Corrosion Resistance of Mild Steel Coated with Orgainc Material Containing Pyrazol Moiety
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Solutions
2.3. Methods
2.3.1. Gravimetric Analysis
2.3.2. Electrochemical Evaluation
2.4. Surface Morphology Evaluation
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. UV-Visible
2.4.3. XPS Analysis
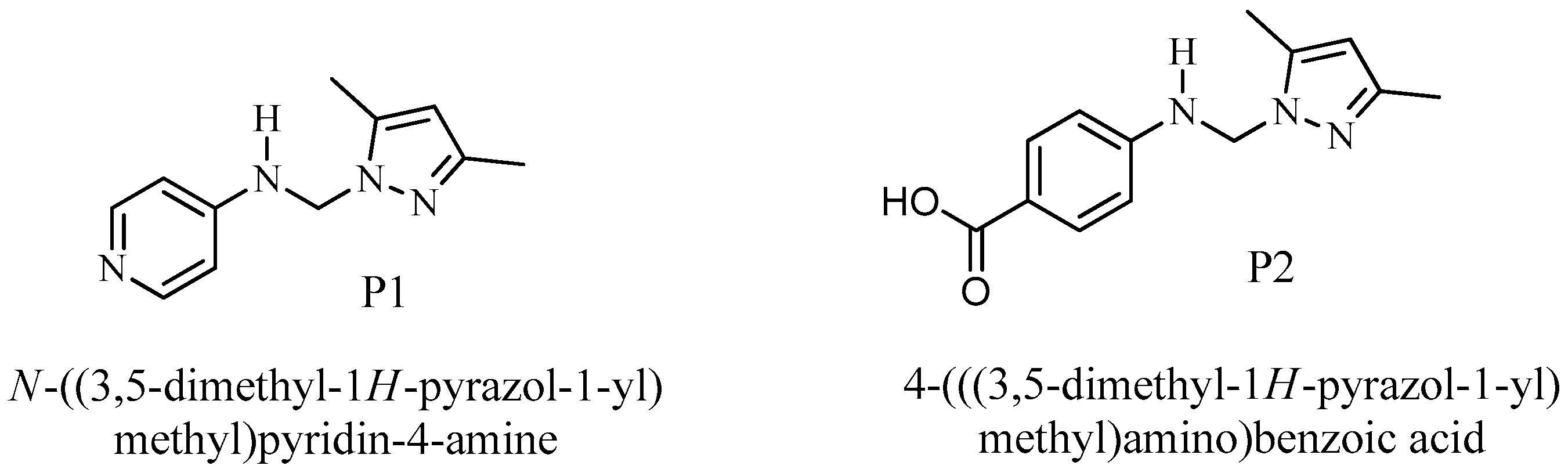
2.5. Quantum Chemical Calculations
3. Results and Discussion
3.1. Gravimetric Analysis
3.1.1. Effect of Pyrazols Concentration
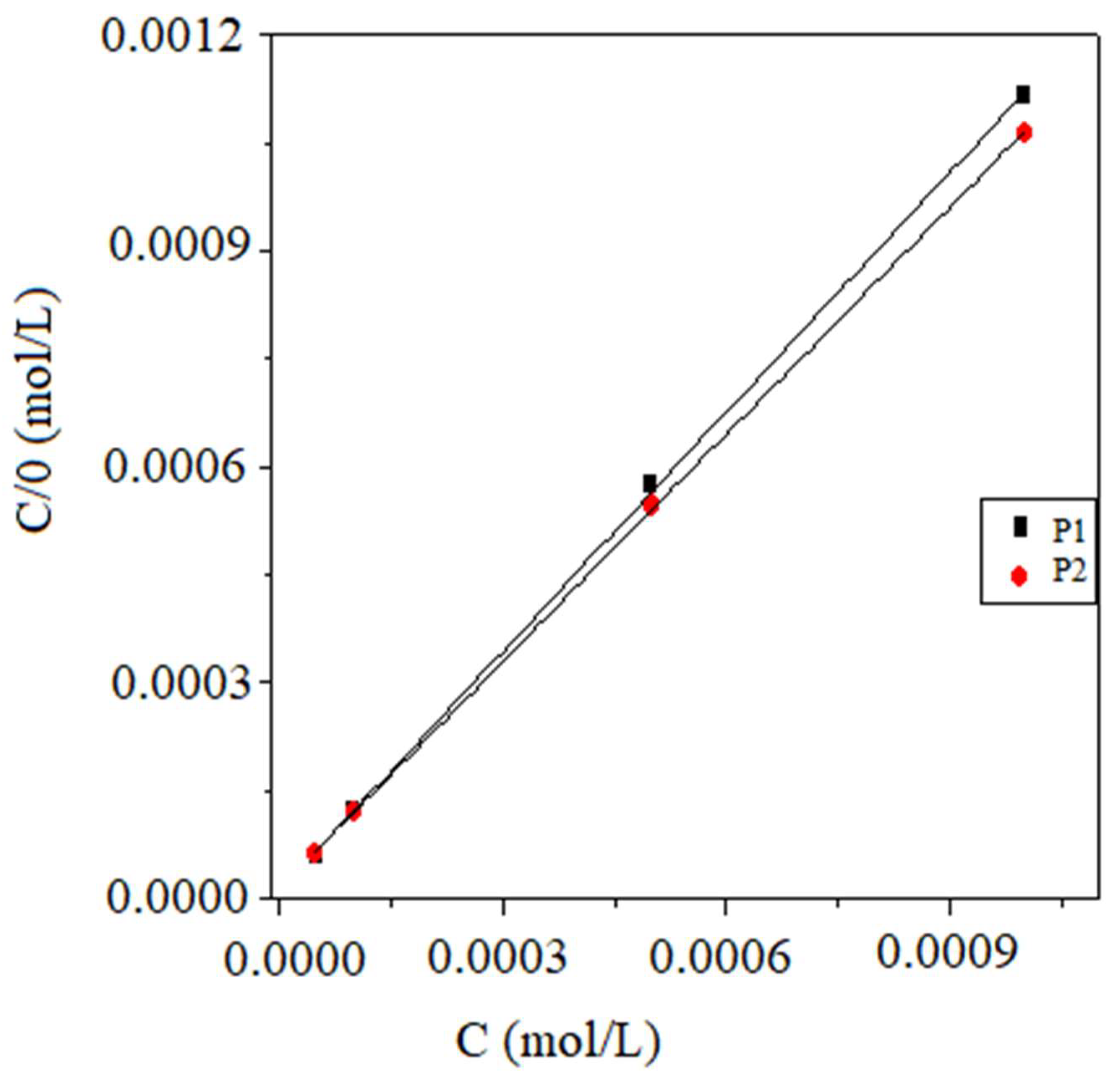
3.1.2. Adsorption Isotherm and Standard Adsorption Free Energy
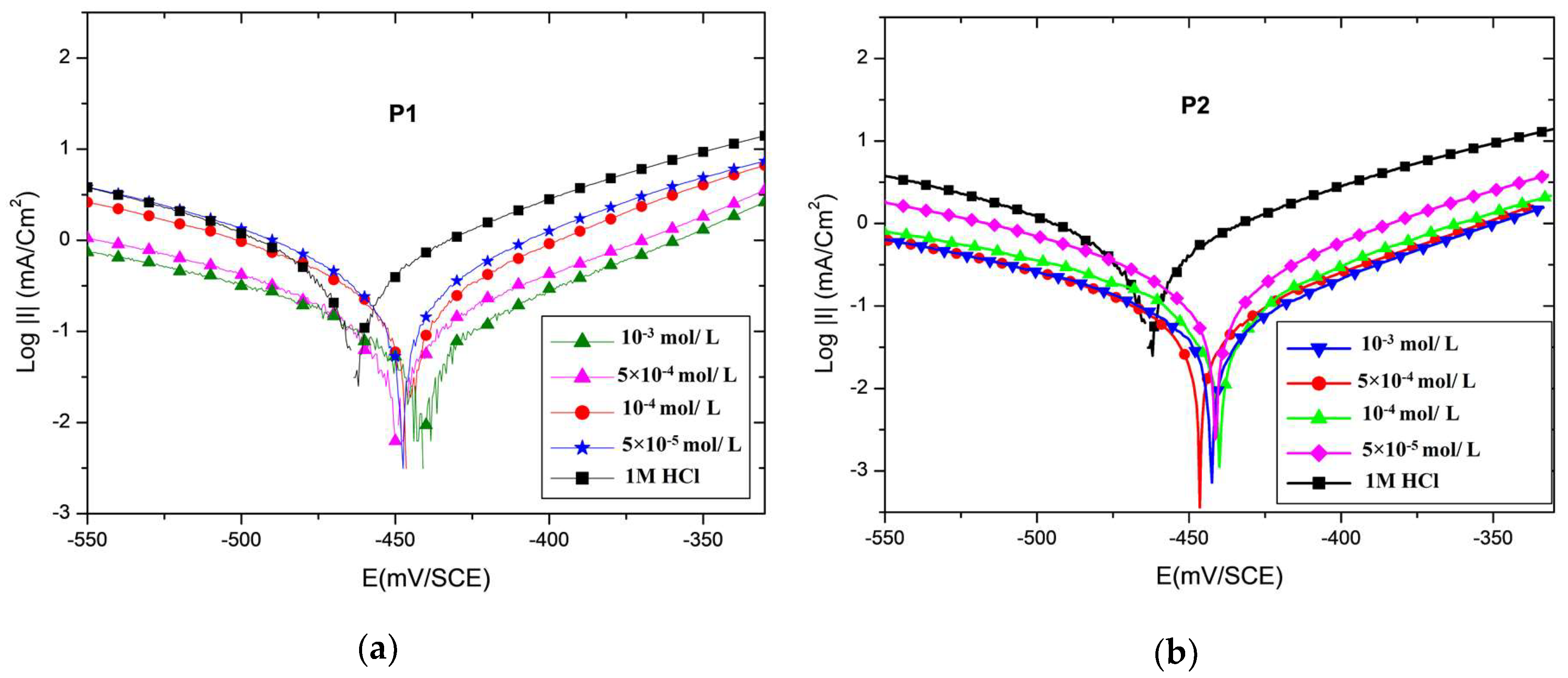
3.2. Potentiodynamic Polarization Curves
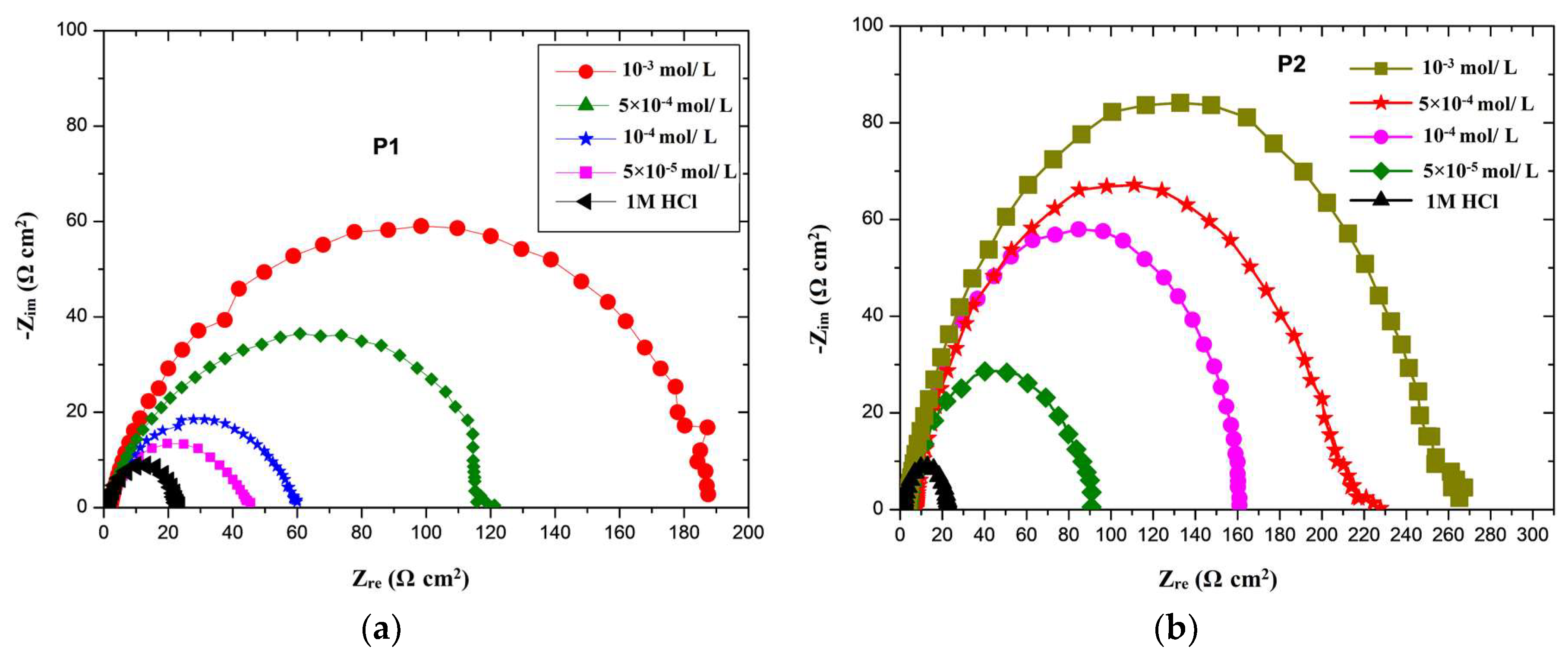
3.3. Electrochemical Impedance Spectroscopy (EIS)
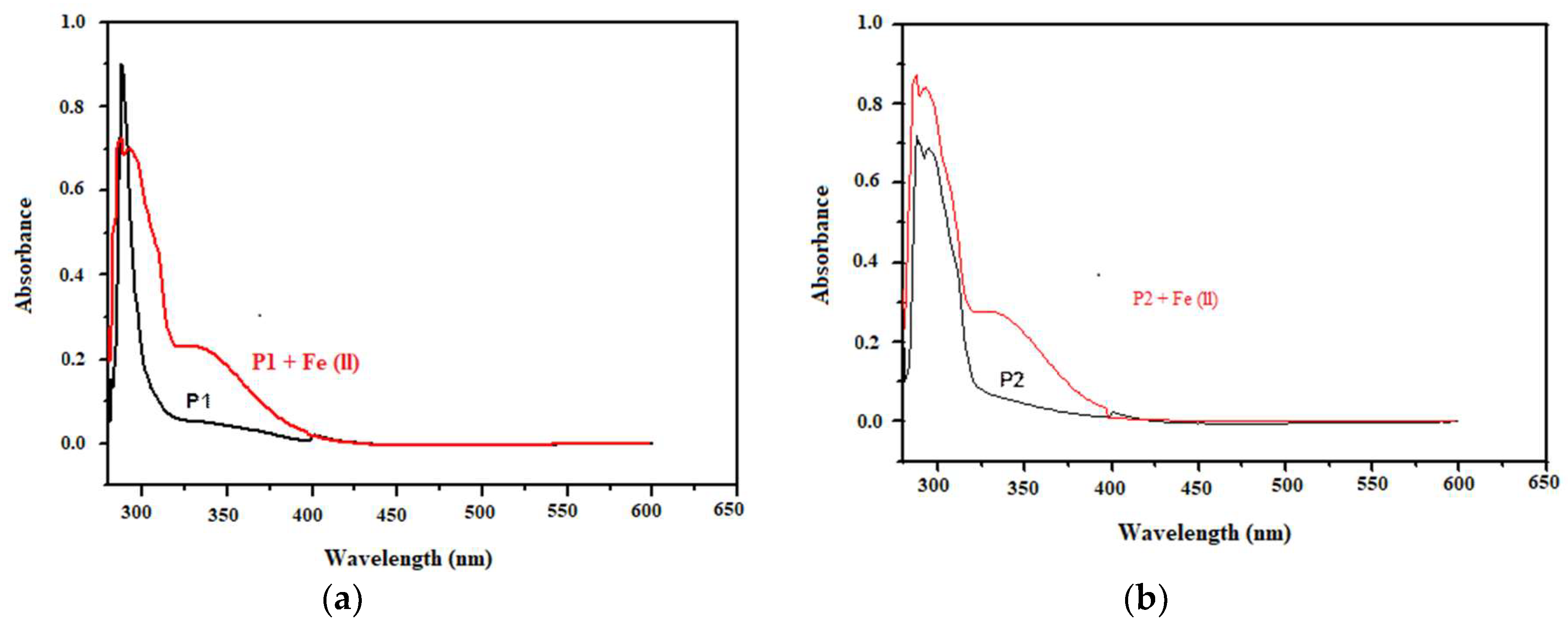
3.4. UV-Visible Spectroscopic Investigation of Inhibitor Solution
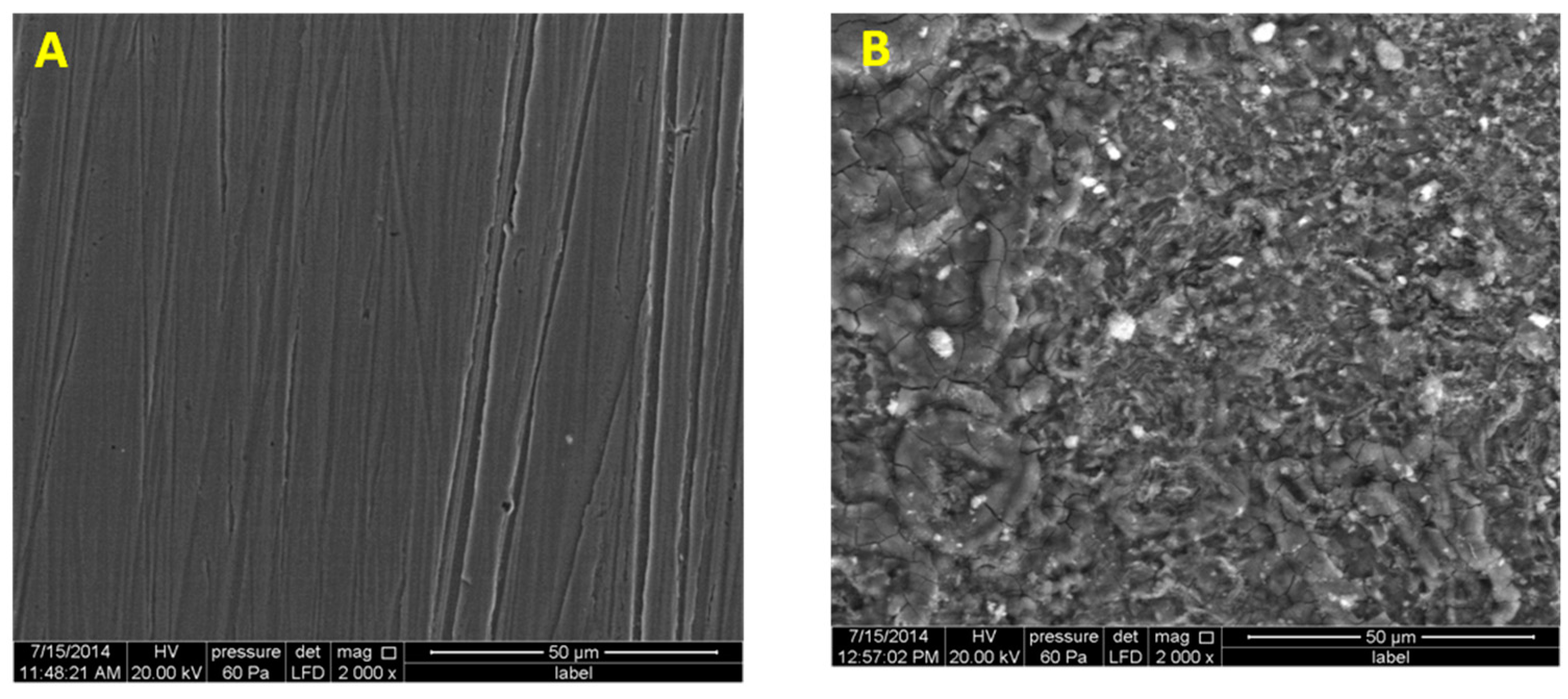
3.5. Scanning Electron Microscopy and XPS Analysis
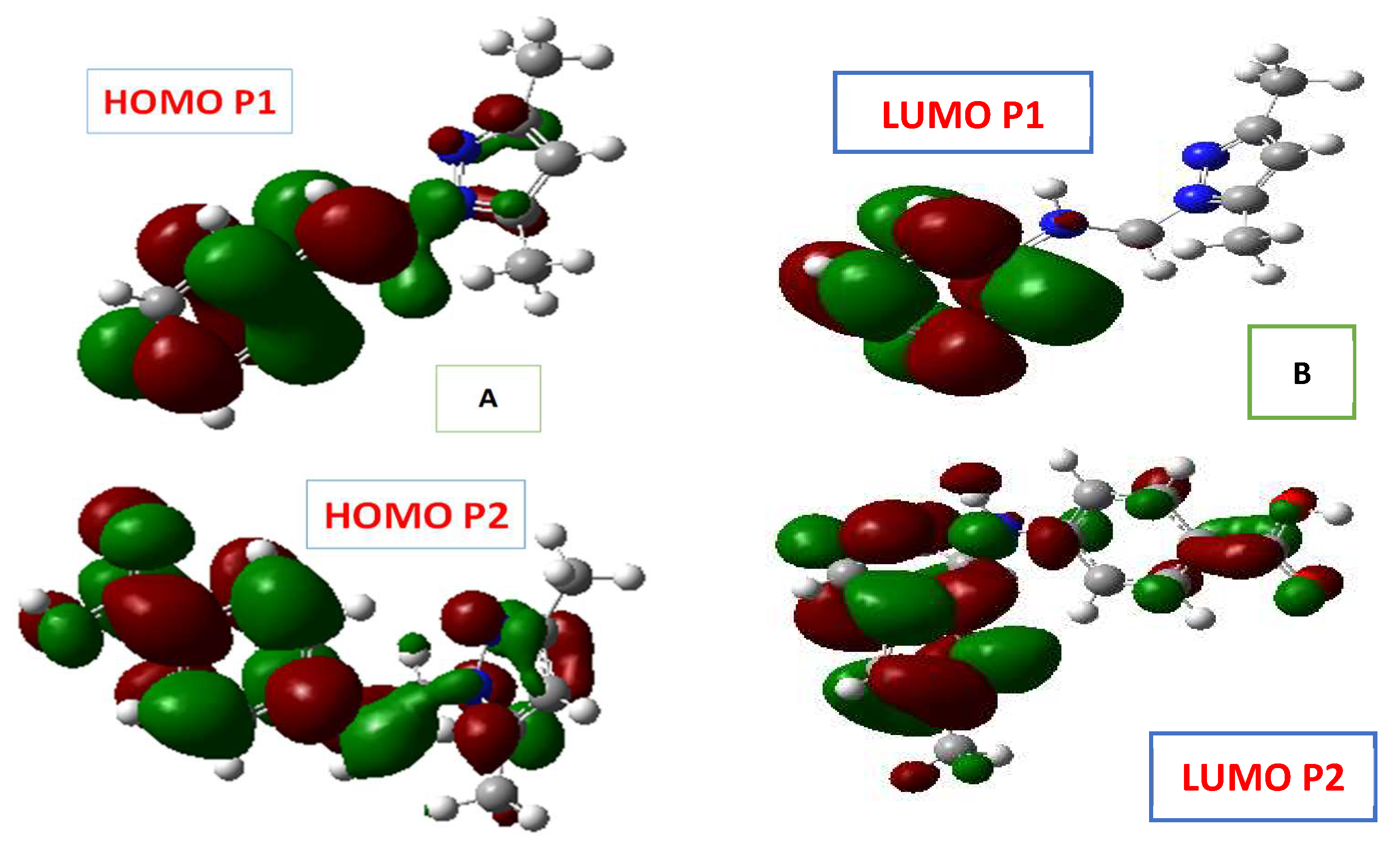
3.6. Quantum Chemical Calculations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bedair, M.A. The effect of structure parameters on the corrosion inhibition effect of some heterocyclic nitrogen organic compound. J. Mol. Liq. 2016, 219, 128–141. [Google Scholar] [CrossRef]
