Dependence of the Molecular Interactions Between Cyanoacrylate and Native Copper Surfaces on the Process Atmosphere
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Characterization Techniques
2.2.1. Atomic Force Microscopy (AFM)
2.2.2. Raman Microscopy
2.2.3. X-Ray Photoelectron Spectroscopy (XPS)
2.2.4. Infrared Reflection Adsorption Spectroscopy (IRRAS)
3. Results and Discussion
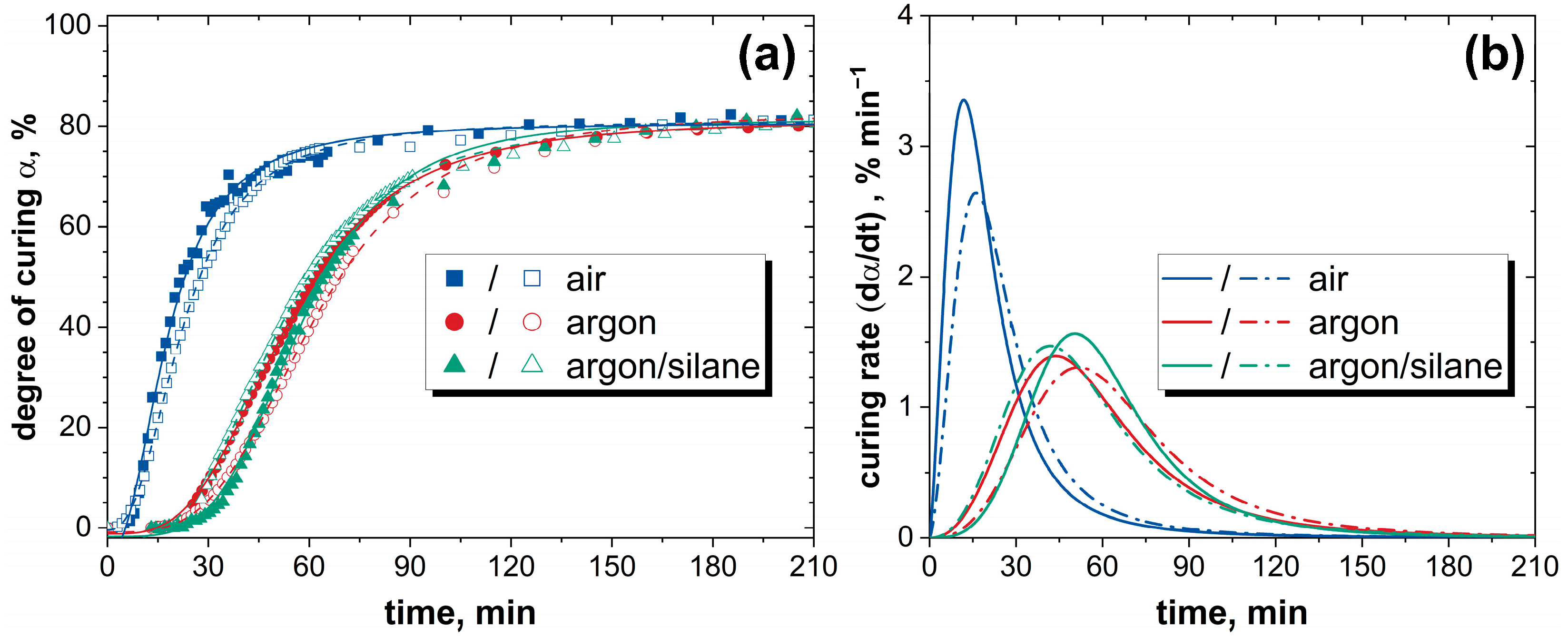
3.1. Influence of the Atmosphere on Curing
3.2. Topography of Thin Cyanoacrylate Films
3.3. Spectroscopic Analysis of the Interface
- The binding energy of the O-C=O species shifts to higher values with decreasing PECA film thickness or concentration. Regardless of the surrounding process atmosphere, there is a shift of approximately 0.2 to 0.3 eV, which is indicated by a slanted dashed line in Figure 5. The higher chemical shift indicates a molecular interaction. The effect can be attributed to hydrogen bonding between the carbonyl oxygen and OH groups on the surface, which is also observed in other studies for comparable polymer/metal oxide interfaces [5,16,25,26]. The change from air to an argon or argon/silane atmosphere presumably does not change the OH groups present on the surface of the oxidized copper, so that the hydrogen bonds can form independently of the process atmosphere.
