Abstract
Wound-induced hair-follicle neogenesis (WIHN) is the phenomenon of regenerating new hair follicles from wounds in mammals. The WIHN involves both developmental and adult wound-healing processes. Moreover, the WIHN is regulated by a variety of factors, particularly multiple molecular signaling pathways produced in several types of cells. Here, the role of multiple signaling in different types of cells in WIHN is comprehensively described. Furthermore, the lack of dermal γδ T cells in the human scalp has hindered the clinical application of WIHN, but the development of drugs such as Wnt signaling activators is increasing the effectiveness of WIHN in humans. Overall, understanding the underlying mechanisms that regulate WIHN may help treat skin diseases, including alopecia.
1. Wound-Induced Hair-Follicle Neogenesis (WIHN) as a Hair-Follicle Regeneration Phenomenon
In mammals, some regenerative organs, such as the skin and alimentary canal, maintain tissue function using tissue-resident stem cells [1]. Skin regeneration, especially, has been studied for a long time to treat various incurable skin diseases [2].
In 2007, a phenomenon of hair-follicle regeneration called wound-induced hair-follicle neogenesis (WIHN) was first reported [3]. The WIHN is a phenomenon in which new hair follicles regenerate from wounds in mammals, and this phenomenon has been observed in mice, rats, rabbits, sheep, and humans [3,4,5,6]. The nascent hair follicle formed by wounding establishes a stem-cell population and ultimately becomes a mature hair follicle by expressing key markers of hair-follicle differentiation [3].
This discovery overturned previous ideas that hair-follicle loss in adults was permanent and that mammalian hair follicles formed only during development [3]. It has been demonstrated that wounding can induce an embryonic phenotype even in adult skin [3]. However, a recent report has shown that embryonic hair-follicle development resurrects after wounding in adult skin but may not necessarily utilize the same molecular signaling used in embryonic development [7]. In other words, the WIHN includes both developmental and adult wound-healing processes [7]. Overall, the WIHN is a hair-follicle regeneration phenomenon that occurs in adults, and subsequent studies have been conducted to find factors that can activate this phenomenon.
2. The Three Major Factors Regulating the WIHN
The WIHN is a regenerative phenomenon that can be exploited in the treatment of alopecia and is regulated by various factors [3,8]. In particular, the WIHN is mainly regulated by the following three factors.
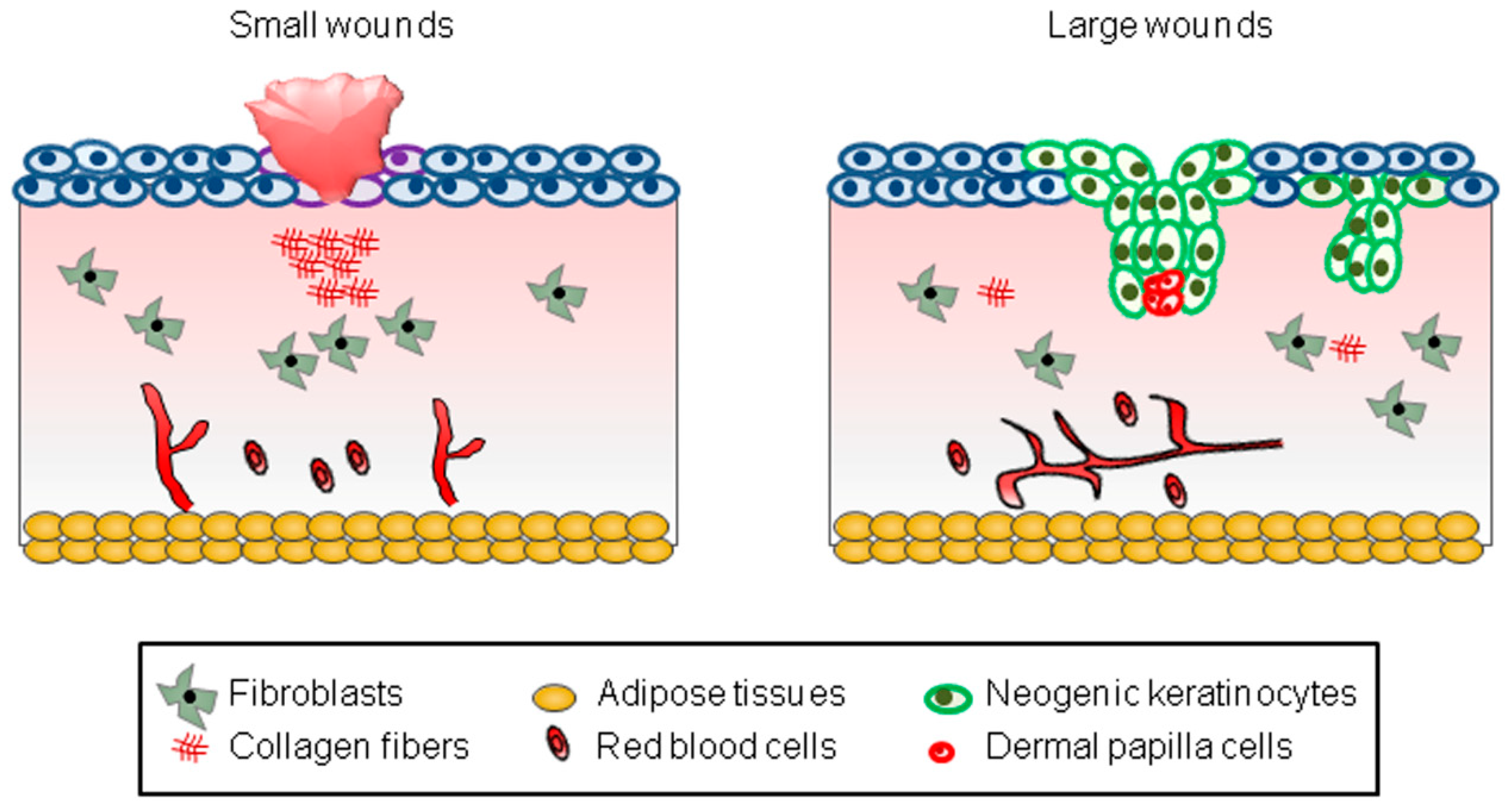
First of all, the WIHN occurs depending on the size of the wound created [3,9,10]. Most small wounds (wounds less than 0.25 cm2 in mice) fail to cause the WIHN, leaving only a scar (Figure 1). On the other hand, most large wounds (wounds larger than 2.25 cm2 in mice) successfully induce the WIHN (Figure 1). However, since it is clinically impossible to create large wounds on the human scalp, efforts should be made to induce the WIHN even in small wounds.
