The Use of the Masquelet Technique in the Treatment of Pathological Distal Third Femoral Fracture Secondary to Chronic Osteomyelitis
Abstract
:1. Introduction
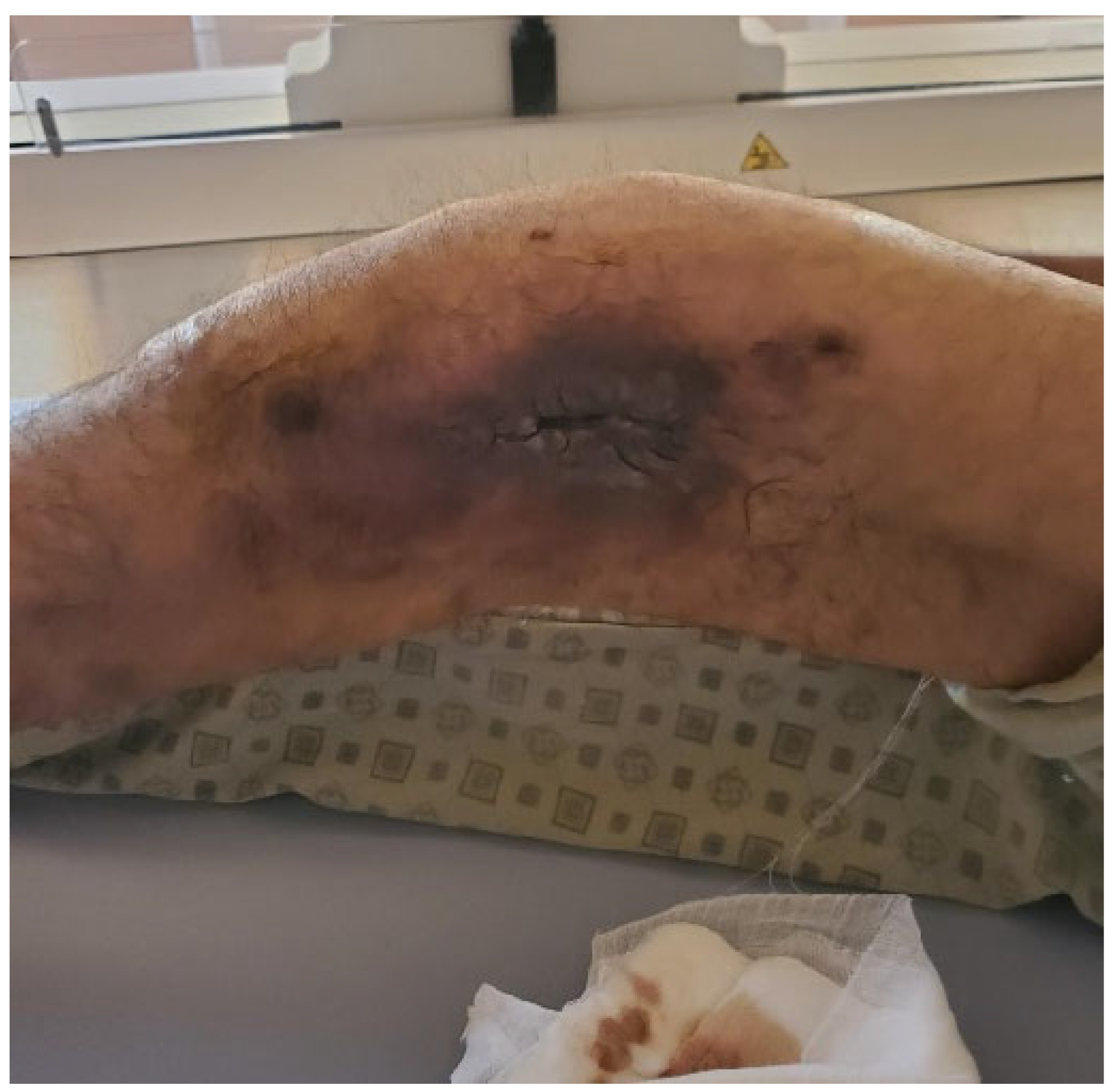
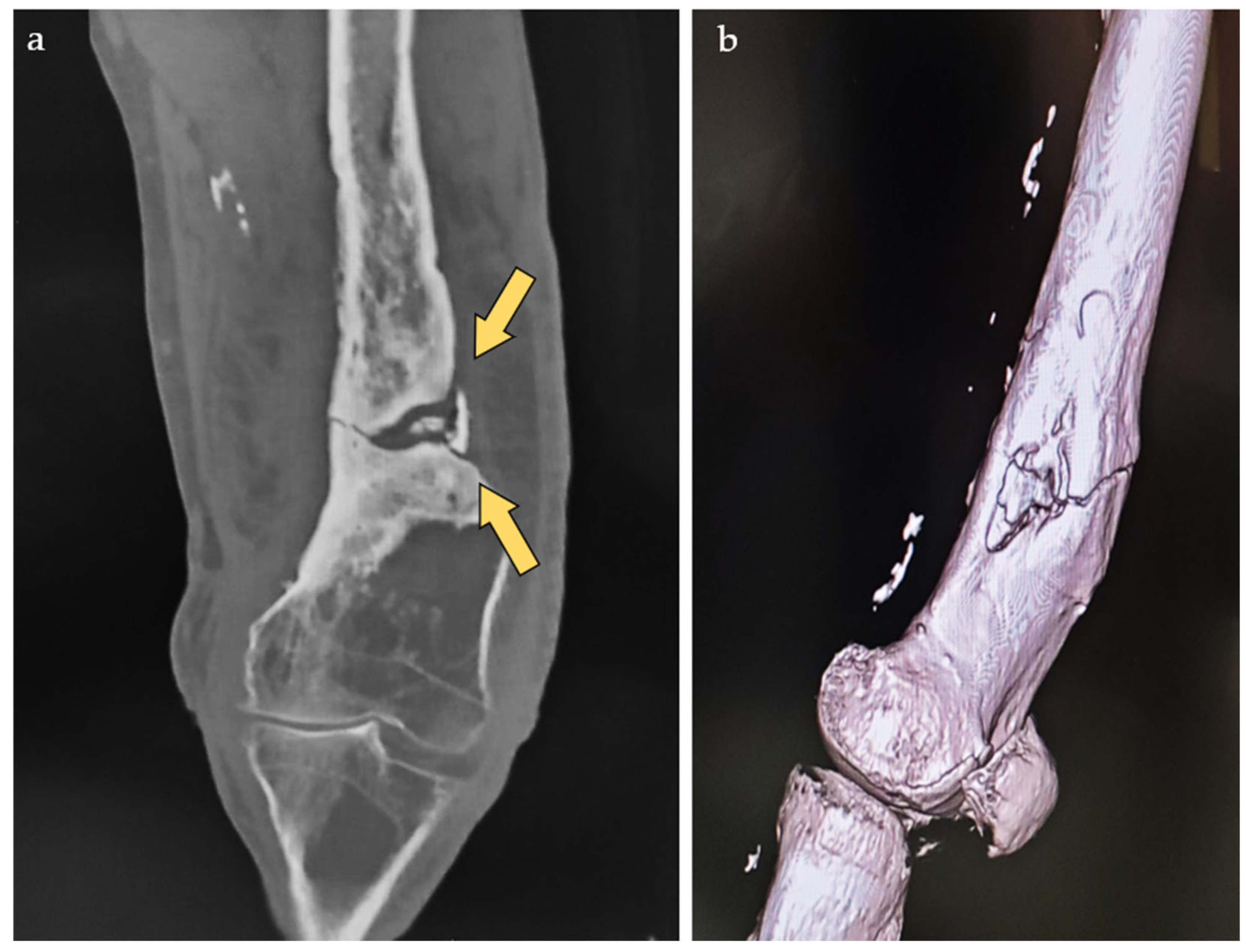
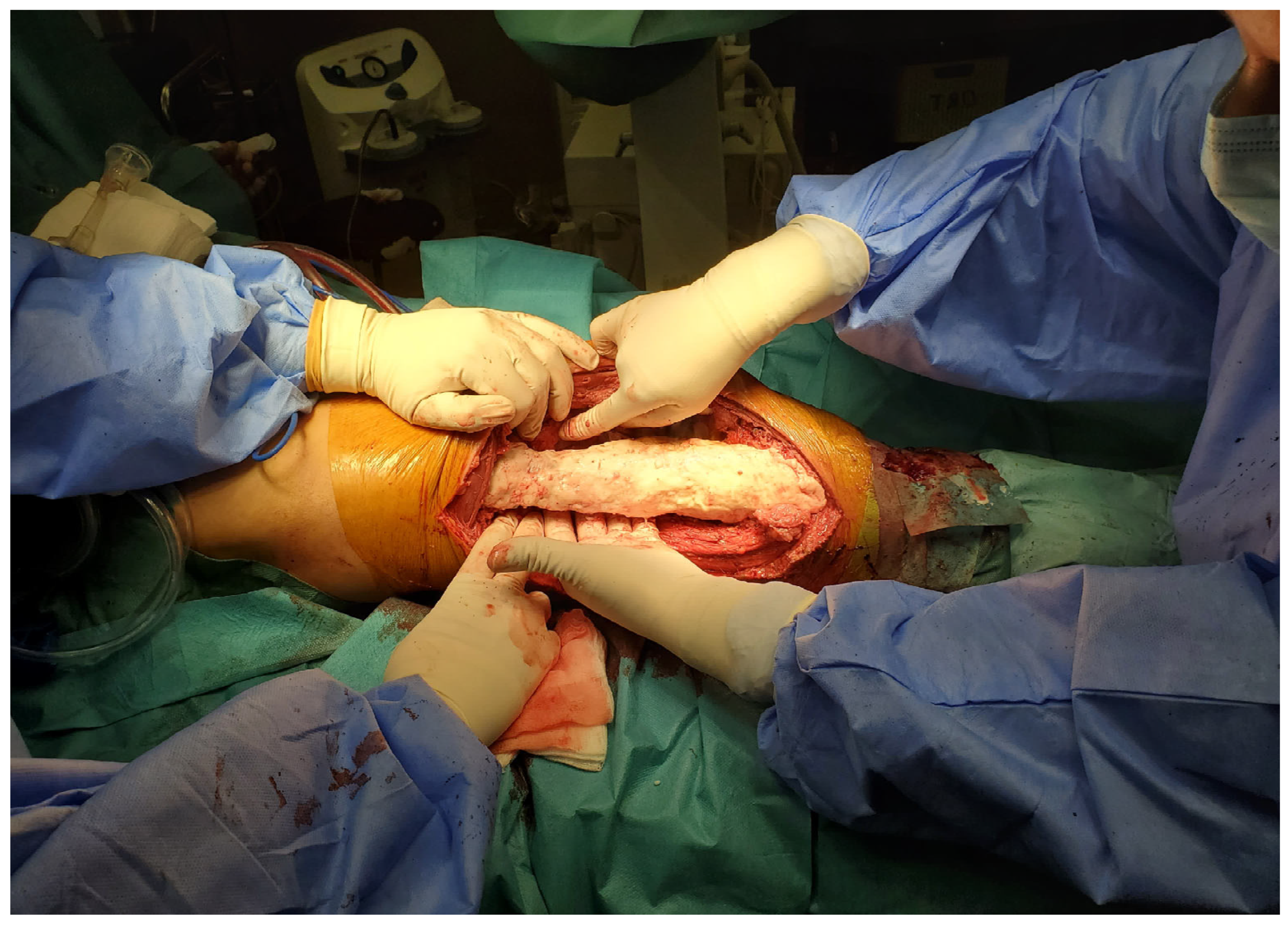
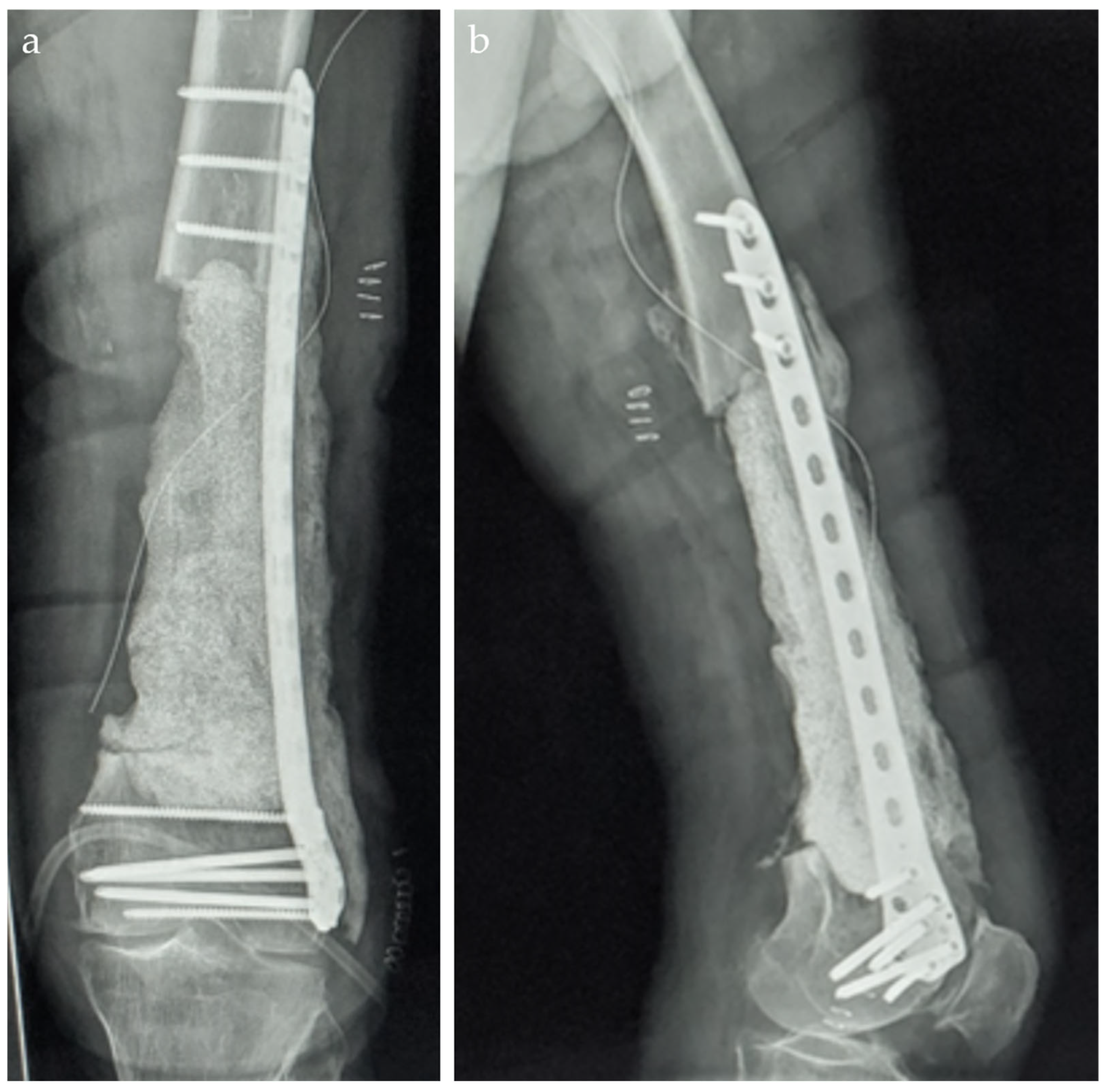
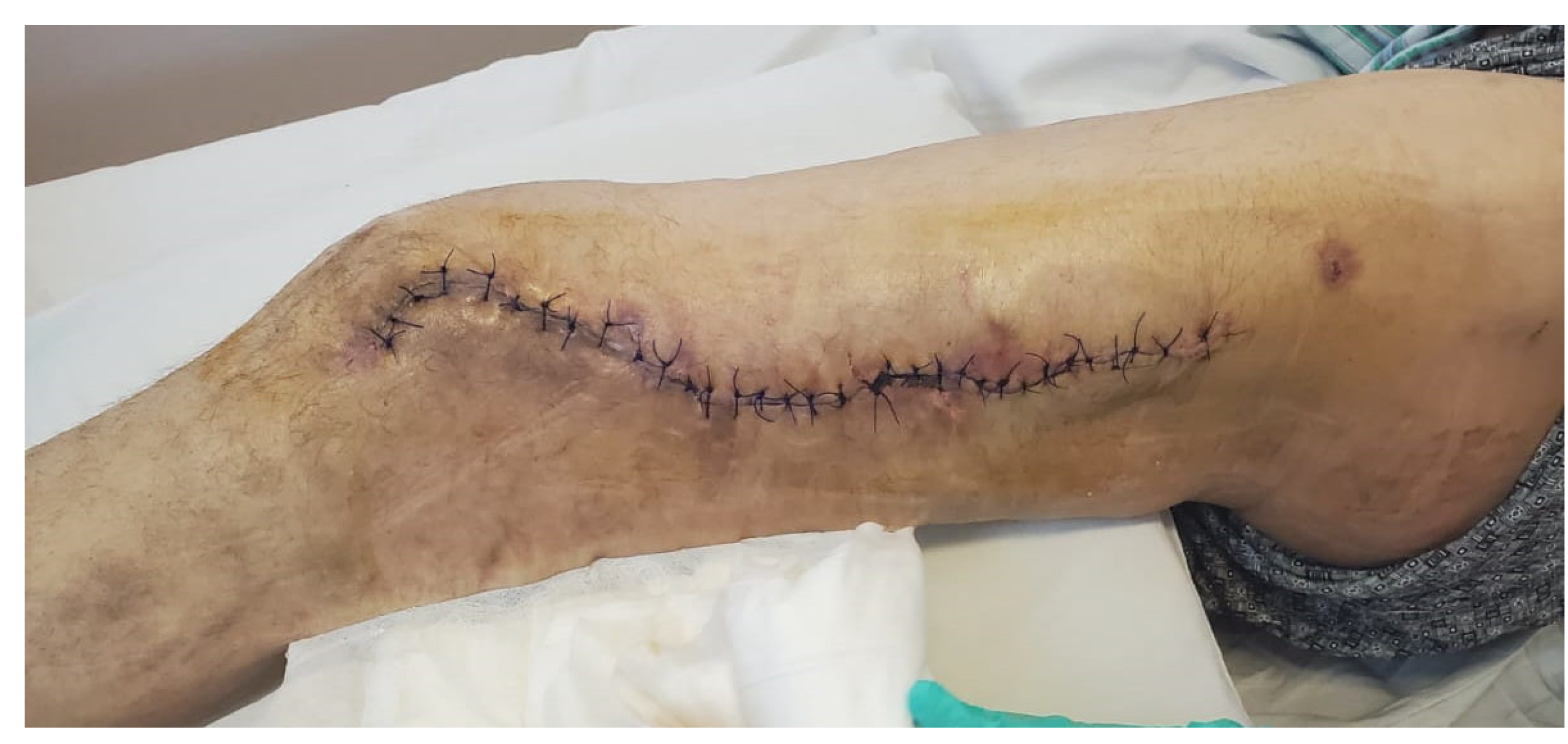
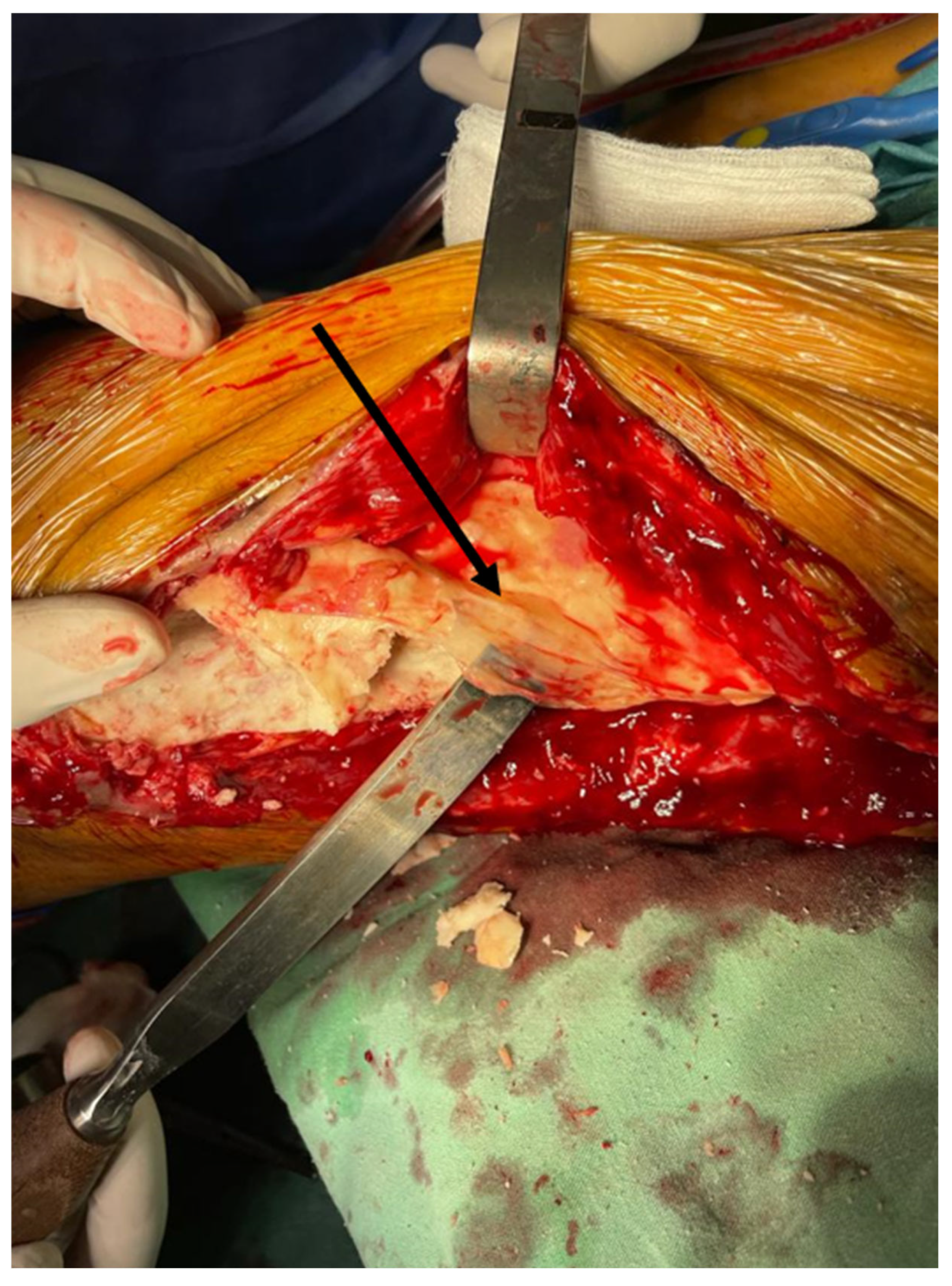
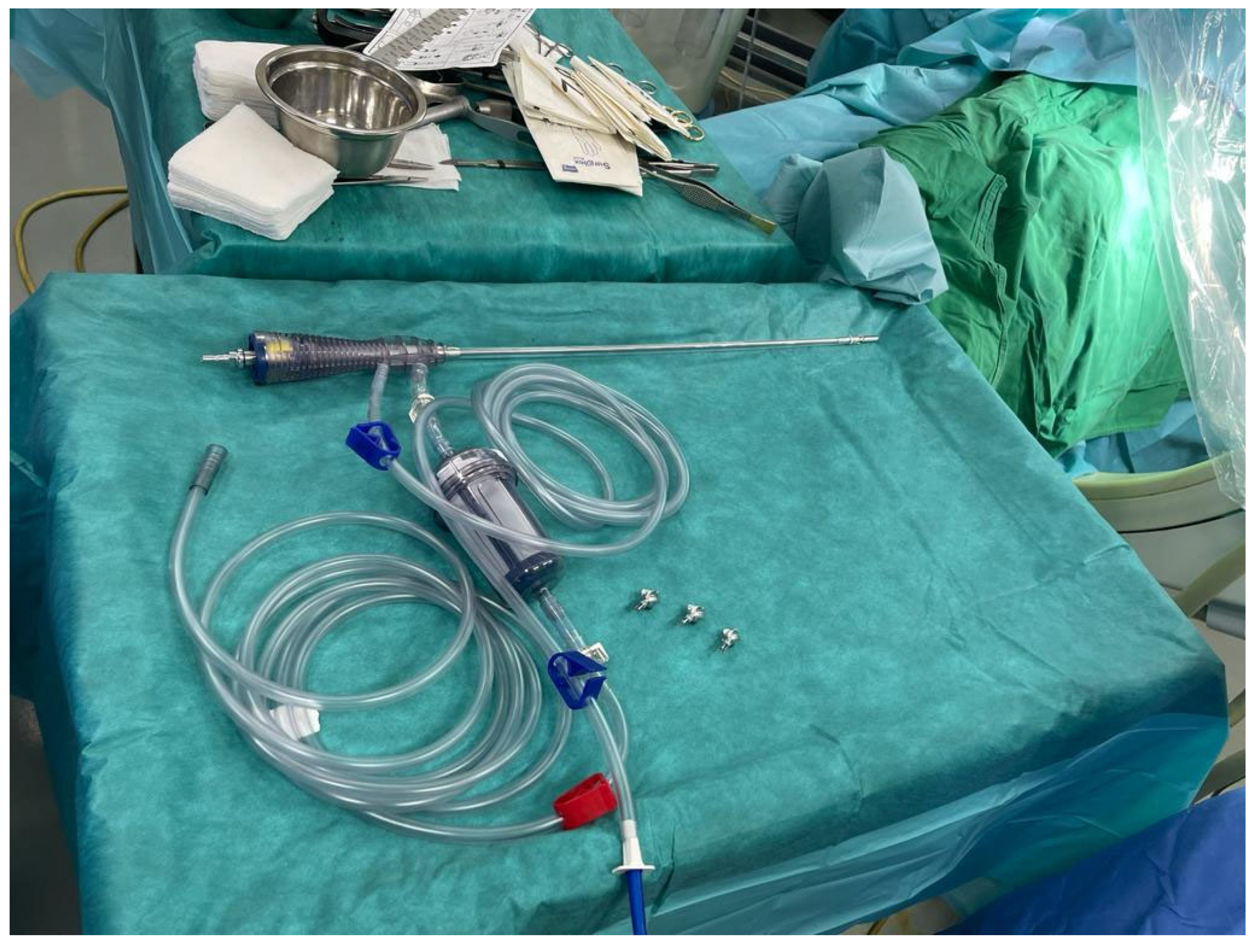
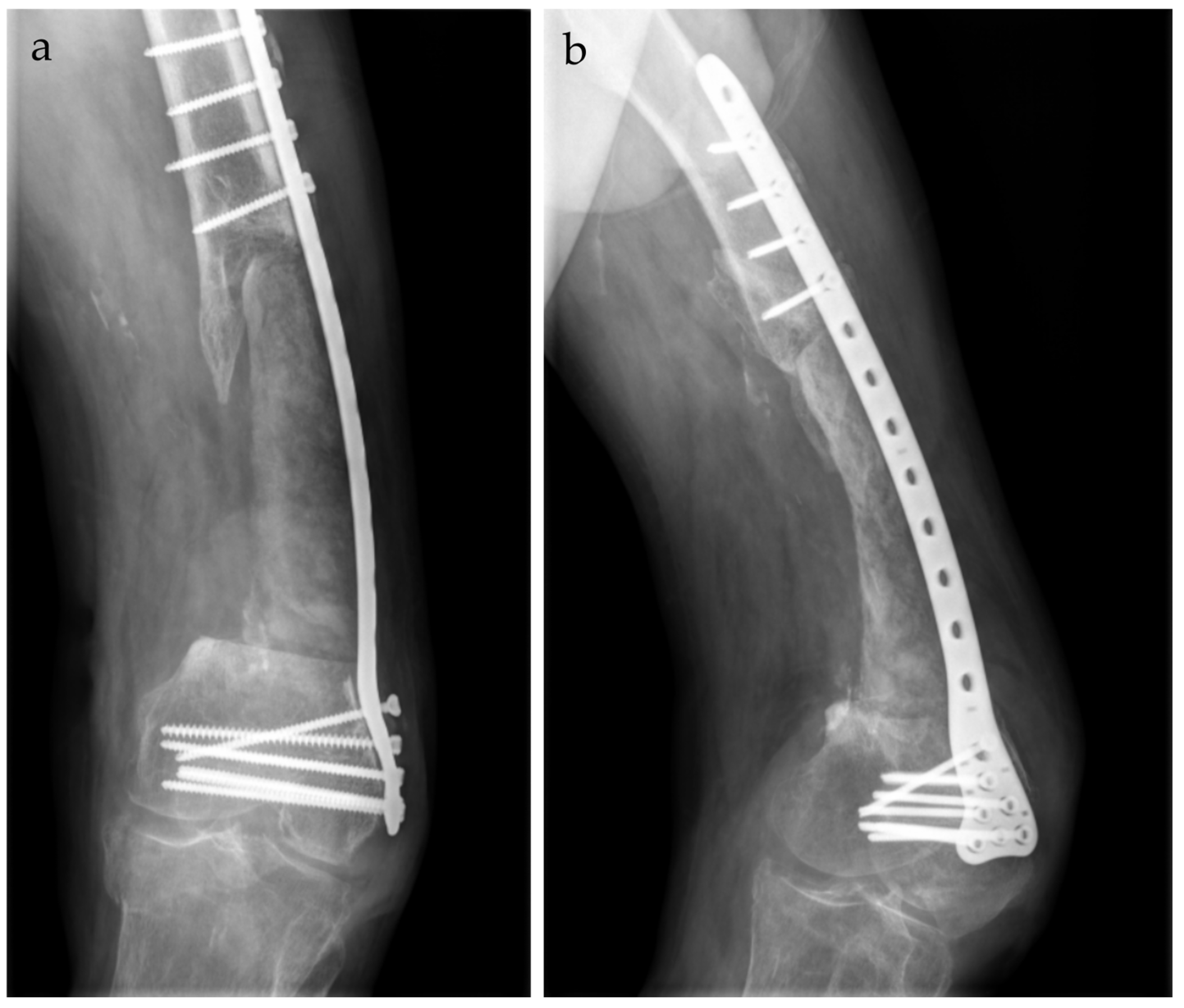
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMP | Bone morphogenetic protein |
| CRP | C-reactive protein |
| CT | Computer tomography |
| ESR | Erythrocyte sedimentation rate |
| Hb | Hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
| MIMT | Masquelet’s induced membrane technique |
| MSSA | Meticilin-sensitive Staphylococcus aureus |
| PLT | Platelets |
| PMMA | Polymethylmethacrylate |
| TGF Beta 1 | Transforming growth factor beta |
| VEGF | Vascular endothelial growth factor |
| WBC | White blood cell |
References
- Panteli, M.; Giannoudis, P.V. Chronic osteomyelitis: What the surgeon needs to know. EFORT Open Rev. 2016, 1, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Momodu, I.I.; Savaliya, V. Osteomyelitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Timofte, D.V.; Tudor, R.C.; Mocanu, V.; Labusca, L. Obesity, Osteoarthritis, and Myokines: Balancing Weight Management Strategies, Myokine Regulation, and Muscle Health. Nutrients 2024, 16, 4231. [Google Scholar] [CrossRef]
