Inhibitory Effect of Astaxanthin on Oxidative Stress-Induced Mitochondrial Dysfunction-A Mini-Review
Abstract
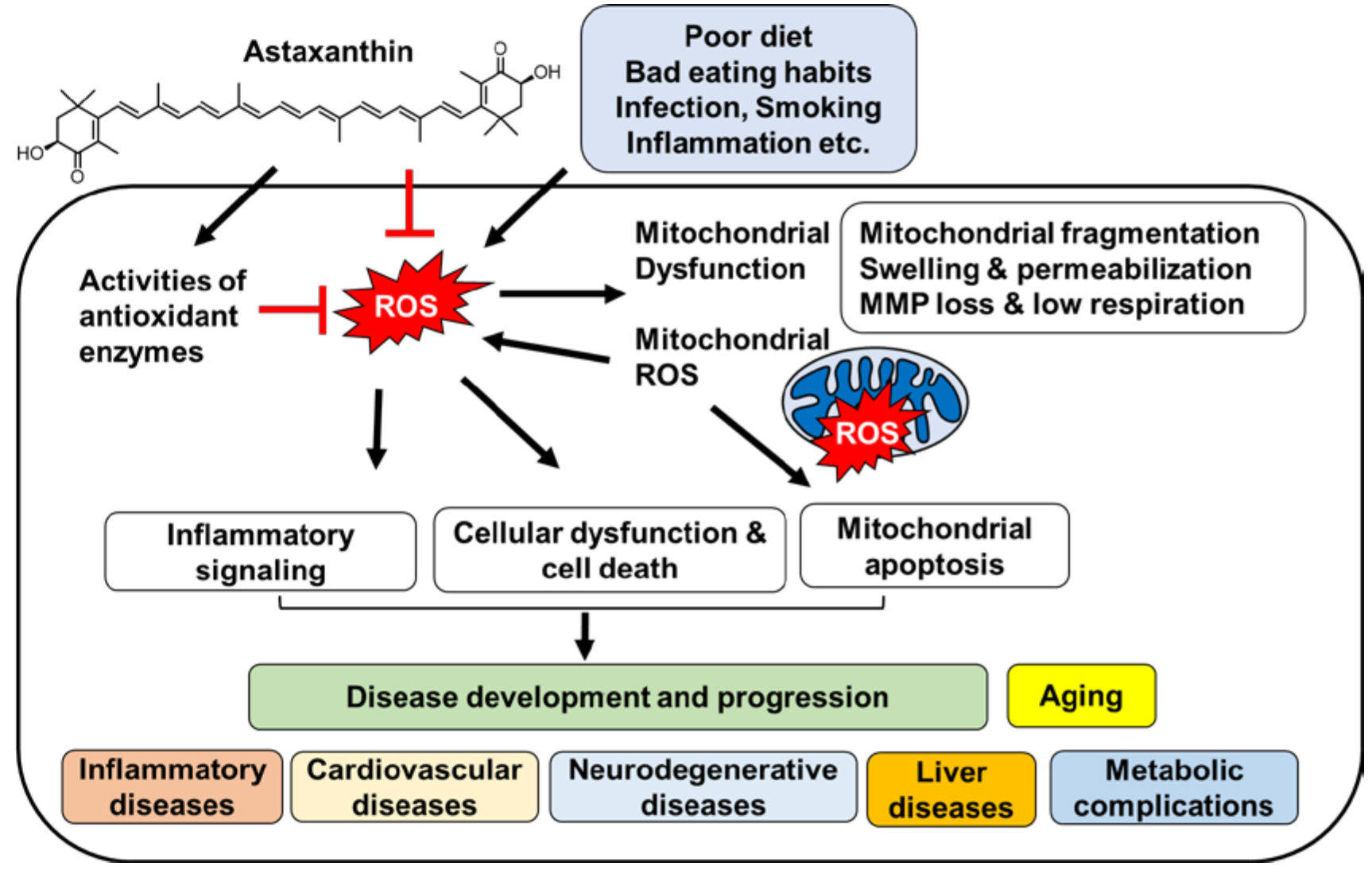
:1. Introduction
2. Oxidative Stress and Mitochondrial Dysfunction
3. Diseases Associated with Oxidative Stress and Mitochondrial Dysfunction
4. Astaxanthin: Biochemistry and Bioactivities
5. Effects of Astaxanthin on Oxidative Stress and Mitochondrial Dysfunction
6. Effect of Astaxanthin on Diseases Associated with Oxidative Stress and Mitochondrial Dysfunction
7. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.M.; Dayem, A.A.; Cho, S.G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, J.S.; Keen, C.L. Influence of Smoking on Markers of Oxidative Stress and Serum Mineral Concentrations in Teenage Girls in Korea. Nutrition 2003, 19, 240–243. [Google Scholar] [CrossRef]
- Chalmers, A. Smoking and oxidative stress. Am. J. Clin. Nutr. 1999, 69, 572. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R. Smoking, oxidative stress and inflammation: Impact on resting energy expenditure in diabetic nephropathy. BMC Nephrol. 2005, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aguilera, A.; Rull, A.; Rodríguez-Gallego, E.; Riera-Borrull, M.; Lucianon-Mateo, F.; Camps, J.; Menéndez, J.A.; Joven, J. Mitochondrial dysfunction: A basic mechanism in inflammation-related non-communicable diseases and therapeutic opportunities. Mediat. Inflamm. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Bullon, P.; Newman, H.N.; Battino, M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: A shared pathology via oxidative stress and mitochondrial dysfunction? Periodontology 2000 2014, 64, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Di Giacomo, M.; Zara, V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J. Gastroenterol. 2017, 23, 4146–4157. [Google Scholar] [CrossRef] [PubMed]
- Mantena, S.K.; King, A.L.; Andringa, K.K.; Eccleston, H.B.; Bailey, S.M. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol-and obesity-induced fatty liver diseases. Free Radic. Biol. Med. 2008, 44, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Runge, M.S. Mitochondrial dysfunction in atherosclerosis. Circ. Res. 2007, 100, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moriano, C.; González-Burgos, E.; Gómez-Serranillos, M.P. Mitochondria-targeted protective compounds in Parkinson’s and Alzheimer’s diseases. Oxid. Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- López-Armada, M.J.; Riveiro-Naveira, R.R.; Vaamonde-García, C.; Valcárcel-Ares, M.N. Mitochondrial dysfunction and the inflammatory response. Mitochondrion 2013, 13, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. 2011, 16, 355–364. [Google Scholar] [PubMed]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Starkov, A.A. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N. Y. Acad. Sci. 2008, 1147, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Grivennikova, V.G.; Vinogradov, A.D. Mitochondrial production of reactive oxygen species. Biochemistry 2013, 78, 1490–1511. