Objective and Subjective Measures of Cognitive Decline in Highly Educated Older Adults: A 10-Year Longitudinal Study
Abstract
1. Introduction
1.1. The Subjective Aspects of Cognitive Decline: SCD and Psychological Distress
1.2. Highly Educated Older Adults
1.3. Neuropsychological Assessment: Longitudinal Study
1.4. Current Research
2. Methods
2.1. Participants
2.2. Procedure
2.3. Assessments
2.3.1. Neuropsychological Tests
2.3.2. Questionnaires
2.4. Data Analyses
3. Results
3.1. Participants Characteristics
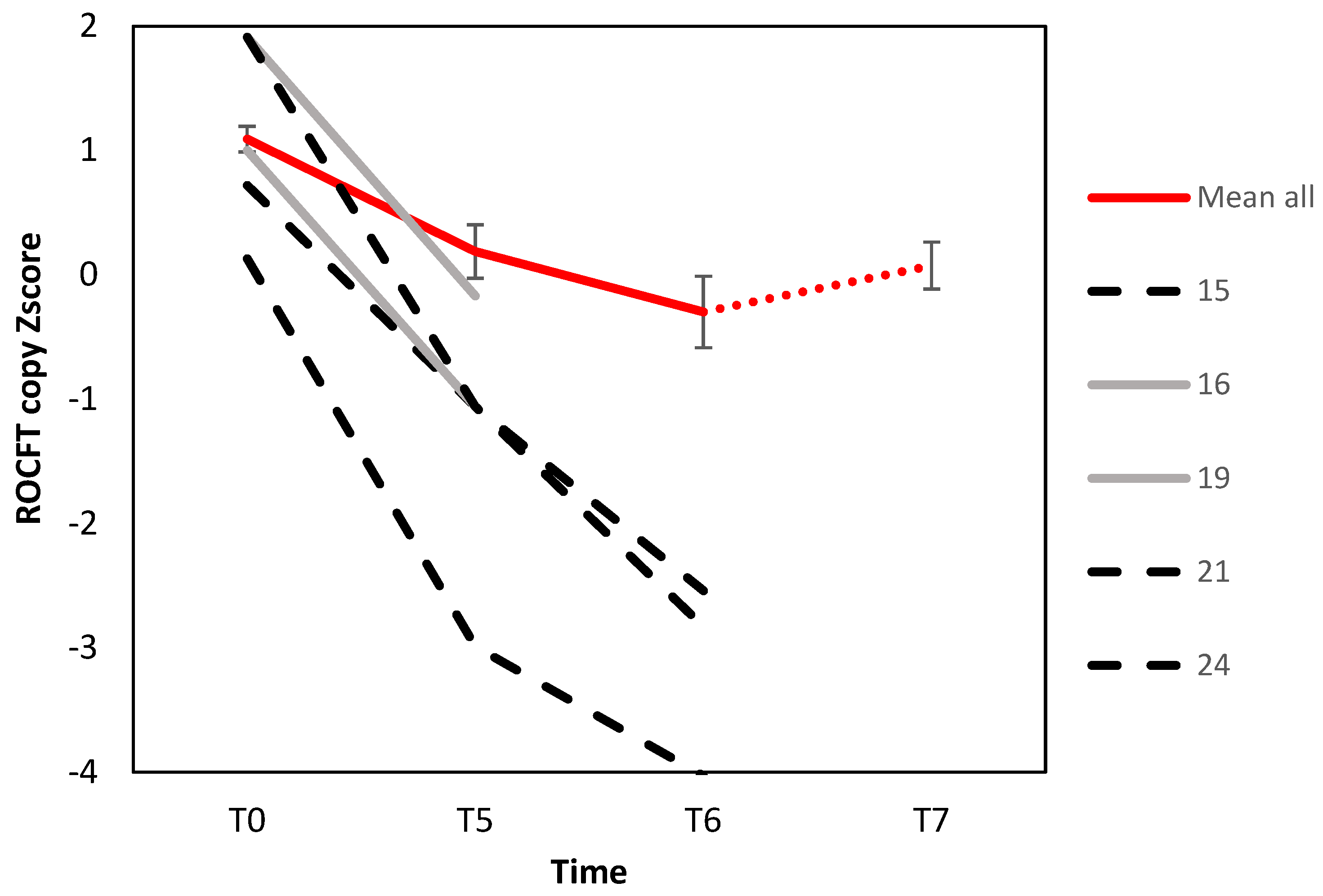
3.2. Change in Objective Assessments
3.3. Change in Subjective Assessments
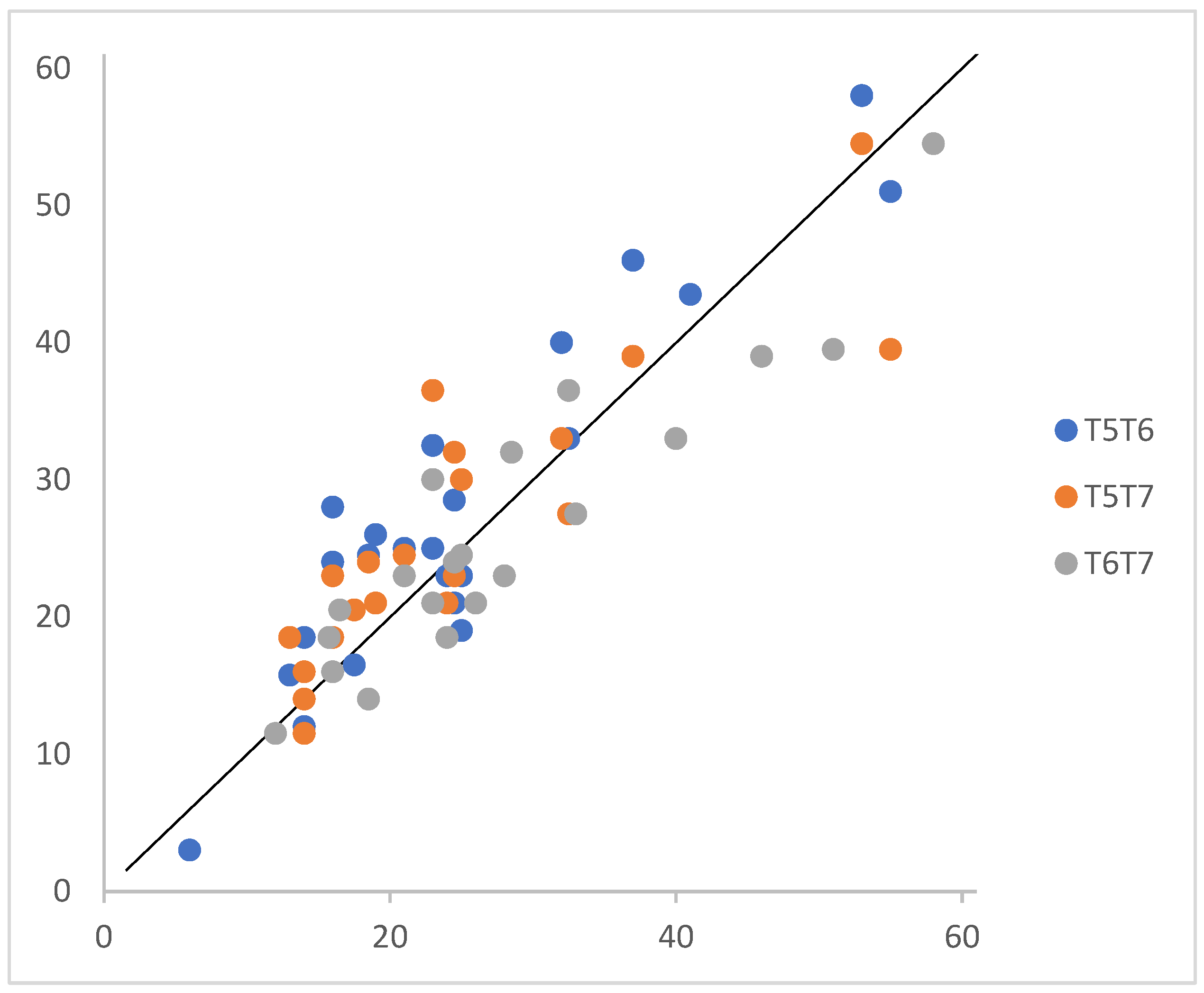
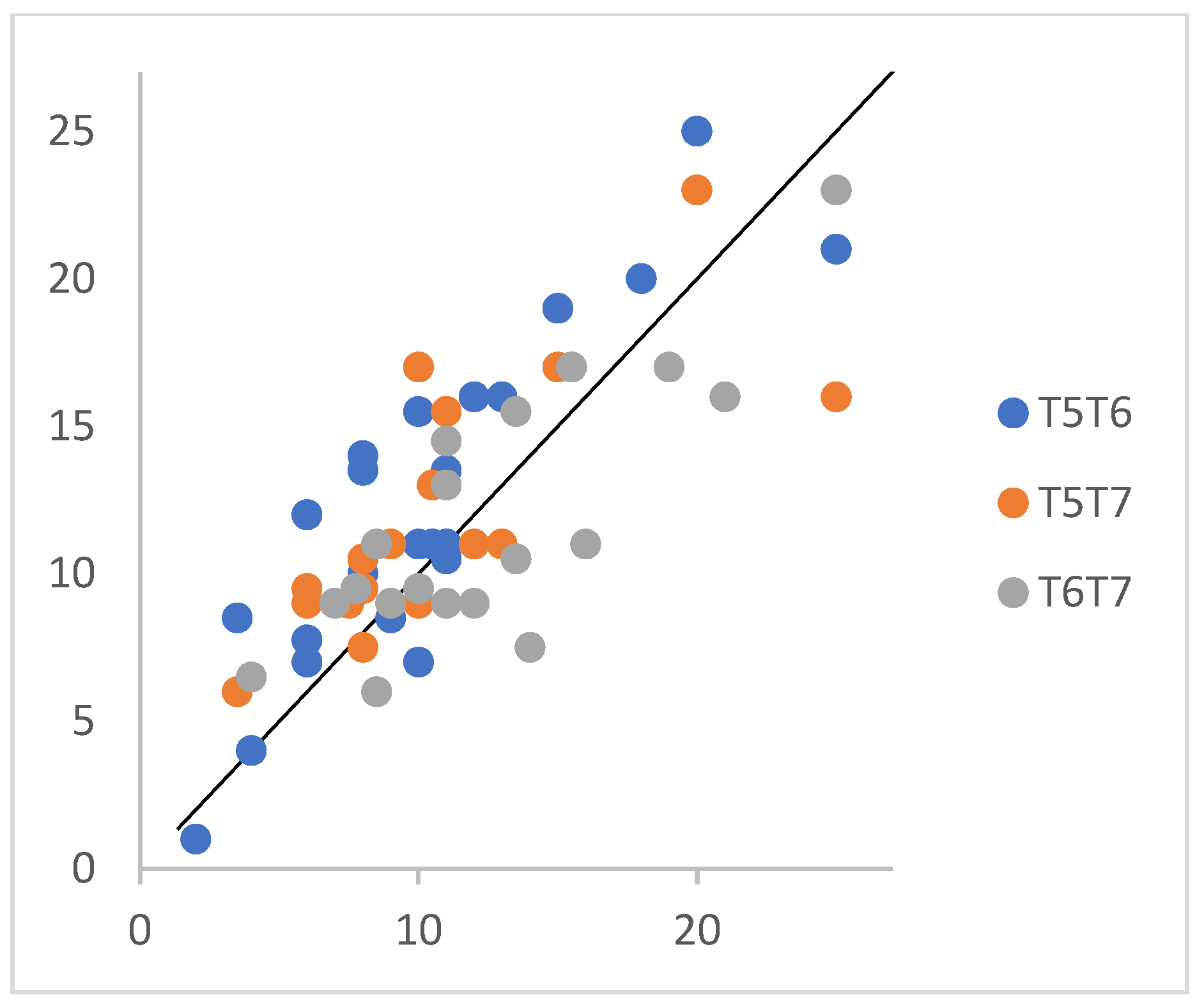
3.4. Preliminary Validation of the Hebrew SCD Questionnaire
4. Discussion
4.1. Objective Assessments
4.2. Subjective Assessments
4.3. The Hebrew SCD Questionnaire
4.4. Clinical and Research Implications
4.5. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Alzheimer’s Association. 2014 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2014, 10, e47–e92. [Google Scholar] [CrossRef]
- Bettio, L.E.; Rajendran, L.; Gil-Mohapel, J. The effects of aging in the hippocampus and cognitive decline. Neurosci. Biobehav. Rev. 2017, 79, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.A.; Wang, T.; Yu, L.; Wilson, R.S.; Dawe, R.; Arfanakis, K.; Schneider, J.A.; Bennett, D.A. To what degree is late life cognitive decline driven by age-related neuropathologies? Brain 2021, 144, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 2002, 8, 448–460. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Manfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446, Erratum in Lancet 2023, 402, 1132. [Google Scholar] [CrossRef]
- Stern, Y.; Arenaza-Urquijo, E.M.; Bartrés-Faz, D.; Belleville, S.; Cantilon, M.; Chetelat, G.; Ewers, M.; Franzmeier, N.; Kempermann, G.; Kremen, W.S.; et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s Dement. 2020, 16, 1305–1311. [Google Scholar] [CrossRef]
- Jessen, F.; Wiese, B.; Cvetanovska, G.; Fuchs, A.; Kaduszkiewicz, H.; Kölsch, H.; Luck, T.; Mösch, E.; Pentzek, M.; Riedel-Heller, S.G.; et al. Patterns of subjective memory impairment in the elderly: Association with memory performance. Psychol. Med. 2007, 37, 1753–1762. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; Van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; van der Flier, W.M.; et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef]
- Lee, S.D.; Ong, B.; Pike, K.E.; Kinsella, G.J. Prospective memory and subjective memory decline: A neuropsychological indicator of memory difficulties in community-dwelling older people. J. Clin. Exp. Neuropsychol. 2018, 40, 183–197. [Google Scholar] [CrossRef]
