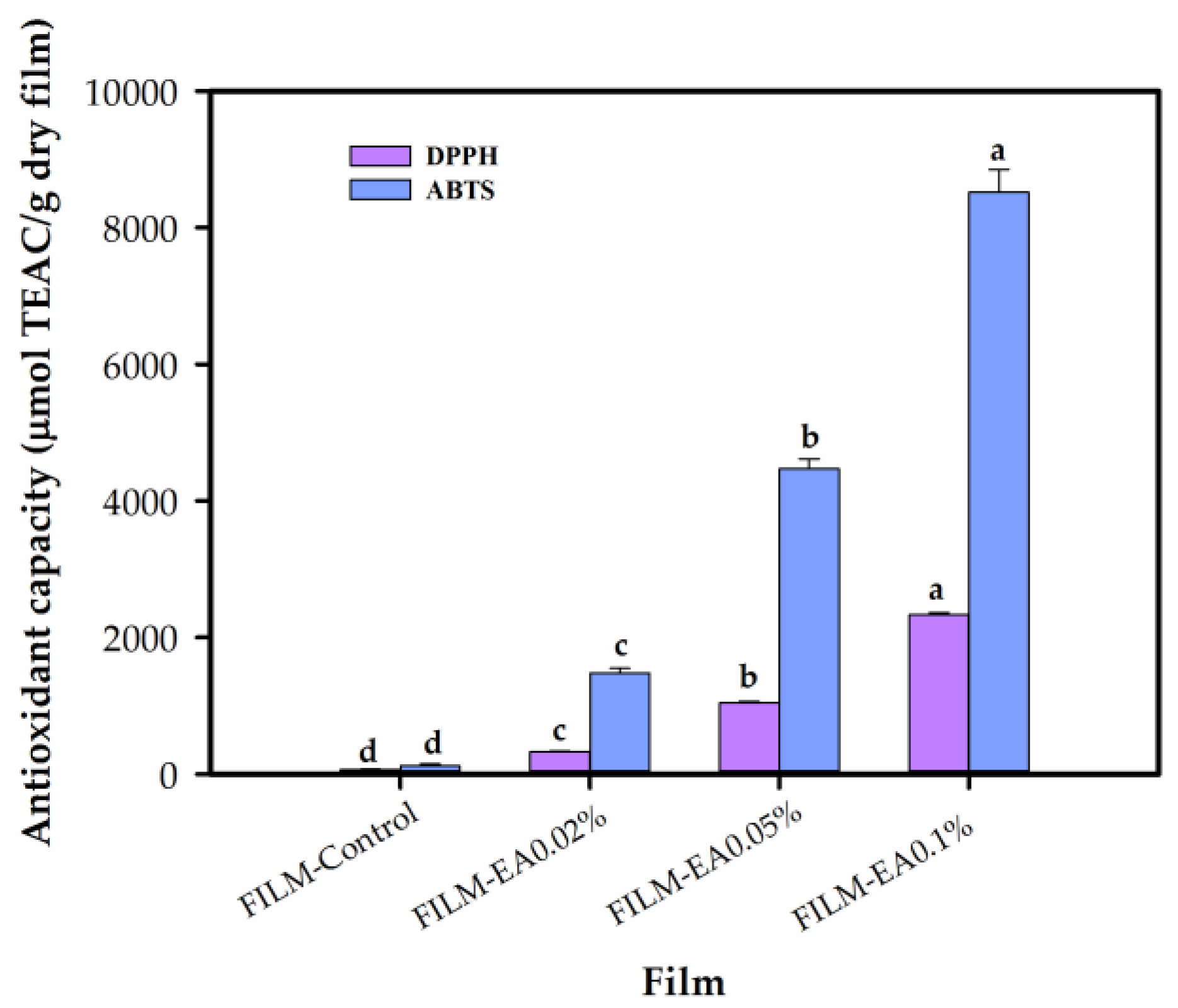
3.2.9. Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Reis, L.C.B.; de Souza, C.O.; da Silva, J.B.A.; Martins, A.C.; Nunes, I.L.; Druzian, J.I. Active biocomposites of cassava starch: The effect of yerba mate extract and mango pulp as antioxidant additives on the properties and the stability of a packaged product. Food Bioprod. Process. 2015, 94, 382–391. [Google Scholar] [CrossRef]
- Oniszczuk, T.; Wójtowicz, A.; Moácicki, L.; Mitrus, M.; Kupryaniuk, K.; Kusz, A.; Bartnik, G. Effect of natural fibres on the mechanical properties of thermoplastic starch. Int. Agrophys. 2016, 30, 211–218. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and biodegradable starch films: A review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- López, O.V.; Castillo, L.A.; García, M.A.; Villar, M.A.; Barbosa, S.E. Food packaging bags based on thermoplastic corn starch reinforced with talc nanoparticles. Food Hydrocoll. 2015, 43, 18–24. [Google Scholar] [CrossRef]
- Ashwar, B.A.; Shah, A.; Gani, A.; Shah, U.; Gani, A.; Wani, I.A.; Wani, S.M.; Masoodi, F.A. Rice starch active packaging films loaded with antioxidants—development and characterization. Starch Stärke 2015, 67, 294–302. [Google Scholar] [CrossRef]
- Zamudio-Flores, P.B.; Bello-Pérez, L.A. Elaboración y caracterización de películas de glicoproteínas obtenidas mediante reacción de Maillard utilizando almidón acetilado y aislado proteico de suero lácteo. Rev. Mex. Ing. Quím. 2013, 12, 401–413. (In Spanish) [Google Scholar]
- Sapper, M.; Chiralt, A. Starch-Based Coatings for Preservation of Fruits and Vegetables. Coatings 2018, 8, 152. [Google Scholar] [CrossRef]
- Yan, Q.; Hou, H.; Guo, P.; Dong, H. Effects of extrusion and glycerol content on properties of oxidized and acetylated corn starch-based films. Carbohydr. Polym. 2012, 87, 707–712. [Google Scholar] [CrossRef]
- Chandra mohan, C.; Rakhavan, K.R.; Sudharsan, K.; Radha krishnan, K.; Babuskin, S.; Sukumar, M. Design and characterization of spice fused tamarind starch edible packaging films. LWT Food Sci. Technol. 2016, 68, 642–652. [Google Scholar] [CrossRef]
- Famá, L.; Flores, S.K.; Gerschenson, L.; Goyanes, S. Physical characterization of cassava starch biofilms with special reference to dynamic mechanical properties at low temperatures. Carbohydr. Polym. 2006, 66, 8–15. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Barrier, mechanical and optical properties of plasticized yam starch films. Carbohydr. Polym. 2004, 56, 129–135. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizer type and concentration on tensile, thermal and barrier properties of biodegradable films based on sugar palm (Arenga pinnata) starch. Polymers 2015, 7, 1106–1124. [Google Scholar] [CrossRef]