- El Faydy, M.; Galai, M.; El Assyry, A.; Tazouti, A.; Touir, R.; Lakhrissi, B.; EbnTouhami, M.; Zarrouk, A. Experimental investigation on the corrosion inhibition of carbon steel by 5-(chloromethyl)-8-quinolinol hydrochloride in hydrochloric acid solution. J. Mol. Liq. 2016, 219, 396–404. [Google Scholar] [CrossRef]
- El-Hajjaji, F.; Messali, M.; Aljuhani, A.; Aouad, M.R.; Hammouti, B.; Belghiti, M.E.; Chauhan, D.S.; Quraishi, M.A. Pyridazinium-based ionic liquids as novel and green corrosion inhibitors of carbon steel in acid medium: Electrochemical and molecular dynamics simulation studies. J. Mol. Liq. 2018, 249, 997–1008. [Google Scholar] [CrossRef]
- Ech-chihbi, E.; Belghiti, M.E.; Salima, R.; Oudda, H.; Taleb, M.; Benchat, N.; Hammouti, B.; El-Hajjaji, F. Experimental and computational studies on the inhibition performance of the organic compound “2-phenylimidazo [1,2-a]pyrimidine-3-carbaldehyde” against the corrosion of carbon steel in 1.0 M HCl solution. Surf. Interfaces 2017, 9, 206–217. [Google Scholar] [CrossRef]
- Ghazoui, A.; Zarrouk, A.; Bencaht, N.; Salghi, R.; Assouag, M.; el Hezzat, M.; Guenbour, A.; Hammouti, B. New possibility of mild steel corrosion inhibition by organic heterocyclic compound. J. Chem. Pharm. Res. 2014, 6, 704–712. [Google Scholar]
