3.1. Chemistry
All melting points were determined on a Stuart melting point apparatus SMP3 (Sigma-Aldrich, Saint Louise, MI, USA). IR spectra were recorded on a Nicolet iS10 FT-IR spectrometer (Thermo Fisher, Waltham, MA, USA) using KBr wafer technique. The
1H-NMR spectra were recorded on Bruker AV500 (400 MHz) (Bruker, Billerica, MA, USA) and Bruker Avance III (400 MHz) spectrometers (Bruker).
1H- and
13C-NMR chemical shifts δ were reported in parts per million (ppm) and were referenced to the solvent peak; CDCl
3 (7.26 ppm for
1H and 76.90 ppm for
13C) and DMSO-
d6 (2.50 ppm for
1H and 39.70 ppm for
13C). Multiplicities are represented by s (singlet), d (doublet), t (triplet), q (quartet), and m (multiplet). Coupling constants (
J) are reported in Hertz (Hz). Mass spectra were taken on a Jeol JMS-600 mass spectrometer (Jeol Inc., Peabody, MA, USA). Elemental analyses were carried out using a Perkin Elmer 240 C Micro analyzer (Perkin Elmer, Waltham, MA, USA) and they were found to be within ±0.4% of the theoretical values. Their results were found in good agreement with the calculated values. Paclitaxel and 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma Aldrich, more detailed IR and NMR data can be found in the
Supplementary Materials.
6-Hydroxy-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyrazine-5-carbonitrile (
6a). Procedure (1): A mixture of compound
1 (3.0 g, 1.5 mmol) and ethyl cyanoacetate (1.8 mL, 1.5 mmol) in dry pyridine (20 mL) was heated under reflux for overnight. After cooling, the solid product obtained was filtered, washed with water, and dried. Recrystallization from ethanol-water mixture (2:1) gave fine yellow needles, yield 0.68 g (68%), m.p. 240–242 °C. Procedure (2): Compound
7 [
13] (0.5 g, 2 mmol) was dissolved in cold H
2SO
4 (5 mL), then a cold sodium nitrite solution (0.4 g in 2 mL H
2O) was added dropwise under stirring to the above solution during 0.5 h in an ice bath at 0–5 °C. The reaction mixture was allowed to stir at room temperature (rt) for 1 h further, then it was poured onto ice-water mixture. The solid product obtained was filtered, washed with water, and recrystallized from ethanol-water mixture (2:1) as yellow crystals, yield 0.3 g (60%), m.p. 240–242 °C. IR (ν, cm
−1): 3197 (OH), 3073 (CH aromatic), 2234 (C≡N).
1H-NMR (400 MHz, DMSO-
d6) δ (ppm): 2.51 (s, 3H, CH
3), 7.38 (t, 1H, phenyl), 7.57 (t, 2H, phenyl), 8.08 (d, 2H, phenyl), 8.43 (s, 1H, OH).
13C-NMR (100 MHz, DMSO-
d6) δ (ppm): 10.99 (CH
3), 114.58 (C), 115.73 (C≡N), 120.28 (2 CH), 126.55 (CH), 129.01 (C), 129.31 (2CH), 137.89 (C), 141.84 (C), 144.48 (C), 160.40 (C) MS:
m/
z (251 M
+). Anal. calcd. for C
13H
9N
5O (251.24): C, 62.15; H, 3.61; N, 27.87. Found: C, 62.30; H, 3.50; N, 27.72%.
6-Hydroxy-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyrazine-5-carboxylic acid (8): The hydroxy cyano compound 6a (0.5 g) was dissolved in EtOH (10 mL), then aqueous solution of NaOH (20%, 10 mL) was added. The reaction mixture was heated under reflux for 5 h, then it was evaporated to half of its volume. After cooling, it was neutralized with dil. HCl, giving a solid precipitate which was filtered off, washed with water, dried, and recrystallized from dioxane as yellow crystals, yield 0.4 g (80%), m.p. 190–192 °C. IR (ν, cm−1): 3493 (OH), 2969, 2920, 2862 (CH aliph.), 3350-2493 (characteristic of COOH), 1667 (C=O). 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 2.57 (s, 3H, CH3), 7.33 (t, 1H, phenyl), 7.55 (t, 2H, phenyl), 8.12 (d, 2H, phenyl), 8.38 (s, 1H, OH). 13C-NMR (100 MHz, DMSO-d6): 11.76 (CH3), 119.67 (2CH, C), 125.59 (CH, C), 129.53 (2CH, C), 139.55 (2C), 144.77 (C), 165.16 (C). Anal. calcd. for C13H10N4O3 (270.24): C, 57.78; H, 3.73; N, 20.73. Found. C, 57.66; H, 3.88; N, 20.56%.
Ethyl 6-hydroxy-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyrazine-5-carboxylate (9): To the hydroxy acid 8 (0.5 g, 1 mmol) in anhydrous ethanol (20 mL), conc. HCl (15 mL) was added, and the mixture was heated under reflux for 6 h. The reaction mixture was then cooled, poured on ice-cold solution of NaHCO3, and the solid precipitate was filtered, washed with water, and dried. Crystallization from dioxane-water mixture (2:1) gave pale brown crystals, yield 0.35 g (70%), m.p. 143–145 °C. IR (ν, cm−1): 3117 (OH), 2974 (CH aliph.), 1677 (C=O). 1H-NMR (400 MHz, CDCl3) δ (ppm): 1.57 (t, J = 7.1 Hz, 3H, CH3), 2.76 (s, 3H, CH3), 4.64 (q, J = 7.1 Hz, 2H, CH2), 7.33 (t, 1H, phenyl), 7.53 (t, 2H, phenyl), 8.25 (d, 2H, phenyl), 12.08 (s, 1H, OH),13C-NMR (100 MHz, CDCl3): 11.64 (CH3), 14.20 (CH3), 63.48 (CH2), 120.32 (2 CH), 123.05 (C) 126.37 (CH), 129.23 (2 CH), 130.58 (C), 138.39 (C), 143.09 (C), 145.8 (C), 161.27 (C), 169.07 (C=O). MS: m/z (298 M+). Anal. calcd. for C15H14N4O3 (298.30): C, 60.40; H, 4.73; N, 18.78. Found: C, 60.30; H, 4.50; N, 18.92%.
5-Acetyl-3-methyl-1-phenyl-1H,6H-imidazo[4,5-c]pyrazole (10): A mixture of compound 1 (1.0 g, 5 mmol) and chloro acetone (0.46 mL, 5 mmol) in dry pyridine (15 mL) was heated under reflux for overnight. After cooling the solid product was filtered, washed with water, and recrystallized from ethanol-dioxane mixture (2:1) as fine yellow needles, yield 0.52 g (98%), m.p. 288–290 °C. IR (ν, cm−1): 3438 (NH), 2922, 2784 (CH aliph.), 1655 (C=O). 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 2.40 (s, 3H, CH3), 2.60 (s, 3H, CH3), 7.33 (t, 1H, phenyl), 7.52 (t, 2H, phenyl), 7.6 (s, 1H, NH), 8.11 (d, 2H, phenyl). 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 11.26 (CH3), 21.17 (CH3), 119.87 (2CH), 125.79 (CH, C), 129.19 (2CH, C), 138.82 (2C), 155.33 (C=O). Anal. calcd. for C13H12N4O (240. 26): C, 64.99; H, 5.03; N, 23.32. Found: C, 64.60; H, 5.21; N, 23.40%.
