Analysis of Modifications to an Outdoor Field-Scale Rotating Algal Biofilm Reactor with a Focus on Biomass Productivity and Power Usage
Abstract
1. Introduction
2. Materials and Methods
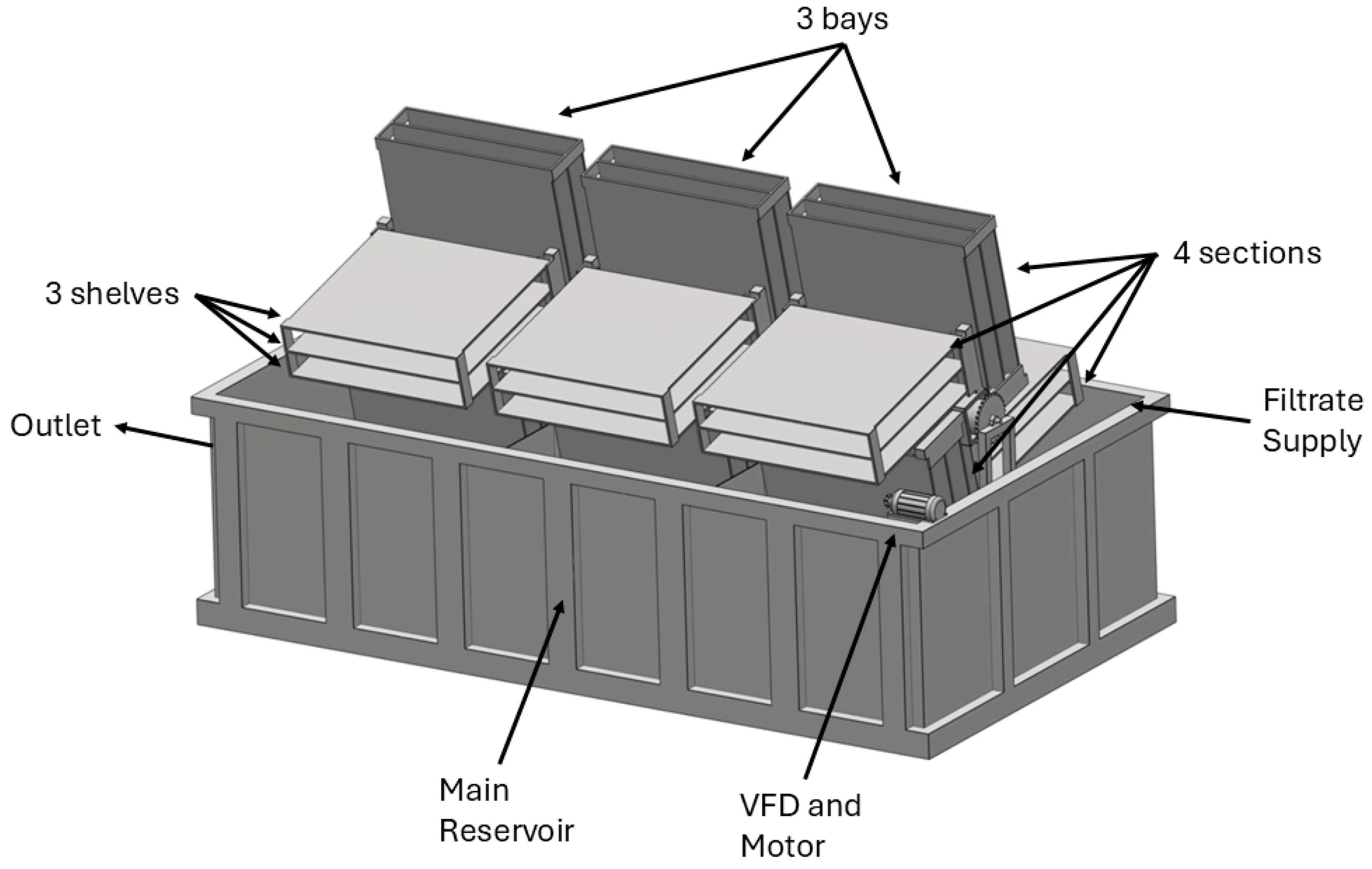
2.1. Modifications
2.2. Sampling
2.3. Harvesting
2.4. Drying