- Pike, K.E.; Zeneli, A.; Ong, B.; Price, S.; Kinsella, G.J. Reduced benefit of memory elaboration in older adults with subjective memory decline. J. Alzheimer’s Dis. 2015, 47, 705–713. [Google Scholar] [CrossRef]
- Pike, K.E.; Cavuoto, M.G.; Li, L.; Wright, B.J.; Kinsella, G.J. Subjective cognitive decline: Level of risk for future dementia and mild cognitive impairment, a meta-analysis of longitudinal studies. Neuropsychol. Rev. 2022, 32, 703–735. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Amariglio, R.E.; Buckley, R.F.; van der Flier, W.M.; Han, Y.; Molinuevo, J.L.; Rabin, L.; Rentz, D.M.; Rodriguez-Gomez, O.; Saykin, A.J.; et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020, 19, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Shulman, M.B.; Torossian, C.; Leng, L.; Zhu, W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimer’s Dement. 2010, 6, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.L.; Mogle, J.; Wion, R.; Munoz, E.; DePasquale, N.; Yevchak, A.M.; Parisi, J.M. Subjective cognitive impairment and affective symptoms: A systematic review. Gerontologist 2016, 56, e109–e127. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An Inventory for Measuring Depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.D. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Gifford, K.A.; Liu, D.; Romano, R.R., III; Jones, R.N.; Jefferson, A.L. Development of a subjective cognitive decline questionnaire using item response theory: A pilot study. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2015, 1, 429–439. [Google Scholar] [CrossRef]
- Hao, L.; Jia, J.; Xing, Y.; Han, Y. An application study-subjective cognitive decline Questionnaire9 in detecting mild cognitive impairment (MCI). Aging Ment. Health 2022, 26, 2014–2021. [Google Scholar] [CrossRef]
- Amieva, H.; Mokri, H.; Le Goff, M.; Meillon, C.; Jacqmin-Gadda, H.; Foubert-Samier, A.; Orgogozo, J.M.; Stern, Y.; Dartigues, J.F. Compensatory mechanisms in higher-educated subjects with Alzheimer’s disease: A study of 20 years of cognitive decline. Brain 2014, 137, 1167–1175. [Google Scholar] [CrossRef]
- Stern, Y. How can cognitive reserve promote cognitive and neurobehavioral health? Arch. Clin. Neuropsychol. 2021, 36, 1291–1295. [Google Scholar] [CrossRef]
- Pettigrew, C.; Soldan, A. Defining cognitive reserve and implications for cognitive aging. Curr. Neurol. Neurosci. Rep. 2019, 19, 1. [Google Scholar] [CrossRef]
- Barulli, D.; Stern, Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn. Sci. 2013, 17, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Beaumont, H.; Ferguson, D.; Yadegarfar, M.; Stubbs, B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatr. Scand. 2014, 130, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Albert, S.M.; Manly, J.J.Y. Education and rates of cognitive decline in incident Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2006, 77, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Calamia, M.; Markon, K.; Tranel, D. Scoring higher the second time around: Meta-analyses of practice effects in neuropsychological assessment. Clin. Neuropsychol. 2012, 26, 543–570. [Google Scholar] [CrossRef]