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Filburn, C.R.; Klotz, L.O.; Zweier, J.L.; Sollott, S.J. Reactive oxygen species (Ros-Induced) Ros release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000, 192, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Siemen, D.; Ziemer, M. What is the nature of the mitochondrial permeability transition pore and what is it not? IUBMB Life 2013, 65, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Ojala, J.; Kaarniranta, K.; Kauppinen, A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: Impact on the aging process and age-related diseases. Cell. Mol. Life Sci. 2012, 69, 2999–3013. [Google Scholar] [CrossRef] [PubMed]
- Novak, E.A.; Mollen, K.P. Mitochondrial dysfunction in inflammatory bowel disease. Front. Cell Dev. Biol. 2015, 1, 62. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Wei, Y.H. Mitochondria and aging. Adv. Exp. Med. Biol. 2012, 942, 311–327. [Google Scholar] [PubMed]
- McManus, M.J.; Murphy, M.P.; Franklin, J.L. Mitochodria-derived reactive oxygen species mediate caspase-dependent and -independent neuronal deaths. Mol. Cell. Neurosci. 2014, 63, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Kluge, M.A.; Fetterman, J.L.; Vita, J.A. Mitochondria and endothelial function. Circ. Res. 2013, 112, 1171–1188. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Oliveira, C.R. Mitochondria as potential targets in antidiabetic therapy. Handb. Exp. Pharmacol. 2011, 203, 331–356. [Google Scholar]
- Nassir, F.; Ibdah, J.A. Role of mitochondria in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 8713–8742. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Li, Q.; Zhang, T.; Wang, D.; Yang, L.; Xu, J.; Wang, Y. The effects of astaxanthin and docosahexaenoic acid-acylated astaxanthin on Alzheimer’s disease in APP/PS1 double transgenic mice. J. Agric. Food Chem. 2018, 66, 4948–4957. [Google Scholar] [CrossRef] [PubMed]
- Chalyk, N.E.; Klochkov, V.A.; Bandaletova, T.Y.; Kyle, N.H.; Petyaev, I.M. Continuous astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res. 2017, 48, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zakerkish, M. Astaxanthin improves glulcose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac. J. Clin. Nutr. 2018, 27, 341–346. [Google Scholar]
- Bi, J.; Cui, R.; Li, Z.; Liu, C.; Zhang, J. Astaxanthin alleviated acute lung injury by inhibiting oxidative/nitrative stress and the inflammatory response in mice. Biomed. Pharmacother. 2017, 95, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Xu, X.C.; Liu, T.; Yuan, S. Mitochondrion-permeable antioxidants to treat ROS-burst-mediated acute diseases. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, T.; Ikeda, S.; Okada, T.; Maoka, T.; Kitamura, A.; Sugimoto, M.; Kume, S. Astaxanthin ameliorates heat stress-induced impairment of blastocyst development in vitro: Astaxanthin colocalization with and action on mitochondria. J. Assist. Reprod. Genet. 2013, 30, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Xuan, R.R.; Niu, T.T.; Chen, H.M. Astaxanthin blocks preeclampsia progression by suppressing oxidative stress and inflammation. Mol. Med. Rep. 2016, 14, 2697–2704. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Mathison, B.D.; Hayek, M.G.; Zhang, J.; Reinhart, G.A.; Chew, B.P. Astaxanthin modulates age-associated mitochondrial dysfunction in healthy dogs. J. Anim. Sci. 2013, 91, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Pongkan, W.; Takatori, O.; Ni, Y.; Xu, L.; Nagata, N.; Chattipakorn, S.C.; Chattipakorn, N. β-Cryptoxanthin exerts greater cardioprotective effects on cardiac ischemia-reperfusion injury than astaxanthin by attenuating mitochondrial dysfunction in mice. Mol. Nutr. Food Res. 2017, 61, 1601077. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, B.; Lin, S.; Jing, L.; Mao, C.; Xu, P.; Zuo, J. Astaxanthin inhibits apoptosis in alveolar epithelial cells type II in vivo and in vitro through the ROS-dependent mitochondrial signaling pathway. J. Cell. Mol. Med. 2014, 18, 2198–2212. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.D.; Sun, J.Y.; Fu, X.T.; Hou, Y.J.; Li, Y.; Yang, M.F.; Fu, X.Y.; Sun, B. Astaxanthin attenuates homocysteine-induced cardiotoxicity in vitro and in vivo by inhibiting mitochondrial dysfunction and oxidative damage. Front. Physiol. 2017, 8, 1041. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Tsuji, S.; Satoh, A.; Ishikura, M.; Shirasawa, T.; Shimizu, T. Protective effects of astaxanthin on 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J. Neurochem. 2008, 107, 1730–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Shibata, T.; Hisaka, S.; Osawa, T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 2009, 1254, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, C.S.; Lee, Y.J. Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food Chem. Toxicol. 2011, 49, 271–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Shamoto-Nagai, M.; Maruyama, W.; Osawa, T.; Naoi, M. Phytochemicals prevent mitochondrial membrane permeabilization and protect SH-SY5Y cells against apoptosis induced by PK11195, a ligand for outer membrane translocator protein. J. Neural Transm. 2017, 124, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.L.; Han, X.D.; Li, Y.; Chu, X.F.; Miao, W.M.; Zhang, J.L.; Fan, S.J. Astaxanthin attenuates total body irradiation-induced hematopoietic system injury in mice via inhibition of oxidative stress and apoptosis. Stem Cell Res. Ther. 2017, 8, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Guo, S.; Zhou, H.; Han, R.; Wu, P.; Han, C. Astaxanthin protects against early burn-wound progression in rats by attenuating oxidative stress-induced inflammation and mitochondria-related apoptosis. Sci. Rep. 2017, 7, 41440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.X.; Zhou, H.L.; Huang, C.L.; You, C.G.; Fang, Q.; Wu, P.; Han, C.M. Astaxanthin attenuates early acute kidney injury following severe burns in rats by ameliorating oxidative stress and mitochondrial-related apoptosis. Mar. Drugs 2015, 13, 2105–2123. [Google Scholar] [CrossRef] [PubMed]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, S58–S68. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.J.; Hazen, S.L.; Lockwood, S.F. Seven day oral supplementation with Cardax TM (disodium disuccinate astaxanthin) provides significant cardioprotection and reduces oxidative stress in rats. Mol. Cell. Biochem. 2006, 283, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Nakao, R.; Nelson, O.L.; Park, J.S.; Mathison, B.D.; Thompson, P.A.; Chew, B.P. Effect of astaxanthin supplementation on inflammation and cardiac function in BALB/c mice. Anticancer Res. 2010, 30, 2721–2725. [Google Scholar] [PubMed]
- Abdelzaher, L.A.; Imaizumi, T.; Suzuki, T.; Tomita, K.; Takashina, M.; Hattori, Y. Astaxanthin alleviates oxidative stress insults-related derangements in human vascular endothelial cells exposed to glucose fluctuations. Life Sci. 2016, 150, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Hossain, M.M.; Rahman, M.M.; Subhan, N.; Mamun, M.A.A.; Ulla, A.; Alam, M.A. Astaxanthin prevented oxidative stress in heart and kidneys of Isoproterenol-administered aged rats. J. Diet. Suppl. 2018, 15, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Rebin, I.; Zicker, S.; Wedekind, K.J.; Paetau-Robinson, I.; Packer, L.; Sohal, R.S. Effect of antioxidant-enriched diets on glutathione redox status in tissue homogenates and mitochondria of the senescence-accelerated mouse. Free Radic. Biol. Med. 2005, 39, 549–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tso, M.O.M.; Lam, T.T. Method of Retarding and Ameliorating Central Nervous System and Eye Damage. U.S. Patent No. 5,527,533, 18 June 1996. [Google Scholar]
- Wu, W.; Wang, X.; Xiang, Q.; Meng, X.; Peng, Y.; Du, N.; Liu, Z.; Sun, Q.; Wang, C.; Liu, X. Astaxanthin alleviates brain aging in rats by attenuating oxidative stress and increasing BDNF levels. Food. Funct. 2014, 5, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, W.; Lee, J.H.; Jeon, S.J.; Choi, Y.H.; Kim, B.W.; Nam, S.W. Astaxanthin inhibits H, O,-mediated apoptotic cell death in mouse neural progenitor cells via modulation of P38 and MEK signaling pathways. J. Microbiol. Biotechnol. 2009, 19, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.P.; Liu, S.Y.; Sun, H.; Wu, X.M.; Li, J.J.; Zhu, L. Neuroprotective effect of astaxanthin on H2O2-induced neurotoxicity in vitro and on focal cerebral ischemia in vivo. Brain Res. 2010, 1360, 40–48. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Full-spectrum antioxidant therapy featuring astaxanthin coupled with lipoprivic strategies and salsalate for management of non-alcoholic fatty liver disease. Med. Hypotheses 2011, 77, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Nagashimada, M.; Zhuge, F.; Zhan, L.; Nagata, N.; Tsutsui, A.; Nakanuma, Y.; Kaneko, S.; Ota, T. Astaxanthin prevents and reverses diet-induced insulin resistance and steatohepatitis in mice: A comparison with vitamin E. Sci. Rep. 2015, 5, 17192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Watanabe, M.; Takimoto, T.; Akiba, Y. Uptake and distribution of astaxanthin in several tissues and plasma lipoproteins in male broiler chickens fed a yeast (Phaffia rhodozyma) with a