2.5. Incineration
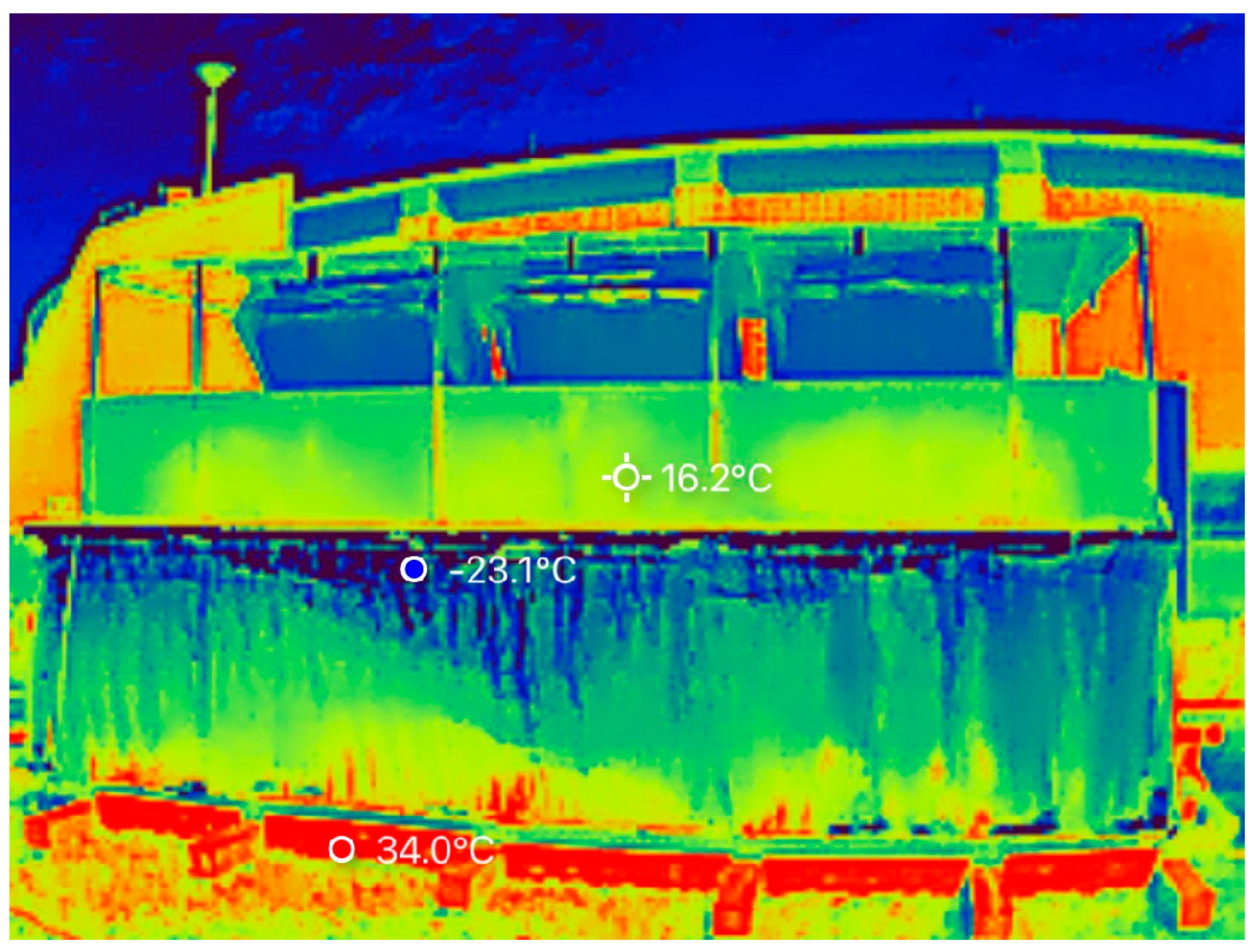
2.6. Insulation
2.7. Biomass Productivity Calculation
3. Results and Discussion
3.1. Performance Evaluation of the Pilot RABR
3.2. Changes in Parameter
- Increase in duty cycle in September 2024 from 25% to 50% to increase biomass productivity.