- Gavett, B.E.; Gurnani, A.S.; Saurman, J.L.; Chapman, K.R.; Steinberg, E.G.; Martin, B.; Chaisson, C.E.; Mez, J.; Tripodis, Y.; Stern, R.A. Practice effects on story memory and list learning tests in the neuropsychological assessment of older adults. PLoS ONE 2016, 11, e0164492. [Google Scholar] [CrossRef]
- Vakil, E.; Blachstein, H. Rey AVLT: Developmental norms for adults and the sensitivity of different memory measures to age. Clin. Neuropsychol. 1997, 11, 356–369. [Google Scholar] [CrossRef]
- Cid, R.E.C.; Loewenstein, D.A. Salient Cognitive Paradigms to Assess Preclinical Alzheimer’s Disease. Neurotherapeutics 2022, 19, 89–98. [Google Scholar] [CrossRef]
- Curiel Cid, R.E.; Matias-Guiu, J.A.; Loewenstein, D.A. A review of novel Cognitive Challenge Tests for the assessment of preclinical Alzheimer’s disease. Neuropsychology 2023, 37, 661. Available online: https://psycnet.apa.org/doi/10.1037/neu0000883 (accessed on 15 May 2025). [CrossRef]
- Wolfsgruber, S.; Kleineidam, L.; Guski, J.; Polcher, A.; Frommann, I.; Roeske, S.; Spruth, E.J.; Franke, C.; Priller, J.; Kilimann, I.; et al. Minor neuropsychological deficits in patients with subjective cognitive decline. Neurology 2020, 95, e1134–e1143. [Google Scholar] [CrossRef]
- Kavé, G.; Knafo-Noam, A. Lifespan development of phonemic and semantic fluency: Universal increase, differential decrease. J. Clin. Exp. Neuropsychol. 2015, 37, 751–763. [Google Scholar] [CrossRef]
- Rami, L.; Mollica, M.A.; García-Sanchez, C.; Saldaña, J.; Sanchez, B.; Sala, I.; Molinuevo, J.L. The subjective cognitive decline questionnaire (SCD-Q): A validation study. J. Alzheimer’s Dis. 2014, 41, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, M.N.; Boada, M.; Borson, S.; Chilukuri, M.; Dubois, B.; Ingram, J.; Iwata, A.; Porsteinsson, A.P.; Possin, K.L.; Rabinovici, G.D.; et al. Early detection of mild cognitive impairment (MCI) in primary care. J. Prev. Alzheimer’s Dis. 2020, 7, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Macoir, J.; Tremblay, P.; Hudon, C. The use of executive fluency tasks to detect cognitive impairment in individuals with subjective cognitive decline. Behav. Sci. 2022, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Favieri, F.; Boncompagni, I.; Agostini, F.; Cantone, M.; Casagrande, M. Executive functions in Alzheimer disease: A systematic review. Front. Aging Neurosci. 2019, 10, 437. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, L.; Barroso, J.; Ferreira, D. Cognitive reserve and network efficiency as compensatory mechanisms of the effect of aging on phonemic fluency. Aging 2020, 12, 23351–23378. [Google Scholar] [CrossRef]