- Desimpel, J.; Posadzy, M.; Vanhoenacker, F. The Many Faces of Osteomyelitis: A Pictorial Review. J. Belg. Soc. Radiol. 2017, 101, 24. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, M.S.; Cleveland, K.O.; Heck, R.K.; Goswami, R. Pathological fracture in acute osteomyelitis of long bones secondary to community-acquired methicillin-resistant Staphylococcus aureus: Two cases and review of the literature. Am. J. Med. Sci. 2006, 332, 357–360. [Google Scholar] [CrossRef]
- Dragosloveanu, Ș.; Dragosloveanu, C.D.M.; Cotor, D.C.; Stoica, C.I. Short vs. long intramedullary nail systems in trochanteric fractures: A randomized prospective single center study. Exp. Ther. Med. 2022, 23, 106. [Google Scholar] [CrossRef]
- Padman, M.; Rosenfeld, S.B.; Belthur, M.V. Pathological Fractures with Osteomyelitis. In Pediatric Musculoskeletal Infections: Principles & Practice; Belthur, M.V., Ranade, A.S., Herman, M.J., Fernandes, J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 383–409. [Google Scholar]
- Botterill, J.; Ghosh, S.; Bhaskaran, A. Pathological Fracture of the Femur Following Chronic Osteomyelitis: A Case Report of This Rare Presentation in Adults. Cureus 2024, 16, e59733. [Google Scholar] [CrossRef]
- Dolitsky, R.; DePaola, K.; Fernicola, J.; Collins, C. Pediatric musculoskeletal infections. Pediatr. Clin. 2020, 67, 59–69. [Google Scholar] [CrossRef]
- Bezstarosti, H.; Metsemakers, W.J.; van Lieshout, E.M.M.; Voskamp, L.W.; Kortram, K.; McNally, M.A.; Marais, L.C.; Verhofstad, M.H.J. Management of critical-sized bone defects in the treatment of fracture-related infection: A systematic review and pooled analysis. Arch. Orthop. Trauma Surg. 2021, 141, 1215–1230. [Google Scholar] [CrossRef]
- Mauffrey, C.; Barlow, B.T.; Smith, W. Management of Segmental Bone Defects. JAAOS-J. Am. Acad. Orthop. Surg. 2015, 23, 143–153. [Google Scholar] [CrossRef]