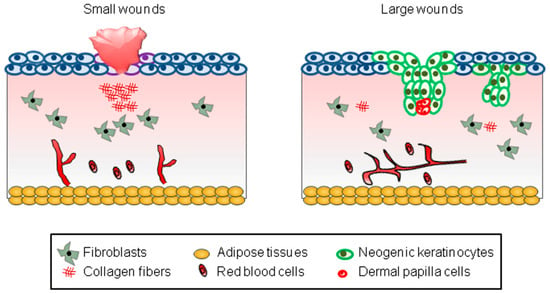
Figure 1.
A schematic model representing WIHN. The schematic pictures show scar formation and WIHN in small and large wounds, respectively. The text in the square box below describes each cell type.
Second, the WIHN is closely related to the age of experimental animals [3]. In young mice, the WIHN occurs frequently (wounds larger than 1 cm2 in 3-week-old mice), but with age, the WIHN becomes limited to large wounds (wounds larger than 2.25 cm2 in 3-week-old mice) [3]. This phenomenon is consistent with the decline in regenerative capacity with age in most tissues in mammals [11]. However, considering that most alopecia occurs in adults, it is important to induce the WIHN in adults.
Finally, multiple molecular signaling pathways are involved in the regulation of WIHN. The importance of these molecular signaling pathways in the WIHN has been proven by previous studies using multiple transgenic mice [3,8]. The roles of multiple signaling pathways in the WIHN will be discussed in detail in Section 3.
3. The Multiple Signaling Pathways Involved in the WIHN
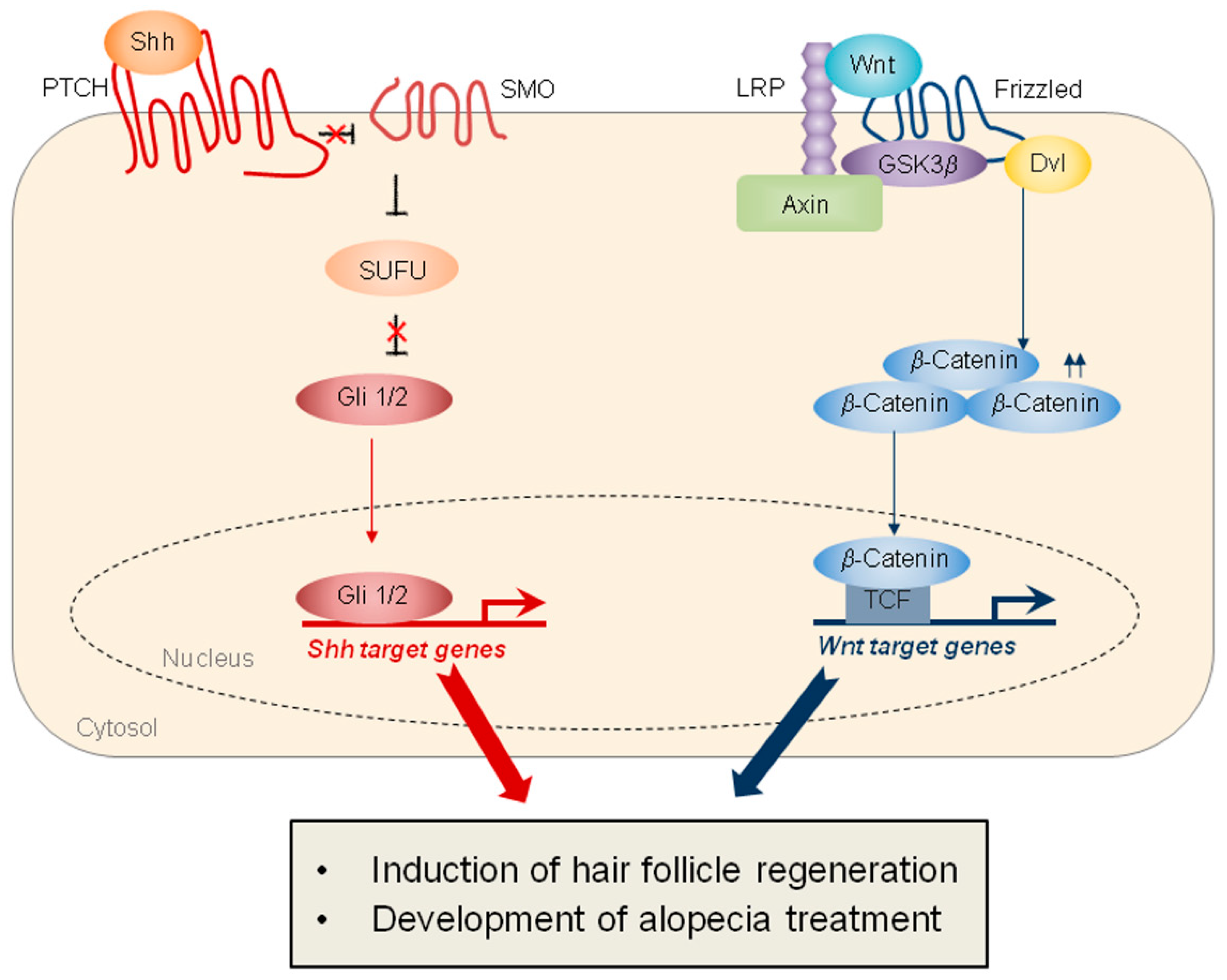
Several signaling pathways are involved in the WIHN, the two main pathways being Wnt signaling and sonic hedgehog (SHH) signaling (Figure 2). Specifically, the epithelial overexpression of Wnt7a, a Wnt ligand, significantly induces the WIHN, while the epithelial overexpression of Dickkopf-1 (DKK-1), an inhibitor of Wnt signaling, markedly inhibits WIHN [3]. Moreover, epithelial SHH overexpression or dermal smoothened (SMO) activation induces extensive WIHN in mouse wounds [8]. Although the crosstalk between Wnt signaling and SHH signaling has been studied in other types of tissue regeneration [12], the cooperation of these two pathways in hair-follicle regeneration is not fully understood (Figure 2). Ultimately, the coordinated regulation of these two major pathways will be key to inducing regenerative healing and hair-follicle regeneration (Table 1).
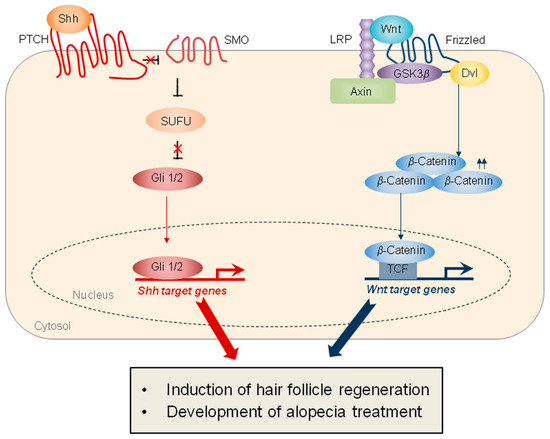
Figure 2.
