Influence of Pyrolysis Temperature on Critical Variables Related to Charcoal Spontaneous Combustion
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Characterization of Charcoal
2.2. Spontaneous Combustion Analysis of Charcoal
2.3. Data Analysis
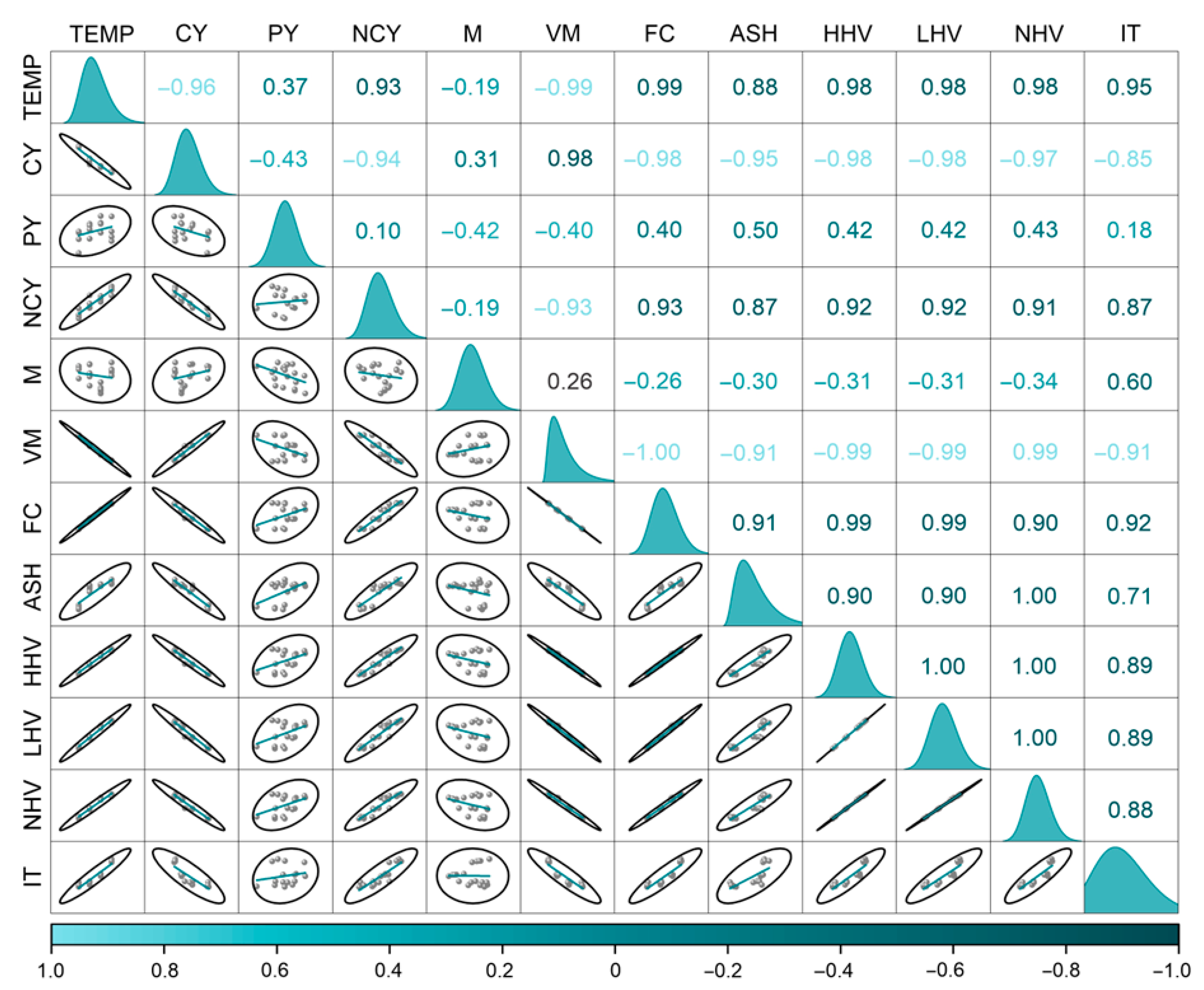
3. Results and Discussion
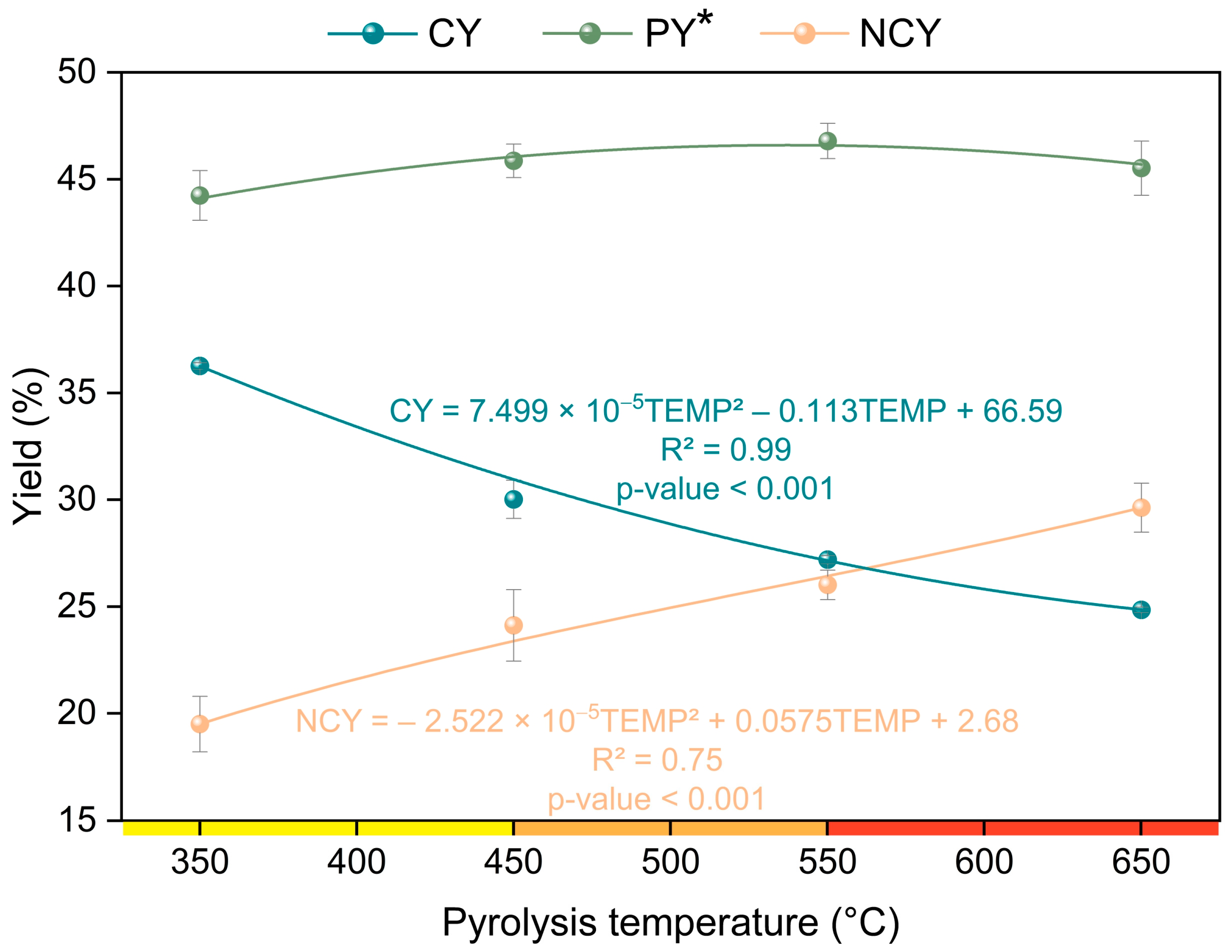
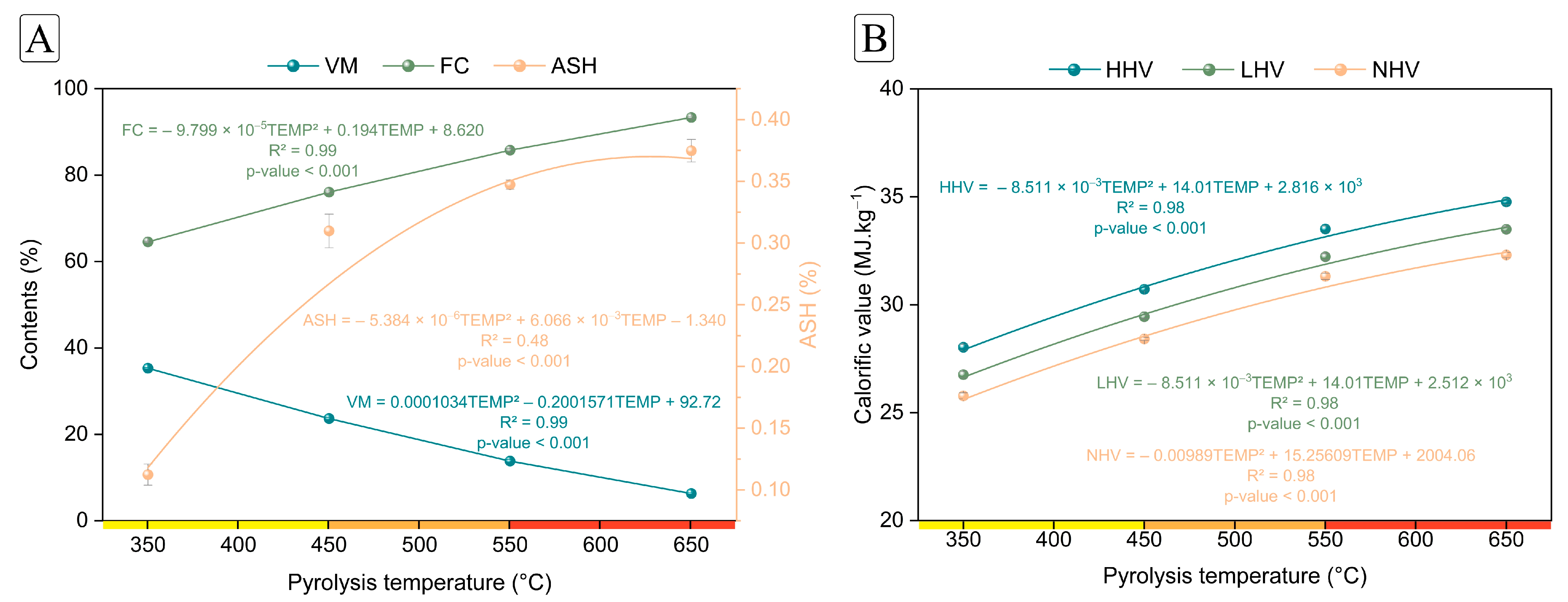
3.1. Pyrolysis Yield and Properties of Charcoal
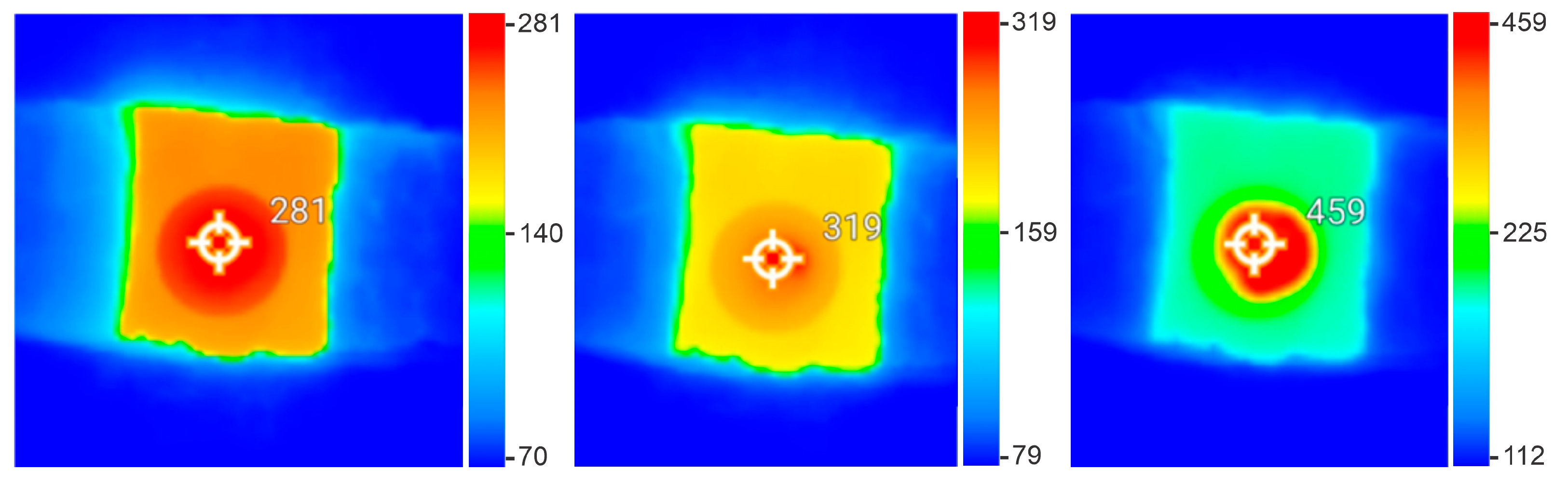
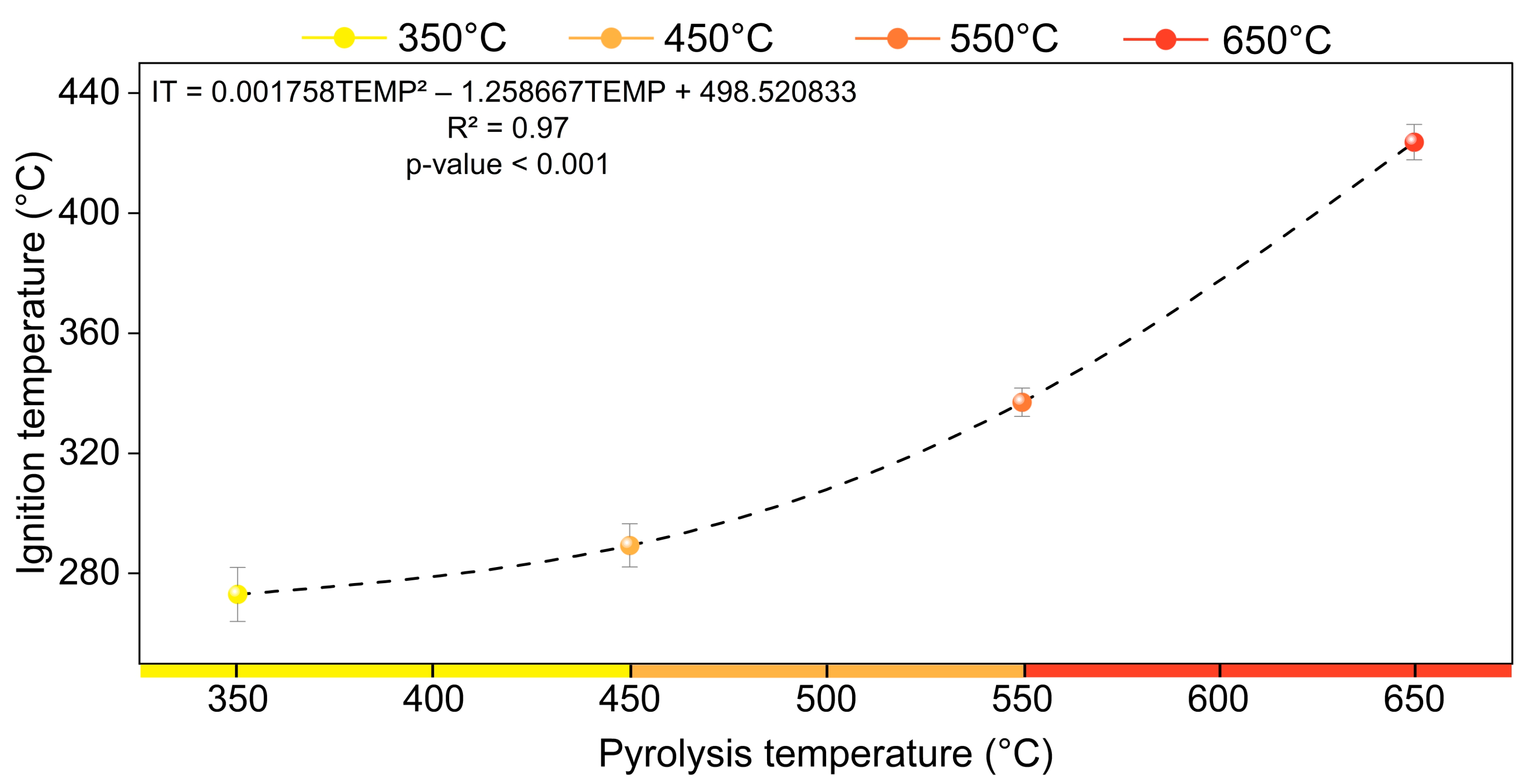
3.2. Ignition Temperature and Its Relationship with Charcoal Properties
3.3. Relevance of the Data and Future Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Appendix A
| Properties | Temperature (°C) | |||
|---|---|---|---|---|
| 350 | 450 | 550 | 650 | |
| Charcoal yield (%) | 36.26 (±0.15) | 30.02 (±0.90) | 27.19 (±0.19) | 24.85 (±0.13) |
| PLY (%) | 44.24 (±1.16) | 45.86 (±0.78) | 46.79 (±0.82) | 45.52 (±1.27) |
| NCY (%) | 19.50 (±1.30) | 24.12 (±1.67) | 26.01 (±0.69) | 29.63 (±1.15) |
| Volatile matter (%) | 35.30 (±0.16) | 23.66 (±0.30) | 13.83 (±0.37) | 6.32 (±0.22) |
| Fixed carbon (%) | 64.57 (±0.17) | 76.07 (±0.30) | 85.78 (±0.35) | 93.36 (±0.25) |
| Ash (%) | 0.11 (±0.1) | 0.31 (±0.1) | 0.35 (±0.0) | 0.37 (±0.0) |
| HHV (MJ.kg–1) | 28.03 (±0.14) | 30.72 (±0.08) | 33.51 (±0.14) | 34.77 (±0.12) |
| LHV (MJ.kg–1) | 26.76 (±0.14) | 29.45 (±0.08) | 32.23 (±0.14) | 33.50 (±0.12) |
| NHV (MJ.kg–1) | 25.78 (±0.14) | 28.42 (±0.07) | 31.32 (±0.14) | 32.31 (±0.12) |
| IT (°C) | 273.00 (±9.02) | 289.33 (±7.17) | 337 (±4.73) | 423.67 (±5.93) |
References
- BRASIL, National Land Transportation Agency. Resolution Nº 5.998, November 3, 2022. Available online: https://www.in.gov.br/web/dou/-/resolucao-n-5.998-de-3-de-novembro-de-2022-441279478 (accessed on 24 July 2025).