high concentration of astaxanthin. Br. Poult. Sci. 2004, 45, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Curek, G.D.; Cort, A.; Yucel, G.; Demir, N.; Ozturk, S.; Elpek, G.O.; Aslan, M. Effect of astaxanthin on hepatocellular injury following ischemia/reperfusion. Toxicology 2010, 267, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, S.; Arunkumar, E.; Viswanathan, P.; Anuradha, C.V. Astaxanthin restricts weight gain, promotes insulin sensitivity and curtails fatty liver disease in mice fed a obesity-promoting diet. Process Biochem. 2010, 45, 1406–1414. [Google Scholar] [CrossRef]
- Islam, M.A.; Al Mamun, M.A.; Faruk, M.; Islam, M.T.U.; Rahman, M.M.; Alam, M.N.; Rahman, A.F.M.T.; Reza, H.M.; Alam, A. Astaxanthin ameliorates hepatic damage and oxidative stress in carbon tetrachloride-administered rats. Pharmacogn. Res. 2017, 9, S84–S91. [Google Scholar]
- Ballinger, S.W. Mitochondrial dysfunction in cardiovascular disease. Free Radical Biol. Med. 2005, 38, 1278–1295. [Google Scholar] [CrossRef] [PubMed]
- Roohbakhsh, A.; Karimi, G.; Iranshahi, M. Carotenoids in the treatment of diabetes mellitus and its complications: A mechanistic review. Biomed. Pharmacother. 2017, 91, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Murillo, A.G.; Fernandez, M.L. Potential of dietary non-provitamin A carotenoids in the prevention and treatment of diabetic microvascular complications. Adv. Nutr. 2016, 7, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Lowell, B.B.; Shulman, G.I. Mitochondrial dysfunction and type 2 diabetes. Science 2005, 307, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Bonnard, C.; Durand, A.; Peyrol, S.; Chanseaume, E.; Chauvin, M.A.; Morio, B.; Rieusset, J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J. Clin. Investig. 2008, 118, 789–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Kim, Y.A.; Yokozawa, T. Protection against oxidative stress, inflammation, and apoptosis of high-glucose-exposed proximal tubular epithelial cells by astaxanthin. J. Agric. Food Chem. 2009, 57, 8793–8797. [Google Scholar] [CrossRef] [PubMed]
- Otton, R.; Marin, D.P.; Bolin, A.P.; dos Santos, R.D.C.M.; Polotow, T.G.; Sampaio, S.C.; de Barros, M.P. Astaxanthin ameliorates the redox imbalance in lymphocytes of experimental diabetic rats. Chem. Biol. Interact. 2010, 186, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Manabe, E.; Handa, O.; Naito, Y.; Mizushima, K.; Akagiri, S.; Adachi, S.; Yoshikawa, T. Astaxanthin protects mesangial cells from hyperglycemia-induced oxidative signaling. J. Cell. Biochem. 2008, 103, 1925–1937. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Uchiyama, K.; Aoi, W.; Hasegawa, G.; Nakamura, N.; Yoshida, N.; Yoshikawa, T. Prevention of diabetic nephropathy by treatment with astaxanthin in diabetic db/db mice. Biofactors 2004, 20, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhu, J.; Yin, W.; Ding, X. Astaxanthin improves cognitive deficits from oxidative stress, nitric oxide synthase and inflammation through upregulation of PI3K/Akt in diabetes rat. Int. J. Clin. Exp. Pathol. 2015, 8, 6083–6094. [Google Scholar] [PubMed]
