Large-Scale Synthesis of Carbon Dots Driven by Schiff Base Reaction at Room Temperature
Abstract
1. Introduction
2. Results and Discussion
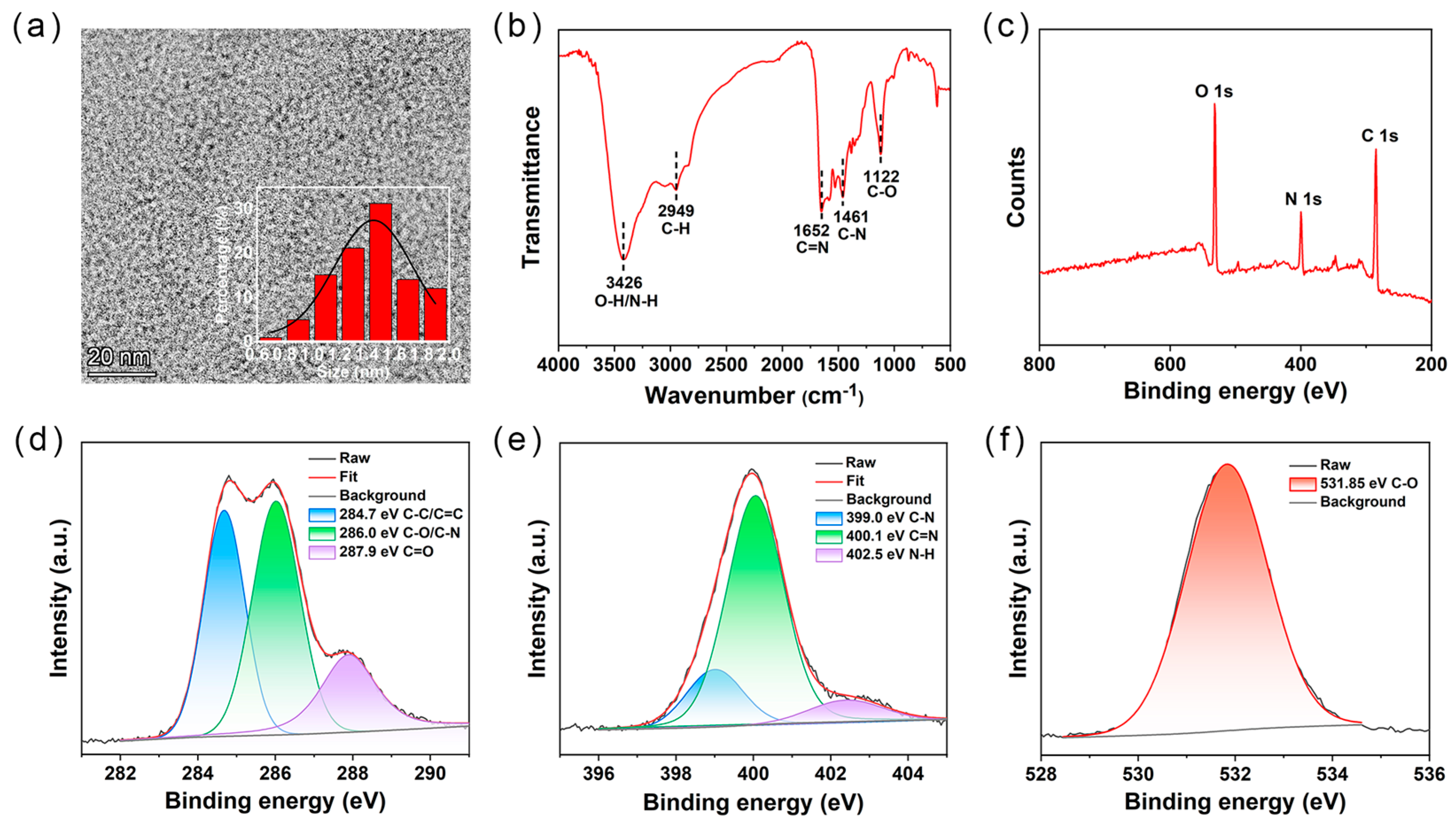
2.1. Formation and Structural Analysis of CDs
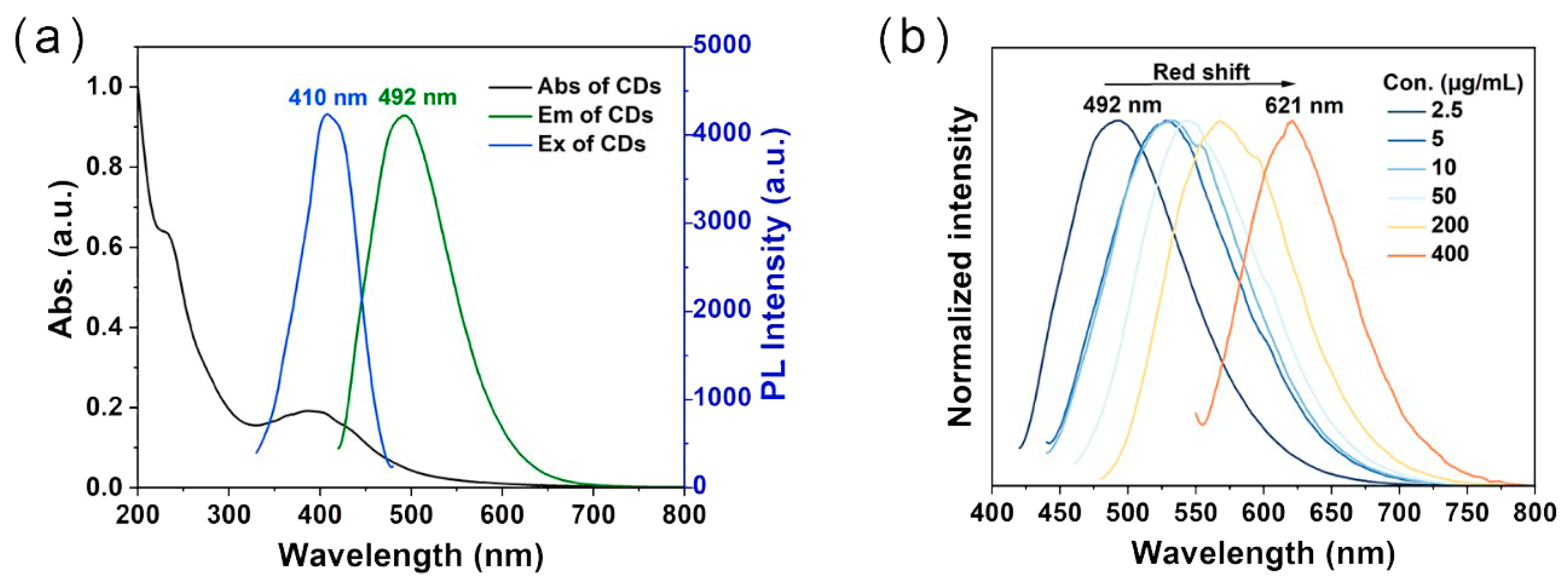
2.2. Photophysical Properties of the Prepared CDs
2.3. Stability Investigation of the Proposed CDs
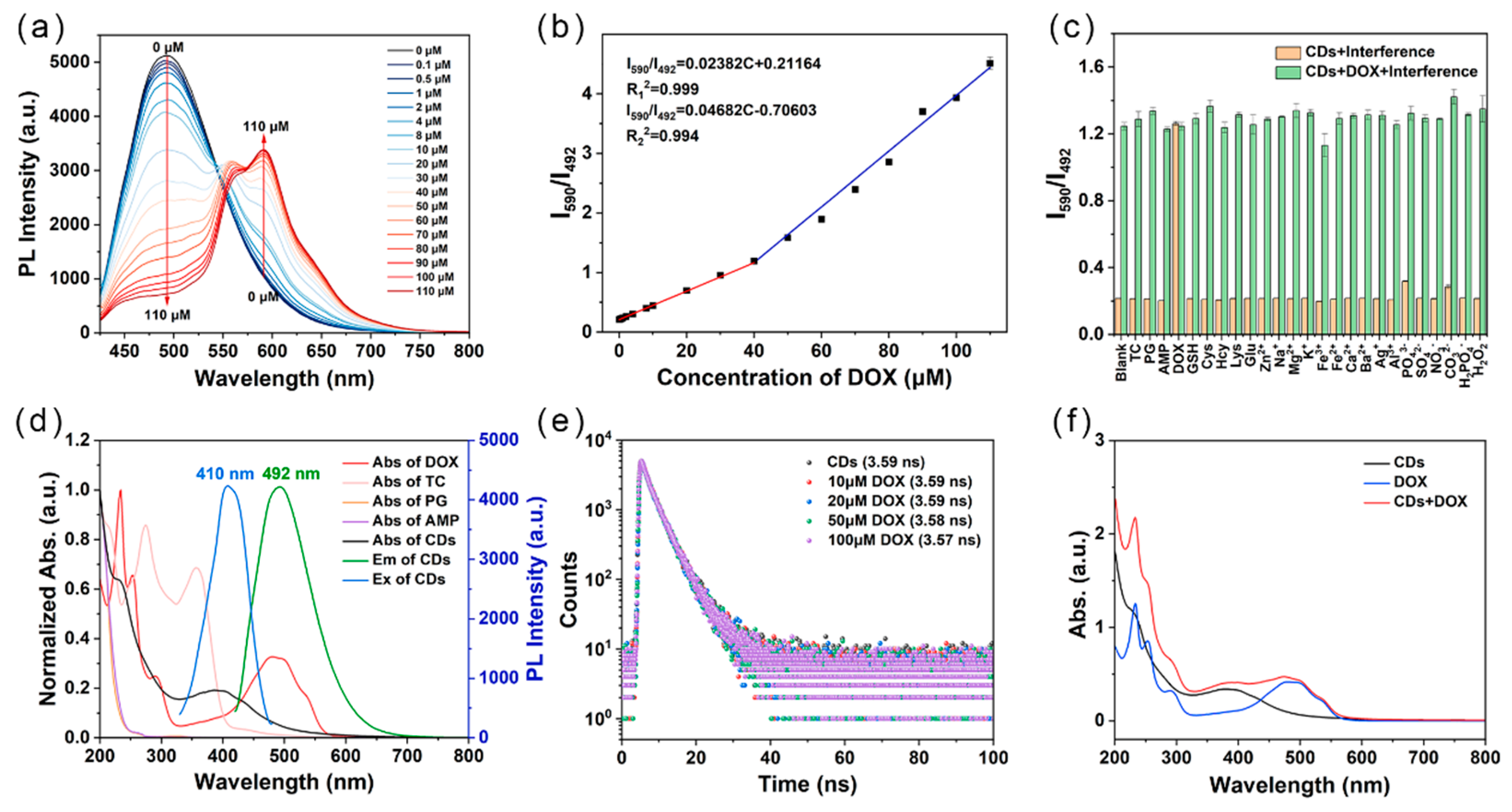
2.4. Detection of DOX by CDs
2.5. Mechanism of the Detection of DOX
3. Materials and Methods
3.1. Reagents
3.2. Apparatus and Characterization
3.3. Preparation and Purification of CDs
3.4. Ratiometric Detection of DOX by CDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.Y.; Ray, R.; Gu, Y.L.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, Z.; Hasanzadeh, M. Nanotechnology-assisted microfluidic systems for chemical sensing, biosensing, and bioanalysis. Trac-Trends Anal. Chem. 2022, 152, 116637. [Google Scholar] [CrossRef]
- Döring, A.; Ushakova, E.; Rogach, A.L. Chiral carbon dots: Synthesis, optical properties, and emerging applications. Light-Sci. Appl. 2022, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Du, J.J.; Xu, N.; Fan, J.L.; Sun, W.; Peng, X.J. Carbon dots for in vivo bioimaging and theranostics. Small 2019, 15, 1805087. [Google Scholar] [CrossRef]
- Marković, Z.M.; Milivojević, D.D.; Kovač, J.; Todorović Marković, B.M. Phloroglucinol-based carbon quantum dots/polyurethane composite films: How structure of carbon quantum dots affects antibacterial and antibiofouling efficiency of composite films. Polymers 2024, 16, 1646. [Google Scholar] [CrossRef]
- Chen, B.-B.; Liu, M.-L.; Gao, Y.-T.; Chang, S.; Qian, R.-C.; Li, D.-W. Design and applications of carbon dots-based ratiometric fluorescent probes: A review. Nano Res. 2023, 16, 1064–1083. [Google Scholar] [CrossRef]