5-Benzoyl-3-methyl-1-phenyl-1H,6H-imidazo[4,5-c]pyrazole (11): A mixture of compound 1 (1.0 g, 5 mmol) and phenacyl bromide (0.46 g, 5 mmol) in dry pyridine (15 mL) was heated under reflux for overnight. After cooling the solid product was filtered, washed with water, dried, and recrystallized from ethanol-dioxane mixture (1:1) as yellow needles, yield 0.8 g (88%), m.p. 304–306 °C. IR (ν, cm−1): 3075 (NH), 2921, 2781 (CH aliph.), 1641 (C=O). 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 2.76 (s, 3H, CH3), 7.33 (t, 1H, phenyl), 7.41 (t, 1H, phenyl), 7.65–7.50 (m, 5H, phenyl), 8.12–8.31 (m, 3H, phenyl), 12.85 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 14.08 (CH3), 120.327 (C), 120.99 (2CH), 123.08 (CH), 126.78 (CH), 127.02 (CH), 127.96 (CH), 128.45 (CH), 129.57 (CH), 129.77 (CH), 129.88 (CH), 131.49 (C), 135.14 (C), 139.11 (C), 154.56 (C), 155.46 (C), 177.73 (C). MS: m/z (302 M+). Anal. calcd. for C18H14N4O (302.33): C, 71.51; H, 4.67; N, 18.53. Found: C, 71.62; H, 4.25; N, 18.72%.
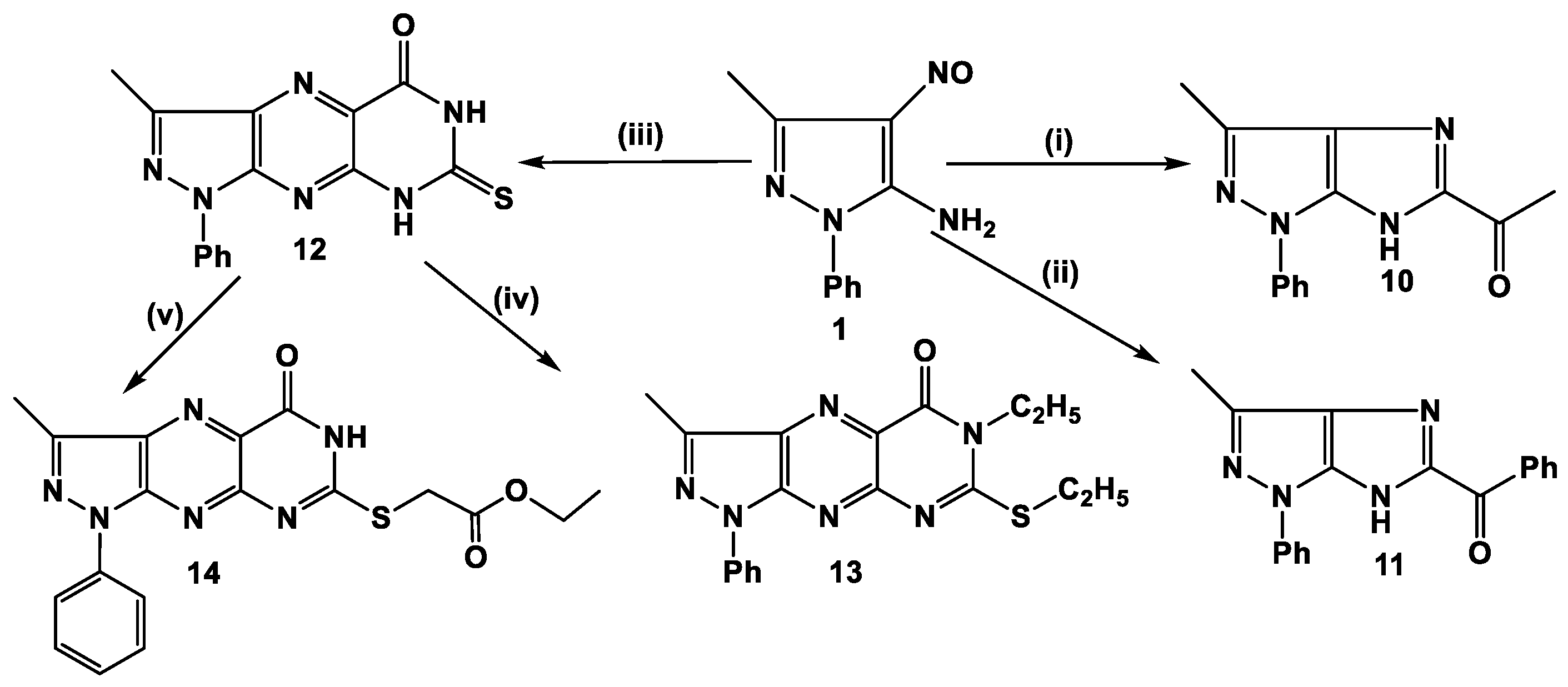
3-Methyl-1-phenyl-7-thioxo-7,8-dihydro-1H-pyrazolo[4,3-g]pteridin-5(6H)-one (12): A mixture of amino nitroso 1 (1.01 g, 5 mmol) and thiobarbituric acid (0.72 g, 5 mmol) in dry pyridine (20 mL) was heated under reflux for overnight. After cooling, the solid product was filtered, washed with water, and recrystallized from ethanol-dioxane mixture (1:3) as fine yellow needles, yield 0.15 g (65%), m.p. 332–334 °C. IR (ν, cm−1): 3416 (NH), 3068 (CH arom.), 2891 (CH aliph.), 1693 (C=O). 1H-NMR (400 MHz, DMSO-d6), δ (ppm): 2.70 (s, 1H, CH3), 7.27 (t, 1H, phenyl), 7.45 (t, 2H, phenyl), 8.13 (d, 2H, phenyl), 11.76 (s, 1H, NH), 11.91 (s, 1H, NH), 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 11.29 (s, 3H, CH3), 119.60 (2CH), 126.36 (CH), 126.99 (C), 129.37 (2CH), 132.78 (C), 138.13 (C), 142.42 (C), 145.60 (C), 147.27 (C), 158.31 (C=O), 175.95 (C=S). MS: m/z (310 M+). Anal. calcd. for C14H10N5OS (310.3): C, 54.18; H, 3.25; N, 27.08 Found: C, 54.02; H, 3.21; N, 26.82%.
6-Ethyl-7-(ethylthio)-3-methyl-1-phenyl-1H-pyrazolo[4,3-g]pteridin-5(6H)-one (13): A mixture of the thione 12 (0.25 g, 0.8 mmol), ethyl iodide (0.24 g, 1.6 mmol), and anhydrous K2CO3 (0.41 g, 3 mmol) in DMF(15 mL) was stirred at 80 °C for 5 h. After cooling the solid product was filtered, washed with water, dried, and recrystallized from ethanol-acetone mixture (2:1) to give yellow crystals, yield 0.25 g (86%), m.p. 238–240 °C. IR (ν, cm−1): 2922, 2784 (CH aliph.), 1655 (C=O). 1H-NMR (400 MHz, CDCl3) δ (ppm): 1.47 (t, J = 7.1 Hz, 3H, CH3), 1.53 (t, J = 7.4 Hz, 3H, CH3), 2.86 (s, 3H, CH3), 3.51 (q, J = 7.4 Hz, 2H, CH2), 4.34 (q, J = 7.0 Hz, 2H, CH2), 7.35 (t, J = 7.3 Hz, 1H, phenyl), 7.57 (t, J = 7.8 Hz, 2H, phenyl), 8.33 (d, J = 8.4 Hz, 2H, phenyl). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.75 (CH3), 12.93 (CH3), 13.68 (CH3), 27.05 (CH2), 40.44 (CH2), 120.25 (2CH), 125.89 (CH), 128.22 (C), 129.21 (2CH), 135.53 (C), 138.67 (C), 144.52 (C), 145.96 (C), 151.08 (C), 160.43 (C), 162.82 (C=O). MS: m/z (366 M+). Anal. calcd. for C18H18N6OS (366.44): C, 59.00; H, 4.95; N, 22.93. Found: C, 59.25; H, 4.72; N, 22.65%.