- Yeh, P.T.; Huang, H.W.; Yang, C.M.; Yang, W.S.; Yang, C.H. Astaxanthin inhibits expression of retinal oxidative stress and inflammatory mediators in streptozotocin-induced diabetic rats. PLoS ONE 2016, 11, e0146438. [Google Scholar] [CrossRef] [PubMed]
| Experimental Model | Effective Dose and Duration | Main Results | Reference | |
|---|---|---|---|---|
| Inflammatory Diseases | Human umbilical vein endothelial cells (HUVECs) treated with H2O2 | 10 μM 48 h | cell viability ↑ reactive oxygen species (ROS) ↓ mitochondrial membrane potential (MMP) ↑ | [40] |
| Preeclamptic pregnant rats | 25 mg/kg 16 days | blood pressure ↓ urinary protein ↓ oxidative stress marker; malondialdehyde (MDA) ↓ serum superoxide dismutase (SOD) ↑ histopathological changes ↓ preeclampsia-associated protein ↓ heme oxygenase-1 ↑ caspase-3 ↓ nuclear factor-κB (NF-κB) ↓ | [40] | |
| Alveolar epithelial cells type II (AECs-II) from rats with bleomycin-induced lung fibrosis | 1, 2 mg/kg 7 days | apoptosis ↓ SOD, catalase activities ↑ mitochondrial membrane integrity ↑ mitochondria swelling ↓ deformed cristae ↓ | [43] | |
| Rat lung epithelial -T-antigen negative (RLE-6TN) cells treated with H2O2 or bleomycin | 8 μM 6–24 h | apoptosis ↓ ROS ↓ SOD, catalase activities ↑ mitochondrial membrane integrity ↑ mitochondria swelling ↓ deformed cristae ↓ mitochondria disarrangement ↓ MMP ↑ pro-apoptotic protein ↓ anti-apoptotic protein ↑ cytochrome c release, caspase activation ↓ nuclear factor erythroid-derived 2-related factor 2 (Nrf2) ↑ p53 ↑ | [43] | |
| A classic “comb” burn model in rats | 5, 10, 20 mg/kg 48 h | burn-associated histological changes ↓ inflammatory cell infiltration ↓ oxidative stress marker (MDA) ↓ SOD, glutathione peroxidase ↑ xanthine oxidase, NADPH oxidase ↓ myeloperoxidase, TNF-α, IL-1β, IL-6 ↓ apoptosis ↓ activated cellular homolog of murine thymoma virus akt8 oncogene (Akt) ↑ inactivated Bcl-2-associated death promoter (Bad) protein ↑ | [50] | |
| Severe burn rat model | 5, 10, 20 mg/kg 24 h | histological and functional damage of kidney ↓ oxidation-reduction potential ↓ oxidative stress marker (MDA) ↓ SOD, catalase ↑ apoptosis ↓ activated Akt, inactivated Bad ↑ cytochrome c, caspases ↓ | [51] | |
| Aging | Geriatric dogs | 20 mg/kg 16 weeks | oxidative stress markers (8-hydroxy-2′-deoxyguanosine, protein carbonyl, nitric oxide) ↓ blood SOD ↑ mitochondrial mass ↑ ATP production ↑ mitochondria Complex III production ↑ | [41] |
| Senescence accelerated mice (SAM) | 8% of antioxidant diet 10 months | plasma glutathione (GSH) ↑ glutathione disulfide (GSSG) ↓ mitochondrial GSH in kidney, heart, brain, skeletal muscle ↑ ,mitochondrial GSSG in liver, kidney, heart, brain ↓ mitochondrial glutathione redox potential ↑ | [57] | |
| Rats with d-galactose-induced brain aging | 0.02% 8 weeks | oxidative stress markers (MDA, 8-hydroxy-2′-deoxyguanosine, protein carbonyls) in brain ↓ brain glutathione peroxidase, SOD activities ↑ total antioxidant capacity ↑ anti-apoptotic protein ↑ pro-apoptotic protein ↓ cyclooxygenase (COX)-2 ↓ brain-derived neurotrophic factor ↓ | [59] | |