- Shao, S.; Wu, C.; Hao, M.; Song, X.; Su, X.; Wang, W.; Li, G.; Shi, B. A Novel Coating Technology for Fast Sealing of Air Leakage in Underground Coal Mines. Int. J. Min. Sci. Technol. 2021, 31, 313–320. [Google Scholar] [CrossRef]
- Barros, D.d.S.; Lima, M.D.R.; Massuque, J.; dos Santos, E.V.; Guirardi, B.D.; Junior, A.F.D.; Bufalino, L.; Silveira, E.A.; Carneiro, A.d.C.O.; Trugilho, P.F.; et al. Advancing Circular Economy in Amazonian Forest Management: A Comparative Study of the Effects of Wood Waste Segregation and Traditional Carbonization on Charcoal Properties, Combustibility, and Spontaneous Combustion Risk. Circ. Econ. 2025, 4, 100152. [Google Scholar] [CrossRef]
- Kumar Mohalik, N.; Mandal, S.; Kumar Ray, S.; Mobin Khan, A.; Mishra, D.; Krishna Pandey, J. TGA/DSC Study to Characterise and Classify Coal Seams Conforming to Susceptibility towards Spontaneous Combustion. Int. J. Min. Sci. Technol. 2022, 32, 75–88. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Tsai, Y.T.; Deng, J.; Shu, C.M. Spontaneous Combustion in Six Types of Coal by Using the Simultaneous Thermal Analysis-Fourier Transform Infrared Spectroscopy Technique. J. Therm. Anal. Calorim. 2016, 126, 1591–1602. [Google Scholar] [CrossRef]
- Méndez, L.B.; Borrego, A.G.; Martinez-Tarazona, M.R.; Menéndez, R. Influence of Petrographic and Mineral Matter Composition of Coal Particles on Their Combustion Reactivity☆. Fuel 2003, 82, 1875–1882. [Google Scholar] [CrossRef]
- Yang, Z.L.; Walvekar, R.; Wong, W.P.; Sharma, R.K.; Dharaskar, S.; Khalid, M. Advances in Phase Change Materials, Heat Transfer Enhancement Techniques, and Their Applications in Thermal Energy Storage: A Comprehensive Review. J. Energy Storage 2024, 87, 111329. [Google Scholar] [CrossRef]
