Simple Summary
Around the world, lichens remain one of the least documented groups of macroogranisms. Many to most places worldwide have never had a formal lichen inventory, and this includes the southern Rocky Mountains of Colorado. Despite the incredible topographic relief and ecological heterogeneity present within Colorado’s borders, expected to harbor a uniquely rich and varied lichen biota, the lichens of the state have never been studied. Here, we describe a new, asexually reproducing lichen species from the cosmopolitan genus Ochrolechia from the Front Range Mountains of Colorado, and then provide a key to help identify all described asexually reproducing species of the genus in western North America. This new species is tentatively ranked as Critically Endangered per IUCN guidelines. Future fieldwork to inventory the lichens of Colorado by ourselves and colleagues, will, we predict, uncover new discoveries of additional populations of this species and other novelties.
Abstract
Ochrolechia is a diverse and charismatic lineage of both sexually and asexually reproducing lichens, with centers of species richness in northern temperate areas of the world, including North America. As part of recent work to comprehensively inventory the lichens of the Indian Peaks Wilderness (Arapaho–Roosevelt National Forest, Front Range Mountains, Colorado), we discovered material of a sorediate member of the genus to which no existing names could be applied. This material was collected in very remote, extremely difficult-to-access mid-montane forests of the west slope of the Indian Peaks Wilderness, in a steep and jagged off-trail drainage (Hell Canyon). Subsequent study of this material along with review of pre-existing collections at the COLO Herbarium revealed it to represent a new scientific species. We here formally describe Ochrolechia raynori, in honor of Seth Raynor who led the Indian Peaks Wilderness lichen inventory. We additionally document the occurrence of Dactylospora parasitica on this new lichen species. Ochrolechia raynori is distinctive for its continuous, smooth, shiny thallus that bears discrete soralia and coarse soredia, its occurrence on mosses and other lichens that overgrow rocks, and its chemistry. We generated a molecular phylogeny of this and other members of Ochrolechia using the nrITS locus and show O. raynori to be sister to the widespread, sexually reproducing species O. upsaliensis. This occurrence of an asexual species that is sister to a sexual species is consistent with the “species pair” hypothesis in lichenology, which suggests an intimate role of reproductive mode divergence in the process of speciation. Examination of the phylogeny yielded evidence of four additional pairs in Ochrolechia, for a total of five species pairs, which indicates that this phenomenon may be a common occurrence in this lineage. IUCN Conservation Assessment of Ochrolechia raynori revealed the species to be best considered as Critically Endangered. However, we expect that continued efforts to inventory the lichens of the southern Rocky Mountains, especially in some of its wildest, most remote regions in similar habitats, will likely result in the discovery of additional populations of this remarkable new species.
1. Introduction
Ochrolechia A. Massal. (Pertusariales M. Choisy ex D. Hawksw. & O.E. Erikss.) is a charismatic genus of crustose lichens consisting of >60 species worldwide, with centers of diversity including temperate regions of North America, Europe, and China [1,2,3,4]. The genus is characterized by a crustose thallus that is most commonly continuous to areolate, a trebouxioid photobiont (but see [5]), typically large and showy apothecia (when fertile), I+ amyloid asci (and hymenium), branched paraphyses, and simple, hyaline, typically large spores [6,7,8].
As currently understood, North America is home to ~38 species of Ochrolechia [1,6,9,10]. Of these, slightly over half reproduce primarily through sexual means (i.e., apothecia, meiotic ascospores) and the remainder, slightly under half, reproduce primarily through lichenized asexual reproductive propagules (e.g., soredia, isidia) which are products of mitosis and co-disperse the fungal, algal, and additional lichen constituents together ([11], Watts et al., ms in prep.). This remarkable relative balance of reproductive modes is unusual for many lineages of lichens, wherein sexual reproduction is much more of a dominant mode compared to asexual means (Watts et al., ms in review). Among these asexual species, chemistry is both diverse and taxonomically important, with frequently produced compounds including orcinol depsides, orcinol depsidones, β-orcinol depsides, fatty acids, and xanthones [3,6,12].
The common occurrence of both sexually and asexually reproducing species in Ochrolechia makes the lineage a natural system in which to test hypotheses of the evolution of “species pairs” ([11] and references therein), wherein sister species or closely related species are divergent in reproductive mode, with one member of the pair reproducing primarily through sexual means and the other reproducing primarily through asexual means. Such species pairs may in fact represent common occurrences in the genus, such as in the present new species and its closest relative.