- Sartori, T.; Menegalli, F.C. Development and characterization of unripe banana starch films incorporated with solid lipid microparticles containing ascorbic acid. Food Hydrocoll. 2016, 55, 210–219. [Google Scholar] [CrossRef]
- Zamudio-Flores, P.B.; Vargas-Torres, A.; Pérez-González, J.; Bosquez-Molina, E.; Bello-Pérez, L.A. Films prepared with oxidized banana starch: Mechanical and barrier properties. Starch Stärke 2006, 58, 274–282. [Google Scholar] [CrossRef]
- Daudt, R.M.; Avena-Bustillos, R.J.; Williams, T.; Wood, D.F.; Külkamp-Guerreiro, I.C.; Marczak, L.D.F.; McHugh, T.H. Comparative study on properties of edible films based on pinhão (Araucaria angustifolia) starch and flour. Food Hydrocoll. 2016, 60, 279–287. [Google Scholar] [CrossRef]
- Tirado-Gallegos, J.M.; Zamudio-Flores, P.B.; Ornelas-Paz, J.d.J.; Rios-Velasco, C.; Acosta-Muñiz, C.H.; Gutiérrez-Meraz, F.; Islas-Hernández, J.J.; Salgado-Delgado, R. Efecto del método de aislamiento y el estado de madurez en las propiedades fisicoquímicas, estructurales y reológicas de almidón de manzana. Rev. Mex. Ing. Quím. 2016, 15, 391–408. (In Spanish) [Google Scholar]
- Stevenson, D.G.; Domoto, P.A.; Jane, J.-L. Structures and functional properties of apple (Malus domestica Borkh) fruit starch. Carbohydr. Polym. 2006, 63, 432–441. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, L.; Zhang, L.; Kang, R.; Yu, Z. Dynamic changes in proteins during apple (Malus x domestica) fruit ripening and storage. Hortic. Res. 2014, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Berlanga Reyes, D.I.; Romo Chacón, A.; Martínez Campos, Á.R.; Guerrero Prieto, V.M. Apple fruit chemical thinning in Chihuahua, Mexico. Rev. Fitotec. Mex. 2008, 31, 243–250. [Google Scholar]
- Rascón-Chu, A.; Martínez-López, A.-L.; Carvajal-Millán, E.; Martínez-Robinson, K.G.; Campa-Mada, A.C. Gelificación iónica de pectina de bajo grado de esterificación extraída de manzanas inmaduras de raleo. Rev. Fitotec. Mex. 2016, 39, 17–24. (In Spanish) [Google Scholar]
- Berlanga-Reyes, D.I.; Rios-Velasco, C.; Romo-Chacón, A.; Guerrero-Prieto, V.M. Raleo químico de flores de manzano (Malus x domestica Borkh.) ‘Golden Delicious’ y ‘RedChief Delicious’. Tecnociencia Chihuah. 2012, 6, 147–157. (In Spanish) [Google Scholar]