- Osterrieth, P.A. Le test de copie d’une figure complexe; contribution a l’etude de la perception et de la memoire. Arch. Psychol. 1944, 30, 206–356. [Google Scholar]
- Rey, A.; Osterrieth, P.A. Translations of excerpts from Andre Rey’s “Psychological examination of traumatic encephalopathy” and P. A. Osterrieth’s “The Complex Figure Copy Test”. Clin. Neuropsychol. 1993, 7, 4–21. [Google Scholar]
- Kwak, Y.T. “Closing-in” phenomenon in Alzheimer’s disease and subcortical vascular dementia. BMC Neurol. 2004, 4, 3. [Google Scholar] [CrossRef]
- Elkana, O.; Eisikovits, O.R.; Oren, N.; Betzale, V.; Giladi, N.; Ash, E.L. Sensitivity of neuropsychological tests to identify cognitive decline in highly educated elderly individuals: 12 months follow up. J. Alzheimer’s Dis. 2016, 49, 607–616. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Wechsler, D. The Wechsler Adult Intelligence Scale, 3rd ed.; The Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Wechsler, D. WMS-III: Wechsler Memory Scale Administration and Scoring Manual; Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Army Individual Test Battery. Manual of Directions and Scoring; War Department, Adjutant General’s Office: Washington, DC, USA, 1944. [Google Scholar]
- Kavé, G. Phonemic fluency, semantic fluency, and difference scores: Normative data for adult Hebrew speakers. J. Clin. Exp. Neuropsychol. 2005, 27, 690–699. [Google Scholar] [CrossRef] [PubMed]
- John, O.P.; Donahue, E.M.; Kentle, R.L. The Big Five Inventory—Versions 4a and 54; University of California, Berkeley, Institute of Personality and Social Research: Berkeley, CA, USA, 1991. [Google Scholar]
- Vakil, E.; Greenstein, Y.; Blachstein, H. Normative data for composite scores for children and adults derived from the Rey Auditory Verbal Learning Test. Clin. Neuropsychol. 2010, 24, 662–677. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Magalhães, S.; Fernandes Malloy-Diniz, L.; Cavalheiro Hamdan, A. Validity convergent and reliability test-retest of the rey auditory verbal learning test. Clin. Neuropsychiatry 2012, 9, 129–137. [Google Scholar]
- Taylor, L.B. Scoring criteria for the Rey-Osterrieth complex figure test. In A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary; Oxford University Press: Oxford, UK, 1998; pp. 350–351. [Google Scholar]
- Berry, D.T.; Allen, R.S.; Schmitt, F.A. Rey-Osterrieth Complex Figure: Psychometric characteristics in a geriatric sample. Clin. Neuropsychol. 1991, 5, 143–153. [Google Scholar] [CrossRef]
- Reedy, S.D.; Boone, K.B.; Cottingham, M.E.; Glaser, D.F.; Lu, P.H.; Victor, T.L.; Ziegler, E.A.; Zeller, M.A.; Wright, M.J. Validation of the Lu and colleagues (2003) Rey-Osterrieth Complex Figure Test effort equation in a large known-group sample. Arch. Clin. Neuropsychol. 2013, 28, 30–37. [Google Scholar] [CrossRef]