- Installation of insulation consisting of fiberglass, polystyrene, and a radiant barrier at the end of October 2024 to reduce heat loss in the above-ground steel tank, thus increasing tank temperature.
- Improvements to the insulation by installing 1.5875 mm (1/16 inch) polycarbonate sheets to cover all sides of the RABR frame in February 2025, as seen in Figure 4. This was performed to reduce impact of winter weather.
- Increase in duty cycle in February 2025 from 50% to 100% to minimize moisture stress on the algae.
- Decrease in HRT from 48 h to 11 h to increase liquid temperature, which resulted from increasing the flow rate to the maximum flow rate possible with the RABR setup of 18 L/min on 28 February 2025. This was performed with the goal of increasing biomass productivity.
- Removal of six shelves from the RABR to fix mechanical issues experienced in January 2025. The reduced surface area is accounted for in substratum productivity calculations. This was performed to prevent the early shutdown of the RABR system.
3.3. Re-Evaluation
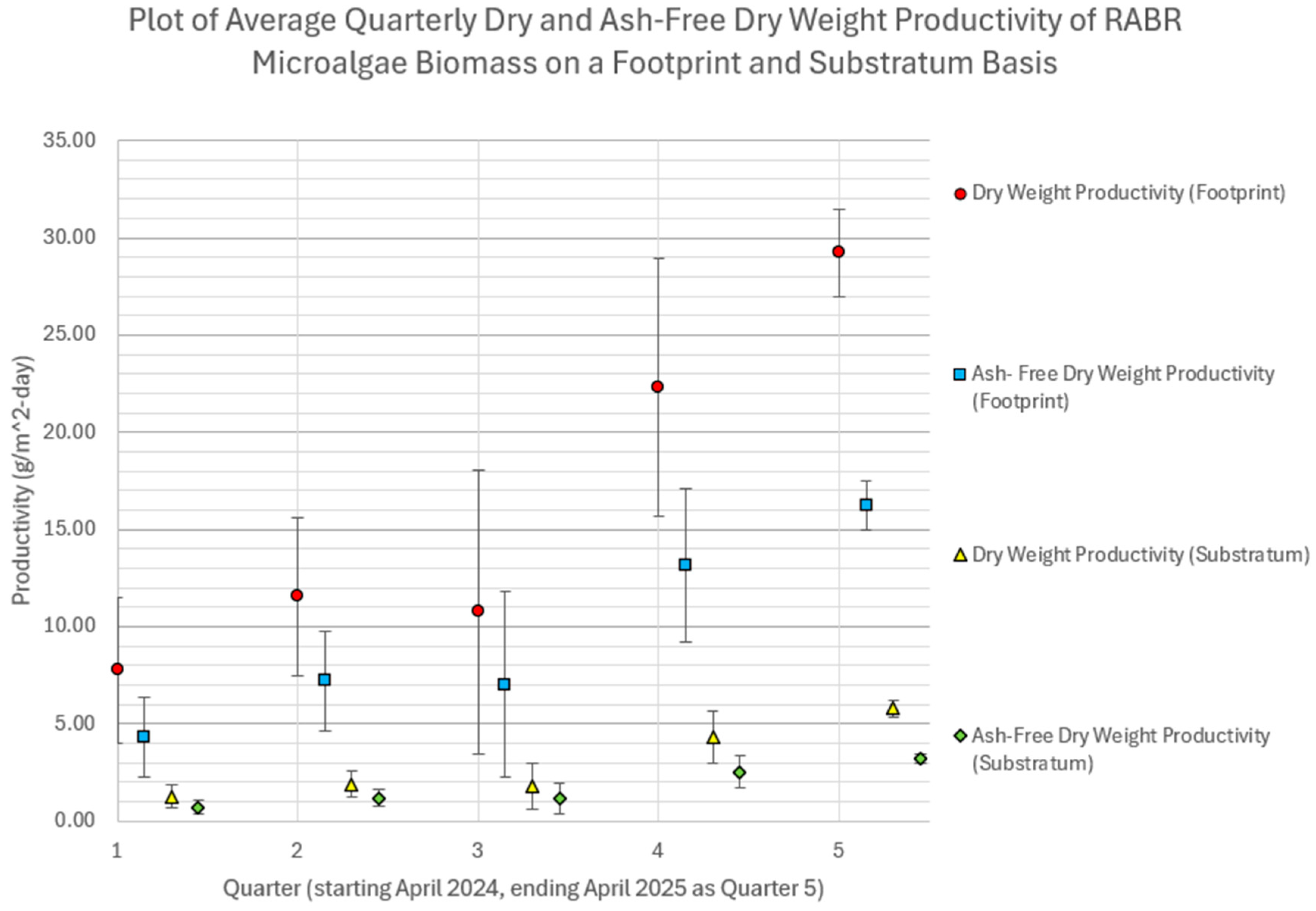
3.4. Discussion of Results
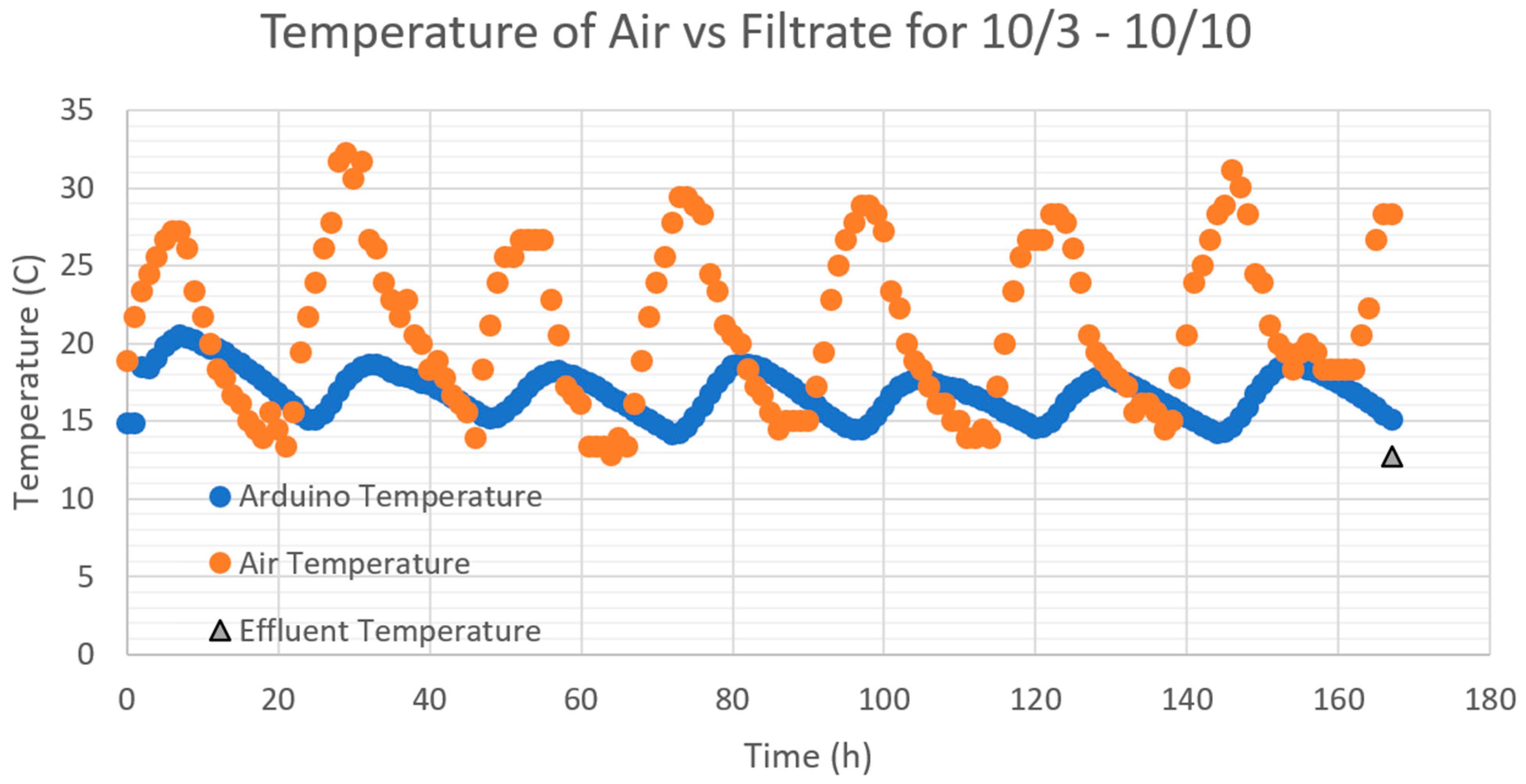
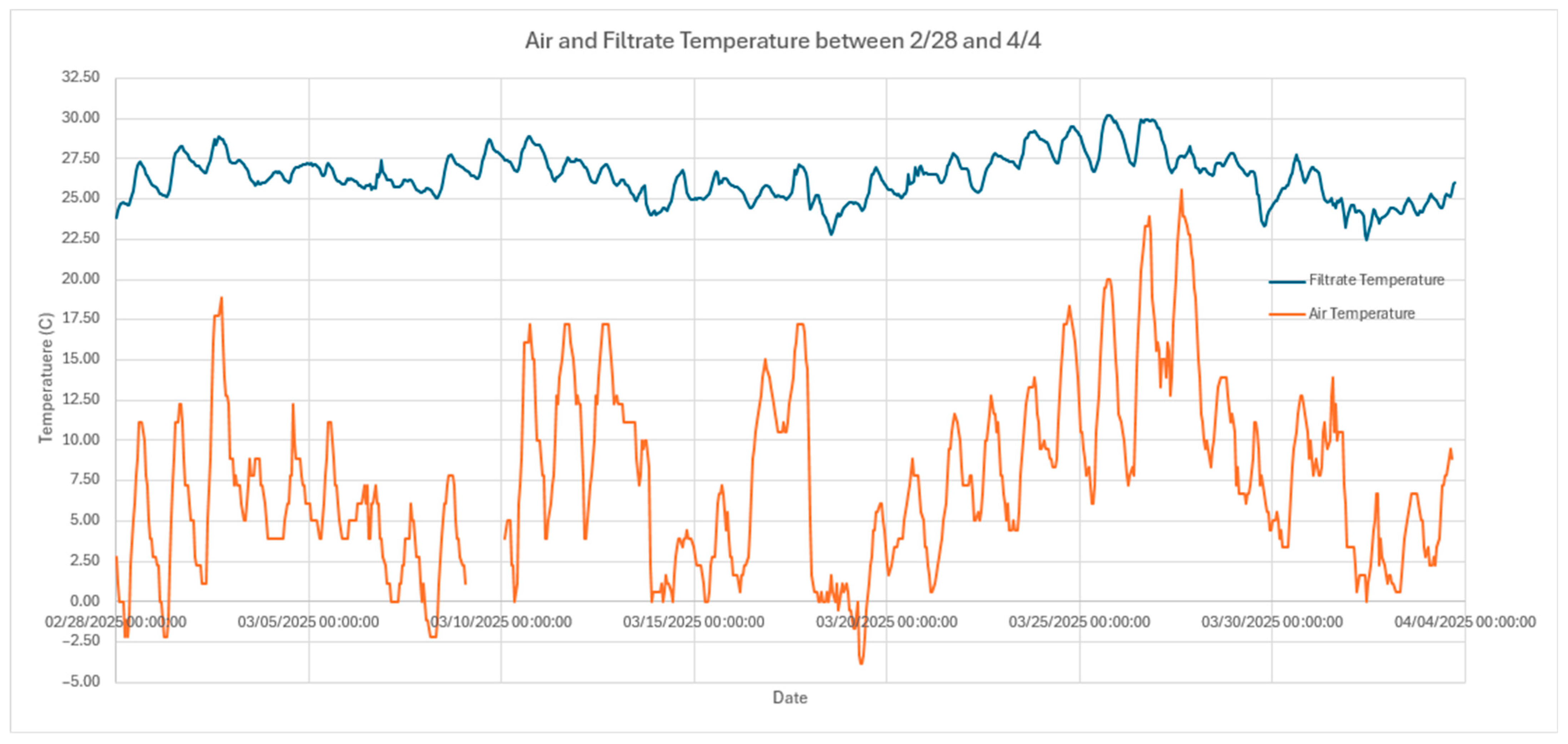