- Xian, Y.M.; Li, K. Hydrothermal synthesis of high-yield red fluorescent carbon dots with ultra-narrow emission by controlled O/N elements. Adv. Mater. 2022, 34, 2201031. [Google Scholar] [CrossRef]
- Tang, L.; Ai, L.; Song, Z.Q.; Sui, L.Z.; Yu, J.K.; Yang, X.; Song, H.Q.; Zhang, B.W.; Hu, Y.S.; Zhang, Y.Q.; et al. Acid-triggered aggregation of carbon dots shifted their emission to give unexpected deep-red lasing. Adv. Funct. Mater. 2023, 33, 2303363. [Google Scholar] [CrossRef]
- Liu, S.; He, Y.; Liu, Y.; Wang, S.; Jian, Y.; Li, B.; Xu, C. One-step hydrothermal synthesis of chiral carbon dots with high asymmetric catalytic activity for an enantioselective direct aldol reaction. Chem. Commun. 2021, 57, 3680–3683. [Google Scholar] [CrossRef]
- Ramírez-Barroso, S.; Jacobo-Martín, A.; Navarro-Baena, I.; Hernández, J.J.; Navio, C.; Rodríguez, I.; Wannemacher, R. On the nature of solvothermally synthesized carbon nanodots. J. Mater. Chem. C 2021, 9, 16935–16944. [Google Scholar] [CrossRef]
- Zhang, D.; Chao, D.; Yu, C.; Zhu, Q.; Zhou, S.; Tian, L.; Zhou, L. One-step green solvothermal synthesis of full-color carbon quantum dots based on a doping strategy. J. Phys. Chem. Lett. 2021, 12, 8939–8946. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Cai, H.; Zhou, W.W.; Li, Z.J.; Zhang, L.; Bi, H. RNA-targeting carbon dots for live-cell imaging of granule dynamics. Adv. Mater. 2023, 35, 2210776. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Song, Y.L.; Fang, Y.Z.; Zhu, K.; Ye, K.; Gao, Y.Y.; Yan, J.; Wang, G.L.; Cao, D.A.X. Microwave-assisted synthesis of carbon dots modified graphene for full carbon-based potassium ion capacitors. Carbon 2021, 178, 1–9. [Google Scholar] [CrossRef]
- Ahmad, K.; Pal, A.; Pan, U.N.; Chattopadhyay, A.; Paul, A. Synthesis of single-particle level white-light-emitting carbon dots via a one-step microwave method. J. Mater. Chem. C 2018, 6, 6691–6697. [Google Scholar] [CrossRef]