Schematics representing SHH and Wnt signaling, which play important roles in hair-follicle regeneration. (Left) SHH signaling is mediated by the Patched (PTCH), which inhibits SMO. The activation of SMO promotes the release of Gli 1/2 proteins from the suppressor of fused (SUFU), thereby inducing the transcription of SHH target genes. (Right) Wnt signaling is mediated by the Fizzled and the lipoprotein receptor-related protein (LRP). In response to Wnt, Wnt receptors recruit Axin and GSK3β, leading to the stabilization of β-catenin. The nuclear β-catenin binds to the T-cell factor (TCF) transcription factor, resulting in the transcription of Wnt target genes. The target proteins activated by both signaling pathways induce hair-follicle regeneration and contribute to the development of treatments for alopecia.

Table 1.
The key molecular signaling pathways involved in the WIHN.
In addition to these two major signaling pathways, various molecular signaling pathways are involved in hair-follicle regeneration (Table 1). For instance, the activation of insulin-like growth factor 1 (IGF1) signaling promotes the WIHN through the contribution of epidermal growth-factor receptor (EGFR)-positive mesenchymal cells [13]. The identification of EGFR-positive cells within dermal fibroblasts enables the potential utilization of this subpopulation in hair-follicle regeneration (Table 1). Bone morphogenetic protein (BMP) signaling has recently been shown to be essential for hair-follicle regeneration, contrary to traditional notions of its influence on hair-follicle cycling [14]. Germline or conditional knockout of Msx2, a bona fide target of BMP signaling, revealed delayed wound healing and the significant inhibition of WIHN in big wounds, indicating that BMP signaling is essential for hair-follicle regeneration (Table 1). Hypoxia-inducible factor 1 alpha (HIF-1α) signaling also promotes the WIHN by stimulating glutamine metabolism [15]. The close relationship of HIF-1α signaling with interleukin-1β (IL-1β), metabolic, and regenerative signaling suggests the significance of pro-regenerative metabolic programs in human regeneration (Table 1). In-depth research on these various molecular signaling pathways has provided an opportunity to further determine the relationship between components within these signaling pathways and hair-follicle regeneration.