- Romero, V.; Borneo, R.; Passalacqua, N.; Aguirre, A. Biodegradable films obtained from triticale (x Triticosecale Wittmack) flour activated with natamycin for cheese packaging. J. Food Packag. Shelf Life 2016, 10, 54–59. [Google Scholar] [CrossRef]
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Development and characterization of active films based on starch-PVA, containing silver nanoparticles. J. Food Packag. Shelf Life 2016, 10, 16–24. [Google Scholar] [CrossRef]
- Shah, U.; Gani, A.; Ashwar, B.A.; Shah, A.; Ahmad, M.; Gani, A.; Wani, I.A.; Masoodi, F.A.; Yildiz, F. A review of the recent advances in starch as active and nanocomposite packaging films. Cogent Food Agric. 2015, 1, 1115640. [Google Scholar] [CrossRef]
- Mehyar, G.F.; Han, J.H. Physical and mechanical properties of high-amylose rice and pea starch films as affected by relative humidity and plasticizer. J. Food Sci. 2004, 69, E449–E454. [Google Scholar] [CrossRef]
- De Araújo, G.K.P.; de Souza, S.J.; da Silva, M.V.; Yamashita, F.; Gonçalves, O.H.; Leimann, F.V.; Shirai, M.A. Physical, antimicrobial and antioxidant properties of starch-based film containing ethanolic propolis extract. Int. J. Food Sci. Technol. 2015, 50, 2080–2087. [Google Scholar] [CrossRef]
- Corrales, M.; Han, J.H.; Tauscher, B. Antimicrobial properties of grape seed extracts and their effectiveness after incorporation into pea starch films. Int. J. Food Sci. Technol. 2009, 44, 425–433. [Google Scholar] [CrossRef]
- Medina, J.C.; Gutiérrez, T.J.; Goyanes, S.; Bernal, C.; Famá, L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr. Polym. 2016, 151, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Liu, Y.; Gaber, M.W.; Bumgardner, J.D.; Haggard, W.O.; Yang, Y. Development of chitosan–ellagic acid films as a local drug delivery system to induce apoptotic death of human melanoma cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Baek, B.; Lee, S.H.; Kim, K.; Lim, H.-W.; Lim, C.-J. Ellagic acid plays a protective role against UV-B-induced oxidative stress by up-regulating antioxidant components in human dermal fibroblasts. Korean J. Physiol. Pharmacol. 2016, 20, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Kilic, I.; Yeşiloğlu, Y.; Bayrak, Y. Spectroscopic studies on the antioxidant activity of ellagic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Bala, I.; Bhardwaj, V.; Hariharan, S.; Kumar, M.R. Analytical methods for assay of ellagic acid and its solubility studies. J. Pharm. Biomed. Anal. 2006, 40, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-Pompa, S.; Jasso-Cantu, D.; Ventura-Sobrevilla, J.; SÁEnz-Galindo, A.; RodrÍGuez-Herrera, R.; Aguilar, C.N. Effect of candelilla wax with natural antioxidants on the shelf life quality of fresh-cut fruits. J. Food Qual. 2007, 30, 823–836. [Google Scholar] [CrossRef]
