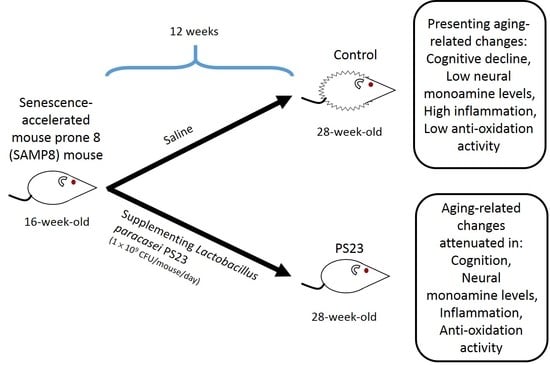
Lactobacillus paracasei PS23 Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. L. paracasei PS23 and Experimental Animals
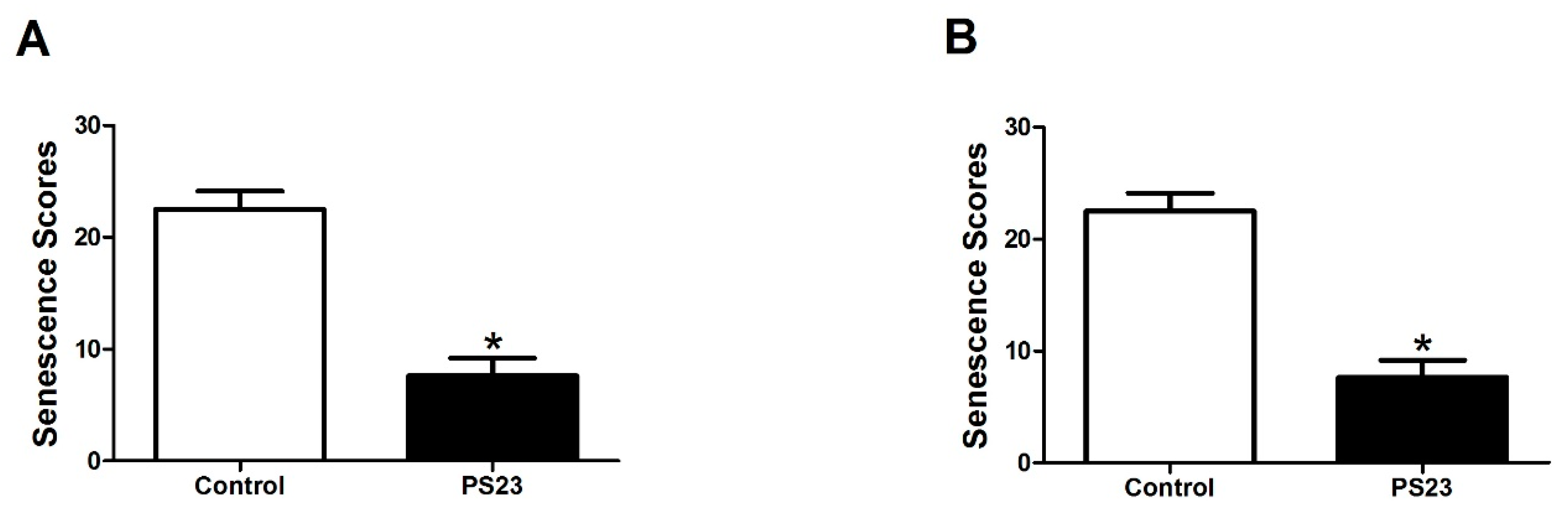
2.2. Evaluation of Senescence
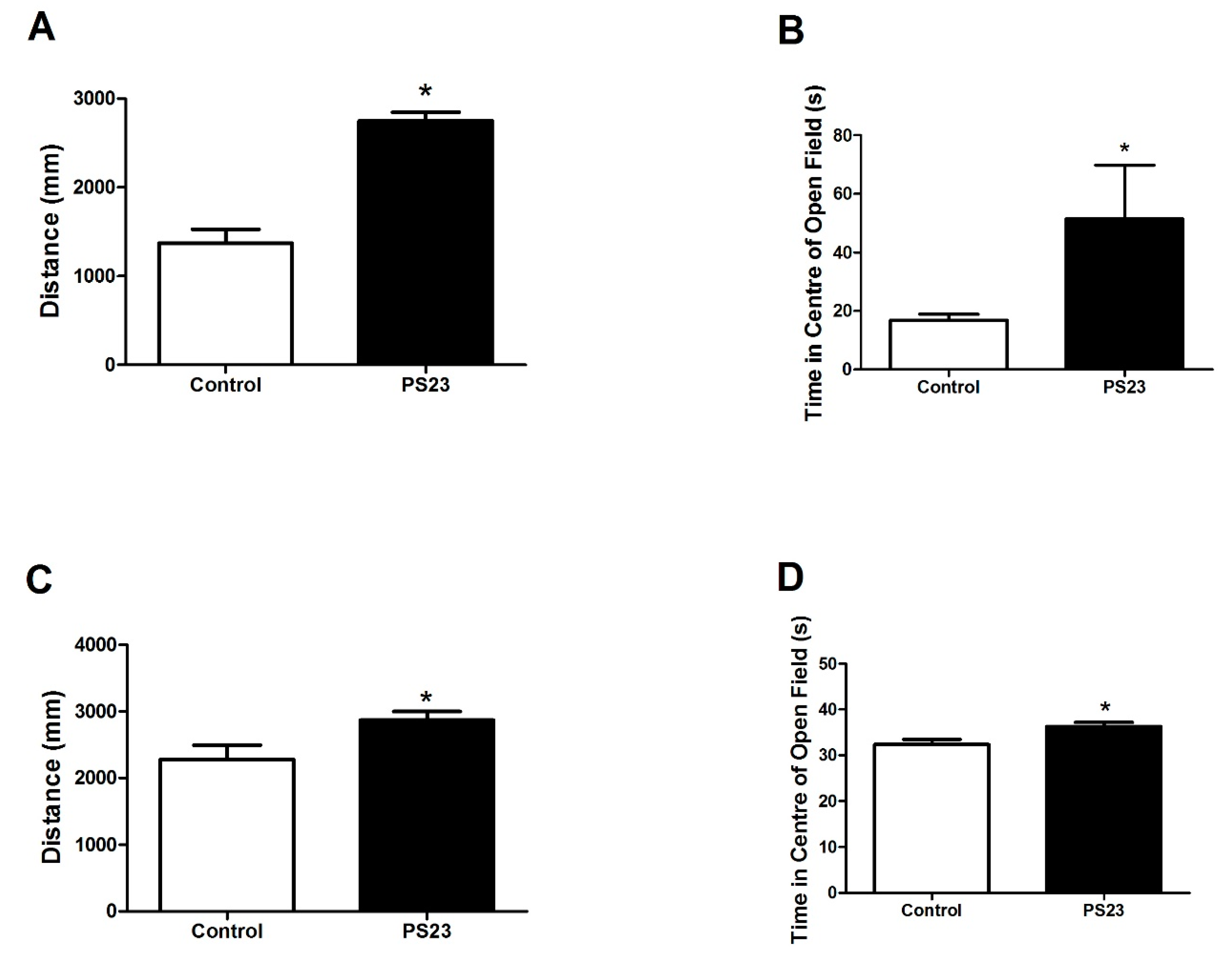
2.3. Open Field Test
2.4. Morris Water Maze Test
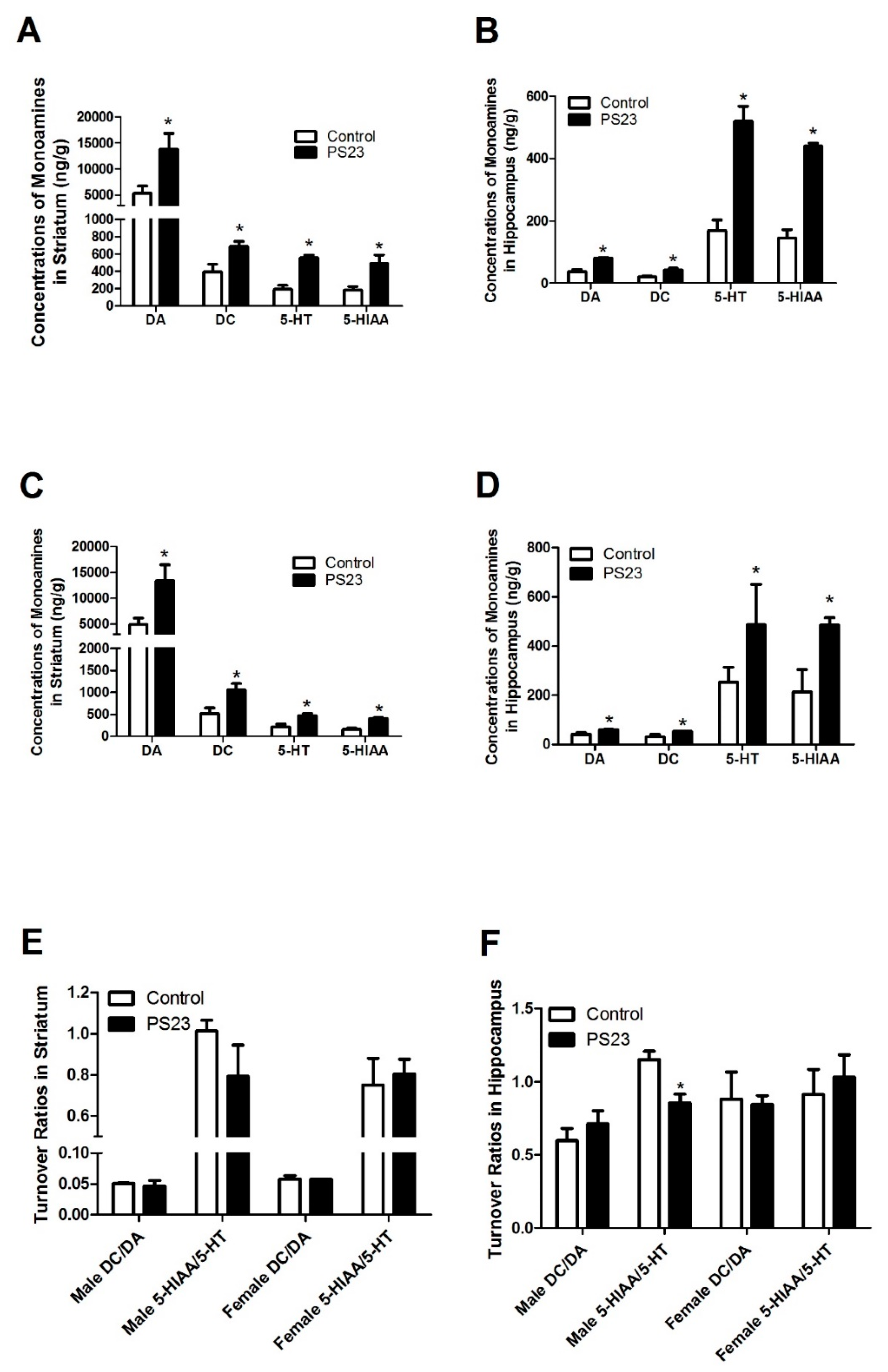
2.5. Quantification of Neuronal Amines and Their Metabolites
2.6. Enzyme-Linked Immunosorbent Assay
2.7. Antioxidative Enzyme Activities
2.8. Statistical Analyses
3. Results
3.1. LPPS23 in the Aged SAMP8 Mice
3.2. Behavioral Changes in the LPPS23-Treated Aged SAMP8 Mice
3.3. Neuronal Monoamine Status in the LPPS23-Treated Aged SAMP8 Mice
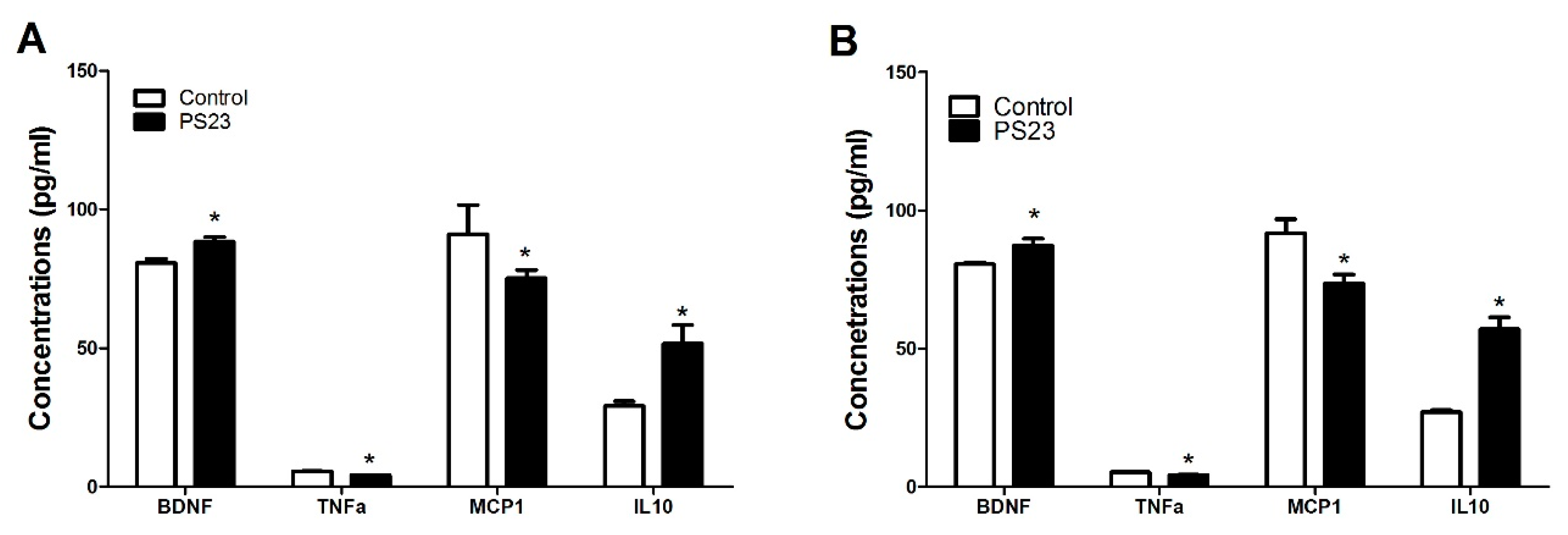
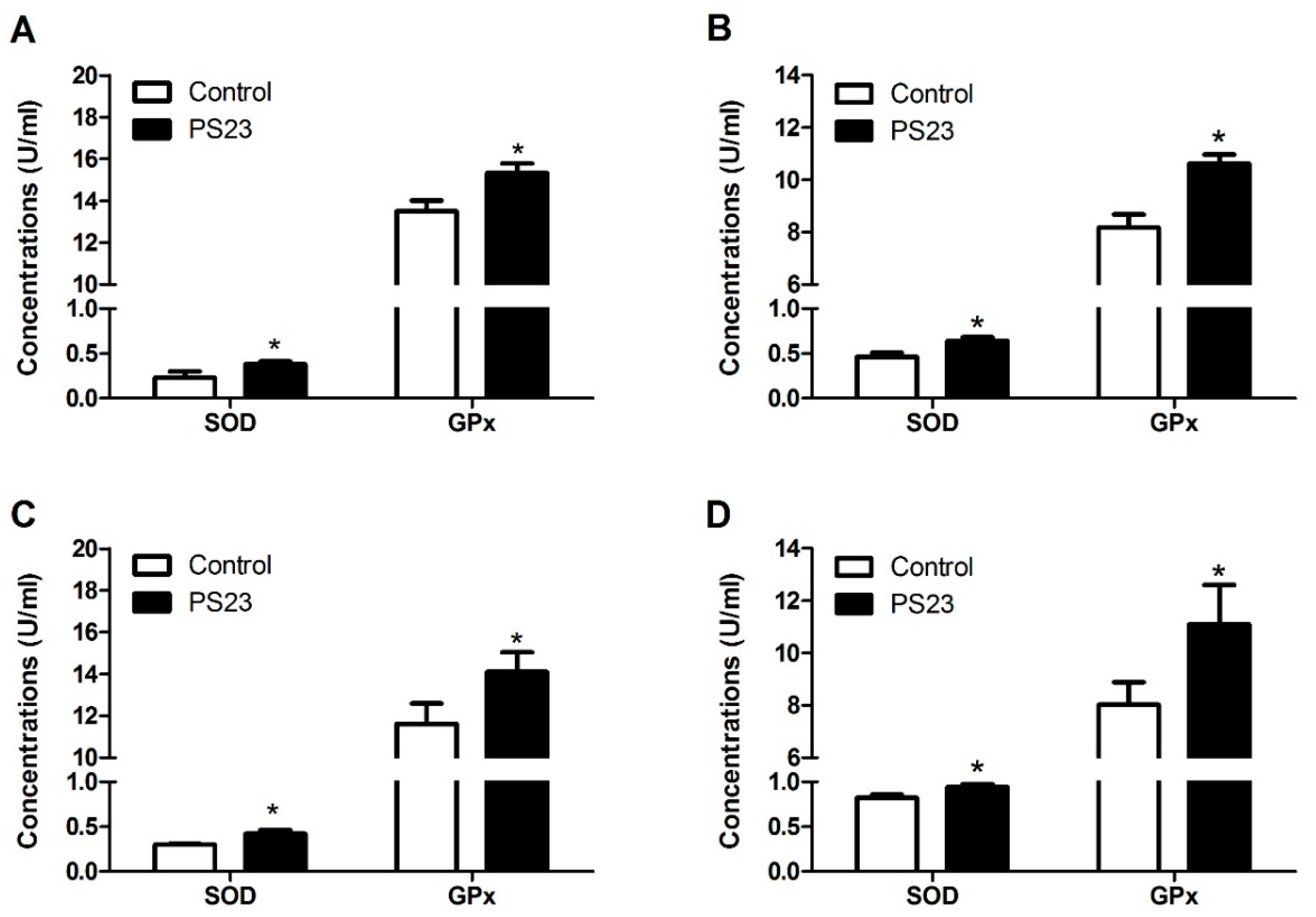
3.4. Inflammatory and Oxidative Statuses in the LPPS23-Treated Aged SAMP8 Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, K. Trillion-dollar brain drain. Nature 2011, 478, 15. [Google Scholar] [CrossRef] [PubMed]
- Hedden, T.; Gabrieli, J.D. Insights into the ageing mind: A view from cognitive neuroscience. Nat. Rev. Neurosci. 2004, 5, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Wolitzky-Taylor, K.B.; Castriotta, N.; Lenze, E.J.; Stanley, M.A.; Craske, M.G. Anxiety disorders in older adults: A comprehensive review. Depress. Anxiety 2010, 27, 190–211. [Google Scholar] [CrossRef] [PubMed]
- Botton, P.H.; Pochmann, D.; Rocha, A.S.; Nunes, F.; Almeida, A.S.; Marques, D.M.; Porciuncula, L.O. Aged mice receiving caffeine since adulthood show distinct patterns of anxiety-related behavior. Physiol. Behav. 