3.5. Temperature
3.6. Section Summary
4. Conclusions and Future Work
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFDW | Ash-Free Dry Weight |
| BETO | Bioenergy Technology Office |
| CVWRF | Central Valley Water Reclamation Facility |
| DLI | Daily Light Integral |
| DW | Dry Weight |
| EERE | Energy Efficiency and Renewable Energy |
| Filtrate | Filtrate from dewatering anaerobically digested biosolids |
| HRT | Hydraulic Retention Time |
| LCA | Life Cycle Analysis |
| MPSP | Minimum Plastic Selling Price |
| Nutrients | Refers to Nitrogen and Phosphorus compounds |
| PPFD | Photosynthetic Photon Flux Density |
| PNNL | Pacific Northwest National Laboratory |
| RABR | Rotating Algal Biofilm Reactor |
| SWBEC | Sustainable Waste to Bioproducts Engineering Center |
| TEA | Techno-Economic Analysis |
| TKN | Total Kjeldahl Nitrogen |
| TP | Total Phosphorus |
| U.S. DOE | United States Department of Energy |
| VFD | Variable Frequency Drive |
| WRRF | Water Resource Recovery Facility |
References
- Water Environment Federation. Algae Biofilm Reactors. In Manual of Practice 35, 2nd ed.; Chapter 6; Boltz, J.P., Sims, R.C., Eds.; Water Environment Federation: Alexandria, VA, USA, 2025; ISBN 978-1-57278-476-5. [Google Scholar]