Indeed, various components within molecular signaling pathways play key roles in hair-follicle regeneration. CXXC-type zinc finger protein 5 (CXXC5) has been identified as a negative regulator of Wnt signaling, and this protein inhibits Wnt signaling and WIHN through interaction with Dishevelled (Dvl) [16,17,18]. Elucidating the function of negative regulators of Wnt signaling, such as CXXC5 and DKK-1, has opened the way to safely regulate Wnt signaling critical for hair-follicle regeneration. The activation of pyruvate kinase M2 (PKM2), one of the key enzymes of tyrosine kinase signaling, also promotes the WIHN through crosstalk with Wnt signaling [19]. Furthermore, OVO homolog-like 1 (OVOL-1), the transcriptional target of Wnt signaling, is closely related to hair-follicle neogenesis [20]. The discovery of molecular targets to induce hair-follicle regeneration has led to the development of drugs to treat hair loss, which will be discussed in Section 7.
4. Role of Multiple Signaling Pathways in Stem Cells During the WIHN
Several types of cells are involved in wound healing and WIHN in the wounded skin [21,22]. Among them, stem cells within the skin play a vital role in skin homeostasis, wound healing, and hair regeneration [21]. Among them, hair-follicle stem cells (HFSCs) and melanocyte stem cells (McSCs) play important roles in maintaining the hair growth cycle and regulating hair pigmentation, respectively [23]. HFSCs and McSCs coordinately regulate pigmented hair regeneration, and HFSCs provide a functional niche for McSCs [23,24].
HFSCs play an important role in inducing hair-follicle regeneration [25]. However, the lineage tracing of keratin 15-positive stem cells in an earlier study demonstrated that keratin 15-positive stem cells make only a minimal contribution to WIHN, indicating a major contribution to WIHN from non-hair-follicle bulge cells [3]. In contrast, Lgr5-positive hair-follicle stem cells were found in almost half of hair follicles regenerated from wounds through lineage studies, and studies using transgenic mice showed that Lgr5-positive hair-follicle stem cells significantly contribute to the WIHN [25]. Overall, it can be seen that the activation of specific populations of HFSCs is important for inducing the WIHN.
McSCs are critical for pigmented hair-follicle regeneration [26,27,28,29]. McSCs migrate from the hair-follicle niche to the epidermis after wounding and contribute to the generation of unpigmented hairs in the wounds [26]. However, because the McSCs of melanocortin 1 receptor (Mc1r)-mutant mice show defects in migrating from the hair-follicle niche to the epidermis, pigmented hair is generated in these mice after wounding [26]. The overexpression of Endothelin-1, which is involved in melanocyte homeostasis, promotes the migration of McSCs and the generation of epidermal melanocytes after wounding [27]. The activation of Wnt signaling also promotes the generation of epidermal melanocytes following wounding [28]. Moreover, overexpression of the melanocyte stimulatory factor Kitl in mice led to pigmented hair regeneration after injury [29]. Collectively, the regulation of multiple signaling pathways within HFSCs and McSCs can induce pigmented hair-follicle regeneration in adults after wounding.
5. The Role of Multiple Signaling Pathways in Immune Cells During the WIHN
Various types of immune cells and signaling pathways activated by these cells are involved in wound healing and WIHN (Table 2). For example, γδ T cells, characterized by T-cell receptors consisting of γ and δ chains, reside in the epidermis and dermis of the skin and secrete various growth factors associated with wound healing and hair-follicle regeneration [30]. Fibroblast growth factor 9 (FGF9), secreted by dermal γδ T cells, promotes the WIHN by activating Wnt signaling [30]. Toll-like receptor 9 activation in γδ T cells also induces the WIHN by up-regulating the expression of amphiregulin (AREG) [31]. Tumor necrosis factor-α (TNF-α) secreted by macrophages also induces the WIHN by activating AKT/β-catenin signaling [25]. Moreover, macrophage-regulated CX3C motif chemokine receptor 1 (CX3CR1) and transforming growth factor-β1 (TGF-β1) signaling play an important role in inducing hair-follicle regeneration [32]. Recent studies on the roles of immune cells in WIHN indicate that various signaling pathways secreted by cutaneous immune cells are important in the regulation of hair-follicle regeneration.

Table 2.
Multiple signaling pathways in immune cells and non-immune cells during the WIHN.
Additionally, non-immune cells, including keratinocytes and neuronal cells, produce cytokines, thereby regulating the WIHN (Table 2). The binding of Toll-like receptor 3 (TLR3), expressed in keratinocytes of injured tissues, and its ligand, double-stranded RNA (dsRNA), induces the WIHN by up-regulating interleukin-6 (IL-6) expression [33]. The self-noncoding dsRNA can also induce retinoic acid (RA) synthesis, which is involved in promoting the WIHN, through up-regulated IL-6 expression either by wounding in mice or by a rejuvenation laser in humans [34]. A recent study has demonstrated that skin-resident bacteria induce IL-1β production in keratinocytes via HIF-1α signaling, leading to the promotion of WIHN [15]. Moreover, interleukin-36α (IL-36α) expressed in keratinocytes induces the WIHN by activating the IL-6/signal transducer and activator of transcription 3 (STAT3) pathway [35]. Overall, the association of WIHN with the immune cells suggests that WIHN and wound healing involving immune cells may be clinically targeted simultaneously.