Ethyl 2-(3-methyl-5-oxo-1-phenyl-5,6-dihydro-1H-pyrazolo[4,3-g]pteridin-7-ylthio)acetate. (14): A mixture of the thione 12 (0.15 g, 0.4 mmol), ethyl chloroacetate (0.1 g, 0.8 mmol), and anhydrous K2CO3 (0.44 g, 3 mmol) in DMF (15 mL) was stirred at 80 °C for 5 h. After cooling the solid product was filtered, washed with water, dried, and recrystallized from dioxane-water mixture (2:1) to give yellow crystals, yield 0.10 g (71.4%), m.p. 243–245 °C. IR (ν, cm−1): 2980, 2923 (CH aliph.), 1737 (C=O), 1686 (C=O). 1H-NMR (400 MHz, CDCl3) δ (ppm): 1.28 (t, J = 17.3, 10.2 Hz, 3H, CH3), 2.78 (s, 3H, CH3), 4.34–4.11 (m, 4H, 2CH2), 7.27 (t, 1H, phenyl), 7.47 (t, 2H, phenyl), 8.23 (d, 2H, phenyl), 9.64 (s, 1H, NH). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.79 (CH3), 14.57 (CH3), 33.31 (CH2), 61.73 (CH2), 120.21 (2CH), 126.65 (CH), 129.78 (2CH), 130.09 (C), 134.73 (C), 138.76 (C), 144.31 (C), 145.79 (C), 160.60 (C), 161.41 (C), 162.100 (C=O), 168.64 (C=O). Anal. calcd. for C18H16N6O3S (396.42): C, 54.54; H, 4.07; N, 21.20. Found: C, 54.66; H, 4.12; N, 21.13%.
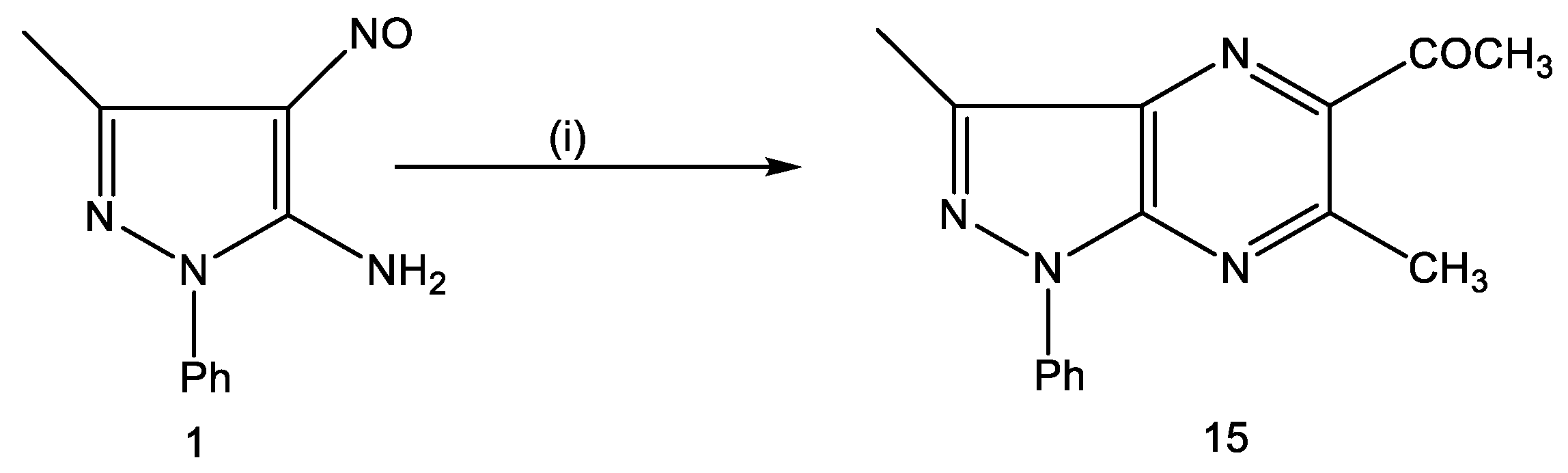
5-Acetyl-3,6-dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyrazine (15): A mixture of amino nitroso compound 1 (6.06 g, 30 mmol) and acetylacetone (3.0 g, 30 mmol) in dry pyridine (20 mL) was heated under reflux for 8 h. The reaction mixture was then evaporated to one-half volume and after cooling it was neutralized with dil. HCl (10%). The solid precipitate was filtered off, washed with water, and recrystallized from ethanol-water mixture (1:3) as pale brown needles, yield 6.4 g (80%), m.p. 130–132 °C. IR (ν, cm−1): 3050 (CH arom.), 2900 (CH aliph.), 1689 (C=O). 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.70 (s, 3H, CH3), 2.80 (s, 3H, CH3), 3.00 (s, 3H, CH3), 7.27–7.23 (t, 1H, phenyl), 7.51–7.56 (t, 2H, phenyl), 8.23–8.31 (d, 2H, phenyl). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.32 (CH3), 25.15 (CH3), 27.79 (CH3), 120.15 (2CH), 126.16 (CH), 129.18 (2CH), 131.83 (C), 138.85 (C), 142.66 (C), 143.18 (C), 144.82 (C), 154.14 (C), 200.54 (C=O). MS: m/z (266 M+). Anal. calcd. for C15H14N4O (266. 12): C, 67.65; H, 5.30; N, 21.04. Found: C, 67.63; H, 5.32; N, 21.08%.
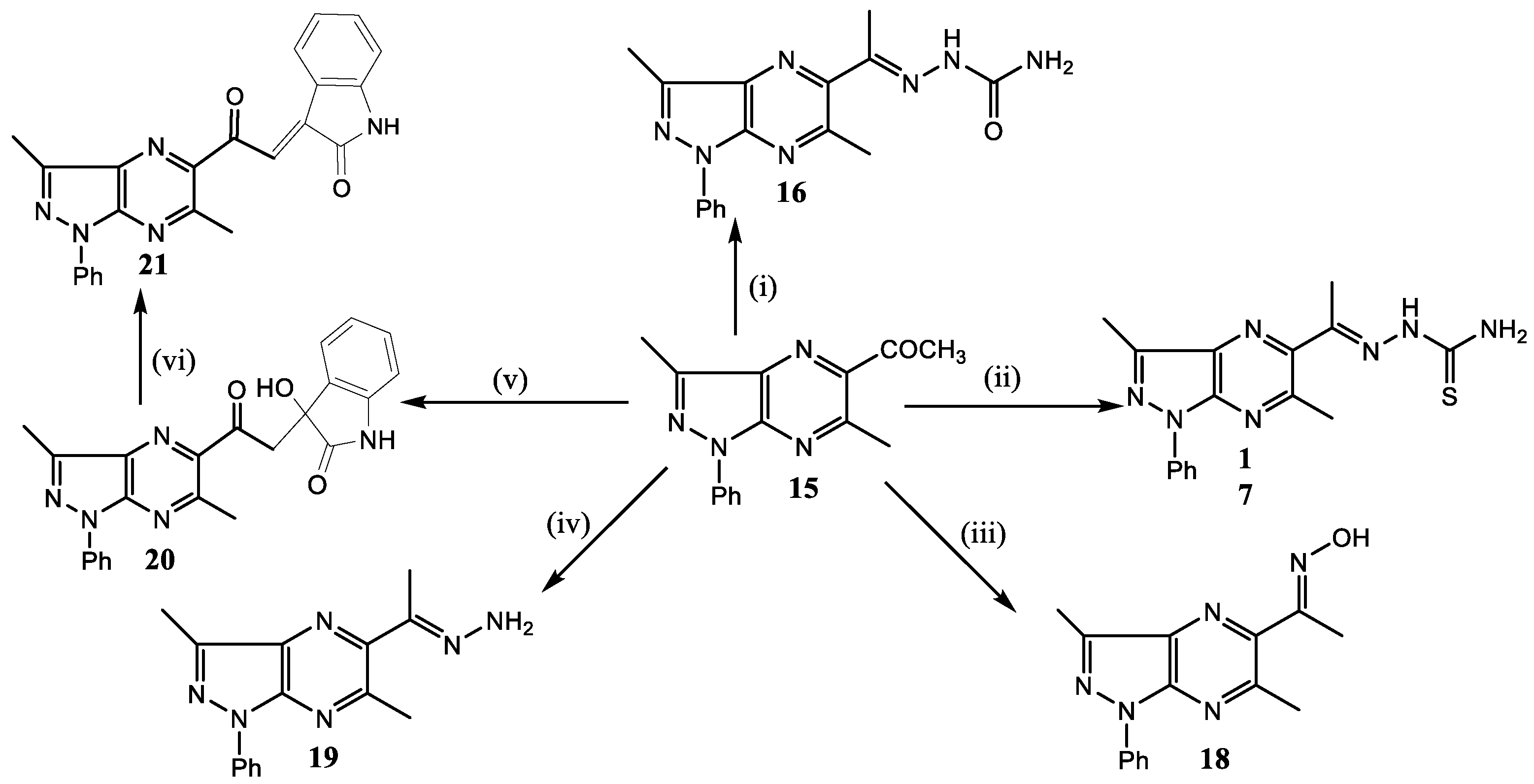
(3,6-Dimethyl-1-phenyl-5-acetyl-1H-pyrazolo[3,4-b]pyrazine)semicarbazone (16): A mixture of the acetyl derivative 5 (0.72 g, 3 mmol), semicarbazide hydrochloride (0.3 g, 3 mmol), and sodium acetate (0.6 g, 1 mol) in ethanol (15 mL) was heated under reflux for 8 h. After cooling, the product was filtered, washed with water, and recrystallized from dioxane-water mixture (3:1) as yellow crystals, yield 0.53 g (93%), m.p. 210–212 °C. IR (ν, cm−1): 3487, 3204 (NH2 + NH), 1739 (C=O). MS: m/z 323 (M+). Anal. calcd. for C16H17N7O (323.35): C, 59.43; H, 5.30; N, 30.32. Found: C, 59.33; H, 5.41; N, 30.26%. No numerical data could be obtained for this compound due its insolubility in deuterated solvents.