- As the concentration decreases, there is a change in the structure of the C 1s signal, which indicates a change in the functional groups or an interaction. The dip in the peak in the range between 288.3 and 288.5 eV is clearly visible in the case of larger film thicknesses or concentrations, in both of the atmospheres shown. In the case of argon, this structure remains approximately the same, even at low film thicknesses. In the measurements in the argon/silane atmosphere, this dip is filled in, and a plateau tends to form. This indicates that an additional binding species is formed in this energy range. Comparable interface studies assign a carboxylate ion (COO-) here, which leads to a stronger ionic interaction between the cyanoacrylate and the oxidized metal surface [5,25,26,27,28]. In an earlier study, a model was established to show how this ionic interaction can develop under the influence of moisture [5]: after forming the hydrogen bonds, the acrylate group (-O-C=O) is hydrolyzed by water, so that, in the case of ethyl cyanoacrylate, a carboxyl group (COOH) is formed with the elimination of ethanol. This carboxyl group is then deprotonated by further influence of water, so that a carboxylate ion (COO-) is formed, which can then undergo a strong ionic interaction with the surface. There is an interaction between the negative ion of the polymer and a positive ion on the metal (oxide) surface. A more detailed description of this mechanism and a graphical representation of the process can be found in the previous study mentioned above [5]. The emergence of carboxylate ions at polymer/oxide interfaces has also been described by Pletincx et al. and Fockaert et al. for other systems [26,27,29].
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klemarczyk, P.; Guthrie, J. Advances in Anaerobic and Cyanoacrylate Adhesives. In Advances in Structural Adhesive Bonding; Elsevier: Amsterdam, The Netherlands, 2010; pp. 96–131. ISBN 978-1-84569-435-7. [Google Scholar]
- Korde, J.M.; Kandasubramanian, B. Biocompatible Alkyl Cyanoacrylates and Their Derivatives as Bio-Adhesives. Biomater. Sci. 2018, 6, 1691–1711. [Google Scholar] [CrossRef]
- Bhat, Y.M.; Banerjee, S.; Barth, B.A.; Chauhan, S.S.; Gottlieb, K.T.; Konda, V.; Maple, J.T.; Murad, F.M.; Pfau, P.R.; Pleskow, D.K.; et al. Tissue Adhesives: Cyanoacrylate Glue and Fibrin Sealant. Gastrointest. Endosc. 2013, 78, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Pantelakis, S.; Tserpes, K.I. Adhesive Bonding of Composite Aircraft Structures: Challenges and Recent Developments. Sci China Phys. Mech. Astron. 2014, 57, 2–11. [Google Scholar] [CrossRef]
- Moritz, P.; Höfft, O.; Wegewitz, L.; Maus-Friedrichs, W. Interaction of Cyanoacrylate with Metal Oxide Surfaces (Cu, Al). ChemPhysChem 2023, 24, e202300076. [Google Scholar] [CrossRef]
- Fifo, O.; Ryan, K.; Basu, B. Investigations on the Effectiveness of Cyanoacrylate Adhesive for Repair of Fibre-Reinforced Polymer Laminates under Different Environmental Conditions. J. Compos. Mater. 2014, 48, 2657–2668. [Google Scholar] [CrossRef]
- Drain, K.F.; Guthrie, J.; Leung, C.L.; Martin, F.R.; Otterburn, M.S. The Effect of Moisture on the Strength of Steel-Steel Cyanoacrylate Adhesive Bonds. J. Adhes. 1984, 17, 71–81. [Google Scholar] [CrossRef]
- Estan-Cerezo, G.; Alonso, D.A.; Martín-Martínez, J.M. Structural and Adhesion Properties of Poly(Ethyl 2-Cyanoacrylate) Post-Cured at Different Temperatures and Times. J. Adhes. Sci. Technol. 2019, 33, 329–345. [Google Scholar] [CrossRef]