The last taxonomic revision of Ochrolechia in North America was by Howard [1], followed by a revision of the corticolous species by Brodo [6]. In this study, we document and describe a new species of Ochrolechia to science from the southern Rocky Mountains of Colorado, then place this new diversity in the context of now 19 known asexually reproducing species in the genus in western North America through molecular phylogenetic analyses and a dichotomous key. This new species was discovered during one grueling field day by the first author together with Seth Raynor, who spearheaded an inventory of the lichens of the Indian Peaks Wilderness (Arapaho-Roosevelt National Forest, Front Range Mountains, Colorado; Raynor & Manzitto-Tripp, ms in revision). The asexually reproducing Ochrolechia raynori is here shown to be sister to the widespread, sexually reproducing species O. upsaliensis, and we hypothesize a “species pair” evolutionary divergence event between the two. We explore the remainder of the phylogeny for evidence of additional species pairs and find instances of four others, for a total of five species pairs. Finally, we document the occurrence of the lichenicolous species Dactylospora parasitica (Flörke) Arnold on this new species, representing its first known occurrence on the genus Ochrolechia and in the state of Colorado to our knowledge.
2. Material and Methods
Field Work. Throughout the summers of 2020–2023, the first author and second author joined colleague Seth Raynor for several field trips throughout the Indian Peaks Wilderness (IPW) of Arapaho–Roosevelt National Forest. This expansive and topographically complex region forms the basis for much of the skyline that lines the Colorado Northern Front Range, ranging in elevation from a low point of ~8400 ft. to a high point of ~13,500 ft. (North Arapaho Peak). During and after this time, collections to comprehensively document the lichen biota of Colorado have been made by all three individuals, throughout the state of Colorado, but with a second emphasis on the lichens of Boulder’s Open Space & Mountain Parks (Watts et al., ms in prep.).
Given a paucity of lichen collections from the west slope of the Continental Divide in the IPW, Raynor and the first author carried out one specific field trip on the west slope (Grand County) to inventory the middle montane habitat. We chose to target an extremely remote and rugged, difficult-to-navigate mesic canyon named Hell Canyon owing to its sheltered and shaded aspect. The canyon itself was so steep and narrow that the lowermost portions likely never receive direct sunlight, and were unlikely to have been surveyed by prior botanists. We sampled all lichen growth forms (i.e., foliose, fruticose, crustose, squamulose, leprose) and all substrates suitable for lichen growth (e.g., rock, bark, lignum, soil, bryophytes, sap, other lichens). Geographical locations of collections were recorded with an iPhone 14 Pro (Apple Inc., Cupertino, CA, USA), along with habitat characteristics. These locations were delimited opportunistically, i.e., based on habitat and biota that appeared interesting to the collectors. On the subsequent day, fieldwork, collections were pre-processed, i.e., stabilized (glued where necessary), then placed in temporary packets to prepare for final identifications (see below). Subsamples were removed for DNA sequencing then stored under refrigeration until returned to freezers in Manzitto-Tripp’s molecular laboratory at The University of Colorado Boulder.
2.1. Herbarium Study
Final identifications of all lichen collections were made at the University of Colorado, Museum of Natural History, COLO Herbarium [13] using an Olympus SZX16 and Olympus BX51 coupled to an Olympus EP50 camera. Samples were hand-sectioned then examined in water mounts along with other media. Spot tests using standard reagents (K, C, KC, P, N) along with UV illumination were conducted at COLO. Additionally, Thin Layer Chromatography and/or DNA sequencing was conducted by the authors at COLO (in Manzitto-Tripp’s molecular lab) on specimens to help confirm their identifications. Thin Layer Chromatography (TLC) utilized Solvent C and silica plates following Culberson & Kristinsson [14] and Culberson [15]. In addition to studying our own newly collected materials, we examined relevant material (i.e., all undetermined material already accessioned under Ochrolechia spp., n = 24 specimens) housed at COLO using similar methods to the above, providing updated identification annotations where necessary. All collections cited below were verified by the authors but, nonetheless, we include a “!” following any of the studied specimen citations, unless in reference to a type specimen housed elsewhere and only photographs were seen (in which case “photo!” is utilized). We referenced the identification of our collections using published literature, floras, field guides, existing herbarium material, and the Consortium of Lichen Herbaria (CLH, Lichen Portal; https://lichenportal.org/portal/, accessed 10 July 2025). Our taxonomy follows [9]. Data and specimens reported in this study are deposited at COLO and data were uploaded to COLO’s database, which is hosted on CLH.
2.2. Molecular Analyses
To place the new species in a molecular genetic context, we generated data from the nuclear ribosomal Internal Transcribed Spacer (nrITS) “barcoding” locus of the new species from the holotype (E. Manzitto-Tripp & S. Raynor 9795) and other members of the lineage (Pertusariales). Genomic DNA was extracted using a modified CTAB protocol [16]. We amplified the nrITS region (including 5.8 s) using ITS4 and ITS5 primers [17]. Resultant successful PCR products were sent to Quintara Biosciences (Cambridge, MA, USA) for product cleanup and bidirectional Sanger Sequencing. We generated a DNA alignment using our newly generated sequences together with pre-existing nrITS data retrieved from GenBank (GenBank numbers are provided in names of taxa/terminals in the phylogeny) and guided by existing literature, including outgroup selection. The final matrix consisted of 96 terminals.