- Timofticiuc, I.-A.; Dragosloveanu, S.; Caruntu, A.; Scheau, A.-E.; Badarau, I.A.; Garofil, N.D.; Didilescu, A.C.; Caruntu, C.; Scheau, C. 3D Bioprinting in Limb Salvage Surgery. J. Funct. Biomater. 2024, 15, 383. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, A.-T.; Troumpata, L.; Dragosloveanu, S.; Timofticiuc, I.-A.; Georgatos-Garcia, S.; Scheau, A.-E.; Periferakis, K.; Caruntu, A.; Badarau, I.A.; et al. Use of Biomaterials in 3D Printing as a Solution to Microbial Infections in Arthroplasty and Osseous Reconstruction. Biomimetics 2024, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Green, S.A. Skeletal defects. A comparison of bone grafting and bone transport for segmental skeletal defects. Clin. Orthop. Relat. Res. 1994, 301, 111–117. [Google Scholar]
- Xie, L.; Huang, Y.; Zhang, L.; Si, S.; Yu, Y. Ilizarov method and its combined methods in the treatment of long bone defects of the lower extremity: Systematic review and meta-analysis. BMC Musculoskelet. Disord. 2023, 24, 891. [Google Scholar] [CrossRef] [PubMed]
- Ilizarov, G.A. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin. Orthop. Relat. Res. 1989, 238, 249–281. [Google Scholar] [CrossRef]
- Malkova, T.A.; Borzunov, D.Y. International recognition of the Ilizarov bone reconstruction techniques: Current practice and research (dedicated to 100(th) birthday of G. A. Ilizarov). World J. Orthop. 2021, 12, 515–533. [Google Scholar] [CrossRef]
- Masquelet, A.C.; Fitoussi, F.; Begue, T.; Muller, G.P. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann. Chir. Plast. Esthet. 2000, 45, 346–353. [Google Scholar]