| Cardiovascular Diseases | BALB/c mice | 0.02, 0.08% 8 weeks | cardiac MMP ↑ TNF-α ↓ contractility of left ventricle ↑ | [54] |
| Human umbilical vein endothelial cells (HUVECs) exposed to glucose fluctuation | 0.05, 0.1, 0.5 μM 3 days | ROS ↓ a component of NADPH oxidase p22phox ↓ endogenous nitric oxide synthase (eNOS) ↑ nitrite ↓ peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α ↑ IL-6, intercellular adhesion molecule-1 ↓ apoptosis ↓ phosphorylation of c-Jun N-terminal kinases (JNK), p-38 ↓ | [55] | |
| Rats with isoproterenol hydrochloride-induced myocardial infarction | 25 mg/kg 2 weeks | heart and kidney wet weight ↓ oxidative stress markers (MDA, nitric oxide) ↓ heart SOD, catalase, GSH ↑ histopathological changes ↓ | [56] | |
| Mice with left anterior descending coronary artery (LAD) occlusion-induced ischemia-reperfusion injury | 50 mg/kg 2 h | infarct size ↓ pro-apoptotic protein ↓ anti-apoptotic protein ↑ mitochondrial ROS ↓ cardiac mitochondria depolarization ↓ cardia mitochondria swelling ↓ oxidative stress marker (MDA) ↓ | [42] | |
| H9c2 rat myocardial cells exposed to homocysteine | 4 μM 6 h | cell viability ↑ apoptosis ↓ MMP ↑ mitochondria fragmentation ↓ pro-apoptotic protein ↓ anti-apoptotic protein ↑ intracellular ROS, mitochondrial ROS ↓ DNA damage ↓ | [44] | |
| Homocysteine administered mice | 5 mg/kg 4 weeks | GSH ↑ oxidative stress marker (MDA) ↓ apoptosis ↓ | [44] | |
| Neuro-degenerative Diseases | Human neuroblastoma SH-SY5Y cells treated with 6-hydroxydopamine | 20 μM 30 min | apoptosis ↓ cytochrome c release, caspase-9 cleavage, caspase-3 activation ↓ p38 ↓ MMP ↑ | [45] |
| Human neuroblastoma SH-SY5Y cells treated with 6-hydroxydopamine or DHA hydroperoxide | 100 nM 4 h | cell viability ↓ apoptosis ↓ cytochrome c release ↓ MMP ↑ oxidative stress marker (protein carbonyls) in mitochondrial fraction ↓ ROS ↓ | [46] | |
| Human neuroblastoma SH-SY5Y cells treated with 1-methyl-4-phenylpyridinium (MPP+) | 50 μM 25 h | cell viability ↑ apoptosis ↓ ROS ↓ SOD, catalase ↑ pro-apoptotic protein ↓ anti-apoptotic protein ↑ cytochrome c release, caspase activation ↓ MMP ↑ | [47] | |
| 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced mouse model of Parkinson’s disease | 30 mg/kg 28 days | dopaminergic neurons ↑ histological hallmarks of Parkinson’s disease ↓ | [47] | |
| Mouse neural progenitor cells treated with H2O2 | 10 ng/mL 8 h | apoptosis ↓ cell proliferation ↑ caspase activation ↓ ATP production ↑ mitochondrial leakage ↓ pro-apoptotic protein ↓ p38 ↑ | [60] | |
| Primary cortical neuron treated with H2O2 | 500 nM 4 h | cell viability ↑ apoptosis ↓ MMP ↑ | [61] | |
| Rats with middle cerebral artery occlusion (MCAO)-induced focal cerebral ischemia | 50, 80 mg/kg 6 h | infarct volume ↓ neurological deficit score ↓ | [61] | |