6. The Role of Multiple Signaling Pathways in Dermal Cells During the WIHN
Recent studies have revealed that dermal cells also play an important role in hair-follicle regeneration [8,36]. For example, the activation of dermal SHH signaling induces extensive WIHN in wounds [8]. Moreover, the epidermal–dermal interactions are crucial for both hair-follicle development and regeneration [37,38]. Importantly, various types of cells in the dermis are involved in hair-follicle regeneration [8,22].
The transition from fibroblasts to myofibroblasts is a key process in wound healing [39]. The myofibroblasts play a key role in wound healing, but their excessive activity can lead to scar formation [40]. Interestingly, recent findings have demonstrated that neogenic follicles can transform myofibroblasts into adipocytes following injury in humans and mice [22]. In particular, neogenic follicles regenerated from the wound activate BMP signaling and induce adipocyte transcription factors such as peroxisome proliferator-activated receptor γ (PPARγ) [22]. Therefore, the overexpression of the BMP antagonist Noggin in hair follicles or the deletion of the BMP receptor in myofibroblasts inhibited the formation of dermal adipocytes in the wounds [22]. Collectively, the regulation of dermal adipocytes through the modulation of BMP signaling may simultaneously inhibit scar formation and induce the WIHN.
7. Development of Drugs to Improve the WIHN
Although there have been attempts to apply the WIHN clinically, they have been relatively ineffective due to the lack of dermal γδ T cells in humans [30]. Therefore, the activation of molecular signaling pathways such as Fgf9 and Wnt signaling, regulated by γδ T cells, was required to induce the WIHN in humans [30]. As described earlier, basic research on the molecular signaling pathways that regulate the WIHN has continued for over a decade, followed by applied research to activate these signaling pathways.
Wnt signaling is an attractive target for drug development to induce hair-follicle regeneration (Table 3). Valproic acid (VPA) activates the Wnt signaling and induces the WIHN by inhibiting glycogen synthase kinase-3β (GSK-3β) [41]. 4-phenyl butyric acid (PBA), a derivative of VPA, also induces hair-follicle regeneration by activating the Wnt signaling [41]. Lithium chloride (LiCl), another GSK-3β inhibitor, promotes the pigmented hair regeneration in wounds [29]. The administration of recombinant IL-6, a target protein of Wnt signaling, also significantly induces the WIHN [33].
Many substances activating Wnt signaling have been tested preclinically to increase the effectiveness of WIHN, but most of them have not been tested clinically because of the worrisome issues of this signaling in relation to cancer [42]. To resolve the issues of Wnt signaling, the ways to safely target this signaling were explored, and one of them was to indirectly activate Wnt signaling by inhibiting the function of negative regulators of Wnt signaling, such as CXXC5 [17,42]. Protein transduction domain–Dishevelled binding motif (PTD-DBM), a peptide that inhibits CXXC5-Dvl interaction, induces the WIHN by activating Wnt signaling [17]. KY19382, a mimetic small molecular compound of PTD-DBM, also significantly induces WIHN by activating Wnt signaling [43].
The pharmacological activation of Wnt signaling through crosstalk with other signaling is also known to markedly increase the WIHN [19,44]. TEPP-46, a selective activator of PKM2, promotes the WIHN, and its combination treatment with Wnt signaling activators synergistically induces the WIHN [19]. RA also induces hair-follicle regeneration and hair-follicle stem cell activation through Wnt signaling [34,44]. In addition, PF573288 and blebbistatin, small molecules that inhibit focal adhesion kinase (FAK) and myosin II, respectively, also significantly induce the WIHN [45]. Specifically, FAK inhibition is known to induce the phosphorylation of STAT3, which is induced by Wnt signaling and promotes WIHN in the wounds [45].
There have been many reports so far on the development of drugs that promote a hair-cycle transition [46,47], but these drugs are unlikely to be the ultimate treatment for alopecia lacking HFSCs [17,43]. Given the regenerative capacity of WIHN, drugs that promote WIHN are desirable but currently unavailable for alopecia treatment, but recent research into the underlying mechanisms of WIHN may ultimately enable the development of drugs that promote WIHN. Overall, developing drugs to increase the effectiveness of WIHN will provide treatment opportunities for many patients suffering from alopecia.

Table 3.
Various drug candidates promoting the WIHN.
Table 3.
Various drug candidates promoting the WIHN.
| Substances | Types | Mechanisms | References |
|---|---|---|---|
| VPA | Small molecule | By inhibiting GSK-3β | [41] |
| PBA | Small molecule | By inhibiting GSK-3β | [41] |
| LiCl | Small molecule | By inhibiting GSK-3β | [29] |
| IL-6 | Recombinant protein | By inducing expression of keratinocyte differentiation | [33] |
| PTD-DBM | Peptide | By inhibiting CXXC5-Dvl interaction | [17] |
| KY19382 | Small molecule | By inhibiting CXXC5-Dvl interaction | [43] |
| TEPP-46 | Small molecule | Through crosstalk with Wnt signaling | [19] |
| RA | Small molecule | By activating Wnt signaling | [34,44] |
| PF573288 | Small molecule | By inhibiting FAK | [45] |
| Blebbistatin | Small molecule | By inhibiting myosin II | [45] |
8. Conclusions and Perspectives
The WIHN is a regenerative phenomenon that can be utilized to treat alopecia [3]. The WIHN involves both developmental and adult wound-healing processes [7], and the WIHN is regulated by a variety of signaling pathways [3,8]. Specifically, WIHN involves both signaling associated with developmental processes, such as Wnt and SHH signaling, and signaling associated with the wound-healing process, such as TGFβ and IL-6 signaling [8,32,33]. The role of multiple signaling pathways in WIHN has been validated by several transgenic mice, and these studies have contributed to the efficient induction of WIHN by the activation of molecular signaling pathways [3,17].