- Gross, M.; Wen, Z. Yearlong Evaluation of Performance and Durability of a Pilot-Scale Revolving Algal Biofilm (RAB) Cultivation System. Bioresour. Technol. 2014, 171, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Mascarenhas, V.; Wen, Z. Evaluating Algal Growth Performance and Water Use Efficiency of Pilot-Scale Revolving Algal Biofilm (RAB) Culture Systems. Biotechnol. Bioeng. 2015, 112, 2040–2050. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Henry, W.; Michael, C.; Wen, Z. Development of a Rotating Algal Biofilm Growth System for Attached Microalgae Growth with in Situ Biomass Harvest. Bioresour. Technol. 2013, 150, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Sebestyén, P.; Blanken, W.; Bacsa, I.; Tóth, G.; Martin, A.; Bhaiji, T.; Dergez, Á.; Kesserű, P.; Koós, Á.; Kiss, I. Upscale of a Laboratory Rotating Disk Biofilm Reactor and Evaluation of Its Performance over a Half-Year Operation Period in Outdoor Conditions. Algal Res. 2016, 18, 266–272. [Google Scholar] [CrossRef]
- Hillman, K.M.; Sims, R.C. Struvite Formation Associated with the Microalgae Biofilm Matrix of a Rotating Algal Biofilm Reactor (RABR) during Nutrient Removal from Municipal Wastewater. Water Sci. Technol. 2020, 81, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Goldsberry, P.; Jeppesen, P.; McLean, J.; Sims, R. An Attached Microalgae Platform for Recycling Phosphorus Through Biologically Mediated Fertilizer Formation and Biomass Cultivation. Clean. Eng. Technol. 2023, 17, 100701. [Google Scholar] [CrossRef]
- Watkins, J.; Zhu, Y.; Valdez, P.; Lords, C.; Zeller, A.; Bohutskyi, P.; Sims, R.C. Techno-Economic Analysis of Bioplastic and Biofuel Production from a High-Ash Microalgae Biofilm Cultivated in Effluent from a Municipal Anaerobic Digester. Algal Res. 2024, 84, 103774. [Google Scholar] [CrossRef]
- Kim, A.H.; Yu, A.C.; El Abbadi, S.H.; Lu, K.; Chan, D.; Appel, E.A.; Criddle, C.S. More than a Fertilizer: Wastewater-Derived Struvite as a High Value, Sustainable Fire Retardant. Green Chem. 2021, 23, 4510–4523. [Google Scholar] [CrossRef]
- Sandia National Laboratories. Algae Scrubbing for Waterway Remediation and Biofuel Production. 2025. Available online: https://www.sandia.gov/app/uploads/sites/223/2024/01/Algae-Scrubbing-for-Waterway-Remediation-SAND2024-00221M.pdf (accessed on 1 July 2025).