- Pelissier, P.; Masquelet, A.C.; Bareille, R.; Pelissier, S.M.; Amedee, J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J. Orthop. Res. 2004, 22, 73–79. [Google Scholar] [CrossRef]
- Bor, N.; Dujovny, E.; Rinat, B.; Rozen, N.; Rubin, G. Treatment of chronic osteomyelitis with antibiotic-impregnated polymethyl methacrylate (PMMA)—The Cierny approach: Is the second stage necessary? BMC Musculoskelet. Disord. 2022, 23, 38. [Google Scholar] [CrossRef]
- van Vugt, T.A.G.; Arts, J.J.; Geurts, J.A.P. Antibiotic-Loaded Polymethylmethacrylate Beads and Spacers in Treatment of Orthopedic Infections and the Role of Biofilm Formation. Front. Microbiol. 2019, 10, 1626. [Google Scholar] [CrossRef]
- Verboket, R.D.; Leiblein, M.; Janko, M.; Schaible, A.; Brune, J.C.; Schröder, K.; Heilani, M.; Fremdling, C.; Busche, Y.; Irrle, T.; et al. From two stages to one: Acceleration of the induced membrane (Masquelet) technique using human acellular dermis for the treatment of non-infectious large bone defects. Eur. J. Trauma Emerg. Surg. 2020, 46, 317–327. [Google Scholar] [CrossRef]
- Alford, A.I.; Nicolaou, D.; Hake, M.; McBride-Gagyi, S. Masquelet’s induced membrane technique: Review of current concepts and future directions. J. Orthop. Res. 2021, 39, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Hoit, G.; Kain, M.S.; Sparkman, J.W.; Norris, B.L.; Conway, J.D.; Watson, J.T.; Tornetta, P., 3rd; Nauth, A. The induced membrane technique for bone defects: Basic science, clinical evidence, and technical tips. OTA Int 2021, 4, e106. [Google Scholar] [CrossRef]