Phylogenetic relationships within Pertusarales were constructed using Maximum Likelihood methods implemented in raxml 2.0 [18] and an ML + transfer bootstrap + consensus algorithm, with a GTR + FO + I + GA model of sequence evolution and 100 bootstrap replicates. We generated a 50% majority rule consensus tree from each matrix then visualized results using FigTree v1.4.4 [19]. We interpreted bootstrap support (BS) values indicative of support for a given phylogenetic relationship as follows: ≥90% = strong support, ≥80–89% = moderate support, ≥70–79% = weak support, and <70% = unsupported. Taxon identification, GenBank number, and collection locations for each specimen included in the phylogeny were displayed at branch tips.
2.3. Conservation Assessments
The conservation status of the new species was assessed following the International Union for Conservation of Nature (IUCN). Given a lack of useful historical data on population sizes and trends through time, which are necessary for criteria A, B, C, and E, we opt to utilize criterion D of the IUCN guidelines version 16 [20]. Under IUCN criterion D, species are classified as Critically Endangered (CR) or Endangered (E) if the total estimated population size is less than 50 individuals or between 51 and 250, respectively. We consider one mature individual as any 1 m2 surface of rock containing an individual of the focal species, following practices recommended by a recent review about conducting conservation assessments of lichens [21].
3. Results and Discussion
That challenging day in Hell Canyon yielded material of a highly unique species of Ochrolechia that we were unable to identify using pre-existing names. As such, Ochrolechia raynori (Figure 1) is formally described below. The west slope of the IPW proved to be exceptionally rich (Figure 2), yielding at least one other new lichen species (Polycauliona pancakeana E. Tripp, Raynor, & J. Watts [22], the holotype of which derived approximately 0.5 miles from the Ochrolechia collection in the primary drainage into which Hell Canyon feeds (Buchanan Creek, Figure 2). Indeed, although we are in only the early phases of our long-term project to comprehensively inventory Colorado’s lichens, the initial discoveries of new species and new records have exceeded our expectations [22,23,24,25,26,27,28,29,30,31,32,33]. As such, it seems reasonable to hypothesize that a steep species accumulation curve will easily characterize the first 5–10 years of our work here.

Figure 1.
(A). Macromorphology of Ochrolechia raynori. (A): Field photograph showing overall gestalt. (B,C): Thallus infected by Dactylospora parasitica. (D): Continuous, smoothy, shiny thallus overgrowing mosses with discrete, almost “pustulose” soralia bearing coarse soredia. (E). Thallus bearing discrete soralia. (F). Muscicolous habit and lichenicolous Dactylospora parasitica. Scale bars: (A) = 10 mm; (B) = 1 mm; (C) = 2 mm; (D) = 0.5 mm; (E) = 0.5 mm; (F) = 1 mm. Photos taken from the holotype: E. Manzitto-Tripp & S. Raynor 9795.

Figure 2.
Habitat, locus classicus, Hell Canyon, Indian Peaks Wilderness, Arapaho–Roosevelt National Forest, Front Range Mountains, Colorado, USA. (A). Seth Raynor and field companion (Pancake) approaching bottom of Hell Canyon. (B). Middle portions of Hell Canyon showing rich and mesic, streamside habitat. (C). Lower portions of Hell Canyon similarly showing rich and mesic, streamside habitat. (D). Buchanan Creek (~1 mile south of type locality) into which Hell Canyon drains.
Phylogenetic analyses resolved our new sorediate Ochrolechia raynori as sister to the widespread and sexually reproducing (“fertile”) species O. upsaliensis with strong support (100% BS; Figure 3). Indeed, O. raynori shares much of its secondary chemistry with O. upsaliensis, as discussed below (Part 1). The “species pair” sister relationship of these two taxa is similarly reflected in four other species-pair groups (for a total of five) reconstructed by our phylogenetic analysis: the isidiate O. yasudae as sister to the fertile O. xanthostoma (74% BS), the sorediate O. gowardii as sister to the fertile O. juvenalis (100% BS), the sorediate O. mahluensis as sister to the fertile O. frigida + O. tartarea. (99% BS), and the sorediate O. bahusiensis as sister to the fertile O. oregonensis (91% BS). Further phylogenetic analyses, with more complete sampling, will likely recover additional fertile-sterile species pairs in Ochrolechia. Wee recognize that the inference of sister relationships is highly susceptible to taxon sampling (hence, no formal character reconstruction analyses were conducted here given the limited nature of our sampling). Nonetheless, together, these five species pairs yield evidence supporting the hypothesis that reproductive mode transitions may be a common occurrence during speciation within the genus Ochrolechia (see [11] and references therein).