- Hou, J.; Zhou, T.Y.; Wang, L.; Zhang, P.; Ding, L. Template-free microwave-assisted fabrication of carbon dots/Zn(OH)2 composites for separation and enhancing chemical sensing. Sens. Actuators B-Chem. 2016, 230, 615–622. [Google Scholar] [CrossRef]
- Che, Y.; Pang, H.; Li, H.; Yang, L.; Fu, X.; Liu, S.; Ding, L.; Hou, J. Microwave-assisted fabrication of copper-functionalized carbon quantum dots for sensitive detection of histidine. Talanta 2019, 196, 442–448. [Google Scholar] [CrossRef]
- Cui, Y.H.; Xue, S.H.; Wang, S.Y.; Chen, X.; Liu, S.J.; Ye, Q.; Zhou, F.; Liu, W.M. Fabrication of carbon dots intercalated MXene hybrids via laser treatment as oil-based additives for synergistic lubrication. Carbon 2023, 205, 373–382. [Google Scholar] [CrossRef]
- Zhu, Z.F.; Wang, S.D.; Chang, Y.J.; Yu, D.B.; Jiang, Y. Direct photodissociation of toluene molecules to photoluminescent carbon dots under pulsed laser irradiation. Carbon 2016, 105, 416–423. [Google Scholar] [CrossRef]
- Liu, M.L.; Xu, Y.H.; Niu, F.S.; Gooding, J.J.; Liu, J.Q. Carbon quantum dots directly generated from electrochemical oxidation of graphite electrodes in alkaline alcohols and the applications for specific ferric ion detection and cell imaging. Analyst 2016, 141, 2657–2664. [Google Scholar] [CrossRef]
- Qiao, Z.A.; Wang, Y.F.; Gao, Y.; Li, H.W.; Dai, T.Y.; Liu, Y.L.; Huo, Q.S. Commercially activated carbon as the source for producing multicolor photoluminescent carbon dots by chemical oxidation. Chem. Commun. 2010, 46, 8812–8814. [Google Scholar] [CrossRef]
- Dordevic, L.; Arcudi, F.; Cacioppo, M.; Prato, M. A multifunctional chemical toolbox to engineer carbon dots for biomedical and energy applications. Nat. Nanotechnol. 2022, 17, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Zhong, X.X.; Rider, A.E.; Furman, S.A.; Ostrikov, K. Fast, energy-efficient synthesis of luminescent carbon quantum dots. Green Chem. 2014, 16, 2566–2570. [Google Scholar] [CrossRef]