- Hartshorn, S.R. Structural Adhesives: Chemistry and Technology, 1st ed.; Topics in Applied Chemistry Ser; Springer: New York, NY, USA, 1986; ISBN 978-1-4684-7781-8. [Google Scholar]
- Habenicht, G. Kleben: Grundlagen, Technologien, Anwendungen, 6th ed.; VDI-Buch; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-85266-7. [Google Scholar]
- Paine, M.; Bandey, H.L.; Bleay, S.M.; Willson, H. The Effect of Relative Humidity on the Effectiveness of the Cyanoacrylate Fuming Process for Fingermark Development and on the Microstructure of the Developed Marks. Forensic Sci. Int. 2011, 212, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, K.J.; Fraser, J.; Friel, L.; Adams, D.; Attard-Montalto, N.; Deacon, P. A Comparison between Atmospheric/Humidity and Vacuum Cyanoacrylate Fuming of Latent Fingermarks. Forensic Sci. Int. 2015, 257, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Moritz, P.; Gerland, S.; Wegewitz, L.; Raatz, A.; Maus-Friedrichs, W. Influence of Different Processing Atmospheres on Curing and Adhesive Strength of Cyanoacrylates. Int. J. Adhes. Adhes. 2023, 124, 103386. [Google Scholar] [CrossRef]
- Udachin, V.; Wegewitz, L.; Dahle, S.; Maus-Friedrichs, W. Reduction of Copper Surface Oxide Using a Sub-Atmospheric Dielectric Barrier Discharge Plasma. Appl. Surf. Sci. 2022, 573, 151568. [Google Scholar] [CrossRef]
- Wegewitz, L.; Maus-Friedrichs, W.; Gustus, R.; Maier, H.J.; Herbst, S. Oxygen-Free Production—From Vision to Application. Adv. Eng. Mater. 2023, 25, 2201819. [Google Scholar] [CrossRef]
- Moritz, P.; Bürger, F.; Höfft, O.; Wegewitz, L.; Maus-Friedrichs, W. Bonding Mechanism of Cyanoacrylates on SiO2 and Au: Spectroscopic Studies of the Interface. J. Phys. Chem. C 2021, 125, 23409–23417. [Google Scholar] [CrossRef]
- Moritz, P.; Bürger, F.; Wegewitz, L.; Maus-Friedrichs, W. Preparation Techniques of Thin Cyanoacrylate Adhesive Films for Interface Analysis. J. Adhes. 2022, 98, 963–978. [Google Scholar] [CrossRef]
- Powell, C.J.; Jablonski, A. Progress in Quantitative Surface Analysis by X-Ray Photoelectron Spectroscopy: Current Status and Perspectives. J. Electron Spectrosc. Relat. Phenom. 2010, 178–179, 331–346. [Google Scholar] [CrossRef]
- Powell, C.J.; Jablonski, A. NIST Electron Inelastic-Mean-Free-Path Database, Version 1.2; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Li, Y.; Xiao, L.; Wang, Z.; Chen, K.; Xue, C.; Yu, M.; Wang, Y.; Kong, F.; Liu, K.; Qin, K. Raman Spectroscopic Characterization of Polymerization Kinetics of Cyanoacrylate Embolic Glues for Vascular Embolization. Polymers 2021, 13, 3362. [Google Scholar] [CrossRef]
- Raheem, K.; Cassidy, J.; Betts, A.; Ryan, B. Use of Confocal Raman Microscopy to Characterise Ethyl Cyanoacrylate Adhesive Depth Curing. Phys. Chem. Chem. Phys. 2020, 22, 23899–23907. [Google Scholar] [CrossRef]
- Dillman, B.; Jessop, J.L.P. Chain Transfer Agents in Cationic Photopolymerization of a Bis-cycloaliphatic Epoxide Monomer: Kinetic and Physical Property Effects. J. Polym. Sci. A Polym. Chem. 2013, 51, 2058–2067. [Google Scholar] [CrossRef]
- Schmitt, M.; Schulze-Pillot, R.; Hempelmann, R. Kinetics of Bulk Polymerisation and Gompertz’s Law. Phys. Chem. Chem. Phys. 2011, 13, 690–695. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Day, J.S. Fourier Transform Raman Spectroscopic Studies of the Curing of Cyanoacrylate Glue. J. Raman Spectrosc. 2004, 35, 555–560. [Google Scholar] [CrossRef]
- Cavezza, F.; Pletincx, S.; Revilla, R.I.; Weaytens, J.; Boehm, M.; Terryn, H.; Hauffman, T. Probing the Metal Oxide/Polymer Molecular Hybrid Interfaces with Nanoscale Resolution Using AFM-IR. J. Phys. Chem. C 2019, 123, 26178–26184. [Google Scholar] [CrossRef]
