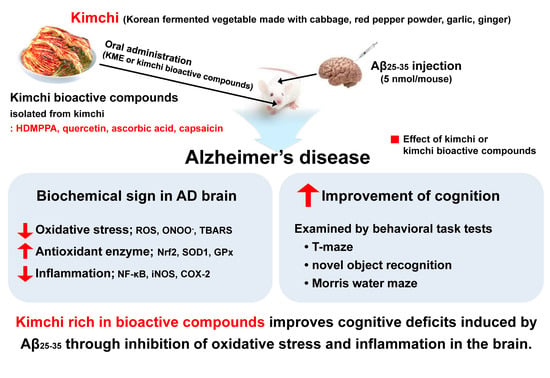
Bioactive Compounds in Kimchi Improve the Cognitive and Memory Functions Impaired by Amyloid Beta
Abstract
1. Introduction
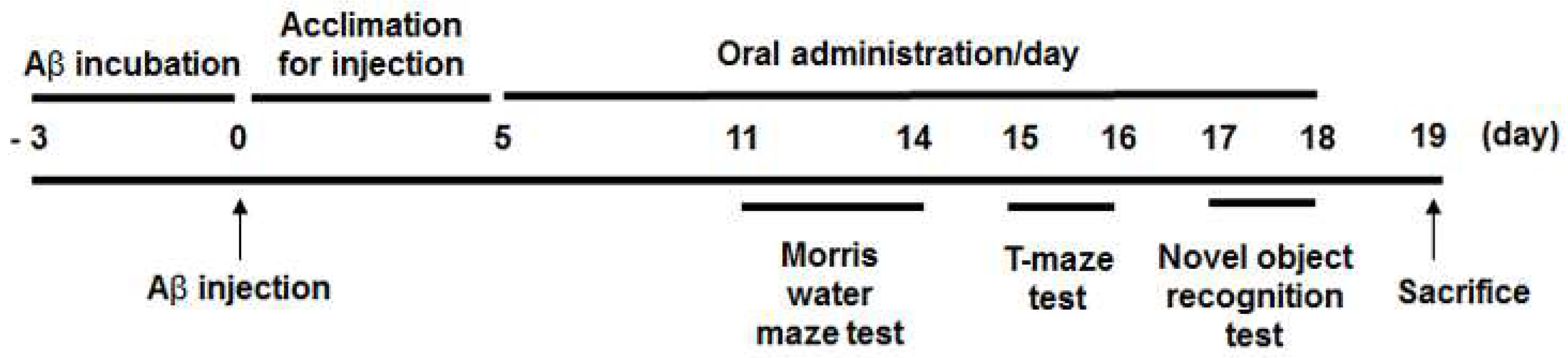
2. Materials and Methods
2.1. Kimchi Bioactive Compounds and Kimchi Methanol Extract
2.2. Animals and Intracerebroventricular Injection of Aβ25-35
2.3. Morris Water Maze Test
2.4. Novel Object Recognition Test
2.5. T-maze Test
2.6. Reactive oxygen species, Peroxynitrite, Thiobarbituric Acid Reactive Substances, and Glutathione Levels in the Brain
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
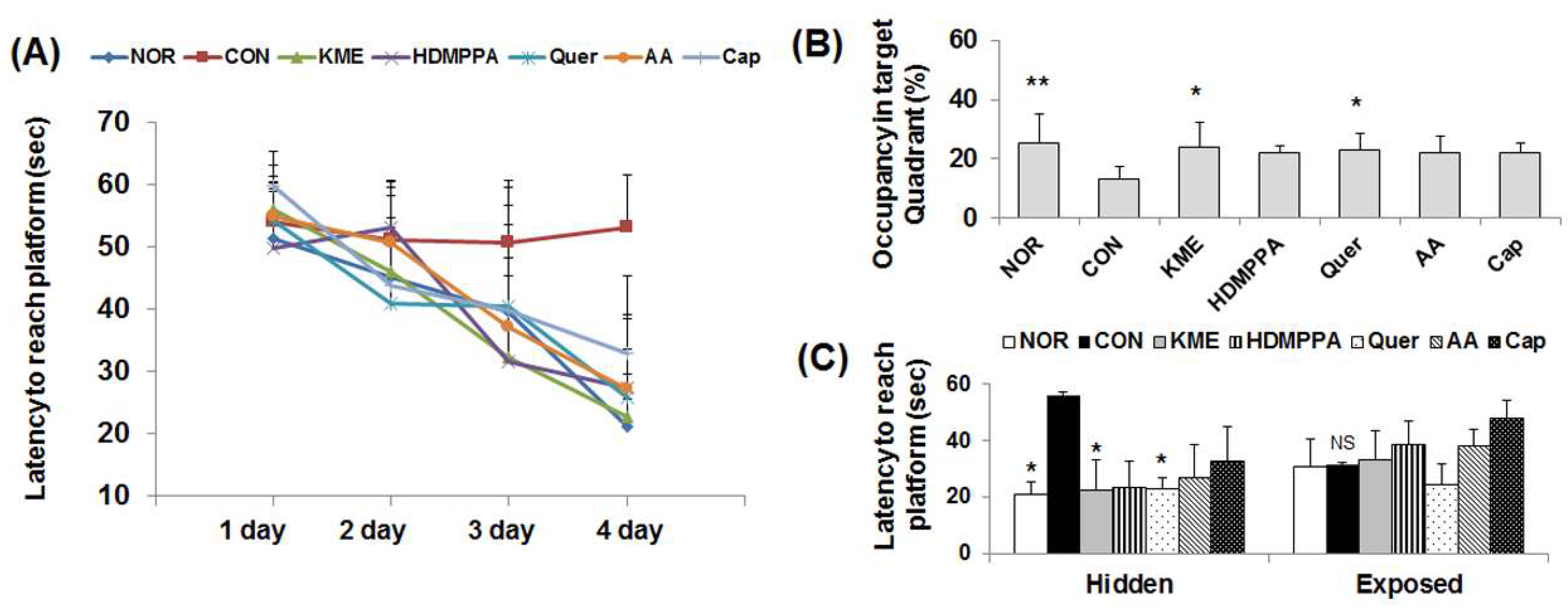
3.1. Morris Water Maze Test
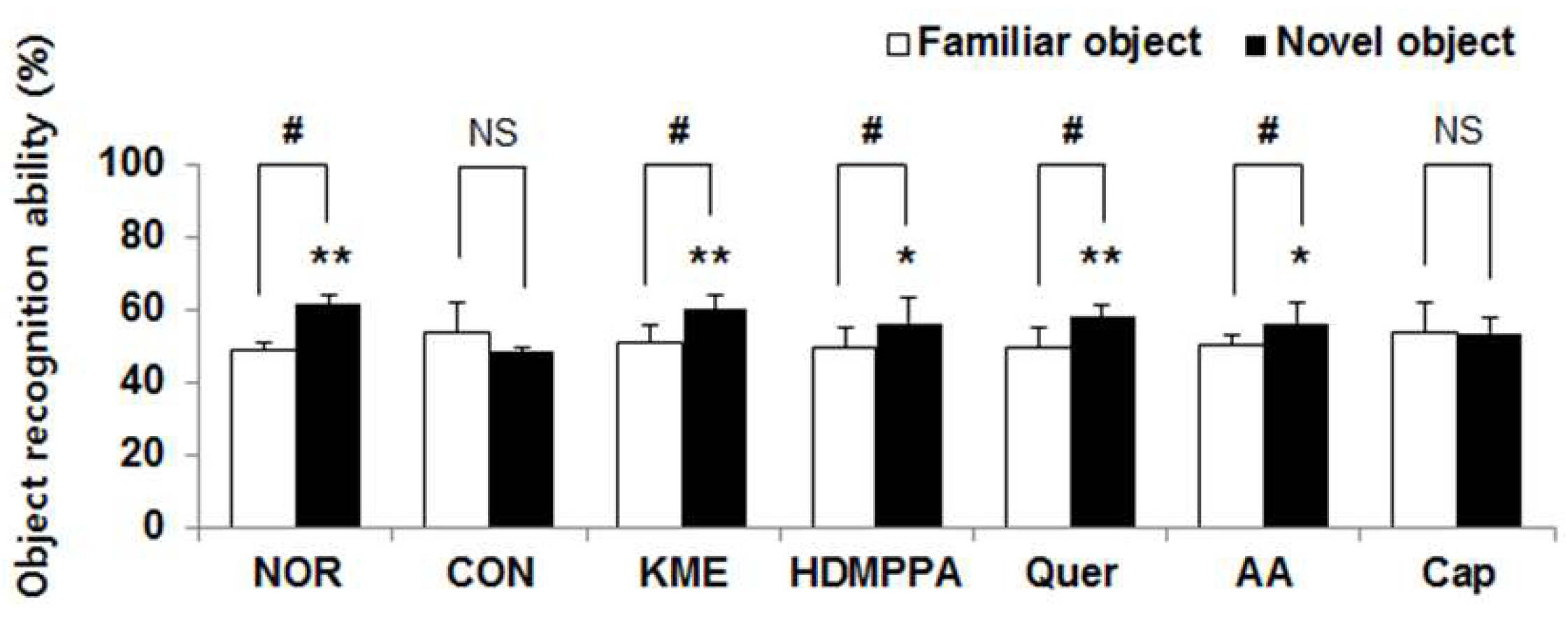
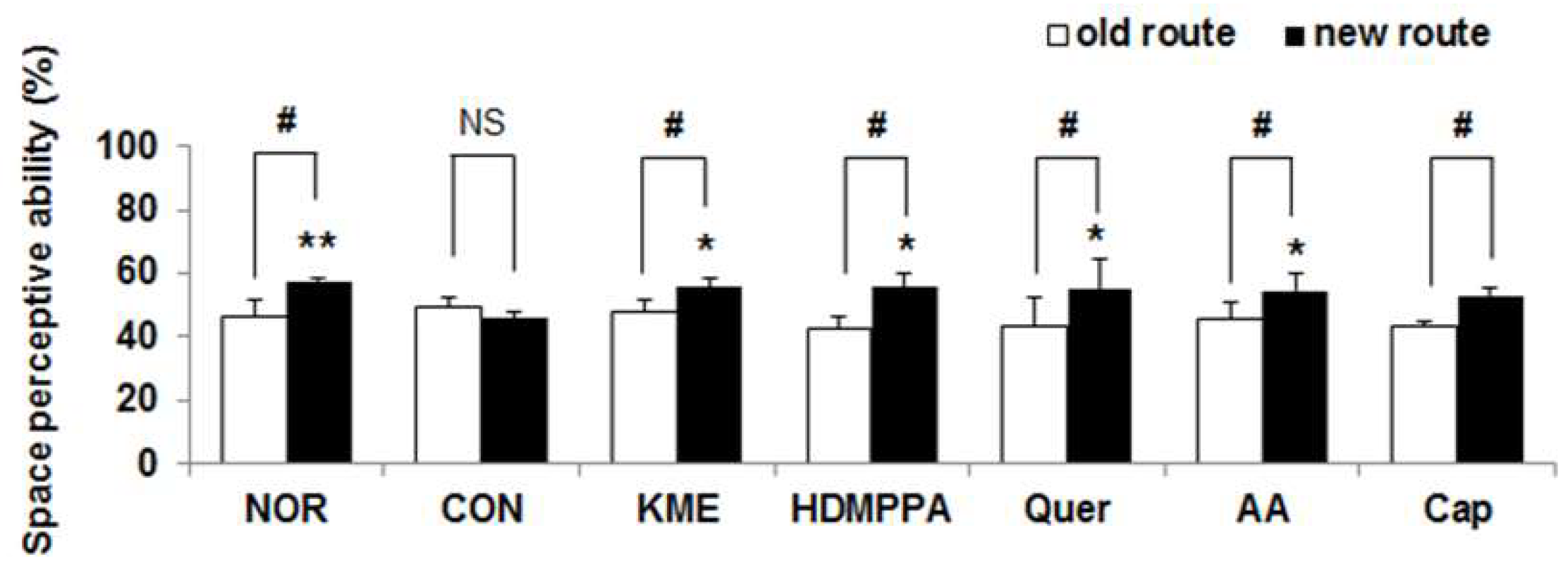
3.2. Novel Object Recognition Task
3.3. T-Maze Test
3.4. Inhibition of Oxidative Stress in the Brain Tissue
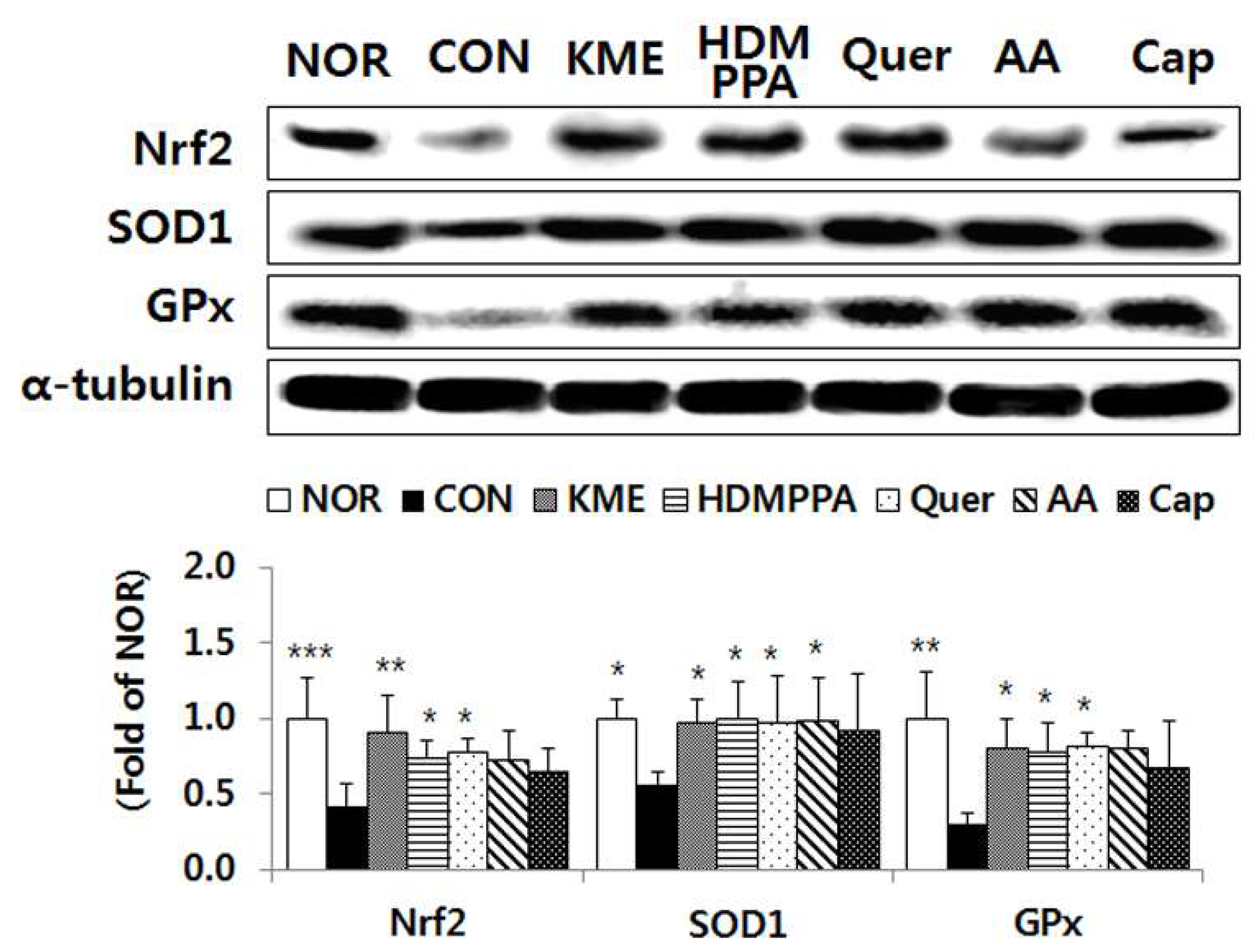
3.5. Elevation of the Antioxidative Status in the Brain Tissue
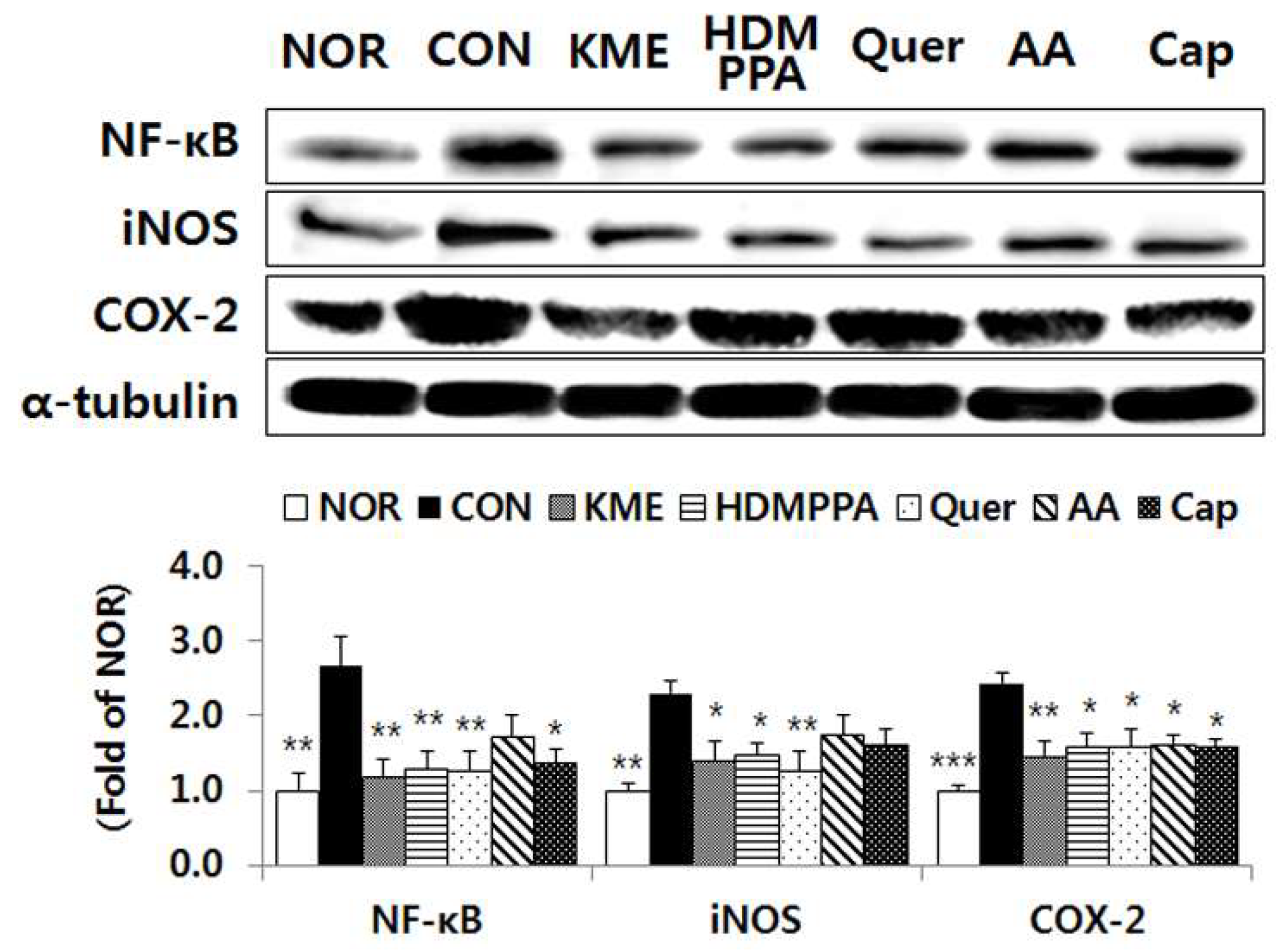
3.6. Suppression of the Inflammatory Response in the Brain Tissue
4. Discussion
Author Contributions
Acknowledgement
Conflicts of Interest
References
- Behl, C.; Moosmann, B. Antioxidant neuroprotection in Alzheimer’s disease as preventive and therapeutic approach. Free Radic. Biol. Med. 2002, 33, 182–191. [Google Scholar] [CrossRef]