2017, 170, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Patki, G.; Ali, Q.; Pokkunuri, I.; Asghar, M.; Salim, S. Grape powder treatment prevents anxiety-like behavior in a rat model of aging. Nutr. Res. 2015, 35, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mesa, Y.; Colie, S.; Corpas, R.; Cristofol, R.; Comellas, F.; Nebreda, A.R.; Gimenez-Llort, L.; Sanfeliu, C. Oxidative stress is a central target for physical exercise neuroprotection against pathological brain aging. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Jenny, N.S. Inflammation in aging: Cause, effect, or both? Discov. Med. 2012, 13, 451–460. [Google Scholar] [PubMed]
- Bonnefoy, M.; Drai, J.; Kostka, T. antioxidants to slow aging, facts and perspectives. Presse Med. 2002, 31, 1174–1184. [Google Scholar] [PubMed]
- Liu, Y.W.; Su, Y.W.; Ong, W.K.; Cheng, T.H.; Tsai, Y.C. Oral administration of lactobacillus plantarum K68 ameliorates DSS-induced ulcerative colitis in BALB/c mice via the anti-inflammatory and immunomodulatory activities. Int. Immunopharmacol. 2011, 11, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Goyarl, A. Antioxidant activity and gamma-aminobutyric acid (GABA) producing ability of probiotic lactobacillus plantarum DM5 isolated from marcha of sikkim. LWT-Food Sci. Technol. 2015, 61, 263–268. [Google Scholar] [CrossRef]
- Chen, L.H.; Chen, Y.H.; Cheng, K.C.; Chien, T.Y.; Chan, C.H.; Tsao, S.P.; Huang, H.Y. Antiobesity effect of lactobacillus reuteri 263 associated with energy metabolism remodeling of white adipose tissue in high-energy-diet-fed rats. J. Nutr. Biochem. 2017, 54, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Liu, W.H.; Wu, C.C.; Juan, Y.C.; Wu, Y.C.; Tsai, H.P.; Wang, S.; Tsai, Y.C. Psychotropic effects of lactobacillus plantarum PS128 in early life-stressed and naive adult mice. Brain Res. 2016, 1631, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vilela, T.C.; Muller, A.P.; Damiani, A.P.; Macan, T.P.; da Silva, S.; Canteiro, P.B.; de Sena Casagrande, A.; Pedroso, G.D.S.; Nesi, R.T.; de Andrade, V.M.; et al. Strength and aerobic exercises improve spatial memory in aging rats through stimulating distinct neuroplasticity mechanisms. Mol. Neurobiol. 