- Goodman, L. Translation of WAIS-III-Wechsler Adult Intelligence Scale; Psychtech: Jerusalem, Israel, 2001. [Google Scholar]
- Iverson, G.L. Interpreting change on the WAIS-III/WMS-III in clinical samples. Arch. Clin. Neuropsychol. 2001, 16, 183–191. [Google Scholar] [CrossRef]
- Tombaugh, T.N. Trail Making Test A and B: Normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Wagner, S.; Helmreich, I.; Dahmen, N.; Lieb, K.; Tadić, A. Reliability of three alternate forms of the trail making tests a and B. Arch. Clin. Neuropsychol. 2011, 26, 314–321. [Google Scholar] [CrossRef]
- Sánchez-Cubillo, I.; Periáñez, J.A.; Adrover-Roig, D.; Rodríguez-Sánchez, J.M.; Ríos-Lago, M.; Tirapu, J.E.E.A.; Barceló, F. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J. Int. Neuropsychol. Soc. 2009, 15, 438–450. [Google Scholar] [CrossRef]
- Salkind, M. Beck depression inventory in general practice. J. R. Coll. Gen. Pract. 1969, 18, 267. [Google Scholar]
- Richter, P.; Werner, J.; Heerlein, A.; Kraus, A.; Sauer, H. On the validity of the Beck Depression Inventory: A review. Psychopathology 1998, 31, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Carbin, M.G. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 1988, 8, 77–100. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Najenson, T. Changes in life patterns and symptoms of low mood as reported by wives of severely brain-injured soldiers. J. Consult. Clin. Psychol. 1976, 44, 881. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.G.; Waal-Manning, H.J.; Spears, G.F. Some norms and reliability data for the State-Trait Anxiety Inventory and the Zung Self-Rating Depression scale. Br. J. Clin. Psychol. 1983, 22, 245–249. [Google Scholar] [CrossRef]
- Kabacoff, R.I.; Segal, D.L.; Hersen, M.; Van Hasselt, V.B. Psychometric properties and diagnostic utility of the Beck Anxiety Inventory and the State-Trait Anxiety Inventory with older adult psychiatric outpatients. J. Anxiety Disord. 1997, 11, 33–47. [Google Scholar] [CrossRef]
- Teichman, Y. Hebrew Version of the State-Trait Anxiety Inventory, Tel-Aviv University: Tel-Aviv, Israel, 1979; manuscript in preparation.
- Etzion, D.; Lasky, S. Personality Traits Questionnaire (Big Five), Faculty of Management, Institute of Business Research, Tel-Aviv University: Tel-Aviv, Israel, 1998; manuscript in preparation.
- Koller, O.M.; Hill, N.L.; Mogle, J.; Bhang, I. Relationships between subjective cognitive impairment and personality traits: A systematic review. J. Gerontol. Nurs. 2019, 45, 27–34. [Google Scholar] [CrossRef]