- Lee, A.Y.; Hwang, B.R.; Lee, M.H.; Lee, S.; Cho, E.J. Perilla frutescens var. japonica and rosmarinic acid improve amyloid-β25-35 induced impairment of cognition and memory function. Nutr. Res. Pract. 2016, 10, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Lee, S.; Park, K.Y.; Kang, S.A.; Cho, E.J. Protective Effect of Kimchi against Aβ25-35-induced Impairment of Cognition and Memory. J. Korean Soc. Food Sci. Nutr. 2014, 43, 360–366. [Google Scholar] [CrossRef]
- Bevins, R.A.; Besheer, J. Object recognition in rats and mice: A one-trial non-matching-to-sample learning task to study “recognition memory”. Nat. Protoc. 2006, 1, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, K.C. A test of two explanations of spontaneous alternation. J. Comp. Physiol. Psychol. 1952, 45, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Essa, M.M.; Vijayan, R.K.; Castellano-Gonzalez, G.; Memon, M.A.; Braidy, N.; Guillemin, G.J. Neuroprotective effect of natural products against Alzheimer’s disease. Neurochem. Res. 2012, 37, 1829–1842. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cheng, X.; Li, H.; Qiu, F.; Yang, N.; Wang, B.; Lu, H.; Wu, H.; Shen, Y.; Wang, Y. Quercetin reduces oxidative stress and inhibits activation of c-Jun N-terminal kinase/activator protein-1 signaling in an experimental mouse model of abdominal aortic aneurysm. Mol. Med. Rep. 2014, 9, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Deng, X.; Liu, N.; Li, M.; Liu, B.; Fu, Q.; Qu, R.; Ma, S. Quercetin attenuates tau hyperphosphorylation and improves cognitive disorder via suppression of ER stress in a manner dependent on AMPK pathway. J. Funct. Foods 2016, 22, 463–476. [Google Scholar] [CrossRef]
- Kim, B.K.; Choi, J.M.; Kang, S.A.; Park, K.Y.; Cho, E.J. Antioxidative effects of Kimchi under different fermentation stage on radical-induced oxidative stress. Nutr. Res. Pract. 2014, 8, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.; Kim, M.; Noh, J.S.; Park, C.H.; Song, Y.O. Preventative activity of kimchi on high cholesterol diet-induced hepatic damage through regulation of lipid metabolism in LDL receptor knockout mice. Food Sci. Biotechnol. 2018, 27, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Song, J.-L.; Ju, J.-H.; Kang, S.-A.; Park, K.-Y. Anticancer effects of kimchi fermented for different times and with added ingredients in human HT-29 colon cancer cells. Food Sci. Biotechnol. 2015, 24, 629–633. [Google Scholar] [CrossRef]