- Xie, J.; Xue, S.; Cheng, W.; Wang, G. Early Detection of Spontaneous Combustion of Coal in Underground Coal Mines with Development of an Ethylene Enriching System. Int. J. Coal Geol. 2011, 85, 123–127. [Google Scholar] [CrossRef]
- Gao, F.; Jia, Z.; Qin, M.L.; Mu, X.G.; Teng, Y.F.; Li, Y.D.; Bai, Q.H. Effects of Organic Sulfur on Oxidation Spontaneous Combustion Characteristics of Coking Coal. Energy Explor. Exploit. 2022, 40, 193–205. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Liu, W.; Yang, X.; Cheng, X.; Qi, C.; Li, C.; Hui, J.; Zhang, L. Research Status and Prospect of Coal Spontaneous Combustion Source Location Determination Technology. Processes 2025, 13, 2305. [Google Scholar] [CrossRef]
- Deng, J.; Ma, X.; Zhang, Y.; Li, Y.; Zhu, W. Effects of Pyrite on the Spontaneous Combustion of Coal. Int. J. Coal Sci. Technol. 2015, 2, 306–311. [Google Scholar] [CrossRef]
- Phounglamcheik, A.; Johnson, N.; Kienzl, N.; Strasser, C.; Umeki, K. Self-Heating of Biochar during Postproduction Storage by O2 Chemisorption at Low Temperatures. Energies 2022, 15, 380. [Google Scholar] [CrossRef]
- Miyawaki, N.; Fukushima, T.; Mizuno, T.; Inoue, M.; Takisawa, K. Effect of Wood Biomass Components on Self-Heating. Bioresour. Bioprocess. 2021, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Cupertino, G.F.M.; da Silva, Á.M.; Pereira, A.K.S.; Delatorre, F.M.; Ucella-Filho, J.G.M.; de Souza, E.C.; Profeti, D.; Profeti, L.P.R.; Oliveira, M.P.; Saloni, D.; et al. Co-Pyrolysis of Biomass and Polyethylene Terephthalate (PET) as an Alternative for Energy Production from Waste Valorization. Fuel 2024, 362, 130761. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, D.; Zhang, Y.; Huang, Y.; Sun, S. Effect of Pyrolysis Temperature on Char Structure and Chemical Speciation of Alkali and Alkaline Earth Metallic Species in Biochar. Fuel Process. Technol. 2016, 141, 54–60. [Google Scholar] [CrossRef]