- Low, L.F.; Harriso, F.; Lackersteen, S.M. Does personality affect risk for dementia? A systematic review and meta-analysis. Am. J. Geriatr. Psychiatry 2013, 21, 713–728. [Google Scholar] [CrossRef]
- Stewart, A.L.; Mills, K.M.; King, A.C.; Haskell, W.L.; Gillis, D.; Ritter, P.L. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Med. Sci. Sports Exerc. 2001, 33, 1126–1141. [Google Scholar] [CrossRef]
- Elkana, O.; Soffer, S.; Eisikovits, O.R.; Oren, N.; Bezalel, V.; Ash, E.L. WAIS Information Subtest as an indicator of crystallized cognitive abilities and brain reserve among highly educated older adults: A three-year longitudinal study. Appl. Neuropsychol. Adult 2019, 27, 525–531. [Google Scholar] [CrossRef]
- Lezak, M.D. Neuropsychological Assessment; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Keppel, G.; Underwood, B.J. Proactive inhibition in short-term retention of single items. J. Verbal Learn. Verbal Behav. 1962, 1, 153–161. [Google Scholar] [CrossRef]
- Burton, R.L.; Lek, I.; Dixon, R.A.; Caplan, J.B. Associative interference in older and younger adults. Psychol. Aging 2019, 34, 558. Available online: https://psycnet.apa.org/doi/10.1037/pag0000361 (accessed on 15 May 2025). [CrossRef] [PubMed]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, B.M.; Eidels, A.; Donkin, C. Effects of aging and distractors on detection of redundant visual targets and capacity: Do older adults integrate visual targets differently than younger adults? PLoS ONE 2014, 9, e113551. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Luppa, M.; Brähler, E.; König, H.H.; Riedel-Heller, S.G. The assessment of changes in cognitive functioning: Reliable change indices for neuropsychological instruments in the elderly–a systematic review. Dement. Geriatr. Cogn. Disord. 2010, 29, 275–286. [Google Scholar] [CrossRef]
- Lezak, M.D.; Howieson, D.B.; Bigler, E.D.; Tranel, D. Neuropsychological Assessment; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Rostamzadeh, A.; Bohr, L.; Wagner, M.; Baethge, C.; Jessen, F. Progression of subjective cognitive decline to MCI or dementia in relation to biomarkers for Alzheimer disease: A meta-analysis. Neurology 2022, 99, e1866–e1874. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, L.; Hernández-Cabrera, J.A.; Westman, E.; Barroso, J.; Ferreira, D. Cognitive compensatory mechanisms in normal aging: A study on verbal fluency and the contribution of other cognitive functions. Aging 2019, 11, 4090–4106. [Google Scholar] [CrossRef]
- Rodriguez, F.S.; Zheng, L.; Chui, H.C. Aging Brain: Vasculature, Ischemia, and Behavior Study. Psychometric characteristics of cognitive reserve: How high education might improve certain cognitive abilities in aging. Dement. Geriatr. Cogn. Disord. 2019, 47, 335–344. [Google Scholar] [CrossRef]