- Saucedo-Pompa, S.; Rojas-Molina, R.; Aguilera-Carbó, A.F.; Saenz-Galindo, A.; Garza, H.d.L.; Jasso-Cantú, D.; Aguilar, C.N. Edible film based on candelilla wax to improve the shelf life and quality of avocado. Food Res. Int. 2009, 42, 511–515. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; Garcia, M.A.; Martino, M.N.; Zaritzky, N.E. Microstructural characterization of yam starch films. Carbohydr. Polym. 2002, 50, 379–386. [Google Scholar] [CrossRef]
- Zamudio-Flores, P.B.; Ochoa-Reyes, E.; Ornelas-Paz, J.D.J.; Tirado-Gallegos, J.M.; Bello-Pérez, L.A.; Rubio-Ríos, A.; Cárdenas-Felix, R.G. Caracterización fisicoquímica, mecánica y estructural de películas de almidones oxidados de avena y plátano adicionadas con betalaínas. Agrociencia 2015, 49, 483–498. (In Spanish) [Google Scholar]
- Luchese, C.L.; Garrido, T.; Spada, J.C.; Tessaro, I.C.; de la Caba, K. Development and characterization of cassava starch films incorporated with blueberry pomace. Int. J. Biol. Macromol. 2018, 106, 834–839. [Google Scholar] [CrossRef] [PubMed]
- López-Mata, M.A.; Ruiz-Cruz, S.; Silva-Beltrán, N.P.; Ornelas-Paz, J.D.J.; Zamudio-Flores, P.B.; Burruel-Ibarra, S.E. Physicochemical, antimicrobial and antioxidant properties of chitosan films incorporated with carvacrol. Molecules 2013, 18, 13735–13753. [Google Scholar] [CrossRef] [PubMed]
- Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemist: Washington, DC, USA, 1995.
- Colla, E.; do Amaral Sobral, P.J.; Menegalli, F.C. Amaranthus cruentus flour edible films: Influence of stearic acid addition, plasticizer concentration and emulsion stirring speed on water vapor permeability and mechanical properties. J. Agric. Food Chem. 2006, 54, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- ASTM-E-96-80 Standard Methods of Test for Water Vapor Transmission of Materials in Sheet Form; ASTM International: West Conshohocken, PA, USA, 2016.
- Mali, S.; Sakanaka, L.S.; Yamashita, F.; Grossmann, M.V.E. Water sorption and mechanical properties of cassava starch films and their relation to plasticizing effect. Carbohydr. Polym. 2005, 60, 283–289. [Google Scholar] [CrossRef]
- ASTM-882-95a Standard Test Methods for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 1995.