- Silva, F.T.M.; Ataíde, C.H. Valorization of Eucalyptus urograndis Wood via Carbonization: Product Yields and Characterization. Energy 2019, 172, 509–516. [Google Scholar] [CrossRef]
- ASTM D1762-84; Standard Method for Chemical Analyses of Wood Charcoal. American Society for Testing Materials: West Conshohocken, PA, USA, 2007.
- DIN EN 14918; Determination of Calorific Value. Deutsches Institut Für Normung (DIN): Berlin, Germany, 2010.
- Dias Junior, A.F.; Arthuso, H.; Lana, A.Q.; Andrade, C.R.; Brito, J.O.; Bortoletto Junior, G. Addition of Eucalyptus sp. Wood to Urban Wood Waste as a Strategy for Energetic Use Adição Da Madeira de Eucalyptus sp. Aos Resíduos Madeireiros Urbanos Como Estratégia Para a Geração de Energia. Sci. For. 2021, 49, e3230. [Google Scholar] [CrossRef]
- Andrade, C.R.; Cupertino, G.F.M.; da Silva, Á.M.; Brito, J.O.; Morais, W.W.C.; Balboni, B.M.; Saloni, D.; Dias Júnior, A.F. The Potential of Wood-Based Urban Waste to Generate Bioenergy and Increase the Energetic Sustainability. Clean. Technol. Environ. Policy 2024, 26, 2885–2898. [Google Scholar] [CrossRef]
- dos Santos, P.L.; Lima, M.D.R.; Bufalino, L.; Hein, P.R.G.; Silveira, E.A.; Candelier, K.; Trugilho, P.F.; Protásio, T. de P. Exploring the Effects of Slow Pyrolysis Temperature and Species on the Quality of Charcoal from Amazonian Woody Wastes. Renew. Energy 2025, 240, 122257. [Google Scholar] [CrossRef]