- Bouoidina, A.; El-Hajjaji, F.; Drissi, M.; Taleb, M.; Hammouti, B.; Chung, I.L.; Jodeh, S.; Lgaz, A.H. Towards a deeper understanding of the anticorrosive properties of hydrazine derivatives in acid medium: experimental, DFT and MD simulation assessment. Metall. Mater. Trans. A 2018, in press. [Google Scholar] [CrossRef]
- El-Hajjaji, F.; Belghiti, M.E.; Hammouti, B.; Jodeh, S.; Hamed, O.; Lgaz, H.; Salghi, R. Adsorption and corrosion inhibition effect of 2-mercaptobenzimidazole (surfactant) on a carbon steel surface in an acidic medium: Experimental and monte carlo simulations. Port. Electrochim. Acta 2018, 36, 197–212. [Google Scholar] [CrossRef]
- Hmamou, D.B.; Aouad, M.R.; Salghi, R.; Zarrouk, A.; Assouag, M.; Benali, O.; Messali, M.; Zarrok, H.; Hammouti, B. Inhibition of C38 steel corrosion in hydrochloric acid solution by 4,5-Diphenyl-1H-Imidazole-2-Thiol: Gravimetric and temperature effects treatment. J. Chem. Pharm. Res. 2012, 4, 3498–3504. [Google Scholar]
- Belayachi, M.; Serrar, H.; Zarrok, H.; el Assyry, A.; Zarrouk, A.; Oudda, H.; Boukhris, S.; Hammouti, B.; Ebenso, E.E.; Geunbour, A. New pyrimidothiazine derivative as corrosion inhibitor for carbon steel in acidic media. Int. J. Electrochem. Sci. 2015, 10, 3010–3025. [Google Scholar]
- Yadav, M.; Sinha, R.R.; Kumar, S.; Bahadur, I.; Ebenso, E.E. Synthesis and application of new acetohydrazide derivatives as a corrosion inhibition of mild steel in acidic medium: Insight from electrochemical and theoretical studies. J. Mol. Liq. 2016, 208, 322–332. [Google Scholar] [CrossRef]