- Gaio, N.; Martino, A.; Toth, Z.; Watson, J.T.; Nicolaou, D.; McBride-Gagyi, S. Masquelet technique: The effect of altering implant material and topography on membrane matrix composition, mechanical and barrier properties in a rat defect model. J. Biomech. 2018, 72, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Masquelet, A.C.; Begue, T. The concept of induced membrane for reconstruction of long bone defects. Orthop. Clin. N. Am. 2010, 41, 27–37. [Google Scholar] [CrossRef]
- Dragosloveanu, Ş.; Cotor, D.C.; Dragosloveanu, C.D.M.; Stoian, C.; Stoica, C.I. Preclinical study analysis of massive magnesium alloy graft for calcaneal fractures. Exp. Ther. Med. 2021, 22, 731. [Google Scholar] [CrossRef]
- Kaneko, Y.; Minehara, H.; Sonobe, T.; Kameda, T.; Sekiguchi, M.; Matsushita, T.; Konno, S.I.; Matsumoto, Y. Differences in macrophage expression in induced membranes by fixation method—Masquelet technique using a mouse’s femur critical-sized bone defect model. Injury 2024, 55, 111135. [Google Scholar] [CrossRef]
- Frese, J.; Schulz, A.P.; Kowald, B.; Gerlach, U.J.; Frosch, K.H.; Schoop, R. Does the extent of bone defects affect the time to reach full weight-bearing after treatment with the Masquelet technique? Biomater. Biosyst. 2024, 15, 100098. [Google Scholar] [CrossRef]
- Kołodziejczyk, K.; Ropielewski, A.; Garlewicz, R.; Złotorowicz, M.; Czubak, J. Clinical Observations of the Effectiveness of the Masquelet Induced Membrane Technique in the Treatment of Critical Long-Bone Defects of the Lower and Upper Extremities. Medicina 2024, 60, 1933. [Google Scholar] [CrossRef]
- Ahmed, H.; Shakshak, M.; Trompeter, A. A review of the Masquelet technique in the treatment of lower limb critical-size bone defects. Ann. R. Coll. Surg. Engl. 2023. [Google Scholar] [CrossRef]