- Park, K.-Y.; Jeong, J.-K.; Lee, Y.-E.; Daily, J.W., III. Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.; Kim, M.; Noh, J.S.; Song, Y.O. Kimchi methanol extracts attenuate hepatic steatosis induced by high cholesterol diet in low-density lipoprotein receptor knockout mice through inhibition of endoplasmic reticulum stress. J. Funct. Foods 2017, 32, 218–225. [Google Scholar] [CrossRef]
- Laursen, S.E.; Belknap, J. Intracerebroventricular injections in mice: Some methodological refinements. J. Pharmacol. Methods 1986, 16, 355–357. [Google Scholar] [CrossRef]
- Ohnuki, K.; Haramizu, S.; Oki, K.; Watanabe, T.; Yazawa, S.; Fushiki, T. Administration of capsiate, a non-pungent capsaicin analog, promotes energy metabolism and suppresses body fat accumulation in mice. Biosci. Biotechnol. Biochem. 2001, 65, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Konopacka, M.; Widel, M.; Rzeszowska-Wolny, J. Modifying effect of vitamins C, E and beta-carotene against gamma-ray-induced DNA damage in mouse cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1998, 417, 85–94. [Google Scholar] [CrossRef]
- Saito, A.; Yamamoto, M. Acute oral toxicity of capsaicin in mice and rats. J. Toxicol. Sci. 1996, 21, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.; Lee, S.; Cho, E.J. Quercetin and quercetin-3-β-d-glucoside improve cognitive and memory function in Alzheimer’s disease mouse. Appl. Biol. Chem. 2016, 59, 721–728. [Google Scholar] [CrossRef]
- Ali, S.; LeBel, C.; Bondy, S. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology 1991, 13, 637–648. [Google Scholar]
- Kooy, N.W.; Royall, J.A.; Ischiropoulos, H.; Beckman, J.S. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic. Biol. Med. 1994, 16, 149–156. [Google Scholar] [CrossRef]
- Jung, K.; Hong, S.H.; Kim, M.; Han, J.-S.; Jang, M.-S.; Song, Y.O. Antiatherogenic effects of Korean cabbage kimchi with added short arm octopus. Food Sci. Biotechnol. 2015, 24, 249–255. [Google Scholar] [CrossRef]
- Woo, M.; Noh, J.S.; Cho, E.J.; Song, Y.O. Bioactive Compounds of Kimchi Inhibit Apoptosis by Attenuating Endoplasmic Reticulum Stress in the Brain of Amyloid β-Injected Mice. J. Agric. Food Chem. 2018, 66, 4883–4890. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood–brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Banerjee, T.; Van der Vliet, A.; Ziboh, V. Downregulation of COX-2 and iNOS by amentoflavone and quercetin in A549 human lung adenocarcinoma cell line. Prostaglandins Leukot. Essent. Fatty Acids 2002, 66, 485–492. [Google