- Dabholkar, V.N.; Ansari, F.Y. Synthesis and characterization of selected fused isoxazole and pyrazole derivatives and their antimicrobial activity. J. Serb. Chem. Soc. 2009, 74, 1219–1228. [Google Scholar] [CrossRef]
- Youssef, M.M.; Mohammed, S.F.; Kotb, E.R.; Salma, M.A. A novel synthesis of some new pyrimidine, thiazolopyrimidine and pyrazole derivatives using diarylepoxypropanones as precursor. World J. Chem. 2009, 4, 149–156. [Google Scholar]
- Priyadarsini, P.; Ujwala, B.; venkata Rao, C.; Madhava Rao, V. Synthesis and antimicrobial activity of some novel pyrazoles. Pharm. Lett. 2012, 4, 1123–1128. [Google Scholar]
- Hassan, S.Y. Synthesis, Antibacterial and antifungal activity of some new pyrazoline and pyrazole derivatives. Molecules 2013, 18, 2683–2711. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, S.A.; Yadav, M.; Pathak, D. Synthesis and characterization of some substituted pyrazoles as analgesics and anti-inflammatory agents. Pharm. Chem. 2011, 3, 215–222. [Google Scholar]
- Bhaskar, V.H.; Mohite, P.B. Design, synthesis, characterization and biological evaluation of some novel 1,5-disubstituted tetrazole as potential anti-inflammatory agent. J. Optoelectron. Biomed. Mater. 2010, 2, 231–237. [Google Scholar]
- Al-Saadi, M.S.M. Synthesis and in vitro antitumour activity of some fused pyrazole and pyrazoline ring systems. Saudi Pharm. J. 2008, 16, 135–145. [Google Scholar]