- Moreno Osorio, J.H.; Pollio, A.; Frunzo, L.; Lens, P.N.L.; Esposito, G. A Review of Microalgal Biofilm Technologies: Definition, Applications, Settings and Analysis. Front. Chem. Eng. 2021, 3, 737710. [Google Scholar] [CrossRef]
- Christenson, L.B.; Sims, R.C. Rotating Algal Biofilm Reactor and Spool Harvester for Wastewater Treatment with Biofuels By-Products. Biotechnol. Bioeng. 2012, 109, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.F. Analysis of an Outdoor Pilot-Scale Rotating Algae Biofilm Reactor for Power Optimization, Ash-Enhanced Productivity, and Nutrient Uptake. Master’s Thesis, Utah State University, Logan, UT, USA, 2024. [Google Scholar]
- Funding Opportunity Announcement 0002203 Synergistic Municipal Wastewater Treatment Using a Rotating Algae Biofilm Reactor. BETO Fiscal Year 2020 Multi-Topic Funding Opportunity Announcement-Project Selections. 2020. Available online: https://www.energy.gov/eere/bioenergy/articles/bioenergy-technologies-office-fiscal-year-2020-multi-topic-funding (accessed on 10 June 2020).
- Zhu, Y.; Schmidt, A.; Valdez, P.; Snowden-Swan, L.; Edmundson, S. Hydrothermal Liquefaction and Upgrading of Wastewater-Grown Microalgae: 2021 State of Technology; Pacific Northwest National Laboratory (PNNL): Richland, WA, USA, 2022. [Google Scholar]
- Lemaire, R.; Christensson, M. Lessons Learned from 10 Years of ANITA Mox for Sidestream Treatment. Processes 2021, 9, 863. [Google Scholar] [CrossRef]
- Janssen, M.; Wijffels, R.H.; Barbosa, M.J. Microalgae Based Production of Single-Cell Protein. Curr. Opin. Biotechnol. 2022, 75, 102705. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Bonnefond, H.; Bernard, O. Rotating Algal Biofilm versus Planktonic Cultivation: LCA Perspective. J. Clean. Prod. 2020, 257, 120547. [Google Scholar] [CrossRef]
- Barlow, J.; Sims, R.C.; Quinn, J.C. Techno-Economic and Life-Cycle Assessment of an Attached Growth Algal Biorefinery. Bioresour. Technol. 2016, 220, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Flavia, M.; Sogand, M.; Miriana, A.; Benedetta, D.C.; Paolo, D.F.; Angela, M.; Mauro, M.; Marianna, V. Coupled Biological and Thermochemical Process for Plastic Waste Conversion Into Biopolymers. Chem. Eng. Trans. 2023, 100, 469–474. [Google Scholar] [CrossRef]
- Witarsa, F.; Yarberry, A.; May, P.; Kangas, P.; Lansing, S. Complementing Energy Production with Nutrient Management: Anaerobic Digestion System for Algal Turf Scrubber Biomass. Ecol. Eng. 2020, 143, 105618. [Google Scholar] [CrossRef]
- Holdmann, C.; Schmid-Staiger, U.; Hirth, T. Outdoor Microalgae Cultivation at Different Biomass Concentrations—Assessment of Different Daily and Seasonal Light Scenarios by Modeling. Algal Res. 2019, 38, 101405. [Google Scholar] [CrossRef]




| Quarter 1: Apr–Jun 2024 | Quarter 2: Jul–Sep 2024 | Quarter 3: Oct–Dec 2024 | Quarter 4: Jan–Mar 2025 | Final Month: Apr 2025 | |
|---|---|---|---|---|---|