Clinical application of WIHN has been difficult due to the lack of dermal γδ T cells in human scalps [30], but drug development including Wnt signaling activators is increasing the effectiveness of WIHN in humans [17,43]. Translational studies using various animal models have also made important contributions to the development of potential drugs to induce WIHN in humans [48]. Importantly, the development of these drugs for practical and clinical purposes may provide many opportunities to treat alopecia. Moreover, given the relationship between scar formation and the WIHN, further studies on the WIHN may offer unprecedented opportunities to treat scarring diseases such as cicatricial alopecia and burns [49,50]. In conclusion, understanding the underlying mechanisms that regulate the WIHN may be beneficial for the treatment of skin disorders, including hair loss.
Funding
This study was supported by National Research Foundation of Korea (NRF) through Creative Challenge Research Foundation Support Project (2022R1I1A1A01073599, RS-2022-NR075558).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Wells, J.M.; Watt, F.M. Diverse mechanisms for endogenous regeneration and repair in mammalian organs. Nature 2018, 557, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, T.; Rothoeft, T.; Teig, N.; Bauer, J.W.; Pellegrini, G.; De Rosa, L.; Scaglione, D.; Reichelt, J.; Klausegger, A.; Kneisz, D.; et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature 2017, 551, 327–332. [Google Scholar] [CrossRef]
- Ito, M.; Yang, Z.; Andl, T.; Cui, C.; Kim, N.; Millar, S.E.; Cotsarelis, G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 2007, 447, 316–320. [Google Scholar] [CrossRef]
- Billingham, R.E.; Russell, P.S. Incomplete wound contracture and the phenomenon of hair neogenesis in rabbits’ skin. Nature 1956, 177, 791–792. [Google Scholar] [CrossRef]
- Brook, A.H.; Short, B.F.; Lyne, A.G. Formation of new wool follicles in the adult sheep. Nature 1960, 185, 51. [Google Scholar] [CrossRef] [PubMed]
- Dann, L.; Glücksmann, A.; Tansley, K. The Healing of Untreated Experimental Wounds. Br. J. Exp. Pathol. 1941, 22, 1–9. [Google Scholar]
- Harn, H.I.; Davidson, J.M.; Chuong, C.M. Bioinspired Strategies for Wound Regeneration. Cold Spring Harb. Perspect. Biol. 2023, 15, a041240. [Google Scholar] [CrossRef]
- Lim, C.H.; Sun, Q.; Ratti, K.; Lee, S.H.; Zheng, Y.; Takeo, M.; Lee, W.; Rabbani, P.; Plikus, M.V.; Cain, J.E.; et al. Hedgehog stimulates hair follicle neogenesis by creating inductive dermis during murine skin wound healing. Nat. Commun. 2018, 9, 4903. [Google Scholar] [CrossRef]
- Choi, S.; Yoon, M.; Choi, K.Y. Approaches for Regenerative Healing of Cutaneous Wound with an Emphasis on Strategies Activating the Wnt/β-Catenin Pathway. Adv. Wound Care 2022, 11, 70–86. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Yun, M.H. Changes in Regenerative Capacity through Lifespan. Int. J. Mol. Sci. 2015, 16, 25392–25432. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, W.; Guo, L.; Zhao, L.; Sun, S.; Li, H. The crosstalk between the Notch, Wnt, and SHH signaling pathways in regulating the proliferation and regeneration of sensory progenitor cells in the mouse cochlea. Cell Tissue Res. 2021, 386, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xu, Z.; Chen, Y.; Yang, Q.; Lu, R.; Dong, Y.; Li, X.; Xie, J.; Xu, R.H.; Jia, H.; et al. EGFR marks a subpopulation of dermal mesenchymal cells highly expressing IGF1 which enhances hair follicle regeneration. J. Cell Mol. Med. 2023, 27, 1697–1707. [Google Scholar] [CrossRef]
- Hughes, M.W.; Jiang, T.X.; Plikus, M.V.; Guerrero-Juarez, C.F.; Lin, C.H.; Schafer, C.; Maxson, R.; Widelitz, R.B.; Chuong, C.M. Msx2 Supports Epidermal Competency during Wound-Induced Hair Follicle Neogenesis. J. Investig. Dermatol. 2018, 138, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Sweren, E.; Andrews, W.; Li, Y.; Chen, J.; Xue, Y.; Wier, E.; Alphonse, M.P.; Luo, L.; Miao, Y.; et al. Commensal microbiome promotes hair follicle regeneration by inducing keratinocyte HIF-1α signaling and glutamine metabolism. Sci. Adv. 2023, 9, eabo7555. [Google Scholar] [CrossRef]