- Kalirajan, R.; Rathore, L.; Jubie, S.; Gowramma, B.; Gomathy, S.; Sankar, S.; Elango, K. Microwave assisted synthesis and biological evaluation of pyrazole derivatives of benzimidazoles. Indian J. Pharm. Edu. Res. 2010, 44, 358–362. [Google Scholar]
- Kini, S.G.; Bhat, A.R.; Bryant, B.; Williamson, J.S.; Dayan, F.E. Synthesis, antitubercular activity and docking study of novel cyclic azole substituted diphenyl ether derivatives. Eur. J. Med. Chem. 2009, 44, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Babu, V.H.; Manna, S.K.; Srinivasson, K.K.; Bhatt, G.V. Synthesis and biological evaluation of 1,3,5-trisubstituted pyrazolines bearing benzofuran. Indian J. Heterocycl. Chem. 2004, 13, 253–256. [Google Scholar]
- Driver, R.; Meakins, R.J. Contrasting behaviour of mild steel and pure iron towards pickling inhibitors. Br. Corros. J. 1977, 12, 46–50. [Google Scholar] [CrossRef]
- Zarrok, H.; Oudda, H.; El Midaoui, A.; Zarrouk, A.; Hammouti, B.; Ebn Touhami, M.; Attayibat, A.; Radi, S.; Touzani, R. Some new bipyrazole derivatives as corrosion inhibitors for C38 steel in acidic medium. Res. Chem. Intermed. 2012, 38, 2051–2063. [Google Scholar] [CrossRef]