- Teodoro, A.P.; Mali, S.; Romero, N.; de Carvalho, G.M. Cassava starch films containing acetylated starch nanoparticles as reinforcement: Physical and mechanical characterization. Carbohydr. Polym. 2015, 126, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Nouri, L.; Mohammadi, N.A. Antibacterial, mechanical and barrier properties of sago starch film incorporated with betel leaves extract. Int. J. Biol. Macromol. 2014, 66, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Medina-Jaramillo, C.; Ochoa-Yepes, O.; Bernal, C.; Famá, L. Active and smart biodegradable packaging based on starch and natural extracts. 2017, 176, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Shiku, Y.; HamaguchI, P.Y.; Tanaka, M. Effect of pH on the preparation of edible films based on fish myofibrillar proteins. Fish. Sci. 2003, 69, 1026–1032. [Google Scholar] [CrossRef]
- García, M.A.; Martino, M.N.; Zaritzky, N.E. Lipid addition to improve barrier properties of edible starch-based films and coatings. J. Food Sci. 2000, 65, 941–944. [Google Scholar] [CrossRef]
- Versino, F.; García, M.A. Cassava (Manihot esculenta) starch films reinforced with natural fibrous filler. Ind. Crops Prod. 2014, 58, 305–314. [Google Scholar] [CrossRef]
- Arulmozhi, V.; Pandian, K.; Mirunalini, S. Ellagic acid encapsulated chitosan nanoparticles for drug delivery system in human oral cancer cell line (KB). Colloids Surf. B Biointerface 2013, 110, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, D.; Panayiotou, C. LDPE/starch blends compatibilized with PE-g-MA copolymers. J. Appl. Polym. Sci. 1998, 70, 1503–1521. [Google Scholar] [CrossRef]
- Wu, Y.-P.; Ji, M.-Q.; Qi, Q.; Wang, Y.-Q.; Zhang, L.-Q. Preparation, structure and properties of starch/rubber composites prepared by co-coagulating rubber latex and starch paste. Macromol. Rapid. Commun. 2004, 25, 565–570. [Google Scholar] [CrossRef]
- Chai, Y.; Wang, M.; Zhang, G. Interaction between amylose and tea polyphenols modulates the postprandial glycemic response to high-amylose maize starch. J. Agric. Food Chem. 2013, 61, 8608–8615. [Google Scholar] [CrossRef] [PubMed]
- Pelissari, F.M.; Andrade-Mahecha, M.M.; do Amaral Sobral, P.J.; Menegalli, F.C. Isolation and characterization of the flour and starch of plantain bananas (Musa paradisiaca). Starch Stärke 2012, 64, 382–391. [Google Scholar] [CrossRef]