- Pereira, A.K.S.; Longue Júnior, D.; da Silva, Á.M.; Cupertino, G.F.M.; de Souza, E.C.; Delatorre, F.M.; Ucella-Filho, J.G.M.; de Oliveira, P.R.S.; Rodrigues, B.P.; Dias Júnior, A.F. How Pyrolysis Conditions Shape the Structural and Functional Properties of Charcoal? A Study of Tropical Dry Forest Biomass. Renew. Energy 2025, 243, 122575. [Google Scholar] [CrossRef]
- Moya, R.; Tenorio, C.; Quesada-Kimzey, J. Charcoal Production from Four Tropical Woods through Slow Pyrolysis under Different Temperatures: Yield of Different Products and Condition of Pyrolysis into the Reactor. Biomass Convers. Biorefinery 2025, 15, 5533–5550. [Google Scholar] [CrossRef]
- Varshney, A.; Mishra, N.K. Optimizing Charcoal Production: Comparative Study of Energy, Exergy, and Emissions from Slow Pyrolysis of Crop Residues. J. Therm. Anal. Calorim. 2025, 50, 13181–13192. [Google Scholar] [CrossRef]
- Delatorre, F.M.; Cupertino, G.F.M.; Oliveira, M.P.; da Silva Gomes, F.; Profeti, L.P.R.; Profeti, D.; Júnior, M.G.; de Azevedo, M.G.; Saloni, D.; Júnior, A.F.D. A Novel Approach to Charcoal Fine Waste: Sustainable Use as Filling of Polymeric Matrices. Polymers 2022, 14, 5525. [Google Scholar] [CrossRef] [PubMed]
- Jerzak, W.; Acha, E.; Li, B. Comprehensive Review of Biomass Pyrolysis: Conventional and Advanced Technologies, Reactor Designs, Product Compositions and Yields, and Techno-Economic Analysis. Energies 2024, 17, 5082. [Google Scholar] [CrossRef]
- Khater, E.S.; Bahnasawy, A.; Hamouda, R.; Sabahy, A.; Abbas, W.; Morsy, O.M. Biochar Production under Different Pyrolysis Temperatures with Different Types of Agricultural Wastes. Sci. Rep. 2024, 14, 2625. [Google Scholar] [CrossRef] [PubMed]
- Birhanu, A.; Hailu, A.M.; Worku, Z.; Tessema, I.; Angassa, K.; Tibebu, S. Optimization of Pyrolysis Conditions for Catha Edulis Waste-Based Biochar Production Using Response Surface Methodology. Bioresour. Bioprocess. 2025, 12, 62. [Google Scholar] [CrossRef]