(3,6-Dimethyl-1-phenyl-5-acetyl-1H-pyrazolo[3,4-b]pyrazine)thiosemicarbazone (17): A mixture of 15 (1.0 g, 4 mmol) and thiosemicarbazide (0.34 g, 4 mmol) in ethanol (10 mL) was heated under reflux for 8 h. After cooling, the solid product was filtered, washed with water, and dried. Crystallization from dioxane-water mixture (1:1) gave yellow crystals, yield 0.28 g (87%); m.p. 230–232 °C; IR (ν, cm−1): 3434, 3237, 3152 (NH2 + NH), 1690 (C=O).1H-NMR (300 MHz, CDCl3) δ (ppm): 2.40 (CH3), 2.82 (CH3), 2.93 (CH3), 6.40 (s, 2H, NH2), 7.32–7.35 (t, 1H, phenyl), 7.51–7.56 (t, 2H, phenyl), 8.27–8.31 (d, 2H, phenyl), 8.88 (s, 1H, NH). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.49 (CH3), 25.00 (CH3), 27.96 (CH3), 120.29 (C, 2CH), 126.7 (CH), 129.7 (2CH), 131 (C), 138 (C), 142.6 (C), 143.8 (C), 144.77 (C), 153.8 (C), 200.19 (C=S). Anal. calcd. for C16H17N7S (339.42): C, 56.62; H, 5.05; N, 28.89. Found: C, 56.33; H, 5.20; N, 28.66%.
(5-Acetyl-3,6-dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyrazine)oxime (18): A mixture of 15 (0.25 g, 0.9 mmol), hydroxyl amine (0.07 g, 1 mmol), and sodium acetate (0.25 g, 3 mmol) in ethanol (10 mL ethanol) was heated under reflux for 3 h. The reaction mixture was then evaporated to half volume and after cooling it was neutralized with dil. HCl. The solid precipitate was filtered off, washed with water, and recrystallized from ethanol-water mixture (3-1) as fine buff needles, yield 0.16 g (89%), m.p. 223–225 °C, IR (ν, cm−1): 3281 broad (OH), 2900 (CH aliph.). 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 2.29 (s, 3H, CH3), 2.63 (s, 3H, CH3) 2.81 (s, 3H, CH3), 7.31–7.36 (t, 1H, phenyl), 7.54–7.59 (t, 2H, phenyl), 8.20–8.23 (d, 2H, phenyl), 11.59 (s, 1H, OH). 13C-NMR (100 MHz, DMSO) δ (ppm): 11.01 (s, 3H, CH3), 13.08 (s, 3H, CH3), 25.11 (s, 3H, CH3), 119.52 (2CH), 125.81 (CH), 129.29 (2CH), 131.11 (C), 138.69 (C), 141.37 (C), 143.08 (C), 146.15 (C), 151.87 (C), 154.14 (C). MS: m/z (281 M+). Anal. calcd. for C15H15N5O (281.31): C, 64.04; H, 5.37; N, 24.90. Found: C, 64.11; H, 5.42; N, 24.97%.
(5-Acetyl-3,6-dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyrazine)hydrazone (19): A mixture of 15 (0.25 g, 0.9 mmol) and hydrazine hydrate (0.5 mL, 80%) in ethanol (10 mL) was heated under reflux for 6 h. After cooling the solid product was filtered, washed with water, and recrystallized from ethanol as buff needles, yield 0.2 g (87%), m.p. 136–138 °C. IR (ν, cm−1): 3453, 3342 (NH2), 3050 (CH arom.). 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.3 (s, 3H, CH3), 2.70 (s, 3H, CH3) 2.90 (s, 3H, CH3), 5.58 (s, 2H, NH2), 7.27–7.31 (t, 1H, phenyl), 7.49–7.54 (t, 2H, phenyl), 8.20–8.31 (d, 2H, phenyl). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.36 (CH3), 12.56 (CH3), 25.65 (CH3), 120.03 (CH), 120.14 (CH), 125.60 (CH), 129.12 (CH), 129.18 (CH), 131.52 (C), 139.37 (C), 141.94 (C), 143.48 (C), 147.35 (C), 147.78 (C), 152.34 (C). MS: m/z (280 M+). Anal. calcd. for C15H16N6 (280.33): C, 64.27; H, 5.75; N, 29.98. Found: C, 64.11; H, 5.42; N, 29.93%.
5-(3-Hydroxy-2,3-dihydroindol-2-on-3-ylacetyl)-3,6-dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyrazine (20): A mixture of 15 (0.25 g, 0.9 mmol) and isatin (0.14 g, 0.9 mmol) and diethyl amine (0.5 mL) in absolute ethanol (15 mL) was stirred at rt for overnight. The solid product obtained was filtered and recrystallized from ethanol-water mixture (1:1) as buff crystals, yield 0.18 g (90%), m.p. 167–169 °C. IR (ν, cm1), 3322 (NH), 1745 (C=O), 1697 (C=O). 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.77 (d, J = 9.7 Hz, 1H, CH2), 2.84 (s, 6H, 2CH3), 3.01 (d, J = 11.4 Hz, 1H, CH2), 6.90 (s, 1H, OH), 7.07 (s, 1H, NH), 7.38–7.29 (m, 2H, Ar-H), 7.44 (d, 2H, Ar-H), 7.55 (t, 2H, Ar-H), 8.34–8.24 (m, 3H, Ar-H). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.25 (CH3), 25.02 (CH3), 27.46 (CH2), 45.61 (C), 110.11 (C), 115.52 (C), 120.31 (CH), 120.40 (CH), 123.03 (CH), 124.52 (CH), 126.19 (CH), 126.41 (CH), 129.20 (2CH), 129.98 (CH), 131.45 (C), 138.65 (C), 142.78 (C), 144.86 (C), 148.28 (C), 151.96 (C), 169.03 (C), 191.12 (C=O). Anal. calcd. for C23H21N5O3 (413.4): C, 66.82; H, 4.63; N, 16.94. Found: C, 66.51; H, 5.43; N, 16.93%.