- Tebbji, K.; Aouniti, A.; Attayibat, A.; Hammouti, B.; Oudda, H.; Benkaddour, M.; Radi, S.; Nahle, A. Inhibition efficiency of two bipyrazole derivatives on steel corrosion in hydrochloric acid media. Indian J. Chem. Technol. 2011, 18, 244–253. [Google Scholar]
- Tebbji, K.; Oudda, H.; Hammouti, B.; Benkaddour, M.; Al-Deyab, S.S.; Aouniti, A.; Radi, S.; Ramdani, A. The effect of 1′,3,5,5′-tetramethyl-1′H-1,3′-bipyrazole on the corrosion of steel in 1.0 M hydrochloric acid. Res. Chem. Intermed. 2011, 37, 985–1007. [Google Scholar] [CrossRef]
- Benabdellah, M.; Touzani, R.; Aouniti, A.; Dafali, A.; el Kadiri, S.; Hammouti, B.; Benkaddour, M. Inhibitive action of some bipyrazolic compounds on the corrosion of steel in 1 M HCl: Part I: Electrochemical study. Mater. Chem. Phys. 2007, 105, 373–379. [Google Scholar] [CrossRef]
- Elayyachy, M.; Elkodadi, M.; Aouniti, A.; Ramdani, A.; Hammouti, B.; Malek, F.; Elidrissi, A. New bipyrazole derivatives as corrosion inhibitors for steel in hydrochloric acid solutions. Mater. Chem. Phys. 2005, 93, 281–285. [Google Scholar] [CrossRef]
- Abrigach, F.; Khoutoul, M.; Benchat, N.; Radi, S.; Draoui, N.; Feron, O.; Riant, O.; Touzani, R. Library of synthetic compounds based on pyrazole unit: Design and screening against breast and colorectal cancer. Lett. Drug Des. Discov. 2012, 11, 1010–1016. [Google Scholar] [CrossRef]
- ASTM G31-72(2004) Standard Practices for Laboratory Immersion Corrosion Testing of Metals; ASTM: West Conshohocken, PA, USA, 1990.