| Footprint Productivity (g/m2/day) | 7.8 ± 3.7 4.3 ± 2.1 (ash-free) | 11.5 ± 4.1 7.2 ± 2.6 (ash-free) | 10.8 ± 7.3 7.2 ± 2.6 (ash-free) | 22.3 ± 6.64 13.2 ± 3.9 (ash-free) | 29.2 ± 2.3 16.3 ± 1.3 (ash-free) |
| Substratum Productivity (g/m2/day) | 1.3 ± 0.6 0.7 ± 0.3 (ash-free) | 1.9 ± 0.7 1.2 ± 0.4 (ash-free) | 1.8 ± 1.2 1.2 ± 0.8 (ash-free) | 4.3 ± 1.4 2.5 ± 0.8 (ash-free) | 5.8 ± 0.4 3.2 ± 0.3 (ash-free) |
| Instantaneous Power Draw (W) | No Data c | No Data c | 49.9 ± 1.4 | 90.8 ± 2.4 | 92.6 ± 1.3 |
| Average Set Flow Rate (L/min) | 3.6 ± 0.8 a | 4.3 ± 0.6 a | 4.3 ± 0.8 a | 17.7 b | 17.7 b |
| Optimal Power Consumption for N (kWh/kg N removed) | No Data c | No Data c | 13.64 ± 33.75 | 19.11 ± 13.51 | 14.89 ± 20.28 |
| Optimal Power Consumption for P (kWh/kg P removed) | No Data c | No Data c | 130.92 ± 129.74 | 63.82 ± 63.66 | 58.52 ± 16.98 |
| Biomass Power Consumption (kWh/kg dry mass) | No Data c | No Data c | 13.45 ± 6.77 20.65 ± 10.40 (ash-free) d | 9.73 ± 5.02 16.51 ± 8.51 (ash-free) | 6.65 ± 0.51 11.96 ± 0.92 (ash-free) |
| Effluent pH | 8.12 ± 0.18 | 7.88 ± 0.38 | 8.16 ± 0.24 | 8.59 ± 0.13 | 8.51 ± 0.14 |
| Biofilm pH | 7.75 ± 0.28 | 7.53 ± 0.38 | 7.95 ± 0.55 | 8.54 ± 0.15 | 8.27 ± 0.09 |
| Organic Percentage | 55.66% ± 3.90% | 62.67% ± 7.02% | 65.15% ± 4.35% | 58.92% ± 3.26% | 55.66% ± 5.08% |
| Percent Ash | 44.34% ± 3.90% | 37.33% ± 7.02% | 34.85% ± 4.35% | 41.08% ± 3.26% | 44.34% ± 5.08% |
| Biomass Light Footprint Productivity (g/mol photons) | 2.7 ± 1.6 1.5 ± 0.89 (ash-free) | 3.5 ± 1.4 2.1 ± 0.88 (ash-free) | 8.0 ± 6.3 d 5.2 ± 4.2 (ash-free) | No Data c | 13.0 ± 6.0 7.2 ± 3.4 (ash-free) |
| Average Photoperiod (h/day) | 15.32 | 14.12 | 11.1 | No Data c | 13.7 |
| Duty Cycle | 25% | 25% (50% in Sep) | 50% | 100% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haag, D.R.; Heck, P.E.; Sims, R.C. Analysis of Modifications to an Outdoor Field-Scale Rotating Algal Biofilm Reactor with a Focus on Biomass Productivity and Power Usage. Bioresour. Bioprod. 2025, 1, 4. https://doi.org/10.3390/bioresourbioprod1010004
Haag DR, Heck PE, Sims RC. Analysis of Modifications to an Outdoor Field-Scale Rotating Algal Biofilm Reactor with a Focus on Biomass Productivity and Power Usage. Bioresources and Bioproducts. 2025; 1(1):4. https://doi.org/10.3390/bioresourbioprod1010004
Chicago/Turabian StyleHaag, Davis R., Phillip E. Heck, and Ronald C. Sims. 2025. "Analysis of Modifications to an Outdoor Field-Scale Rotating Algal Biofilm Reactor with a Focus on Biomass Productivity and Power Usage" Bioresources and Bioproducts 1, no. 1: 4. https://doi.org/10.3390/bioresourbioprod1010004
APA StyleHaag, D. R., Heck, P. E., & Sims, R. C. (2025). Analysis of Modifications to an Outdoor Field-Scale Rotating Algal Biofilm Reactor with a Focus on Biomass Productivity and Power Usage. Bioresources and Bioproducts, 1(1), 4. https://doi.org/10.3390/bioresourbioprod1010004