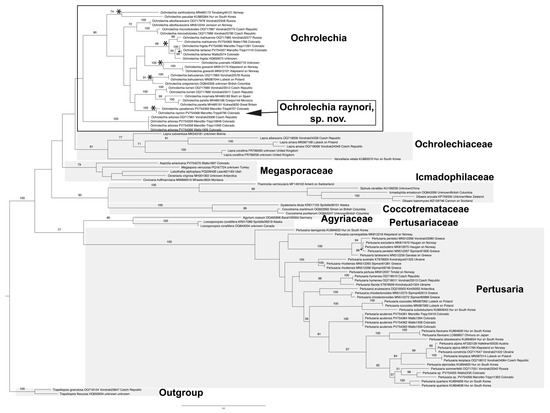
Figure 3.
Phylogenetic analysis of Ochrolechia raynori using maximum likelihood methods to resolve evolutionary relationship of new species to close relatives. Labels of terminals include taxon name, collector (lead collector, only) and collection number, GenBank reference number, and geographical origin of sample (where known). Asterisks (*) highlight five hypothesized “species pairs” resolved in this study, i.e., instances where sister taxa diverge in reproductive mode (i.e., sexually vs. asexually reproducing species).
Combing of existing collections of Ochrolechia revealed only one additional collection of the species, which was made immediately adjacent to (north of) the IPW, i.e., Rocky Mountain National Park, but from the east slope (R. Anderson 3840 [COLO!], Boulder County; Figure 4). Additional fieldwork in similar habitats and at comparable elevations is needed to more fully reveal the range of this species. Following description and discussion of Ochrolechia raynori (Part 1), we present a key to identify asexually reproducing species of Ochrolechia in western North America (Part 2).
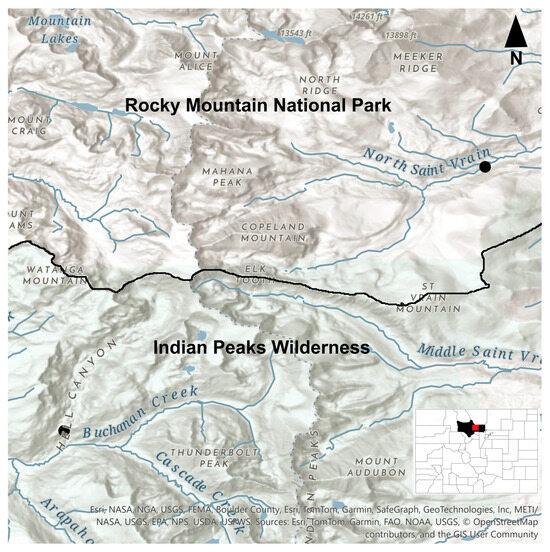
Figure 4.
A distribution map of collections of Ochrolechia raynori, so far known from two collections and endemic to the Front Range Mountains of Colorado, North America. We expect continued fieldwork inventory efforts of the lichens of the southern Rocky Mountains will yield a broader geographical distribution for this and other recently discovered species. The red circle indicates the type locality of O. raynori, the black line indicates the border between Rocky Mountain National Park and Indian Peaks Wilderness, and the gray dotted line indicates the continental divide. The map was made using ArcGis (https://www.esri.com/en-us/arcgis/geospatial-platform/overview, accessed 10 July 2025).
Part 1: TAXONOMY
Ochrolechia raynori E. Tripp & J. Watts, sp. nov.
TYPE: USA. Colorado. Grand County, Arapaho–Roosevelt National Forest, Indian Peaks Wilderness, Hell Canyon, extremely steep, mesic, and rocky slopes immediately bordering creek, with Acer glabrum, Alnus incana, Ceanothus velutinus, Picea engelmannii, Populus tremuloides, and Pseudotsuga menziesii, saxicolous and overgrowing mosses on rocks above creek, 9153 ft. elev., 40.11465–105.714750, 2 July 2023, E. Manzitto-Tripp & S. Raynor 9795 (Holotype: COLO!; Isotype: KANU!).
Mycobank #: 859527
Description. Thallus crustose, continuous, moderately thick, smooth except for swelling in the vicinity of developing soralia or as a function of underlying substrate (e.g., verruculose where underlying moss leaves depart from main axis), shiny, continuously corticate, whitish gray to faintly bluish gray. Prothallus absent. Upper cortex of two distinct layers, epinecral layer up to 35 µm thick, the eu-cortex paraplectenchymatous and composed of irregularly isodiametrical cells, up to 25 µm thick. Algal layer continuous, 35–75 µm thick. Medulla well-developed, up to 175 µm thick directly underneath a developing soralium or up to 50 µm thick when not associated with a soralium. Soralia round and regular in shape, discrete, sometimes coalescing with age but always remaining conspicuously distinct from one another, never entirely covering the upper surface. Soredia light grayish white, almost always slightly lighter in color than that of the thallus, 63–125 µm in diameter. Apothecia and associated sexual reproductive structures not seen.