- Pletincx, S.; Marcoen, K.; Trotochaud, L.; Fockaert, L.-L.; Mol, J.M.C.; Head, A.R.; Karslioğlu, O.; Bluhm, H.; Terryn, H.; Hauffman, T. Unravelling the Chemical Influence of Water on the PMMA/Aluminum Oxide Hybrid Interface in Situ. Sci. Rep. 2017, 7, 13341. [Google Scholar] [CrossRef] [PubMed]
- Pletincx, S.; Trotochaud, L.; Fockaert, L.-L.; Mol, J.M.C.; Head, A.R.; Karslıoğlu, O.; Bluhm, H.; Terryn, H.; Hauffman, T. In Situ Characterization of the Initial Effect of Water on Molecular Interactions at the Interface of Organic/Inorganic Hybrid Systems. Sci. Rep. 2017, 7, 45123. [Google Scholar] [CrossRef] [PubMed]
- Pletincx, S.; Fockaert, L.-L.I.; Meeusen, M.; Mol, J.M.C.; Terryn, H.; Hauffman, T. In Situ Methanol Adsorption on Aluminum Oxide Monitored by a Combined ORP-EIS and ATR-FTIR Kretschmann Setup. J. Phys. Chem. C 2018, 122, 21963–21973. [Google Scholar] [CrossRef]
- Fockaert, L.-L.I.; Ganzinga-Jurg, D.; Versluis, J.; Boelen, B.; Bakker, H.J.; Terryn, H.; Mol, J.M.C. Studying Chemisorption at Metal–Polymer Interfaces by Complementary Use of Attenuated Total Reflection–Fourier Transform Infrared Spectroscopy (ATR-FTIR) in the Kretschmann Geometry and Visible–Infrared Sum-Frequency Generation Spectroscopy (SFG). J. Phys. Chem. C 2020, 124, 7127–7138. [Google Scholar] [CrossRef]
- Vouvoudi, E.C.; Morfis, P.D.; Verros, G.D.; Achilias, D.S. Polymerisation Kinetics on FT-IR and Colorimetric Changes under UV Irradiation for a Commercial Polycyanoacrylate Adhesive, Addressed to Glass Restoration. Coatings 2022, 12, 490. [Google Scholar] [CrossRef]
- Sain, S.; Ray, D.; Mukhopadhyay, A.; Sengupta, S.; Kar, T.; Ennis, C.J.; Rahman, P.K.S.M. Synthesis and Characterization of PMMA-cellulose Nanocomposites by in Situ Polymerization Technique. J. Appl. Polym. Sci 2012, 126 (Suppl. S1), E127–E134. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Chichester, UK, 2010; ISBN 978-0-470-09307-8. [Google Scholar]
- Suffner, J.; Schechner, G.; Sieger, H.; Hahn, H. In-Situ Coating of Silica Nanoparticles with Acrylate-Based Polymers. Chem. Vap. Depos. 2007, 13, 459–464. [Google Scholar] [CrossRef]
- Burunkova, J.A.; Denisyuk, I.Y.; Arefieva, N.N.; Semina, S.A. Influence of SiO2 Nanoaddition on the Self-Organization via UV-Polymerization of Acrylate Nanocomposites. Mol. Cryst. Liq. Cryst. 2011, 536, 242–248. [Google Scholar] [CrossRef]
- Szafarska, M.; Olszok, V.; Holländer, U.; Gustus, R.; Weber, A.P.; Maus-Friedrichs, W. Gas Phase Reaction of Silane with Water at Different Temperatures and Supported by Plasma. ACS Omega 2023, 8, 8388–8396. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moritz, P.; Wegewitz, L.; Maus-Friedrichs, W. Dependence of the Molecular Interactions Between Cyanoacrylate and Native Copper Surfaces on the Process Atmosphere. Adhesives 2025, 1, 1. https://doi.org/10.3390/adhesives1010001
Moritz P, Wegewitz L, Maus-Friedrichs W. Dependence of the Molecular Interactions Between Cyanoacrylate and Native Copper Surfaces on the Process Atmosphere. Adhesives. 2025; 1(1):1. https://doi.org/10.3390/adhesives1010001
Chicago/Turabian StyleMoritz, Philipp, Lienhard Wegewitz, and Wolfgang Maus-Friedrichs. 2025. "Dependence of the Molecular Interactions Between Cyanoacrylate and Native Copper Surfaces on the Process Atmosphere" Adhesives 1, no. 1: 1. https://doi.org/10.3390/adhesives1010001
APA StyleMoritz, P., Wegewitz, L., & Maus-Friedrichs, W. (2025). Dependence of the Molecular Interactions Between Cyanoacrylate and Native Copper Surfaces on the Process Atmosphere. Adhesives, 1(1), 1. https://doi.org/10.3390/adhesives1010001