2017, 54, 7928–7937. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Maudsley, S.; Martin, B. BDNF and 5-HT: A dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004, 27, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Backman, L.; Nyberg, L.; Lindenberger, U.; Li, S.C.; Farde, L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci. Biobehav. Rev. 2006, 30, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lee, I.S.; Braun, C.; Enck, P. Effect of probiotics on central nervous system functions in animals and humans: A systematic review. J. Neurogastroenterol. Motil. 2016, 22, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Ohland, C.L.; Kish, L.; Bell, H.; Thiesen, A.; Hotte, N.; Pankiv, E.; Madsen, K.L. Effects of lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrino 2013, 38, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, T.; Liang, S.; Hu, X.; Li, W.; Jin, F. Ingestion of lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat. Sci. China Life Sci. 2014, 57, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Savignac, H.M.; Tramullas, M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav. Brain Res. 2015, 287, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Hosokawa, M.; Takeshita, S.; Irino, M.; Higuchi, K.; Matsushita, T.; Tomita, Y.; Yashuhira, K.; Hamamoto, H.; Shimizu, K.; et al. A new murine model of accelerated senescence. Mech. Ageing Dev. 1981, 17, 183–194. [Google Scholar] [CrossRef]
- Miyamoto, M.; Kiyota, Y.; Yamazaki, N.; Nagaoka, A.; Matsuo, T.; Nagawa, Y.; Takeda, T. Age-related-changes in learning and memory in the senescence-accelerated mouse (SAM). Physiol. Behav. 1986, 38, 399–406. [Google Scholar] [CrossRef]