- Chen, B.B.; Chang, S.; Jiang, L.; Lv, J.; Gao, Y.T.; Wang, Y.; Qian, R.C.; Li, D.W.; Hafez, M.E. Reversible polymerization of carbon dots based on dynamic covalent imine bond. J. Colloid Interface Sci. 2022, 621, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Chen, B.B.; Gao, Y.T.; Zheng, Y.H.; Shi, J.F.; Qian, R.C.; Li, D.W. Carbon dots with hydrogen bond-controlled aggregation behavior. Analyst 2023, 148, 507–511. [Google Scholar] [CrossRef]
- Li, T.Z.; Shuang, E.; Wang, J.H.; Chen, X.W. Regulating the properties of carbon dots via a solvent-involved molecule fusion strategy for improved sensing selectivity. Anal. Chim. Acta 2019, 1088, 107–115. [Google Scholar] [CrossRef]
- Huang, X.S.; Liu, Z.K.; Huang, Y.Q.; Zong, Y.; Yang, X.Y.; Hu, Z.Q.; Zeng, C. One-pot room temperature synthesis of orange-emitting carbon dots for highly-sensitive vitamin B12 sensing. Spectrochim. Acta A 2022, 276, 121239. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.S.; de Luca, A.C.; Pelendritis, M.; Terenghi, G.; Downes, S.; Schroeder, S.L.M. Quantitative analysis of complex amino acids and RGD peptides by X-ray photoelectron spectroscopy (XPS). Surf. Interface Anal. 2013, 45, 1238–1246. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Duong, L.V.; Wood, B.J.; Frost, R.L. XPS study of the major minerals in bauxite: Gibbsite, bayerite and (pseudo-)boehmite. J. Colloid Interface Sci. 2006, 296, 572–576. [Google Scholar] [CrossRef]
- Bai, L.H.; Yan, H.X.; Feng, Y.B.; Feng, W.X.; Yuan, L.Y. Multi-excitation and single color emission carbon dots doped with silicon and nitrogen: Synthesis, emission mechanism, Fe3+ probe and cell imaging. Chem. Eng. J. 2019, 373, 963–972. [Google Scholar] [CrossRef]