- Razzak, S.A. Municipal Solid and Plastic Waste Derived High-Performance Biochar Production: A Comprehensive Review. J. Anal. Appl. Pyrolysis 2024, 181, 106622. [Google Scholar] [CrossRef]
- Gao, L.; Ma, X.; Cheng, J.; Liu, Y. The Effect of Preparation Process on Combustibility of Robinia pseudoacacia Charcoal and Its Structure-Performance Relationship. Sci. Rep. 2025, 15, 14563. [Google Scholar] [CrossRef]
- Elnour, A.Y.; Alghyamah, A.A.; Shaikh, H.M.; Poulose, A.M.; Al-Zahrani, S.M.; Anis, A.; Al-Wabel, M.I. Effect of Pyrolysis Temperature on Biochar Microstructural Evolution, Physicochemical Characteristics, and Its Influence on Biochar/Polypropylene Composites. Appl. Sci. 2019, 9, 1149. [Google Scholar] [CrossRef]
- López, F.A.; Centeno, T.A.; García-Díaz, I.; Alguacil, F.J. Textural and Fuel Characteristics of the Chars Produced by the Pyrolysis of Waste Wood, and the Properties of Activated Carbons Prepared from Them. J. Anal. Appl. Pyrolysis 2013, 104, 551–558. [Google Scholar] [CrossRef]
- Hwangdee, P.; Junsiri, C.; Sudajan, S.; Laloon, K. Fuel Potential Values of Biomass Charcoal Powder. Biomass Convers. Biorefin 2023, 13, 5721–5730. [Google Scholar] [CrossRef]
- Lubwama, M.; Yiga, V.A.; Ssempijja, I.; Lubwama, H.N. Thermal and Mechanical Characteristics of Local Firewood Species and Resulting Charcoal Produced by Slow Pyrolysis. Biomass Convers. Biorefin 2023, 13, 6689–6704. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Z.; Wang, Z.; Kong, W.; Feng, S.; Shen, B.; Mu, L. Integration of Torrefaction and In-Situ Pelletization for Biodried Products Derived from Municipal Organic Wastes: The Influences of Temperature on Fuel Properties and Combustion Behaviours. Fuel 2022, 313, 122845. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, E. Numerical Investigation of the Influence of Particle Shape, Pretreatment Temperature, and Coal Blending on Biochar Combustion in a Blast Furnace. Fuel 2022, 313, 123016. [Google Scholar] [CrossRef]
- Bouesso, B.; González Martínez, M.; Nzihou, A. Co-Combustion of Anthracite Coal and Biomass-Derived Biocarbons: Reaction Kinetics and Combustion Performance Indicators. Biomass Bioenergy 2025, 201, 108117. [Google Scholar] [CrossRef]
- Putri, R.W.; Rahmatullah; Santoso, B.; Savitri, A.; Zahara, M. Effect of Drying Temperature of Sawdust Biobriquettes with Used Lubricant Oil Adhesive Volume Variation over Carbonization Process. J. Ecol. Eng. 2024, 25, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Uzoagba, C.E.J.; Bello, A.; Ngasoh, F.O.; Onwualu, A.P. Effect of Pyrolysis Temperature on Physicochemical Characteristics of Biochar Derived from Rapid Pyrolysis of Prosopis africana Biomass. Biofuels 2025, 1–8. [Google Scholar] [CrossRef]
- Batista, R.R.; Gomes, M.M. Effects of Chemical Composition and Pyrolysis Process Variables on Biochar Yields: Correlation and Principal Component Analysis. Floresta Ambiente 2021, 28, e20210007. [Google Scholar] [CrossRef]
- Schettini, B.L.S.; Jacovine, L.A.G.; Torres, C.M.M.E.; Carneiro, A.d.C.O.; Villanova, P.H.; da Rocha, S.J.S.S.; Rufino, M.P.M.X.; Silva, L.B.; Castro, R.V.O. Furnace-Kiln System: How Does the Use of New Technologies in Charcoal Production Affect the Carbon Balance? Ind. Crops Prod. 2022, 187, 115330. [Google Scholar] [CrossRef]