2,3-Dihydroindol-2-on-3-ylideneacetyl-3,6-dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyrazine (21): Compound 20 (0.18 g, 4 mmol) was heated in a mixture of ethanol (7.5 mL) and conc. HCl (2.5 mL) for 15 min. After cooling the solid product obtained was filtered, washed with water, and recrystallized from ethanol-dioxane mixture (1:3) as orange needles, yield 0.13 g (81%), m.p. 248–250 °C. IR (ν, cm−1), 3444 (NH), 3163, 3074 (CH arom.), 1716 (C=O), 1670 (CO-NH). 1H-NMR (300 MHz, CDCl3) δ (ppm): 2.90 (s, 3H, CH3), 3.20 (s, 3H, CH3), 6.85–6.89 (d, 1H, phenyl), 7.10 (t, 1H, phenyl), 7.34–7.37 (t, 2H, phenyl), 7.52–7.55 (t, 2H, phenyl), 7.50 (s, 1H, NH), 8.30–8.33 (d, 2H, phenyl), 8.56 (s, 1H, CH), 8.68–8.71 (d, 1H, phenyl). 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 11.72 (CH3), 25.32 (CH3), 110.80 (CH), 120.43 (CH), 120.58 (2CH), 122.29 (CH), 126.73 (CH), 126.87 (CH), 128.37 (CH), 129.87 (2CH), 131.56 (C), 133.90 (C), 137.79 (C), 138.71 (C), 142.55 (C), 144.31 (C), 145.09 (C), 145.88 (C), 155.16 (C), 168.97 (C=O), 190.75 (C=O) MS: m/z (395 M+). Anal. calcd. for C23H19N5O2 (395.4): C, 69.86; H, 4.33; N, 17.71. Found: C, 69.43; H, 4.64; N, 17.83%.
N-(4-Methyl-2,3-dihydrothiazol-2-ylidene)-(5-acetyl-3,6-dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyrazine) hydrazine (22): A mixture of the thiosemicarbazone 17 (0.4 g, 1 mmol), chloro acetone (0.1 mL, 1 mmol), and anhydrous sodium acetate (0.5 g) in absolute ethanol was heated under reflux for 5 h. After cooling, the solid product obtained was filtered the reaction mixture was cooled, washed with water, and recrystallized from acetone-water mixture (1:1) as buff crystals, yield 0.05 g (17%), m.p. 222–224 °C, IR (ν, cm−1): 3178 (NH), 3067 (CH arom.), 2921, 2852 (CH aliph.). 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.36 (s, 3H, CH3), 2.59 (s, 3H, CH3), 2.74 (s, 3H, CH3), 3.06 (s, 3H, CH3), 5.66 (s, 1H, NH), 6.29 (s, 1H, CH), 7.35–7.27 (m, 1H, phenyl), 7.54 (t, 2H, phenyl), 8.33 (d, 2H, phenyl). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.46 (CH3), 14.02 (CH3), 16.71 (CH3), 26.43 (CH3), 103.13 (CH), 120.08 (2CH), 125.76 (CH), 128.74 (2CH), 131.52 (C), 141.26 (C), 139.02 (C), 143.79 (C), 145.19 (C), 146.05 (C), 149.26 (C), 152.70 (C), 169.00 (C). Anal. calcd. for C19H19N7S (377.47): C, 60.46; H, 5.07; N, 25.98. Found: C, 60.33; H, 5.11; N, 25.21%.
N-(4-Phenyl-2,3-dihydrothiazol-2-ylidene)-(5-acetyl-3,6-dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyrazine) hydrazine (23): A mixture of thiosemicarbazide 17 (0.25 g, 0.7 mmol), phenacyl bromide (0.3 g, 1 mmol), and anhydrous sodium acetate (0.25 g) in absolute ethanol (15 mL) was heated under reflux for 5 h. After cooling, the solid product was filtered, washed with water, and recrystallized from ethanol-dioxane mixture (3:1) as brown crystals, yield 0.11 g (40%), m.p. 256–258 °C. IR (ν, cm−1): 3435 (NH), 3065 (CH arom.), 2924 (CH aliph.). 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.53 (s, 3H, CH3), 2.73 (s, 3H, CH3), 3.09 (s, 3H, CH3), 6.90 (s, 1H, CH), 7.30–7.35 (t, 2H, phenyl), 7.51–7.56 (t, 2H, phenyl), 7.39–7.44 (t, 2H, phenyl), 7.82–7.84 (d, 2H, phenyl), 8.31–8.33 (d, 2H, phenyl) 8.90 (bs, 1H, NH). MS: m/z (439 M+). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.49 (CH3), 24.85 (CH3), 29.07 (CH3), 114.30 (CH), 120.23 (2CH), 121.02 (CH), 126.13 (CH), 127.81 (CH), 129.19 (2CH), 130.52 (2CH), 131.67 (C), 138.92 (C), 142.67 (C), 144.47 (2C), 144.59 (C), 144.71 (C), 154.59 (2C), 161.76 (C). Anal. calcd. for C24 H21 N7 S (439.54): C, 65.58; H, 4.82; N, 22.31%. Found: C, 65.51; H, 4.75; N, 22.21%.
N-(Thiazolidin-4-on-2-ylidene)-(5-Acetyl-3,6-dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyrazine) hydrazine (24): A mixture of 17 (0.5 g, 1 mmol), chloro acetic acid (0.1 g, 0.8 mol), and anhydrous sodium acetate (0.5 g) in absolute ethanol was heated under reflux for 5 h. After cooling, the solid product obtained was filtered, washed with water, and dried. Recrystallization from ethanol-dioxane mixture (1:3) gave buff crystals, yield 0.12 g (50%), m.p. 308–310 °C. IR (ν, cm−1): 3446 (NH), 2850 (CH aliph.), 1705 (C=O). MS: m/z (379 M+). Anal. calcd. for C18H17N7OS (379.44): C, 56.98; H, 4.52; N, 25.84. Found: C, 56.88; H, 4.65; N, 25.91%.
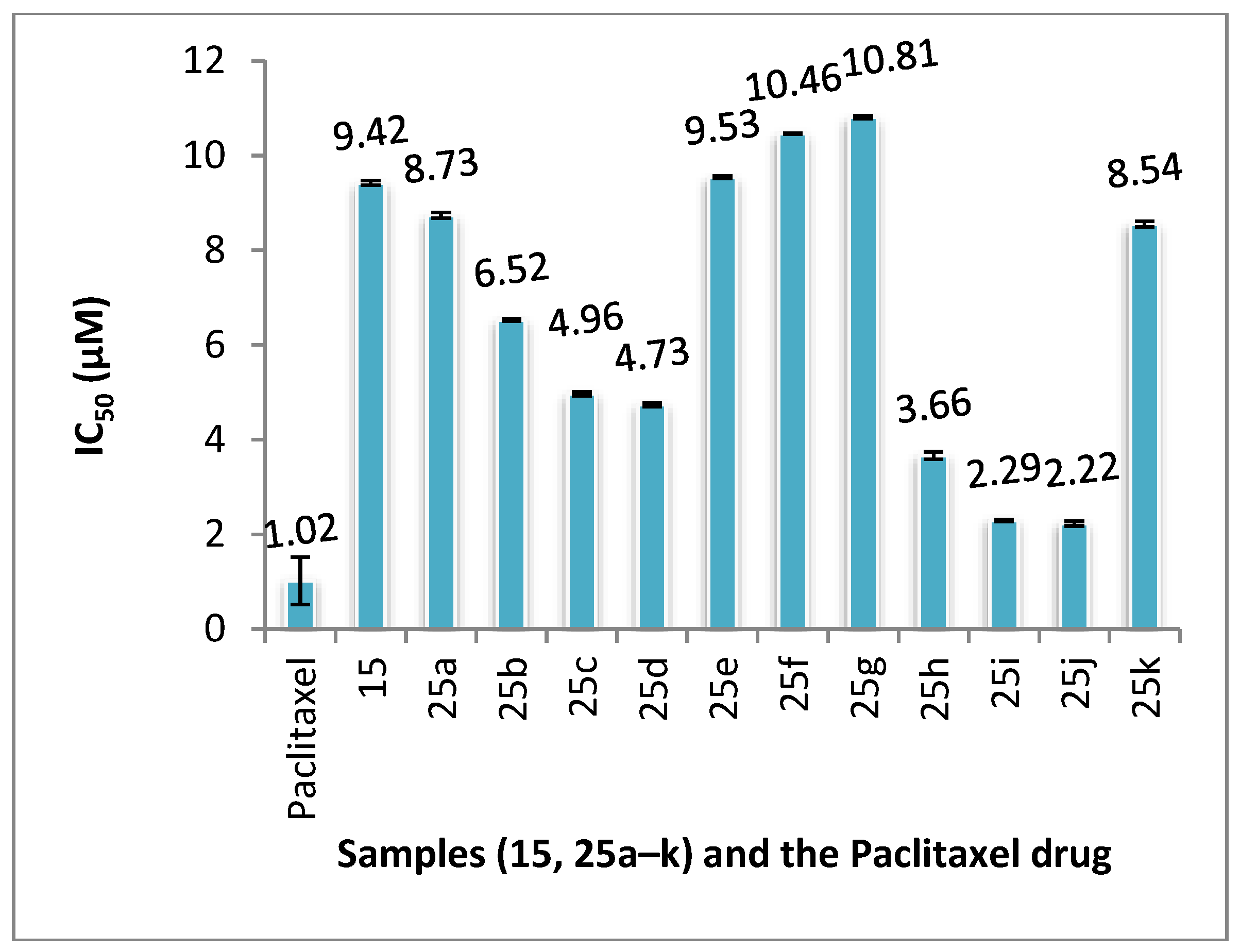
General procedure for the synthesis of 5-arylideneacetyl-3,6-dimethyl-1-phenyl-1H-pyrazolo[3,4-b] pyrazine (25a–k): To a mixture of equimolar amounts (0.27, 1 mmol) of 5-acetyl-3,6-dimethyl-1-phenyl-1H- pyrazolo[3,4-b]pyrazine (15) and an aromatic aldehyde in ethanol (15 mL), aqueous NaOH (0.5 mL, 25%) was added. The reaction mixture was stirred at room temperature for overnight, then it was poured onto ice-cold water. The solid precipitate formed was collected by filtration, washed with water, and recrystallized from dioxane-water mixture.