Etymology. Ochrolechia raynori is named in honor of our dear colleague Seth Raynor, graduate student at the University of Colorado (EBIO Department), who spearheaded an impressive inventory of the lichens of the Indian Peaks Wilderness where this new species was discovered. The species was found in Hell Canyon, a remote and difficult-to-traverse drainage on the west slope of the range, where both the primary collector and co-collector (Raynor) endured considerable hardship during fieldwork. Seth’s unwavering dedication to understanding the lichen biota of Colorado combined with his resilience and remarkable work ethic in the field and herbarium have led to significant contributions to the study of lichens in the region. His ongoing research continues to expand and enrich our knowledge of Colorado’s diverse lichen flora. We are deeply grateful for Seth’s tireless efforts, collaboration, and most excellent companionship during our journey to understand these fascinating organisms in the southern Rocky Mountains.
Chemistry. Spot Tests: K-, C-, KC-, P-, UV- (thallus and soredia). Thin Layer Chromatography: murolic acid complex unknown (charring brown, below gyrophoric, above variolaric), unknown fatty acid (just above or level with the previous chemical), variolaric acid.
Distribution. Ochrolechia raynori, as currently understood, has a highly restricted range and is so far known only from the west slope (Grand County) and east slope (Boulder County) of the Front Range Mountains of Colorado. The first derived from the Indian Peaks Wilderness of Arapaho–Roosevelt National Forest and the second derived from Rocky Mountain National Park, immediately north of and adjacent to the IPW.
Substrate and Habitat. Ochrolechia raynori has been found overgrowing mosses and lichens on rocks on the banks immediately above Hell Canyon Creek in a mesic upper montane forest. On the east slope of the continental divide in Wild Basin, it was also found overgrowing saxicolous lichens in a mesic upper montane forest, suggesting a narrow niche.
Notes. Ochrolechia raynori is distinguished by its shiny, continuous, smooth thallus, discrete (almost pustulose) soralia that bear very coarse soredia, occurrence overgrowing mosses and lichens on rocks, and chemistry. Chemically, it is the only species with an unknown fatty acid plus an unknown compound in the murolic acid complex and variolaric acid, the latter two compounds shared with Colorado material of Ochrolechia upsaliensis (e.g., E. Tripp 5112 [COLO]), to which the new species was resolved as sister, phylogenetically.
Of sterile species of Ochrolechia in North America that produce variolaric acid, Ochrolechia raynori is unique for its production of secondary metabolites in the murolic acid complex, its negative spot tests, and its occurrence on moss and detritus rather than bark and wood, which is more common for species in this group. In general, TLC is needed for reliable identification of species of Ochrolechia containing variolaric acid. Perhaps most similar to the new species is Ochrolechia alboflavescens (Wulfen) Zahlbr.; however, the latter produces lichesterinic and protolichesterinic acids rather than murolic acid complex compounds. Ochrolechia farinacea Howard similarly produces murolic acid complex compounds, but occurs on bark, especially that of Quercus, has coalescing pale yellow soredia, and produces abundant UV+ bright yellow apothecia. Ochrolechia turneri (Sm.) Hasselrot differs by its occurrence on bark and lack of the murolic acid complex. Ochrolechia microstictoides Räsänen is known only from maritime New England and Nova Scotia in North America and differs in its production of lichesterinic acid, which results in a UV+ white reaction of the thallus. Ochrolechia gowardii Brodo produces variolaric acid and gyrophoric acid, yielding a C+ reaction in the soralia. Two additional species fail to produce either variolaric or gyrophoric acid: Ochrolechia sorediosa Howard differs by its occurrence on bark and its diffuse, coalescing soralia, that are P+ yellow. Ochrolechia minuta (Degel.) T. Sprib. occurs on bark and produces alectoronic acid, reacting KC+ pale red and UV+ white. We provide a key to sterile Ochrolechia in western North America to aid in the identification of the new species with respect to relatives. For more detailed information and useful taxonomic treatments of Ochrolechia in North America, see Howard [1] for a revision of all species, and Brodo [6] for a treatment of corticolous species and more detailed chemistry.
The holotype of Ochrolechia raynori (E. Manzitto-Tripp & S. Raynor 9795) is furthermore noteworthy for its lichenicolous ascomycete Dactylospora parasitica (Flörke ex Sprengel) Zopf. So far, this has not yet been documented to occur on Ochrolechia but rather is known from species of Pertusaria (Pertusariales). This is additionally the first documented occurrence of Dactylospora parasitica in Colorado.
In most clades of lichenized fungi, fertile species appear to never produce asexual lichenized propagules (in contrast, asexual species can sometimes manufacture sexual, fruiting bodies). However, in Ochrolechia, workers have noted the apparently increased tendency for fertile species to occasionally produce soredia [3,6]. The phenomenon is interesting and warrants further study, as it may ultimately impact stable taxonomy in Ochrolechia. In the case of O. raynori, however, we are unaware of any reports of sorediate in O. upsaliensis, despite it being common and widespread throughout the arctic. Additionally, the holotype of O. raynori is wholly sorediate and bears no fruiting bodies. We therefore here treat O. raynori at the species level rather than a sorediate form of O. upsaliensis.