- Flood, J.F.; Morley, J.E. Early onset of age-related impairment of aversive and appetitive learning in the SAM-P/8 mouse. J. Gerontol. 1992, 47, B52–B59. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M. Characteristics of age-related behavioral changes in senescence-accelerated mouse SAMP8 and SAMP10. Exp. Gerontol. 1997, 32, 139–148. [Google Scholar] [CrossRef]
- Perez-Caceres, D.; Ciudad-Roberts, A.; Rodrigo, M.T.; Pubill, D.; Camins, A.; Camarasa, J.; Escubedo, E.; Pallas, M. Depression-like behavior is dependent on age in male SAMP8 mice. Biogerontology 2013, 14, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Rhea, E.M.; Banks, W.A. The SAMP8 mouse for investigating memory and the role of insulin in the brain. Exp. Gerontol. 2017, 94, 64–68. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Collins, S.M.; Bercik, P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microbes 2014, 5, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Verdu, E.F.; Bercik, P.; Bergonzelli, G.E.; Huang, X.X.; Blennerhasset, P.; Rochat, F.; Fiaux, M.; Mansourian, R.; Corthesy-Theulaz, I.; Collins, S.M. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology 2004, 127, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Distrutti, E.; O’Reilly, J.A.; McDonald, C.; Cipriani, S.; Renga, B.; Lynch, M.A.; Fiorucci, S. Modulation of intestinal microbiota by the probiotic VSL#3 resets brain gene expression and ameliorates the age-related deficit in LTP. PLoS ONE 2014, 9, e106503. [Google Scholar]
- Liu, C.F.; Pan, T.M. In vitro effects of lactic acid bacteria on cancer cell viability and antioxidant activity. J. Food Drug Anal. 2010, 18, 77–86. [Google Scholar]