- Kalantar, S.H.; Saffar, H.; Hoveidaei, A.H. Bone reconstruction with modified Masquelet technique in open distal femoral fractures: A case series. BMC Musculoskelet. Disord. 2024, 25, 26. [Google Scholar] [CrossRef]
- Wang, P.; Wu, Y.; Rui, Y.; Wang, J.; Liu, J.; Ma, Y. Masquelet technique for reconstructing bone defects in open lower limb fracture: Analysis of the relationship between bone defect and bone graft. Injury 2021, 52, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Dragosloveanu, S.; Dragosloveanu, C.D.M.; Stanca, H.T.; Cotor, D.C.; Dragosloveanu, C.I.; Stoica, C.I. A new perspective towards failure of gamma nail systems. Exp. Ther. Med. 2020, 20, 216. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.A.; Makaram, N.; Simpson, A.; Keating, J.F. Fracture nonunion in long bones: A literature review of risk factors and surgical management. Injury 2021, 52 (Suppl. S2), S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, P.; Xie, S. The risk of loosening of extramedullary fracture fixation devices. Injury 2019, 50 (Suppl. S1), S66–S72. [Google Scholar] [CrossRef]
- Worgul, C.A.; Lentine, B.; Dicken, Q.G.; Freccero, D.M. Catastrophic Failure of the Tibial Component After Total Knee Arthroplasty: Fracture and Dissociation Between the Baseplate and Stem. Arthroplast. Today 2022, 14, 194–198. [Google Scholar] [CrossRef]
- Akilapa, O.; Gaffey, A. Hydroxyapatite pins for external fixation: Is there sufficient evidence to prove that coated pins are less likely to be replaced prematurely? Acta Orthop. Traumatol. Turc. 2015, 49, 410–415. [Google Scholar] [CrossRef]