Conservation Assessment. Ochrolechia raynori is here assessed as Critically Endangered (CR) under IUCN criterion D because of its small population size (two mature individuals currently known) and its highly restricted niche of near-pristine, riparian, mesic, upper montane forests of the northern Front Range Mountains. We note, however, that this assessment should be considered provisional given that much of the landscape remains unexplored from a lichenological perspective. New fieldwork (along with searches of relevant herbarium holdings), especially work that targets suitable habitat, may recover additional known populations of the species.
Additional Specimens Examined. USA. Colorado. Boulder County. Rocky Mountain National Park, Wild Basin, southwest of campground, 8600–8800 ft. elevation, 26 April 1963, R. Anderson 3840 (COLO!).
| PART 2: Key to sterile Ochrolechia in western North America (north of Mexico) | ||
| 1a. | Thallus isidiate or producing isidia-like spines | 2 |
| 1b. | Thallus sorediate, not producing isidia-like spines | 6 |
| 2a. | True isidia absent, isidia-like spines present, soredia sometimes present, usually | |
| terricolous, arctic-boreal distribution | 3 | |
| 2b. | True isidia present, isidia-like spines absent, soredia sometimes present, substrate | |
| and distribution various | 4 | |
| 3a. | Upper portions of cortex C+ yellow, containing variolaric acid, lower portions of | |
| cortex C+ red, containing gyrophoric acid | Ochrolechia alaskana (Verseghy) Kukwa | |
| 3b. | Entire cortex C+ red, containing gyrophoric acid | Ochrolechia frigida f. lapuensis (Vain.) Coppins |
| 4a. | Isidia soon breaking down into granules to form a granular crust, in arid habitats, | |
| usually on Juniper wood | Ochrolechia subisidiata Brodo | |
| 4b. | Isidia not breaking down into granules, in humid maritime habitats, on wood 5 | |
| 5a. | Isidia densely branched, occurring in clusters, usually growing on conifers, known | |
| from coastal southern Alaska | Ochrolechia cooperi T. Sprib. | |
| 5b. | Isidia thick, cylindrical, occasionally branched, occurring uniformly across the thallus, | |
| growing on hardwoods, distribution primarily eastern, but also reported | ||
| from coastal Washington and Oregon | Ochrolechia yasudae Vain. | |
| 6a. | Cortex, medulla, and/or soralia C+ red, KC+ red, containing gyrophoric and/or | |
| lecanoric acids | 7 | |
| 6b. | Cortex, medulla, and/or soralia C+ yellow or C−, KC− or KC+ yellow, or KC+ | |
| fleeting pink/purple, containing variolaric acid, alectoronic acid, or unknown | ||
| substances | 13 | |
| 7a. | Soralia (and cortex) UV+ vibrant yellow-orange, containing lichexanthone | Ochrolechia arborea (Kreyer) Almb. |
| 7b. | Soralia UV− or UV+ white, lacking lichexanthone | 8 |
| 8a. | Cortex C−, soralia and epihymenium C+ red | Ochrolechia gowardii Brodo |
| 8b. | Cortex C+ red, containing gyrophoric and/or lecanoric acids, soralia and | |
| epihymenium spot test various | 9 | |
| 9a. | Cortex K+ yellow, containing atranorin, always terricolous, distribution | |
| arctic-boreal | 10 | |
| 9b. | Cortex K−, lacking atranorin, usually corticolous or lignicolous, distribution | |
| various | 11 | |
| 10a. | Thallus thick, well developed | Ochrolechia gyalectina (Nyl.) Zahlbr. |
| 10b. | Thallus thin, poorly developed | Ochrolechia inaequatula (Nyl.) Zahlbr. |
| 11a. | Thallus thin, rimose-areolate, lacking accessory compounds by TLC | Ochrolechia mahluensis Räsänen |
| 11b. | Thallus thin or thick, containing accessory compounds (other than gyrophoric and | |
| lecanoric acids) by TLC | 12 | |
| 12a. | Thallus usually thin, containing fatty acids of the murolic acid complex, lacking | |
| hiascic acid | Ochrolechia bahusiensis H. Magn. | |
| 12b. | Thallus usually thick and bullate-verrucose, lacking murolic acid complex fatty acids, | |
| containing other unknown fatty acids and sometimes hiascic acid | Ochrolechia androgyna (Hoffm.) Arnold | |
| 13a. | Cortex C+ yellow | 14 |
| 13b. | Cortex C− | 15 |
| 14a. | Apothecial margins and disks UV−, soralia spot tests variable, containing variolaric | |
| acid in low concentrations | Ochrolechia alboflavescens (Wulfen) Zahlbr. | |
| 14b. | Apothecial margins and disks UV+ yellow orange, soralia C+ yellow, containing an | |
| unknown UV flourescent compound and variolaric acid in high | ||
| concentrations | Ochrolechia farinacea Howard | |
| 15a. | On mosses and detritus over rock, containing unknown compounds of the murolic | |
| acid complex | Ochrolechia raynori E. Tripp & J. Watts | |
| 15b. | On bark, lacking murolic acid complex compounds | 16 |
| 16a. | Soralia C+ yellow, UV+ glaucus orange or UV−, P− | Ochrolechia turneri (Sm.) Hasselrot |