- Chiang, S.S.; Liu, C.F.; Tseng, K.C.; Mau, J.L.; Pan, T.M. Immunomodulatory effects of dead lactobacillus on murine splenocytes and macrophages. Food Agric. Immunol. 2012, 23, 183–202. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of lactobacillus strain regulates emotional behavior and central gaba receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Savignac, H.M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol. Motil. 2014, 26, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.C.; Kuo, J.S.; Huang, H.M.; Yang, D.Y.; Wu, T.F.; Tsai, T.H. Determination of catecholamines in pheochromocytoma cell (PC-12) culture medium by microdialysis-microbore liquid chromatography. J. Chromatogr. A 2000, 870, 405–411. [Google Scholar] [CrossRef]
- Hashimoto, K. Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry Clin. Neurosci. 2010, 64, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Byers, A.L.; Yaffe, K.; Covinsky, K.E.; Friedman, M.B.; Bruce, M.L. High occurrence of mood and anxiety disorders among older adults the national comorbidity survey replication. Arch. Gen. Psychiatry 2010, 67, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Douillard-Guilloux, G.; Guilloux, J.P.; Lewis, D.A.; Sibille, E. Anticipated brain molecular aging in major depression. Am. J. Geriatr. Psychiatry 2013, 21, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chang, L.C.; Wang, X.; Guilloux, J.P.; Parrish, J.; Oh, H.; French, B.J.; Lewis, D.A.; Tseng, G.C.; Sibille, E. Molecular and genetic characterization of depression: Overlap with other psychiatric disorders and aging. Mol. Neuropsychiatry 2015, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lenze, E.J.; Wetherell, J.L. Anxiety disorders: New developments in old age. Am. J. Geriatr. Psychiatry 2011, 19, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Perna, G.; Iannone, G.; Alciati, A.; Caldirola, D. Are anxiety disorders associated with accelerated aging? A focus on neuroprogression. Neural Plast. 2016, 2016, 8457612. [Google Scholar] [CrossRef] [PubMed]