- Gupta, N.K.; Verma, C.; Quraishi, M.A.; Mukherjee, A.K. Schiff’s bases derived from llysine and aromatic aldehydes as green corrosion inhibitors for mild steel: Experimental and theoretical studies. J. Mol. Liq. 2016, 215, 47–57. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Ju, H.; Kai, Z.P.; Li, Y. Aminic nitrogen-bearing polydentate Schiff base compounds as corrosion inhibitors for iron in acidic media: A quantum chemical calculation. Corros. Sci. 2008, 50, 865–871. [Google Scholar] [CrossRef]
- Lgaz, H.; Salghi, R.; Jodeh, S.; Hammouti, B. Insights into corrosion inhibition behavior of three chalcone derivatives for mild steel in hydrochloric acid solution. J. Mol. Liq. 2017, 225, 271–280. [Google Scholar] [CrossRef]
- Bilgic, S.; Sahin, M. The corrosion inhibition of austenitic chromium-nickel steel in H2SO4 by 2-butyn-1-ol. Mater. Chem. Phys. 2001, 70, 290–295. [Google Scholar] [CrossRef]
- Gharebaa, S.; Omanovic, S. Interaction of 12-aminododecanoic acid with a carbon steel surface: Towards the development of green corrosion inhibitors. Corros. Sci. 2010, 52, 2104–2113. [Google Scholar] [CrossRef]
- Noor, E.A. Temperature effects on the corrosion inhibition of mild steel in acidic solutions by aqueous extract of fenugreek leaves. Int. J. Electrochem. Sci. 2007, 2, 996–1017. [Google Scholar]
- Fouda, A.S.; Elewady, Y.A.; El-Aziz, H.K.A. Corrosion inhibition of carbon steel by cationic surfactants in 0.5 M HCl solution. J. Chem. Sci. Technol. 2012, 1, 45–53. [Google Scholar]
- Li, X.; Deng, S.; Fu, H.; Mu, G. Inhibition effect of 6-benzylaminopurine on the corrosion of cold rolled steel in H2SO4 solution. Corros. Sci. 2009, 51, 620–634. [Google Scholar] [CrossRef]
- Amar, H.; Tounsi, A.; Makayssi, A.; Derja, A.; Benzakour, J.; Outzourhit, A. Corrosion inhibition of Armco iron by 2-mercaptobenzimidazole in sodium chloride 3% media. Corros. Sci. 2007, 49, 2936–2945. [Google Scholar] [CrossRef]
- Scendo, M.; Uznanska, J. The effect of ionic liquids on the corrosion inhibition of copper in acidic chloride solutions. Int. J. Corros. 2011, 2011, 718626. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, S.; Li, W.; Gong, M.; Yin, L. Experimental and theoretical studies of two imidazolium-based ionic liquids as inhibitors for mild steel in sulfuric acid solution. Corros. Sci. 2015, 95, 168–179. [Google Scholar] [CrossRef]
- Lopez, D.A.; Simison, S.N.; de Sánchez, S.R. The influence of steel microstructure on CO2 corrosion. EIS studies on the inhibition efficiency of benzimidazole. Electrochim. Acta 2003, 48, 845–854. [Google Scholar] [CrossRef]
- Stoynov, Z.B.; Grafov, B.M.; Savova-Stoynova, B.S.; Elkin, V.V. “Electrochemical Impedance”, Moscow: Nauka, 1991, 336p. Rus. J. Electrochem. 1994, 30, 1075–1076. [Google Scholar]
- Amin, M.A.; Khaled, K.F.; Mohsen, Q.; Arida, H.A. A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros. Sci. 2010, 52, 1684–1695. [Google Scholar] [CrossRef]
- Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S. Experimental and theoretical study on the inhibition performance of triazole compounds for mild steel corrosion. Corros. Sci. 2010, 52, 3331–3340. [Google Scholar] [CrossRef]
- Jacob, K.S.; Parameswaran, G. Corrosion inhibition of mild steel in hydrochloric acid solution by Schiff base furoin thiosemicarbazone. Corros. Sci. 2010, 52, 224–228. [Google Scholar] [CrossRef]
- Labjar, N.; Lebrini, M.; Bentiss, F.; Chihib, N.; ElHajjaji, S.; Jama, C. Corrosion inhibition of carbon steel and antibacterial properties of aminotris-(methylenephosphonic) acid. Mater. Chem. Phys. 2010, 119, 330–336. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, S.; Li, W.; Yin, L.; He, J.; Wua, J. Investigation of 1-butyl-3-methyl-1H-benzimidazolium iodide as inhibitor for mild steel in sulfuric acid solution. Corros. Sci. 2014, 80, 383–392. [Google Scholar] [CrossRef]
- Mallaiya, K.; Subramaniam, R.; Srikandan, S.S.; Gowri, S.; Rajasekaran, R.; Selvaraj, A. Electrochemical characterization of the protective film formed by the unsymmetrical Schiff’s base on the mild steel surface in acid media. Electrochim. Acta 2011, 56, 3857–3863. [Google Scholar] [CrossRef]
- Ozcan, M.; Karadag, F.; Dehri, I. Investigation of adsorption characteristics of methionine at mild steel/sulfuric acid interface: An experimental and theoretical study. Colloids Surf. A 2008, 316, 55–61. [Google Scholar] [CrossRef]
- Musa, A.Y.; Jalgham, R.T.T.; Mohamad, A.B. Molecular dynamic and quantum chemical calculations for phthalazine derivatives as corrosion inhibitors of mild steel in 1 M HCl. Corros. Sci. 2012, 56, 176–183. [Google Scholar] [CrossRef]
- Lebrini, M.; Lagreneé, M.; Vezin, H.; Traisnel, M.; Bentiss, F. Experimental and theoretical study for corrosion inhibition of mild steel in normal hydrochloric acid solution by some new macrocyclic polyether compounds. Corros. Sci. 2007, 49, 2254–2269. [Google Scholar] [CrossRef]
- Helmholtz, H. Studies on electrical boundary layers. Ann. Phys. 1879, 243, 337–382. [Google Scholar] [CrossRef]