5-Benzylideneacetyl-3,6-dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyrazine (25a): Yield 1.68 g (84%), m.p. 167–169 °C. IR (ν, cm−1): 3059 (CH arom.), 2900 (CH aliph.), 1670 (C=O). 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.80 (s, 3H, CH3), 3.06 (s, 3H, CH3), 7.20–8.30 (m, 10H, phenyl),13C-NMR (100 MHz, CDCl3) δ (ppm): 11.49 (CH3), 24.91 (CH3), 120.34 (2CH), 123.29 (CH), 126.23 (CH), 128.74 (2CH), 128.95 (2CH), 129.23 (2CH), 130.60 (CH, C), 135.09 (2C), 142.69 (C), 144.55 (CH, C), 145.82 (2C), 190.23 (C=O). MS: m/z (354 M+). Anal. calcd. for C22H18N4O (354): C, 74.56; H, 5.12; N, 15.81. Found: C, 74.63; H, 5.14; N, 15.60%.
3,6-Dimethyl-5-(2-hydroxylbenzylideneacetyl)-1-phenyl-1H-pyrazolo[3,4-b]pyrazine (25b): Yield 0.14 g (70%), m.p. 194–196 °C. IR (ν, cm−1): 3213 (OH), 2923.40 (CH aliph.), 1657 (C=O). 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 2.69 (s, 3H, CH3), 2.89 (s, 3H, CH3), 6.90 (t, 1H, Ar-H), 6.96 (d, J = 8.1 Hz, 1H, CH), 7.30 (t, 1H, Ar-H), 7.37 (t, 1H, Ar-H), 7.59 (t, 2H, Ar-H), 7.70 (d, J = 7.6 Hz, 1H, CH), 8.03 (s, 2H, Ar-H), 8.23 (d, 2H, phenyl), 10.32 (s, 1H, OH).13C-NMR (100 MHz, DMSO-d6): 11.59 (CH3), 24.68 (CH3), 116.80 (C), 120.00 (CH), 120.31(2CH), 121.73 (C), 123.33 (CH), 126.67 (CH), 129.61 (CH), 129.82 (2CH), 131.49 (CH), 132.70 (C), 138.87 (C), 140.71 (C), 142.60 (C), 144.73 (CH), 145.18 (C), 154.09 (C), 157.95 (CH), 190.71 (C=O). Anal. calcd. for C22H18N4O2 (370.40): C, 71.34; H, 4.90; N, 15.13. Found: C, 71.23; H, 4.85; N, 15.25%.
3,6-Dimethyl-5-(2-nitrobenzylideneacetyl)-1-phenyl-1H-pyrazolo[3,4-b]pyrazine (25c): Yield 0.17 g (85%), m.p. 263–265 °C. IR (ν, cm−1): 3066 (CH arom.), 1672 (C=O). 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.84 (s, 3H, CH3), 3.10 (s, 3H, CH3), 7.37 (d, J = 6.5 Hz, 2H, CH, Ar-H), 7.57 (t, 2H, Ar-H), 7.90 (t, 3H, Ar-H), 8.41–8.30 (m, 4H, CH, Ar-H). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.52 (CH3), 25.11 (CH3), 120.38 (2CH), 124.20 (2CH), 126.40 (CH), 126.85 (CH), 129.18 (2CH), 129.26 (2CH, C), 138.76 (C), 140.86 (2C), 141.31 (C), 144.93 (CH, C), 155.35 (2C), 189.20 (C=O). Anal. calcd. For C22H17N5O3 (399.40): C, 66.16; H, 4.29; N, 17.53. Found: C, 66.22; H, 4.18; N, 17.41%.
3,6-Dimethyl-5-(4-nitrobenzylideneacetyl)-1-phenyl-1H-pyrazolo[3,4-b]pyrazine (25d): Yield 0.12 g (80%), m.p. 263–265 °C. IR (ν, cm−1): 3051 (CH arom.), 1672 (C=O). 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.84 (s, 3H, CH3), 3.10 (s, 3H, CH3), 7.35–7.37 (t, J= 7.4 Hz, 1H, CH), 7.55–7.59 (t, 2H, Ar-H), 7.87–7.93 (t, J = 11.9 Hz, 2H, CH, Ar-H), 8.32–8.40 (m, 5H, Ar-H). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.52 (CH3), 25.11 (CH3), 120.37 (2CH), 124.21 (2CH), 126.40 (CH), 126.86 (CH), 129.18 (2CH), 129.26 (2CH), 131.89 (C), 138.75 (C), 140.86 (CH), 141.31 (C), 142.67 (C), 143.25 (C), 144.94 (C), 148.57 (C), 155.35 (C), 189.20 (C=O). Anal. calcd. for C22H17N5O3 (399.40): C, 66.16; H, 4.29; N, 17.53. Found: C, 66.11; H, 4.17; N, 17.42%.
3,6-Dimethyl-5-(3-nitrobenzylideneacetyl)-1-phenyl-1H-pyrazolo[3,4-b]pyrazine (25e): Yield 0.15 g (75%); m.p. 223–225 °C; IR (ν, cm−1): 3086 (CH arom.), 2925 (CH aliph.), 1674 (C=O); 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.85 (s, 3H, CH3), 3.10 (s, 3H, CH3), 7.36 (t, 1H, Ar-H), 7.57 (t, 2H, Ar-H), 7.66 (t, 1H, Ar-H), 7.91 (d, J = 16.0 Hz, 1H, CH), 8.02 (d, J = 7.5 Hz, 1H, CH), 8.42–8.27 (m, 4H, Ar-H), 8.59 (s, 1H, Ar-H).13C-NMR (100 MHz, CDCl3) δ (ppm): 11.58 (CH3), 25.09 (CH3), 120.36 (2CH), 122.80 (CH), 124.64 (CH), 125.79 (CH), 126.35 (CH), 129.25 (2CH), 129.97 (CH), 131.89 (CH), 134.38 (C), 136.93 (C), 138.73 (C), 141.06 (C), 142.67 (C), 143.36 (C), 144.98 (C), 148.97 (C), 155.25 (C), 189.29 (C=O). Anal. calcd. For C22H17N5O3 (399.40): C, 66.16; H, 4.29; N, 17.53. Found: C, 66.24; H, 4.17; N, 17.66%.