- United Nations. Committee of Experts on the Transport of Dangerous Goods. Recommendations on the Transport of Dangerous Goods: Manual of Tests and Criteria; United Nations Publications: Geneva, Switzerland, 2009. [Google Scholar]
- Rohde, G.M. Evidências Científicas Da Impossibilidade Da Combustão Espontânea Do Carvão Vegetal Produzido No Estado Do Rio Grande Do Sul; Cientec: Porto Alegre, Brazil, 2005. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, T.R.; da Silva, Á.M.; Cupertino, G.F.M.; Delatorre, F.M.; Amorim, G.A.; de Souza, M.P.; Brito, J.O.; Dias Júnior, A.F. Influence of Pyrolysis Temperature on Critical Variables Related to Charcoal Spontaneous Combustion. Bioresour. Bioprod. 2025, 1, 6. https://doi.org/10.3390/bioresourbioprod1020006
Oliveira TR, da Silva ÁM, Cupertino GFM, Delatorre FM, Amorim GA, de Souza MP, Brito JO, Dias Júnior AF. Influence of Pyrolysis Temperature on Critical Variables Related to Charcoal Spontaneous Combustion. Bioresources and Bioproducts. 2025; 1(2):6. https://doi.org/10.3390/bioresourbioprod1020006
Chicago/Turabian StyleOliveira, Tayná Rebonato, Álison Moreira da Silva, Gabriela Fontes Mayrinck Cupertino, Fabíola Martins Delatorre, Gabriela Aguiar Amorim, Marina Passos de Souza, José Otávio Brito, and Ananias Francisco Dias Júnior. 2025. "Influence of Pyrolysis Temperature on Critical Variables Related to Charcoal Spontaneous Combustion" Bioresources and Bioproducts 1, no. 2: 6. https://doi.org/10.3390/bioresourbioprod1020006
APA StyleOliveira, T. R., da Silva, Á. M., Cupertino, G. F. M., Delatorre, F. M., Amorim, G. A., de Souza, M. P., Brito, J. O., & Dias Júnior, A. F. (2025). Influence of Pyrolysis Temperature on Critical Variables Related to Charcoal Spontaneous Combustion. Bioresources and Bioproducts, 1(2), 6. https://doi.org/10.3390/bioresourbioprod1020006