- Sundaravel, K.; Suresh, E.; Palaniandavar, M. Iron(III) complexes of tridentate N3 ligands as models for catechol dioxygenases: Stereoelectronic effects of pyrazole coordination. Inorg. Chim. Acta 2010, 363, 2768–2777. [Google Scholar] [CrossRef]
- Li, W.; Rodríguez-Castellón, E.; Bandosz, T.J. Photosensitivity of g-C3N4/S-doped carbon composites: Study of surface stability upon exposure to CO2 and/or water at ambient light. J. Mater Chem. A 2017, 5, 24880–24891. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Musa, A.Y.; Kadhum, A.H.; Mohamad, A.B.; Rohoma, A.B.; Mesmari, H. Electrochemical and quantum chemical calculations on 4,4-dimethyloxazolidine-2-thione as inhibitor for mild steel corrosion in hydrochloric acid. J. Mol. Struct. 2010, 969, 233–237. [Google Scholar] [CrossRef]
- Gece, G.; Bilgic, S. Quantum chemical study of some cyclic nitrogen compounds as corrosion inhibitors of steel in NaCl media. Corros. Sci. 2009, 51, 1876–1878. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Wang, H.; Wang, L.; Liu, A. DFT study of new bipyrazole derivatives and their potential activity as corrosion inhibitors. J. Mol. Model. 2007, 13, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Obot, I.B.; Obi-Egbedi, N.O.; Umoren, S.A. Adsorption characteristics and corrosion inhibitive properties of clotrimazole for aluminium corrosion in hydrochloric acid. Int. J. Electrochem. Sci. 2009, 4, 863–877. [Google Scholar]
- Breket, G.; Hur, E.; Ogretir, C. Quantum chemical studies on some imidazole derivatives as corrosion inhibitors for iron in acidic medium. J. Mol. Struct. THEOCHEM 2002, 578, 79–88. [Google Scholar] [CrossRef]
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions; John Wiley & Sons: Hoboken, NJ, UAS, 1976. [Google Scholar]
- Herrag, L.; Hammouti, B.; Elkadiri, S.; Aouniti, A.; Jama, C.; Vezin, H.; Bentiss, F. Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: Experimental and theoretical investigations. Corros. Sci. 2010, 52, 3042–3051. [Google Scholar] [CrossRef]



| Inhibitor | Conc. (M) | w (mg cm−2 h−1) | IE (%) ± 1% | θ |
|---|---|---|---|---|
| P1 | 1 × 10−3 | 0.081 | 89.5 | 0.895 |
| 5 × 10−4 | 0.125 | 87.0 | 0.870 | |
| 1 × 10−4 | 0.158 | 83.3 | 0.833 | |
| 5 × 10−5 | 0.160 | 83.6 | 0.836 | |
| P2 | 1 × 10−3 | 0.058 | 94.0 | 0.940 |
| 5 × 10−4 | 0.082 | 91.4 | 0.914 | |
| 1 × 10−4 | 0.147 | 84.0 | 0.840 | |
| 5 × 10−5 | 0.175 | 81.8 | 0.818 |
| Inhibitors | Slopes | Kads (M−1) | R2 | ΔG0ads (kJ mol−1) |
|---|---|---|---|---|
| P1 | 1.11 | 111884.23 | 0.99973 | −40.05 |
| P2 | 1.05 | 77893.75 | 0.99981 | −39.13 |
| Medium | Conc. (M) | −Ecorr (mVSCE) | −βa (mV dec−1) | −βc (mV dec−1) | Icorr (μA cm−2) | IE (%) |
|---|---|---|---|---|---|---|
| HCl | 1 | 450 | 105 | 207 | 1870.0 | − |
| P1 | 1 × 10−3 | 442 | 78 | 165 | 115.2 | 93 |
| 5 × 10−4 | 450 | 99 | 180 | 338.3 | 82 | |
| 1 × 10−4 | 449 | 95 | 140 | 455.2 | 75 | |
| 5 × 10−5 | 447 | 96 | 150 | 529.3 | 71 | |
| P2 | 1 × 10−3 | 442 | 79 | 145 | 66.5 | 96 |
| 5 × 10−4 | 446 | 79 | 135 | 125.0 | 93 | |
| 1 × 10−4 | 439 | 85 | 165 | 187.8 | 90 | |
| 5 × 10−5 | 441 | 92 | 172 | 253.0 | 86 |
| Medium | Rs (Ω cm2) | n | Rct/Fit (Ω cm2) | Rct/Exp (Ω cm2) | Cdl (μF cm−2) | τ (ms) | IE (%) | |
|---|---|---|---|---|---|---|---|---|
| Blank | 2.02 | 0.80 | 23.26 | 20.35 | 158.89 | 3.23 | − | |
| P1 | 5 × 10−5 M | 2.00 | 0.74 | 45.1 | 60.20 | 121.23 | 7.30 | 66.2 |
| 1 × 10−4 M | 1.97 | 0.76 | 60 | 72.50 | 114.56 | 8.30 | 71.9 | |
| 5 × 10−4 M | 1.45 | 0.74 | 120.8 | 117.60 | 75.33 | 8.86 | 82.7 | |
| 1 × 10−3 M | 1.82 | 0.77 | 187.5 | 183.10 | 71.95 | 13.17 | 88.9 | |
| P2 | 5 × 10−5 M | 2.50 | 0.77 | 90 | 76.48 | 87.55 | 6.70 | 73.4 |
| 1 × 10−4 M | 2.52 | 0.79 | 161 | 152.60 | 74.02 | 11.29 | 86.7 | |
| 5 × 10−4 M | 2.61 | 0.78 | 225 | 173.30 | 70.90 | 12.29 | 88.3 | |
| 1 × 10−3 M | 2.76 | 0.79 | 262 | 248.00 | 58.18 | 14.43 | 91.8 | |
| Inhibitor | EHOMO (eV) | ELUMO (eV) | ΔE (eV) | µ (Debye) |
|---|---|---|---|---|
| P2 | −8.54134 | −4.39552 | 4.14582 | 3.6319 |
| P1 | −5.80012 | −0.89434 | 4.90579 | 3.9252 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hajjaji, F.; Abrigach, F.; Hamed, O.; Hasan, A.R.; Taleb, M.; Jodeh, S.; Rodríguez-Castellón, E.; Martínez de Yuso, M.D.V.; Algarra, M. Corrosion Resistance of Mild Steel Coated with Orgainc Material Containing Pyrazol Moiety. Coatings 2018, 8, 330. https://doi.org/10.3390/coatings8100330
El Hajjaji F, Abrigach F, Hamed O, Hasan AR, Taleb M, Jodeh S, Rodríguez-Castellón E, Martínez de Yuso MDV, Algarra M. Corrosion Resistance of Mild Steel Coated with Orgainc Material Containing Pyrazol Moiety. Coatings. 2018; 8(10):330. https://doi.org/10.3390/coatings8100330
Chicago/Turabian StyleEl Hajjaji, Fadoua, Farid Abrigach, Othman Hamed, Abdelfatah Rasem Hasan, Mustapha Taleb, Shehdeh Jodeh, Enrique Rodríguez-Castellón, María Del Valle Martínez de Yuso, and Manuel Algarra. 2018. "Corrosion Resistance of Mild Steel Coated with Orgainc Material Containing Pyrazol Moiety" Coatings 8, no. 10: 330. https://doi.org/10.3390/coatings8100330