3,6-Dimethyl-5-(4-cyanobenzylideneacetyl)-1-phenyl-1H-pyrazolo[3,4-b]pyrazine (25f): Yield 0.16 g (80%), m.p. 254–256 °C. IR (ν, cm−1): 3079 (CH arom.), 2224 (CN), 1672 (C=O). 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.84 (s, 3H, CH3), 3.09 (s, 3H, CH3), 7.37 (t, 1H, Ar-H), 7.57 (d, J = 7.4 Hz, 2H, CH), 7.81 (m, 6H, Ar-H), 8.39–8.30 (d, 2H, Ar-H). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.50 (CH3), 25.10 (CH3), 113.45 (C),118.46 (C≡N), 120.32 (2CH), 126.16 (CH), 126.94 (CH), 128.94 (CH), 129.24 (2CH), 130.02 (CH), 131.85 (CH), 132.66 (CH), 138.77 (C), 139.45 (C), 140.34 (C), 141.42 (C), 142.64 (C), 143.32 (C), 144.89 (CH), 155.28 (C), 189.27 (C=O). Anal. calcd. for C23H17N5O (379.41): C, 72.81; H, 4.52; N, 18.46. Found: C, 72.76; H, 4.40; N, 18.55%.
3,6-Dimethyl-5-(4-methoxybenzylideneacetyl)-1-phenyl-1H-pyrazolo[3,4-b]pyrazine (25g): Yield 0.18 g (90%), m.p. 210–212 °C. IR (ν, cm−1): 3069 (CH arom.), 2934, 2836 (CH aliph.), 1663 (C=O); 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.82 (s, 3H, CH3), 3.05 (s, 3H, CH3), 3.89 (s, 3H, CH3), 6.98 (d, 2H, Ar-H), 7.34 (t, 1H, Ar-H), 7.56 (t, 2H, Ar-H), 7.69 (d, 2H, Ar-H), 7.84 (d, 1H, CH), 8.00 (d, J = 15.9 Hz, 1H, CH), 8.34 (d, 2H, Ar-H). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.49 (CH3), 24.85 (CH3), 55.41 (CH3), 114.40 (2CH), 120.23 (2CH), 121.02 (C), 126.13 (CH), 127.81 (CH), 129.19 (2CH), 130.52 (2CH), 131 (C), 138.92 (C), 142.67 (C), 144.47 (CH), 144.59 (C), 144.71 (C), 154.59 (C), 161.76 (C), 190.26 (C=O). Anal. calcd. for C23H20N4O2 (384.43): C, 71.86; H, 5.24; N, 14.57. Found: C, 71.75; H, 5.33; N, 14.63%.
3,6-Dimethyl-5-(4-N,N-dimethylaminobenzylideneacetyl)-1-phenyl-1H-pyrazolo[3,4-b]pyrazine (25h): Yield 0.14 g (70%), m.p. 227–229 °C. IR (ν, cm−1): 2921 (CH aliph.), 1659 (C=O); 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.81 (s, 3H, CH3), 3.03 (s, 3H, CH3), 3.08 (s, 6H, 2CH3), 6.79 (d, 2H, Ar-H), 7.38–7.25 (t, 1H, Ar-H), 7.69–7.50 (m, 4H, CH, Ar-H), 7.83 (s, 2H, Ar-H), 8.34 (d, 2H, phenyl). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.48 (CH3), 24.64 (CH3), 40.08 (2CH3), 111.78 (2C), 118.36 (C), 120.17 (2CH), 122.77 (CH), 126.02 (CH), 129.16 (2CH), 130.75 (2CH), 131.57 (C), 139.06 (C), 142.81 (C), 144.61 (C), 146.06 (2C), 152.12 (CH), 154.100 (C), 190.60 (C=O). Anal. calcd. For C24H23N5O (397.47): C, 72.52; H, 5.83; N, 17.62. Found: C, 72.63; H, 5.94; N, 17.45%.
3,6-Dimethyl-5-(4-chlorobenzylideneacetyl)-1-phenyl-1H-pyrazolo[3,4-b]pyrazine (25i): Yield 0.23 g (92%), m.p. 228–230 °C; IR (ν, cm−1): 3081 (CH arom.), 1669 (C=O); 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.82 (s, 3H, CH3), 3.07 (s, 3H, CH3), 7.33–7.37 (t, 1H, Ar-H), 7.43–7.45 (d, 2H, Ar-H), 7.54–7.58 (t, 2H, Ar-H), 7.65–7.67 (d, 2H, Ar-H), 7.81–7.85 (d, J = 16.0 Hz, 1H, CH), 8.14–8.18 (d, J = 16.0 Hz, 1H, CH), 8.32–8.34 (d, 2H, Ar-H).13C-NMR (100 MHz, CDCl3) δ (ppm): 11.50 (CH3), 24.98 (CH3), 120.31 (2CH), 123.61 (CH), 126.26 (CH), 129.23 (2CH), 129.63 (2CH), 129.86 (2CH), 131.77 (C), 133.60 (C), 136.46 (C), 138.85 (C), 142.67 (C), 142.85 (C), 43.92 (C), 144.82 (CH), 154.96 (C), 189.83 (C). Anal. calcd. for C22H17ClN4O (388.85): C, 67.95; H, 4.41; Cl, 9.12; N, 14.41. Found: C, 67.8; H, 4.55; Cl, 9.07; N, 14.54%.
3,6-Dimethyl-5-(3,4-Dimethoxybenzylideneacetyl)-1-phenyl-1H-pyrazolo[3,4-b]pyrazine (25j): Yield 0.17 g (85%), m.p. 199–201 °C. IR (ν, cm−1): 3078 (CH arom.), 2988, 2837 (CH aliph.), 1663 (C=O); 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.82 (s, 3H, CH3), 3.05 (s, 3H, CH3), 3.98 (d, J = 9.8 Hz, 6H, 2CH3), 6.95 (d, J = 8.2 Hz, 1H, CH), 7.40–7.24 (m, 3H, CH, Ar-H), 7.56 (t, 2H, Ar-H), 7.82 (d, 1H, Ar-H), 7.96 (d, 1H, Ar-H), 8.34 (d, 2H, Ar-H). Anal. calcd. for C24H22N4O3 (414.46): C, 69.55; H, 5.35; N, 13.52. Found: C, 69.43; H, 5.47; N, 13.67%. No 13C-NMR data could be obtained due to the precipitation of the compound while running the measurement.
3,6-Dimethyl-5-(Ethene-2-ylbenzylideneacetyl)-1-phenyl-1H-pyrazolo[3,4-b]pyrazine (25k): Yield 0.17 g (85%), m.p. 211–213 °C, IR (ν, cm−1): 2922 (CH aliph.), 1662 (C=O); 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.81 (s, 3H, CH3), 3.05 (s, 3H, CH), 7.13 (m, 2H, CH, Ar-H), 7.43–7.33 (m, 4H, CH, Ar-H), 7.55 (d, 4H, CH, Ar-H), 7.70 (t, 2H, Ar-H), 8.33 (d, 2H, Ar-H). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.45 (CH3), 24.88 (CH3), 120.32 (2CH), 126.20 (CH), 126.84 (CH), 127.35 (2CH), 128.86 (2CH), 129.22 (2CH), 136.20 (CH), 138.90 (C), 142.02 (CH, C), 144.29 (2C), 144.58 (CH, C),144.78 (CH, C), 154.72 (C), 189.32 (C=O). Anal. calcd. for C24H20N4O for (380.44): C, 75.77; H, 5.30; N, 14.73. Found: C, 75.83; H, 5.18; N, 14.66%.