Scholar] [CrossRef] [PubMed]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Mishra, M.; Ghosh, S.; Tewari, R.; Basu, A.; Seth, P.; Sen, E. Modulation of interleukin-1β mediated inflammatory response in human astrocytes by flavonoids: Implications in neuroprotection. Brain Res. Bull. 2007, 73, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.W.; Yu, H.Q.; Zhang, H.; Zheng, Y.L.; Wang, J.J.; Luo, L. Quercetin attenuates spontaneous behavior and spatial memory impairment in d-galactose–treated mice by increasing brain antioxidant capacity. Nutr. Res. 2007, 27, 169–175. [Google Scholar] [CrossRef]
- Noh, J.S.; Choi, Y.H.; Song, Y.O. Beneficial effects of the active principle component of Korean cabbage kimchi via increasing nitric oxide production and suppressing inflammation in the aorta of apoE knockout mice. Br. J. Nutr. 2013, 109, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic. Biol. Med. 2001, 30, 433–446. [Google Scholar] [CrossRef]
- Heo, J.-H.; Hyon-Lee; Lee, K.-M. The possible role of antioxidant vitamin C in Alzheimer’s disease treatment and prevention. Am. J. Alzheimer’s Dis. 2013, 28, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Noh, J.S.; Kim, H.J.; Kwon, M.J.; Song, Y.O. Active principle of kimchi, 3-(4′-hydroxyl-3′, 5′-dimethoxyphenyl) propionic