- Borgeest, G.S.; Henson, R.N.; Shafto, M.; Samu, D.; Cam-CAN; Kievit, R.A. Greater lifestyle engagement is associated with better age-adjusted cognitive abilities. PLoS ONE 2020, 15, e0230077. [Google Scholar] [CrossRef]
- Webb, L.M.; Chen, C.Y. The COVID-19 pandemic’s impact on older adults’ mental health: Contributing factors, coping strategies, and opportunities for improvement. Int. J. Geriatr. Psychiatry 2022, 37, 1–10. [Google Scholar] [CrossRef]
- Colombo, B.; Hamilton, A.; Telazzi, I.; Balzarotti, S. The relationship between cognitive reserve and the spontaneous use of emotion regulation strategies in older adults: A cross-sectional study. Aging Clin. Exp. Res. 2023, 35, 1505–1512. [Google Scholar] [CrossRef]
- Daly, M.; Robinson, E. Depression and anxiety during COVID-19. Lancet 2022, 399, 518. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, R.F.; Direk, N.; Mirza, S.S.; Hofman, A.; Koudstaal, P.J.; Tiemeier, H.; Ikram, M.A. Anxiety is not associated with the risk of dementia or cognitive decline: The Rotterdam Study. Am. J. Geriatr. Psychiatry 2014, 22, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Hebert, L.E.; Scherr, P.A.; Barnes, L.L.; Mendes de Leon, C.F.; Evans, D.A. Educational attainment and cognitive decline in old age. Neurology 2009, 72, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Zahodne, L.B.; Glymour, M.M.; Sparks, C.; Bontempo, D.; Dixon, R.A.; MacDonald, S.W.; Manly, J.J. Education does not slow cognitive decline with aging: 12-year evidence from the victoria longitudinal study. J. Int. Neuropsychol. Soc. JINS 2011, 17, 1039–1046. [Google Scholar] [CrossRef]
- Liew, T.M. Depression, subjective cognitive decline, and the risk of neurocognitive disorders. Alzheimer’s Res. Ther. 2019, 11, 70. [Google Scholar] [CrossRef]
- Wolfsgruber, S.; Wagner, M.; Schmidtke, K.; Frölich, L.; Kurz, A.; Schulz, S.; Hampel, H.; Heuser, I.; Peters, O.; Reischies, F.M.; et al. Memory concerns, memory performance and risk of dementia in patients with mild cognitive impairment. PLoS ONE 2014, 9, e100812. [Google Scholar] [CrossRef]
| Characteristic | Values |
|---|---|
| Age (years) Gender | M = 80.45 (SD = 4.78) 10 men/10 female |
| Education (years) | M = 17.6 (SD = 3.41) |
| BMI a | M = 26 (SD = 2.82) |
| Engagement in physical exercise b (number of times per week) | M = 2.8 (SD = 1.39) |
| Leisure activity c (times per week) Social gathering d (times per week) Living with a partner or alone? | M = 2.2 (SD = 1.57) M = 1.9 (SD = 1.14) 5 alone/15 with a partner |
| Country of birth, N | Israel-12 (60%) Poland-2 (10%) Rumania-2 (10%) England; Germany; Russia; Argentina-4 (20%) |
| Age of immigration, years | M = 10.43 (SD = 10.61) |
| Native language, n | Hebrew-12 (60%) Yiddish-4 (20%) English; Hungarian; Russian; Spanish; Romanian-5 (20%) |
| Time Point/ Test | T0 M (SD) | T5 M (SD) | T6 M (SD) | T7 M (SD) | F(57) | p | ηp2 |