3,6-Dimethyl-5-(5-phenyl-4,5-dihydro-1H-pyrazol-3-yl)-1-phenyl-1H-pyrazolo[3,4-b]pyrazine (26): A mixture of chalcone 25a (0.25 g, 7 mmol), hydrazine hydrate (80%, 0.3 mL), in ethanol (15 mL) was heated under reflux for 10 h. After cooling the solid product obtained was filtered, washed with water, and recrystallized from dioxane-water mixture (3:1) as yellow crystals, yield 0.23 g (50%), m.p. 146–148 °C. IR (ν, cm−1): 3351 (NH), 2921 (CH aliph.). 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.63 (s, 3H, CH3), 3.10 (s, 3H, CH3), 3.73 (s, 3H, CH, CH2), 5.08 (s, 1H, NH), 7.33 (m, 3H, phenyl), 7.45–7.37 (m, 3H, phenyl), 7.55 (t, 2H, phenyl), 8.32 (d, 2H, phenyl). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.27 (CH3), 25.00 (CH3), 43.00 (CH2), 67.10 (CH), 120.04 (2CH), 125.69 (2CH), 126.3 (2CH), 128.86 (2CH), 129.1 (2CH), 129.8 (C), 131 (C), 139.7 (C), 142 (C), 143.3 (C), 143.5 (C), 144.5 (C), 154 (C). Anal. calcd. for C22H20N6 (368.43): C, 71.72; H, 5.47; N, 22.81. Found: C, 71.63; H, 5.53; N, 22.60%.
3,6-Dimethyl-5-(5-phenyl-4,5-dihydro-1-phenyl-1H-pyrazol-3-yl)-1-phenyl-1H-pyrazolo[3,4-b]pyrazine (27): A mixture of chalcone 25a (0.25 g, 7 mmol), phenyl hydrazine (0.1 g, 7 mmol) in ethanol (15 mL) was heated under reflux for 15 min. After cooling, the reaction mixture was filtered, washed with water, and recrystallized from ethanol as yellow crystals, yield 0.15 g (62%), m.p. 162–164 °C. IR (ν, cm−1): 2900 (CH aliph.); 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.81 (s, 3H, CH3), 3.06 (s, 3H, CH3), 3.70 (t, 1H, CH), 7.34–7.44 (d, 2H, phenyl), 7.45–7.46 (t, 2H, phenyl), 7.52–7.58 (t, 2H, phenyl), 7.71–7.74 (m, 6H, phenyl), 7.85 (d, 1H, CH2), 8.15 (d, 1H, CH2), 8.30–8.33 (d, 3H, phenyl). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.50 (CH3), 24.93 (CH3), 38.13 (CH2), 59.51 (CH), 102.18 (C), 120.33 (2CH), 123.25 (2CH), 126.23 (2CH), 128.73 (2CH), 128.94 (2CH), 129.23 (2CH), 130.60 (2CH), 131.75 (CH), 135.06 (C), 138.86 (C), 142.68 (C), 144.20 (C), 144.55 (2C), 144.82 (C), 154.82 (C). MS: m/z (443 M+-1). Anal. calcd. for C28H24N6 (444.5): C, 75.65; H, 5.44; N, 18.91. Found: C, 75.41; H, 5.21; N, 19.20%.
3,6-Dimethyl-5-(5-phenyl-4,5-dihydro[
1,
2]
oxazol-3-yl)-1-phenyl-1H-pyrazolo[3,4-b]pyrazine (
28): A mixture of chalcone
25a (0.25 g, 7 mmol), hydroxylamine hydrochloride (0.1 g, 7 mmol), and sodium acetate (0.3 g, 2 mmol) in ethanol (10 mL) was heated under reflux for 6 h. The reaction mixture was then evaporated to half of its volume. After cooling, it was neutralized with dil. HCl and the solid precipitate was filtered off, washed with water, and recrystallized from ethanol-water mixture (1:1), yield 0.17 g (74%), m.p. 200–202 °C. IR (ν, cm
−1): 2926 (CH aliph.).
1H-NMR (400 MHz, CDCl
3) δ (ppm): 2.69 (s, 3H, CH
3), 2.73 (s, 3H, CH
3), 2.79 (d, 1H, CH
2), 3.14 (d, 1H, CH
2), 3.72–3.77 (t, 1H, CH), 7.28–8.33 (m, 10H, Ar-H).
13C-NMR (100 MHz, CDCl
3) δ (ppm): 11.38 (CH
3), 23.15 (CH
3), 46.08 (CH
2), 102.88 (CH cyclic), 106.66 (C), 120.33 (2CH), 123 (CH), 125.95 (CH), 126.17 (CH), 126.95 (CH), 128.71 (CH), 128.92 (CH), 129.00 (CH), 129.19 (CH), 130 (C), 139.01 (C), 144.53 (C), 151.42 (C), 156.52 (C), 162.43 (C), 169.64 (C). Anal. calcd. for C
22H
19N
5O (369.42): C, 71.53; H, 5.18; N, 18.96. Found: C, 71.41; H, 5.21; N, 18.91%.
5-Phenyl-4,5-dihydro-3-(3,6-dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyrazine-5-yl) pyrazole-1-carbothioamide (29): A mixture of compound 25a (0.25 g, 7 mmol) and thiosemicarbazide (0.1 g, 7 mmol) in ethanolic NaOH (25%, 10 mL) was heated under reflux for 3 h. After cooling, the reaction mixture was filtered, washed with water, and recrystallized from ethanol-dioxane mixture (1:1) as yellow crystals, yield 0.08 g (50%), m.p. 234–236 °C. IR (ν, cm−1): 3407, 3280 (NH2), 3165 (CH arom.), 2851 (CH aliph.). 1H-NMR (400 MHz, CDCl3) δ (ppm): 2.67 (s, 3H, CH3), 2.70 (s, 3H, CH3), 3.09–3.22 (dd, J = 27.5, 21.8 Hz, 2H), 7.03 (s, 1H, CH), 7.31–7.39 (m, 6H, phenyl), 7.52–7.60 (m, 2H, phenyl), 8.30–8.40 (d, 2H, phenyl), 9.06 (2H, NH2). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.48 (CH3), 26.86 (CH3), 44.7(cyclic CH2), 66.85 (CH cyclic), 120.25 (2CH), 125.7 (CH, C), 126.5 (2CH), 127.4 (CH, C), 129.02 (2CH), 129.8 (2CH), 131 (C), 138 (C), 141.60 (C), 155.80 (C), 156.27 (C), 165 (C), 178 (C=S). Anal. calcd. for C23H19N7S (425.5): C, 64.92; H, 4.50; N, 23.04. Found: C, 64.94; H, 4.44; N, 23.30%.
2-Amino-4-(4-phenyl)-6-(3,6-dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyrazine-5-yl)pyrimidine (30): A mixture of chalcone 25a (7 mmol), guanidine sulfate (0.12 g, 7 mmol) in ethanolic KOH (30%, 5 mL) was heated under reflux for 3–4 h. The reaction mixture was then evaporated to half volume and after cooling it was neutralized with dil. HCl. The solid precipitate was filtered off, washed with water, and recrystallized from dioxane-acetone mixture (1:3) as yellow crystals, yield 0.1 g (40%), m.p. 150–152 °C. IR (ν, cm−1): 3302, 3182 (NH2), 3061.37 (CH arom.), 2919, 2849 (CH aliph.).1H-NMR (400 MHz, CDCl3) δ (ppm): 2.77 (s, 3H, CH3), 2.99 (s, 3H, CH3), 5.27 (d, 2H, NH2), 7.34 (t, 3H, phenyl), 7.53 (t, 3H, phenyl), 7.67 (s, 1H, CH), 8.23 (d, 2H, phenyl), 8.34–8.36 (d, 2H, phenyl). 13C-NMR (100 MHz, CDCl3) δ (ppm): 11.11 (CH3), 24.58 (CH3), 107.81 (C), 119.92 (CH), 120.21 (2CH), 125.86 (CH), 127.22 (2CH), 128.77 (2CH), 129.14 (2CH), 130.66 (CH), 132.31 (C), 137.40 (C), 139.23 (C), 142.91 (C), 144.02 (C), 146.52 (C), 152.54 (C), 162.87 (C), 166.64 (C). Anal. calcd. for C23H21N7 (395): C, 69.85; H, 5.35; N, 24.79. Found: C, 69.63; H, 5.44; N, 24.60%.