- Yanai, S.; Endo, S. Early onset of behavioral alterations in senescence-accelerated mouse prone 8 (SAMP8). Behav. Brain Res. 2016, 308, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, H.; Cohen, N.J. Memory, Amnesia, and the Hippocampal System; MIT Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Squire, L.S.D. The Neuropsychology of Memory; Guilford Press: New York, NY, USA, 2002. [Google Scholar]
- DeCoteau, W.E.; Thorn, C.; Gibson, D.J.; Courtemanche, R.; Mitra, P.; Kubota, Y.; Graybiel, A.M. Learning-related coordination of striatal and hippocampal theta rhythms during acquisition of a procedural maze task. Proc. Natl. Acad. Sci. USA 2007, 104, 5644–5649. [Google Scholar] [CrossRef] [PubMed]
- Shivarama Shetty, M.; Sajikumar, S. ‘Tagging’ along memories in aging: Synaptic tagging and capture mechanisms in the aged hippocampus. Ageing Res. Rev. 2017, 35, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Bessinger, K.; Hellmann, J.; Murakami, S. Manipulation of serotonin signal suppresses early phase of behavioral aging in caenorhabditis elegans. Neurobiol. Aging 2008, 29, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Volpi-Abadie, J.; Kaye, A.M.; Kaye, A.D. Serotonin syndrome. Ochsner. J. 2013, 13, 533–540. [Google Scholar] [PubMed]
- Haberzettl, R.; Bert, B.; Fink, H.; Fox, M.A. Animal models of the serotonin syndrome: A systematic review. Behav. Brain Res. 2013, 256, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.L.; Kahn, R.S.; Ko, G.; Davidson, M. Dopamine in schizophrenia: A review and reconceptualization. Am. J. Psychiatry 1991, 148, 1474–1486. [Google Scholar] [PubMed]