- Lee, S.H.; An, S.; Ryu, Y.C.; Seo, S.H.; Park, S.; Lee, M.J.; Cho, S.W.; Choi, K.Y. Adhesive Hydrogel Patch-Mediated Combination Drug Therapy Induces Regenerative Wound Healing through Reconstruction of Regenerative Microenvironment. Adv. Healthc. Mater. 2023, 12, e2203094. [Google Scholar] [CrossRef]
- Lee, S.H.; Seo, S.H.; Lee, D.H.; Pi, L.Q.; Lee, W.S.; Choi, K.Y. Targeting of CXXC5 by a Competing Peptide Stimulates Hair Regrowth and Wound-Induced Hair Neogenesis. J. Investig. Dermatol. 2017, 137, 2260–2269. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.Y.; Kim, H.Y.; Lee, Y.M.; Kim, H.; Nam, K.A.; Roh, M.R.; do Min, S.; Chung, K.Y.; Choi, K.Y. The Dishevelled-binding protein CXXC5 negatively regulates cutaneous wound healing. J. Exp. Med. 2015, 212, 1061–1080. [Google Scholar] [CrossRef]
- Ryu, Y.C.; Kim, Y.R.; Park, J.; Choi, S.; Ryu, W.J.; Kim, G.U.; Kim, E.; Hwang, Y.; Kim, H.; Han, G.; et al. Pyruvate Kinase M2 Promotes Hair Regeneration by Connecting Metabolic and Wnt/β-Catenin Signaling. Pharmaceutics 2022, 14, 2774. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, D.; Hwang, J.; Kim, M.K.; Kim, J.C.; Sung, Y.K. OVO homolog-like 1, a target gene of the Wnt/beta-catenin pathway, controls hair follicle neogenesis. J. Investig. Dermatol. 2014, 134, 838–840. [Google Scholar] [CrossRef]
- Blanpain, C.; Fuchs, E. Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol. 2006, 22, 339–373. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Guerrero-Juarez, C.F.; Ito, M.; Li, Y.R.; Dedhia, P.H.; Zheng, Y.; Shao, M.; Gay, D.L.; Ramos, R.; His, T.C.; et al. Regeneration of fat cells from myofibroblasts during wound healing. Science 2017, 355, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, P.; Takeo, M.; Chou, W.; Myung, P.; Bosenberg, M.; Chin, L.; Taketo, M.M.; Ito, M. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell 2011, 145, 941–955. [Google Scholar] [CrossRef]
- Tanimura, S.; Tadokoro, Y.; Inomata, K.; Binh, N.T.; Nishie, W.; Yamazaki, S.; Nakauchi, H.; Tanaka, Y.; McMillan, J.R.; Sawamura, D.; et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell 2011, 8, 177–187. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Tian, R.; Zhang, Y.; Drutskaya, M.S.; Wang, C.; Ge, J.; Fan, Z.; Kong, D.; Wang, X.; et al. Macrophages induce AKT/beta-catenin-dependent Lgr5(+) stem cell activation and hair follicle regeneration through TNF. Nat. Commun. 2017, 8, 14091. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.C.; Takeo, M.; Rabbani, P.; Hu, H.; Lee, W.; Chung, Y.R.; Carucci, J.; Overbeek, P.; Ito, M. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat. Med. 2013, 19, 924–929. [Google Scholar] [CrossRef]
- Takeo, M.; Lee, W.; Rabbani, P.; Sun, Q.; Hu, H.; Lim, C.H.; Manga, P.; Ito, M. EdnrB Governs Regenerative Response of Melanocyte Stem Cells by Crosstalk with Wnt Signaling. Cell Rep. 2016, 15, 1291–1302. [Google Scholar] [CrossRef]
- Sun, Q.; Rabbani, P.; Takeo, M.; Lee, S.H.; Lim, C.H.; Noel, E.S.; Taketo, M.M.; Myung, P.; Millar, S.; Ito, M. Dissecting Wnt Signaling for Melanocyte Regulation during Wound Healing. J. Investig. Dermatol. 2018, 138, 1591–1600. [Google Scholar] [CrossRef]
- Yuriguchi, M.; Aoki, H.; Taguchi, N.; Kunisada, T. Pigmentation of regenerated hairs after wounding. J. Dermatol. Sci. 2016, 84, 80–87. [Google Scholar] [CrossRef]
- Gay, D.; Kwon, O.; Zhang, Z.; Spata, M.; Plikus, M.V.; Holler, P.D.; Ito, M.; Yang, Z.; Treffeisen, E.; Kim, C.D.; et al. Fgf9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nat. Med. 2013, 19, 916–923. [Google Scholar] [CrossRef]
- Li, X.; An, T.; Yang, Y.; Xu, Z.; Chen, S.; Yi, Z.; Deng, C.; Zhou, F.; Man, Y.; Hu, C. TLR9 activation in large wound induces tissue repair and hair follicle regeneration via γδT cells. Cell Death Dis. 2024, 15, 598. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, W.; Liu, Y.; Rosin, N.L.; Kline, A.; Raharjo, E.; Yoon, J.; Stratton, J.A.; Sinha, S.; Biernaskie, J. Macrophages Promote Wound-Induced Hair Follicle Regeneration in a CX(3)CR1- and TGF-beta1-Dependent Manner. J. Investig. Dermatol. 2018, 138, 2111–2122. [Google Scholar] [CrossRef]