- Mano, J.F.; Koniarova, D.; Reis, R.L. Thermal properties of thermoplastic starch/synthetic polymer blends with potential biomedical applicability. J. Mater. Sci. Mater. Med. 2003, 14, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Jha, S.; Kumar, K.J. Isolation and release characteristics of starch from the rhizome of Indian Palo. Int. J. Biol. Macromol. 2015, 72, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.Z.; Al Ali, S.H.; Zainal, Z.; Hakim, M.N. Development of antiproliferative nanohybrid compound with controlled release property using ellagic acid as the active agent. Int. J. Nanomed. 2011, 6, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiu, C.; Ji, N.; Sun, C.; Xiong, L.; Sun, Q. Mechanical, barrier and morphological properties of starch nanocrystals-reinforced pea starch films. Carbohydr. Polym. 2015, 121, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Perazzo, K.K.N.C.L.; de Vasconcelos Conceição, A.C.; dos Santos, J.C.P.; de Jesus Assis, D.; Souza, C.O.; Druzian, J.I. Properties and antioxidant action of actives cassava starch films incorporated with green tea and palm oil extracts. PLoS ONE 2014, 9, e105199. [Google Scholar] [CrossRef] [PubMed]

| Analyzed Variable | Film | |||
|---|---|---|---|---|
| FILM-60 | FILM-70 | FILM-80 | FILM-90 | |
| Moisture (%) | 23.11 ± 0.49 a | 21.56 ± 0.45 b | 22.84 ± 0.39 ab | 21.33 ± 0.33 b |
| Solubility (%) | 18.55 ± 1.01 ab | 19.89 ± 0.49 a | 21.36 ± 0.89 a | 15.62 ± 0.62 b |
| Thickness (µm) | 102.30 ± 4.39 a | 100.28 ± 2.74a | 99.33 ± 1.94 a | 96.73 ± 1.48 a |
| Transparency | 1.14 ± 0.02 b | 1.18 ± 0.01 b | 1.39 ± 0.04 a | 1.06 ± 0.02b c |
| Color | − | − | − | − |
| L* | 95.73 ± 0.09 a | 95.81 ± 0.14 a | 95.77 ± 0.11 a | 95.46 ± 0.19 a |
| a* | 0.21 ± 0.01 ab | 0.19 ± 0.01 b | 0.18 ± 0.01 b | 0.25 ± 0.01 a |
| b* | 2.04 ± 0.05 a | 2.14 ± 0.05 a | 2.15 ± 0.04 a | 2.02 ± 0.04 a |
| Mechanical properties | − | − | − | − |
| TS (MPa) | 4.70 ± 0.15 b | 8.12 ± 0.36 a | 7.37 ± 0.02 a | 7.14 ± 0.35 a |
| %E (%) | 54.99 ± 4.04 a | 52.12 ± 4.30 a | 56.59 ± 0.56 a | 56.35 ± 2.79 a |
| EM (MPa) | 0.92 ± 0.14 c | 3.10 ± 0.27 a | 2.03 ± 0.11 ab | 1.71 ± 0.19 bc |
| Crystallinity (%) | 28.29 ± 1.25 a | 29.66 ± 2.52 a | 33.50 ± 1.82 a | 28.47 ± 1.20 a |
| WVP × 10−11 (g m−1 s−1 Pa−1) | 11.97 ± 1.09 a | 6.77 ± 0.85 b | 7.36 ± 0.29 b | 9.71 ± 0.25 ab |
| Analyzed Variable | Film | |||
|---|---|---|---|---|
| FILM-Control | FILM-EA0.02% | FILM- EA0.05% | FILM-EA0.1% | |
| Transparency | 1.51 ± 0.09 d | 3.73 ± 0.27 c | 8.46 ± 0.194 b | 17.45 ± 0.55 a |
| Color | − | − | − | − |
| L* | 95.87 ± 0.07 a | 95.11 ± 0.13 b | 94.13 ± 0.17 c | 92.32 ± 0.14 d |
| a* | 0.22 ± 0.01 a | −0.82 ± 0.03 b | −1.24 ± 0.03 c | −1.31 ± 0.02 c |
| b* | 2.73 ± 0.06 d | 6.77 ± 0.20 c | 10.52 ± 0.29 b | 14.48 ± 0.18 a |
| WI | 95.04 ± 0.08 a | 91.60 ± 0.24 b | 87.89 ± 0.33 c | 83.8 ± 83.56 d |
| Moisture (%) | 23.83 ± 0.88 a | 21.52 ± 0.49 ab | 22.92 ± 0.49 ab | 21.23 ± 0.32 b |
| Solubility (%) | 20.51 ± 0.12 b | 21.79 ± 0.43 ab | 22.02 ± 0.93 a | 23.79 ± 0.20 a |
| Thickness (µm) | 102.79 ± 3.67 a | 102.79 ± 3.67 a | 104.42 ± 3.61 a | 103. 53 ± 4.17 a |