acid, retards fatty streak formation at aortic sinus of apolipoprotein E knockout mice. J. Med. Food 2009, 12, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Pyun, C.W.; Kim, J.H.; Han, K.H.; Hong, G.E.; Lee, C.H. In vivo protective effects of dietary curcumin and capsaicin against alcohol-induced oxidative stress. BioFactors 2014, 40, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Bhupathiraju, S.N.; Tucker, K.L. Variety in fruit and vegetable intake and cognitive function in middle-aged and older Puerto Rican adults. Br. J. Nutr. 2013, 109, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guáqueta, A.M.; Munoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Ono, K.; Yamada, M. Curcumin and Alzheimer’s disease. CNS Neurosci. Ther. 2010, 16, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Li, M.; Hu, J.; Mahaman, Y.A.; Bao, J.; Huang, F.; Xia, Y.; Liu, X.; Wang, Q.; Wang, J.-Z. Ginkgo biloba extract EGb761 attenuates Hyperhomocysteinemia-induced AD like tau hyperphosphorylation and cognitive impairment in rats. Curr. Alzheimer Res. 2018, 15, 89–99. [Google Scholar] [CrossRef] [PubMed]
| Group | ROS | Peroxynitrite | TBARS | GSH |
|---|---|---|---|---|
| (Flu/min/mg Tissue) | (mM/g Tissue) | |||