- Wang, C.; Hu, T.T.; Wen, Z.q.; Zhou, J.D.; Wang, X.J.; Wu, Q.; Wang, C.X. Concentration-dependent color tunability of nitrogen-doped carbon dots and their application for iron(III) detection and multicolor bioimaging. J. Colloid Interface Sci. 2018, 521, 33–41. [Google Scholar] [CrossRef]
- Chang, S.; Chen, B.B.; Lv, J.; Fodjo, E.K.; Qian, R.C.; Li, D.W. Label-free chlorine and nitrogen-doped fluorescent carbon dots for target imaging of lysosomes in living cells. Microchim. Acta 2020, 187, 435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, L.; Yu, S.H. A selective sensor for cyanide ion (CN−) based on the inner filter effect of metal nanoparticles with photoluminescent carbon dots as the fluorophore. Sci. Bull. 2015, 60, 785–791. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y.L.; Wang, J.H. Inner filter effect-based fluorescent sensing systems: A review. Anal. Chim. Acta 2018, 999, 13–26. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Chang, S.; Gao, Y.; Lv, J.; Qian, R.; Chen, B.; Li, D. Large-Scale Synthesis of Carbon Dots Driven by Schiff Base Reaction at Room Temperature. Inorganics 2024, 12, 310. https://doi.org/10.3390/inorganics12120310
Shi J, Chang S, Gao Y, Lv J, Qian R, Chen B, Li D. Large-Scale Synthesis of Carbon Dots Driven by Schiff Base Reaction at Room Temperature. Inorganics. 2024; 12(12):310. https://doi.org/10.3390/inorganics12120310
Chicago/Turabian StyleShi, Jifen, Shuai Chang, Yating Gao, Jian Lv, Ruocan Qian, Binbin Chen, and Dawei Li. 2024. "Large-Scale Synthesis of Carbon Dots Driven by Schiff Base Reaction at Room Temperature" Inorganics 12, no. 12: 310. https://doi.org/10.3390/inorganics12120310
APA StyleShi, J., Chang, S., Gao, Y., Lv, J., Qian, R., Chen, B., & Li, D. (2024). Large-Scale Synthesis of Carbon Dots Driven by Schiff Base Reaction at Room Temperature. Inorganics, 12(12), 310. https://doi.org/10.3390/inorganics12120310