- Cousins, D.A.; Butts, K.; Young, A.H. The role of dopamine in bipolar disorder. Bipolar Disord. 2009, 11, 787–806. [Google Scholar] [CrossRef] [PubMed]
- Granholm, A.C.; Boger, H.; Emborg, M.E. Mood, memory and movement: an age-related neurodegenerative complex? Curr. Aging Sci. 2008, 1, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D. Extracellular superoxide dismutase (EC-SOD) quenches free radicals and attenuates age-related cognitive decline: Opportunities for novel drug development in aging. Curr. Alzheimer Res. 2005, 2, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.H.; Johnson, L.A.; Zuloaga, D.G.; Limoli, C.L.; Raber, J. Enhanced hippocampus-dependent memory and reduced anxiety in mice over-expressing human catalase in mitochondria. J. Neurochem. 2013, 125, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Butters, M.A.; Bhalla, R.K.; Andreescu, C.; Wetherell, J.L.; Mantella, R.; Begley, A.E.; Lenze, E.J. Changes in neuropsychological functioning following treatment for late-life generalised anxiety disorder. Br. J. Psychiatry 2011, 199, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Mohanty, D. Psychobiotics: A new approach for treating mental illness? Crit. Rev. Food Sci. Nutr. 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Verdu, E.F.; Bercik, P.; Verma-Gandhu, M.; Huang, X.X.; Blennerhassett, P.; Jackson, W.; Mao, Y.; Wang, L.; Rochat, F.; Collins, S.M. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut 2006, 55, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Chunchai, T.; Thunapong, W.; Yasom, S.; Wanchai, K.; Eaimworawuthikul, S.; Metzler, G.; Lungkaphin, A.; Pongchaidecha, A.; Sirilun, S.; Chaiyasut, C.; et al. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflamm. 