- Grün, W.; Hansen, E.J.J.; Andreassen, G.S.; Clarke-Jenssen, J.; Madsen, J.E. Functional outcomes and health-related quality of life after reconstruction of segmental bone loss in femur and tibia using the induced membrane technique. Arch. Orthop. Trauma Surg. 2023, 143, 4587–4596. [Google Scholar] [CrossRef]
- Mathieu, L.; Mourtialon, R.; Durand, M.; de Rousiers, A.; de l’Escalopier, N.; Collombet, J.-M. Masquelet technique in military practice: Specificities and future directions for combat-related bone defect reconstruction. Mil. Med. Res. 2022, 9, 48. [Google Scholar] [CrossRef]
- Cristea, S.; Predescu, V.; Dragosloveanu, S.; Cuculici, S.; Marandici, N. Surgical Approaches for Total Knee Arthroplasty; IntechOpen: London, UK, 2016; pp. 25–47. [Google Scholar]
- Morelli, I.; Drago, L.; George, D.; Gallazzi, E.; Scarponi, S.; Romanò, C. Masquelet technique: Myth or reality? A systematic review and meta-analysis. Injury 2016, 47, S68–S76. [Google Scholar] [CrossRef]
- Toth, Z.; Roi, M.; Evans, E.; Watson, J.T.; Nicolaou, D.; McBride-Gagyi, S. Masquelet Technique: Effects of Spacer Material and Micro-topography on Factor Expression and Bone Regeneration. Ann. Biomed. Eng. 2019, 47, 174–189. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Kobbe, P.; Laubach, M.; Hutmacher, D.W.; Alabdulrahman, H.; Sellei, R.M.; Hildebrand, F. Convergence of scaffold-guided bone regeneration and RIA bone grafting for the treatment of a critical-sized bone defect of the femoral shaft. Eur. J. Med. Res. 2020, 25, 70. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Han, Q.; Wang, C.; Liu, Y.; Chen, B.; Wang, J. Porous Scaffold Design for Additive Manufacturing in Orthopedics: A Review. Front. Bioeng. Biotechnol. 