| Liver Diseases | Nonalcoholic steatohepatitis (NASH) mice fed high-fat, cholesterol, and chocolate diet | 0.02% 12 weeks | liver AST, ALT ↓ triglyceride, total cholesterol, non-esterified fatty acid ↓ hepatic lipid accumulation ↓ oxidative stress marker (MDA) ↓ lipogenic gene expression ↓ glucose intolerance ↓ hyperinsulinemia ↓ hepatic insulin signaling proteins ↓ JNK, p38, NF-κB ↓ infiltration and activation of Kupffer cells ↓ hepatic fibrosis ↓ | [63] |
| Rat model of ischemia-reperfusion injury | 5 mg/kg 14 days | Histopathological score ↓ cell damage ↓ xanthine dehydrogenase: xanthine oxidase ratio ↑ mitochondrial swelling ↓ rough endoplasmic reticulum disarrangement ↓ | [65] | |
| High fat- high fructose diet -induced mice obesity model | 6 mg/kg 45 days | body weight ↓ hepatomegaly ↓ plasma glucose ↓ plasma liver lipid ↓ oxidative stress markers (MDA, nitrite nitrosothiol) ↓ SOD, catalase, glutathione peroxidase, glutathione s-transferase ↑ TGF-β1 ↓ histological abnormality ↓ | [66] | |
| Rats intoxicated with CCL4 | 10 mg/kg 2 weeks | liver AST, ALT, alkaline phosphatase ↓ oxidative stress markers (MDA, nitric oxide) ↓ SOD, catalase activities ↑ myeloperoxidase ↓ inflammatory cell infiltration ↓ liver tissue necrosis ↓ hepatic fibrosis ↓ | [67] | |
| Metabolic Complications | Porcine proximal tubular epithelial cells (PTECs) exposed to high glucose | 5, 10 μg/mL 24–48 h | cell viability ↑ cytotoxicity ↓ pro-apoptotic protein ↓ anti-apoptotic protein ↑ reactive nitrogen species (RNS) (•O2, NO•, ONOO–) ↓ oxidative stress marker (MDA) ↓ COX-2, inducible nitric oxide synthase (iNOS), NF-κB ↓ | [73] |
| Alloxan-induced diabetic rat model | 20 mg/kg 30 days | blood glucose, blood triglyceride ↓ pro-reducing redox balance of plasmalymphocyte oxidative stress marker (MDA) ↓ lymphocyte ROS/RNS (H2O2, •O2, NO•) ↓ calcium influx of lymphocytes ↓ | [74] | |
| Normal human mesangial cells (NHMCs) treated with high glucose | 10−6 M 24 h | mitochondrial ROS ↓ activator protein-1 activation ↓ monocyte chemoattractant peptide-1, COX-1, TGF-β1 ↓ lipid peroxidation in mitochondria ↓ mitochondrial protein adducts ↓ NF-κB ↓ | [75] | |
| Streptozotocin-induced diabetic rats | 10, 20, 40 mg/kg 5 days | body weight ↓ blood glucose ↓ oxidative stress marker (MDA) in cerebral cortex and hippocampus ↓ SOD, GSH ↑ eNOS, iNOS ↓ NF-κB, TNF-α, IL-1β, IL-6 ↓ caspase ↓ phosphoinositide 3-kinase/Akt ↑ | [77] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Kim, H. Inhibitory Effect of Astaxanthin on Oxidative Stress-Induced Mitochondrial Dysfunction-A Mini-Review. Nutrients 2018, 10, 1137. https://doi.org/10.3390/nu10091137
Kim SH, Kim H. Inhibitory Effect of Astaxanthin on Oxidative Stress-Induced Mitochondrial Dysfunction-A Mini-Review. Nutrients. 2018; 10(9):1137. https://doi.org/10.3390/nu10091137
Chicago/Turabian StyleKim, Suhn Hyung, and Hyeyoung Kim. 2018. "Inhibitory Effect of Astaxanthin on Oxidative Stress-Induced Mitochondrial Dysfunction-A Mini-Review" Nutrients 10, no. 9: 1137. https://doi.org/10.3390/nu10091137
APA StyleKim, S. H., & Kim, H. (2018). Inhibitory Effect of Astaxanthin on Oxidative Stress-Induced Mitochondrial Dysfunction-A Mini-Review. Nutrients, 10(9), 1137. https://doi.org/10.3390/nu10091137