| 16b. | Soralia C−, UV+ bright white or UV−, P+ pale yellow or P− | 17 |
| 17a. | Soralia UV+ bright white, P−, containing alectoronic acid, distribution | |
| coastal | Ochrolechia minuta (Degel.) T. Sprib. | |
| 17b. | Soralia UV−, P+ pale yellow, containing an unknown compound, distribution | |
| inland | Ochrolechia sorediosa Howard | |
Author Contributions
E.A.M.-T. and J.L.W. conceived the project. E.A.M.-T. conducted new fieldwork to advance this discovery, and E.A.M.-T. and J.L.W. advanced additional fieldwork throughout the state. E.A.M.-T. and J.L.W. generated and analyzed morphological, chemical, anatomical, and molecular data presented here. E.A.M.-T. wrote the manuscript, and J.L.W. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Indianapolis Zoo, Indian Peaks Wilderness Alliance, Boulder County Nature Association, Colorado Native Plant Society, Botanical Society of America, American Society of Plant Taxonomists, the International Association for Plant Taxonomy, and University of Colorado’s Museum of Natural History along with Department of Ecology and Evolutionary Biology. We are grateful to these organizations who have supported our fieldwork and subsequent discoveries.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
GenBank data are available publicly at https://www.ncbi.nlm.nih.gov (accessed on 10 July 2025).
Acknowledgments
The authors acknowledge that much of Colorado and the surrounding southern Rocky Mountains are the traditional and ancestral homelands of the Hinono’ei (Arapaho), Tsistsistas (Cyenne), and Nuuchu (Ute) Nations, among other peoples. We recognize the Indigenous peoples as original stewards of this land and are honored to research the incredible biodiversity that lies therein. We thank our Colorado lichen colleague Seth Raynor for his extensive fieldwork and contributions to enlighten the lichen biota of the Indian Peaks Wilderness (Front Range Mountains), and other portions of Colorado. We are grateful to the following Collections Managers and staff at the University of Colorado Herbarium (COLO) for their assistance with specimen curation and support of this project: Dina Clark, James Ryan, Amber Horning, and the late Tim Hogan. We thank staff members at the United States National Forest Service (Region 2), in particular Rick Stumpf of the Arapaho–Roosevelt National Forest, for their/his continued support of our research and field permits.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Howard, G.E. The lichen genus Ochrolechia in North America north of Mexico. Bryologist 1970, 73, 93–130. [Google Scholar] [CrossRef]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Ainsworth & Bisby’s Dictionary of the Fungi, 10th ed.; CABI Publishing: Oxfordshire, UK, 2008. [Google Scholar]
- Kukwa, M. The Lichen Genus Ochrolechia in Europe; Fundacja Rozwoju Uniwersytetu Gdanskiego: Gdańsk, Poland, 2011; 390p. [Google Scholar]
- Ren, Q. A revision of the lichen genus Ochrolechia in China. Lichenologist 2017, 49, 67–84. [Google Scholar] [CrossRef]
- Ertz, D.; Fryday, A.; Schmitt, I.; Charrier, M.; Dudek, M.; Kukwa, M. Ochrolechia kerguelensis sp. nov. from the southern Hemisphere and O. antarctica reinstated from the synonym of O. parallela. Phytotaxa 2016, 280, 129–140. [Google Scholar]
- Brodo, I.M. Studies in the lichen genus Ochrolechia. 2. Corticolous species of North America. Can. J. Bot. 1991, 69, 733–772. [Google Scholar] [CrossRef]
- Schmitt, I.; Yamamoto, Y.; Lumbsch, H.T. Phylogeny of Pertusariales (Ascomycotina): Resurrection of Ochrolechiaceae and new circumscription of Megasporaceae. J. Hattori Bot. Lab. 2006, 100, 753–764. [Google Scholar]
- Schmitt, I.; Lumbsch, H.T. Molecular phylogeny of the Pertusariaceae supports secondary chemistry as an important systematic character set in lichen-forming ascomycetes. Mol. Phylogenetics Evol. 2004, 33, 43–55. [Google Scholar] [CrossRef]
- Esslinger, T. A cumulative checklist for the lichen-forming, lichenicolous and allied fungi of the continental United States and Canada, Version 24. Opusc. Philolichenum 2021, 20, 100–394. [Google Scholar] [CrossRef]
- Verseghy, D. Die Gattung Ochrolechia. Beiheftze zur Nova Hedwigia 1962, 50, 76–85. [Google Scholar]
- Tripp, E.A. Is asexual reproduction and evolutionary dead end in lichens? Lichenologist 2016, 48, 559–580. [Google Scholar] [CrossRef]
- Messuti, M.I.; Lumbsch, H.T. A revision of the genus Ochrolechia in southern South America. Bibl. Lichenol. 2000, 75, 33–46. [Google Scholar]
- Thiers, B. Index Herbariorum: A Global Director of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. 2016. Available online: http://sweetgum.nybg.org/science/ih (accessed on 20 June 2025).