| Mechanical properties | − | − | − | − |
| TS (MPa) | 6.51 ± 0.18 b | 8.98 ± 0.28 a | 9.63 ± 0.52 a | 8.21 ± 0.45 a |
| %E (%) | 65.11 ± 2.98 a | 62.48 ± 2.98 ab | 56.91 ± 3.01 ab | 52.44 ± 1.21 b |
| EM (MPa) | 1.79 ± 0.19 c | 2.78 ± 0.13 bc | 4.58 ± 0.42 a | 3.63 ± 0.32 ab |
| Crystallinity (%) | 28.81 ± 1.69 a | 28.63 ± 0.64 a | 28.99 ± 0.27 a | 31.39 ± 1.66 a |
| WVP × 10−11 (g m−1 s−1 Pa−1) | 6.59 ± 0.28 b | 7.46 ± 0.34 a | 6.65 ± 0.19 ab | 6.35 ± 0.22 b |
| Film | T90 (°C) | MC100 (%) | Tonset (°C) | Tmax (°C) | RM800 (%) |
|---|---|---|---|---|---|
| FILM-Control | 176.83 ± 1.08 a | 4.11 ± 0.22 a | 291.20 ± 0.76 a | 313.77 ± 0.83 a | 1.63 ± 0.62 a |
| FILM-0.02%EA | 170.86 ± 6.89 a | 5.02 ± 0.47 a | 280.63 ± 2.33 b | 309.16 ± 1.27 b | 4.61 ± 0.15 b |
| FILM-0.05%EA | 180.30 ± 3.10 a | 3.95 ± 0.21 a | 278.37 ± 0.42 b | 302.63 ± 0.33 c | 5.31 ± 0.33 b |
| FILM-0.10%EA | 179.86 ± 14.68 a | 3.70 ± 1.22 a | 278.50 ± 0.80 b | 301.29 ± 0.01 c | 7.58 ± 0.29 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirado-Gallegos, J.M.; Zamudio-Flores, P.B.; Ornelas-Paz, J.d.J.; Rios-Velasco, C.; Olivas Orozco, G.I.; Espino-Díaz, M.; Baeza-Jiménez, R.; Buenrostro-Figueroa, J.J.; Aguilar-González, M.A.; Lardizábal-Gutiérrez, D.; et al. Elaboration and Characterization of Active Apple Starch Films Incorporated with Ellagic Acid. Coatings 2018, 8, 384. https://doi.org/10.3390/coatings8110384
Tirado-Gallegos JM, Zamudio-Flores PB, Ornelas-Paz JdJ, Rios-Velasco C, Olivas Orozco GI, Espino-Díaz M, Baeza-Jiménez R, Buenrostro-Figueroa JJ, Aguilar-González MA, Lardizábal-Gutiérrez D, et al. Elaboration and Characterization of Active Apple Starch Films Incorporated with Ellagic Acid. Coatings. 2018; 8(11):384. https://doi.org/10.3390/coatings8110384
Chicago/Turabian StyleTirado-Gallegos, Juan Manuel, Paul Baruk Zamudio-Flores, José de Jesús Ornelas-Paz, Claudio Rios-Velasco, Guadalupe Isela Olivas Orozco, Miguel Espino-Díaz, Ramiro Baeza-Jiménez, José Juan Buenrostro-Figueroa, Miguel Angel Aguilar-González, Daniel Lardizábal-Gutiérrez, and et al. 2018. "Elaboration and Characterization of Active Apple Starch Films Incorporated with Ellagic Acid" Coatings 8, no. 11: 384. https://doi.org/10.3390/coatings8110384
APA StyleTirado-Gallegos, J. M., Zamudio-Flores, P. B., Ornelas-Paz, J. d. J., Rios-Velasco, C., Olivas Orozco, G. I., Espino-Díaz, M., Baeza-Jiménez, R., Buenrostro-Figueroa, J. J., Aguilar-González, M. A., Lardizábal-Gutiérrez, D., Hernández-González, M., Hernández-Centeno, F., & López-De la Peña, H. Y. (2018). Elaboration and Characterization of Active Apple Starch Films Incorporated with Ellagic Acid. Coatings, 8(11), 384. https://doi.org/10.3390/coatings8110384