2020, 8, 609. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tudor, R.C.; Timofte, D.V.; Forna, N.; Badulescu, O.V.; Savin, L.; Pinteala, T.; Mihailescu, D.; Ciobotariu, T.; Ciobica, A.; Sirbu, M.T.; et al. The Use of the Masquelet Technique in the Treatment of Pathological Distal Third Femoral Fracture Secondary to Chronic Osteomyelitis. Life 2025, 15, 537. https://doi.org/10.3390/life15040537
Tudor RC, Timofte DV, Forna N, Badulescu OV, Savin L, Pinteala T, Mihailescu D, Ciobotariu T, Ciobica A, Sirbu MT, et al. The Use of the Masquelet Technique in the Treatment of Pathological Distal Third Femoral Fracture Secondary to Chronic Osteomyelitis. Life. 2025; 15(4):537. https://doi.org/10.3390/life15040537
Chicago/Turabian StyleTudor, Razvan Cosmin, Daniel Vasile Timofte, Norin Forna, Oana Viola Badulescu, Liliana Savin, Tudor Pinteala, Dan Mihailescu, Tudor Ciobotariu, Alin Ciobica, Mihnea Theodor Sirbu, and et al. 2025. "The Use of the Masquelet Technique in the Treatment of Pathological Distal Third Femoral Fracture Secondary to Chronic Osteomyelitis" Life 15, no. 4: 537. https://doi.org/10.3390/life15040537
APA StyleTudor, R. C., Timofte, D. V., Forna, N., Badulescu, O. V., Savin, L., Pinteala, T., Mihailescu, D., Ciobotariu, T., Ciobica, A., Sirbu, M. T., Sirbu, P. D., Dragosloveanu, S., Capitanu, B. S., Cergan, R., & Scheau, C. (2025). The Use of the Masquelet Technique in the Treatment of Pathological Distal Third Femoral Fracture Secondary to Chronic Osteomyelitis. Life, 15(4), 537. https://doi.org/10.3390/life15040537