- Culberson, C.F.; Kristinsson, H.-D. A standardized method for the identification of lichen products. J. Chromatogr. A 1970, 46, 85–93. [Google Scholar] [CrossRef]
- Culberson, C.F. Improved conditions and new data for identification of lichen products by standardized thin-layer chromatographic method. J. Chromatogr. A 1972, 72, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bulltein 1987, 10, 11–15. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogentics. In PCR Protocols: A Ghuide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0, a graphical interface and toolkit for phylogenetic analysis using RaxML. Methods Ecol. Evol. 2020, 12, 373–377. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1.4.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2010; Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 20 June 2025).
- IUCN Standards and Petitions Committee. Guidelines for Using the IUCN Red List Categories and Criteria. Version 16. Prepared by the Standards and Petitions Committee. 2024. Available online: https://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 22 April 2025).
- Yahr, R.; Allen, J.L.; Atienza, V.; Burgartz, F.; Chrismas, N.; Forno, M.D.; Degtjarenko, P.; Ohmura, Y.; Pérez-Ortega, S.; Randlane, T.; et al. Red listing lichenized fungi: Best practices and future prospects. Lichenologist 2024, 56, 345–362. [Google Scholar] [CrossRef]
- Raynor, S.J.; Watts, J.L.; Manzitto-Tripp, E.A. Polycauliona pancakeana, a new species to science from the southern Rocky Mountains. Bryologist 2025, in press. [Google Scholar]
- Tripp, E.A. Lichen inventory of White Rocks Open Space (City of Boulder, Colorado). West. North Am. Nat. 2015, 75, 301–310. [Google Scholar] [CrossRef]
- Tripp, E.A. Field Guide to the Lichens of White Rocks (Boulder, Colorado); University Press of Colorado: Denver, CO, USA, 2017. [Google Scholar]
- Tripp, E.A.; Morse, C.A.; Keepers, K.G.; Stewart, C.A.; Pogoda, C.S.; White, K.H.; Hoffman, J.R.; Kane, N.C.; McCain, C.M. Evidence of substrate endemism of lichens on Fox Hills Sandstone: Discovery and description of Lecanora lendemeri as new to science. Bryologist 2019, 122, 246–259. [Google Scholar] [CrossRef]
- Raynor, S.J.; Kesler, J.; Allen, J.R.; Manzitto-Tripp, E.A. New and noteworthy reports on Colorado lichens and lichen allies, 2, Biatoropsis hirtae and B. minuta. West. North Am. Nat. 2023, 83, 454–461. [Google Scholar] [CrossRef]
- Raynor, S.J.; Watts, J.L.; Manzitto-Tripp, E.A. Sarea cirrhendocarpa, a fungus species new to science from the southern Rocky Mountains. Phytotaxa 2024, 671, 87–97. [Google Scholar] [CrossRef]
- Díaz, V.; Manzitto-Tripp, E.A. A synopsis of the yellow-green, usnic-acid producing Xanthoparmelia in Colorado. Opuscula Philolichenum 2023, 22, 1–40. [Google Scholar] [CrossRef]
- Manzitto-Tripp, E.A.; Raynor, S.J.; Watts, J.L. New and noteworthy reports of lichens and allied fungi to Colorado, including descriptions of two species new to science. J. Bot. Res. Inst. Tex. 2025, in press. [Google Scholar]
- Watts, J.L.; Raynor, S.J.; Li, Y.; Meier, R.; Cook, C.; Casini, G.; Chadwick, E.; Manzitto-Tripp, E.A. Lecanora exspersa: A new lichen record for North America and a key to sorediate Lecanora (Lecanoraceae) in western North America. Bryologist 2024, 127, 427–440. [Google Scholar] [CrossRef]
- Watts, J.L.; Raynor, S.J.; Manzitto-Tripp, E.A. Character evolution in Heterodermia s.l. (Physciaceae; Caliciales) and two new species from the southern Rocky Mountains, USA. Phytotaxa 2025, 698, 61–81. [Google Scholar] [CrossRef]
- Manzitto-Tripp, E.A.; Watts, J.L. The thin horizon of a plan is almost clear: Towards a lichen biodiversity inventory of the southern Rocky Mountains. Phytotaxa. 2025, in press. [Google Scholar]
- Manzitto-Tripp, E.A.; Agabani, R.; McMullin, R.T. New and noteworthy reports of Colorado lichens and lichen allies, 1. Opuscula Philolichenum 2018, 17, 362–367. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).