|---|---|---|---|---|---|---|---|
| ROCFT copy | 1.1 (0.52) | 0.4 (0.79) | 0.1 (0.93) | 0.1 (0.84) | 9.05 | <0.001 | 0.32 |
| ROCFT delay | 0.8 (0.71) | 1 (0.8) | 0.9 (1.03) | 1 (0.95) | 0.51 | 0.67 | 0.03 |
| PF | 0.2 (0.92) | 0.4 (1.03) | 0.6 (0.97) | 0.6 (0.83) | 1.26 | 0.29 | 0.06 |
| SF | 0.5 (1.18) | 0.4 (0.92) | 0.5 (1.29) | 0.4 (1.3) | 0.10 | 0.90 | 0.01 |
| RAVLT trial six | 0.6 (1.34) | 0.3 (0.88) | 0.1 (1.12) | −0.2 (1.23) | 2.16 | 0.10 | 0.10 |
| TMT B | 0.7 (0.53) | 0.8 (0.46) | 0.4 (0.47) | 0.1 (0.74) | 5.81 | <0.001 | 0.26 |
| Time Point/ Questionnaire | T5 M (SD) | T6 M (SD) | T7 M (SD) | F (38) | p | ηp2 |
|---|---|---|---|---|---|---|
| BDI (0–62) | 5.2 (3.74) | 6 (3.59) | 5.6 (2.54) | 2.17 | 0.13 | 0.11 |
| STAI (20–80) | 29.1 (7.96) | 30.8 (8.94) | 28.9 (9.45) | 0.73 | 0.49 | 0.04 |
| SCD50 (0–61) | 24.7 (12.04) | 28.1 (12.26) | 26.4 (10.38) | 4.54 | 0.02 | 0.19 |
| SCD21 (0–27) | 10.2 (5.18) | 12.7 (5.08) | 11.72 (4.3) | 7.06 | <0.01 | 0.27 |
| Measure | SCD21_T5 | SCD50_T5 | SCD21_T6 | SCD50_T6 | SCD21_T7 | SCD50_T7 |
|---|---|---|---|---|---|---|
| SCD21_T5 | - | 0.98 ** | 0.87 ** | 0.89 ** | 0.78 ** | 0.85 ** |
| SCD50_T5 | - | 0.80 ** | 0.80 ** | 0.78 ** | 0.89 ** | |
| SCD21_T6 | - | 0.97 ** | 0.80 ** | 0.93 ** | ||
| SCD50_T6 | - | 0.78 ** | 0.93 ** | |||
| SCD21_T7 | - | 0.94 ** | ||||
| SCD50_T7 | - |
| Measure | SCD21_T5 | SCD50_T5 | SCD21_T6 | SCD50_T6 | SCD21_T7 | SCD50_T7 |
|---|---|---|---|---|---|---|
| Δ ROCFT copy T0–T7 | 0.34 | 0.42 | 0.52 * | 0.55 * | 0.44 | 0.64 ** |
| Agreeableness BFI (T7) | −0.08 | 0.09 | 0.02 | 0.06 | 0.05 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkana, O.; Levy, M.; Tal Bicovsky, Y.; Tal, N.; Oren, N.; Ash, E.L. Objective and Subjective Measures of Cognitive Decline in Highly Educated Older Adults: A 10-Year Longitudinal Study. J. Dement. Alzheimer's Dis. 2025, 2, 18. https://doi.org/10.3390/jdad2020018
Elkana O, Levy M, Tal Bicovsky Y, Tal N, Oren N, Ash EL. Objective and Subjective Measures of Cognitive Decline in Highly Educated Older Adults: A 10-Year Longitudinal Study. Journal of Dementia and Alzheimer's Disease. 2025; 2(2):18. https://doi.org/10.3390/jdad2020018
Chicago/Turabian StyleElkana, Odelia, Meitav Levy, Yael Tal Bicovsky, Noy Tal, Noga Oren, and Elissa L. Ash. 2025. "Objective and Subjective Measures of Cognitive Decline in Highly Educated Older Adults: A 10-Year Longitudinal Study" Journal of Dementia and Alzheimer's Disease 2, no. 2: 18. https://doi.org/10.3390/jdad2020018
APA StyleElkana, O., Levy, M., Tal Bicovsky, Y., Tal, N., Oren, N., & Ash, E. L. (2025). Objective and Subjective Measures of Cognitive Decline in Highly Educated Older Adults: A 10-Year Longitudinal Study. Journal of Dementia and Alzheimer's Disease, 2(2), 18. https://doi.org/10.3390/jdad2020018