| NOR | 999 ± 82 ** | 582 ± 91 * | 78 ± 27 *** | 24.8 ± 0.5 ** |
| CON | 2143 ± 336 | 1338 ± 103 | 138 ± 20 | 23.2 ± 0.7 |
| KME | 1258 ± 192 * | 654 ± 193 * | 90 ± 16 *** | 24.6 ± 0.2 * |
| HDMPPA | 1287 ± 321 * | 693 ± 228 * | 107 ± 7 * | 23.9 ± 0.4 |
| Quer | 1228 ± 335 * | 637 ± 346 * | 107 ± 8 * | 24.8 ± 0.8 * |
| AA | 1539 ± 466 | 648 ± 157 * | 116 ± 14 | 24.1 ± 1.2 |
| Cap | 1650 ± 604 | 774 ± 465 | 120 ± 4 | 23.3 ± 0.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, M.; Kim, M.J.; Song, Y.O. Bioactive Compounds in Kimchi Improve the Cognitive and Memory Functions Impaired by Amyloid Beta. Nutrients 2018, 10, 1554. https://doi.org/10.3390/nu10101554
Woo M, Kim MJ, Song YO. Bioactive Compounds in Kimchi Improve the Cognitive and Memory Functions Impaired by Amyloid Beta. Nutrients. 2018; 10(10):1554. https://doi.org/10.3390/nu10101554
Chicago/Turabian StyleWoo, Minji, Mi Jeong Kim, and Yeong Ok Song. 2018. "Bioactive Compounds in Kimchi Improve the Cognitive and Memory Functions Impaired by Amyloid Beta" Nutrients 10, no. 10: 1554. https://doi.org/10.3390/nu10101554
APA StyleWoo, M., Kim, M. J., & Song, Y. O. (2018). Bioactive Compounds in Kimchi Improve the Cognitive and Memory Functions Impaired by Amyloid Beta. Nutrients, 10(10), 1554. https://doi.org/10.3390/nu10101554