- Nelson, A.M.; Reddy, S.K.; Ratliff, T.S.; Hossain, M.Z.; Katseff, A.S.; Zhu, A.S.; Chang, E.; Resnik, S.R.; Page, C.; Kim, D.; et al. dsRNA Released by Tissue Damage Activates TLR3 to Drive Skin Regeneration. Cell Stem Cell 2015, 17, 139–151. [Google Scholar] [CrossRef]
- Kim, D.; Chen, R.; Sheu, M.; Kim, N.; Kim, S.; Islam, N.; Wier, E.M.; Wang, G.; Li, A.; Park, A.; et al. Noncoding dsRNA induces retinoic acid synthesis to stimulate hair follicle regeneration via TLR3. Nat. Commun. 2019, 10, 2811. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Xiao, J.; Li, X.; Li, Y.; Gao, X.; Xu, X. IL-36alpha Promoted Wound Induced Hair Follicle Neogenesis via Hair Follicle Stem/Progenitor Cell Proliferation. Front. Cell Dev. Biol. 2020, 8, 627. [Google Scholar] [CrossRef]
- Rognoni, E.; Gomez, C.; Pisco, A.O.; Rawlins, E.L.; Simons, B.D.; Watt, F.M.; Driskell, R.R. Inhibition of beta-catenin signalling in dermal fibroblasts enhances hair follicle regeneration during wound healing. Development 2016, 143, 2522–2535. [Google Scholar]
- Briggaman, R.A. Epidermal-dermal interactions in adult skin. J. Investig. Dermatol. 1982, 79, 21s–24s. [Google Scholar] [CrossRef] [PubMed]
- Jahoda, C.A.; Reynolds, A.J. Dermal-epidermal interactions--follicle-derived cell populations in the study of hair-growth mechanisms. J. Investig. Dermatol. 1993, 101, 33S–38S. [Google Scholar] [CrossRef]
- D’Urso, M.; Kurniawan, N.A. Mechanical and Physical Regulation of Fibroblast-Myofibroblast Transition: From Cellular Mechanoresponse to Tissue Pathology. Front. Bioeng. Biotechnol. 2020, 8, 609653. [Google Scholar] [CrossRef]
- Sarrazy, V.; Billet, F.; Micallef, L.; Coulomb, B.; Desmoulière, A. Mechanisms of pathological scarring: Role of myofibroblasts and current developments. Wound Repair Regen. 2011, 19, s10–s15. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoon, J.; Shin, S.H.; Zahoor, M.; Kim, H.J.; Park, P.J.; Park, W.S.; do Min, S.; Kim, H.Y.; Choi, K.Y. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS ONE 2012, 7, e34152. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014, 13, 513–532. [Google Scholar] [CrossRef]
- Ryu, Y.C.; Lee, D.H.; Shim, J.; Park, J.; Kim, Y.R.; Choi, S.; Bak, S.S.; Sung, Y.K.; Lee, S.H.; Choi, K.Y. KY19382, a novel activator of Wnt/β-catenin signalling, promotes hair regrowth and hair follicle neogenesis. Br. J. Pharmacol. 2021, 178, 2533–2546. [Google Scholar] [CrossRef]
- Wen, L.; Fan, Z.; Huang, W.; Miao, Y.; Zhang, J.; Liu, B.; Zhu, D.; Dai, D.; Zhang, J.; Le, D.; et al. Retinoic acid drives hair follicle stem cell activation via Wnt/beta-catenin signalling in androgenetic alopecia. J. Eur. Acad. Dermatol. Venereol. 2025, 39, 189–201. [Google Scholar] [CrossRef]
- Harn, H.I.; Chiu, P.Y.; Lin, C.H.; Chen, H.Y.; Lai, Y.C.; Yang, F.S.; Wu, C.C.; Tang, M.J.; Chuong, C.M.; Hughes, M.W. Topological Distribution of Wound Stiffness Modulates Wound-Induced Hair Follicle Neogenesis. Pharmaceutics 2022, 14, 1926. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kang, J.I.; Yoon, H.S.; Choi, Y.K.; Go, J.S.; Oh, S.K.; Ahn, M.; Kim, J.; Koh, Y.S.; Hyun, J.W.; et al. HNG, A Humanin Analogue, Promotes Hair Growth by Inhibiting Anagen-to-Catagen Transition. Int. J. Mol. Sci. 2020, 21, 4553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, H.; Jing, J.; Yu, L.; Wu, X.; Lu, Z. Morroniside regulates hair growth and cycle transition via activation of the Wnt/beta-catenin signaling pathway. Sci. Rep. 2018, 8, 13785. [Google Scholar]
- Xue, Y.; Lim, C.H.; Plikus, M.V.; Ito, M.; Cotsarelis, G.; Garza, L.A. Wound-Induced Hair Neogenesis Model. J. Investig. Dermatol. 2022, 142, 2565–2569. [Google Scholar] [CrossRef]
- Wier, E.M.; Garza, L.A. Through the lens of hair follicle neogenesis, a new focus on mechanisms of skin regeneration after wounding. Semin. Cell Dev. Biol. 2020, 100, 122–129. [Google Scholar] [CrossRef]
- Dutta, A.; Saha, D.; Jamora, C. Approaches to Study Wound-Induced Hair Neogenesis (WIHN). Methods Mol. Biol. 2024, 2849, 31–44. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).