2018, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Tanida, M.; Shen, J.; Maeda, K.; Horii, Y.; Yamano, T.; Fukushima, Y.; Nagai, K. High-fat diet-induced obesity is attenuated by probiotic strain lactobacillus paracasei ST11 (NCC2461) in rats. Obes. Res. Clin. Pract. 2008, 2, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Jasarevic, E.; Morrison, K.E.; Bale, T.L. Sex differences in the gut microbiome-brain axis across the lifespan. Philos. Trans. R. Soc. Lond. Ser. B 2016, 371, 20150122. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kober, M.M.; Bowe, W.P. Anti-aging effects of probiotics. J. Drugs Dermatol. 2016, 15, 9–12. [Google Scholar] [PubMed]
- Rockville, M. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Adult Healthy Volunteer; USFDA: Silver Spring, MD, USA, 2005. [Google Scholar]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-Y.; Chen, L.-H.; Wang, M.-F.; Hsu, C.-C.; Chan, C.-H.; Li, J.-X.; Huang, H.-Y. Lactobacillus paracasei PS23 Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice. Nutrients 2018, 10, 894. https://doi.org/10.3390/nu10070894
Huang S-Y, Chen L-H, Wang M-F, Hsu C-C, Chan C-H, Li J-X, Huang H-Y. Lactobacillus paracasei PS23 Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice. Nutrients. 2018; 10(7):894. https://doi.org/10.3390/nu10070894
Chicago/Turabian StyleHuang, Shih-Yi, Li-Han Chen, Ming-Fu Wang, Chih-Chieh Hsu, Ching-Hung Chan, Jia-Xian Li, and Hui-Yu Huang. 2018. "Lactobacillus paracasei PS23 Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice" Nutrients 10, no. 7: 894. https://doi.org/10.3390/nu10070894
APA StyleHuang, S.-Y., Chen, L.-H., Wang, M.-F., Hsu, C.-C., Chan, C.-H., Li, J.-X., & Huang, H.-Y. (2018). Lactobacillus paracasei PS23 Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice. Nutrients, 10(7), 894. https://doi.org/10.3390/nu10070894