Gold-Based Medicine: A Paradigm Shift in Anti-Cancer Therapy?
Abstract
1. Introduction
2. General Perspectives
3. Gold(I) and Gold(III) Compounds with Promising Anti-Cancer Potential
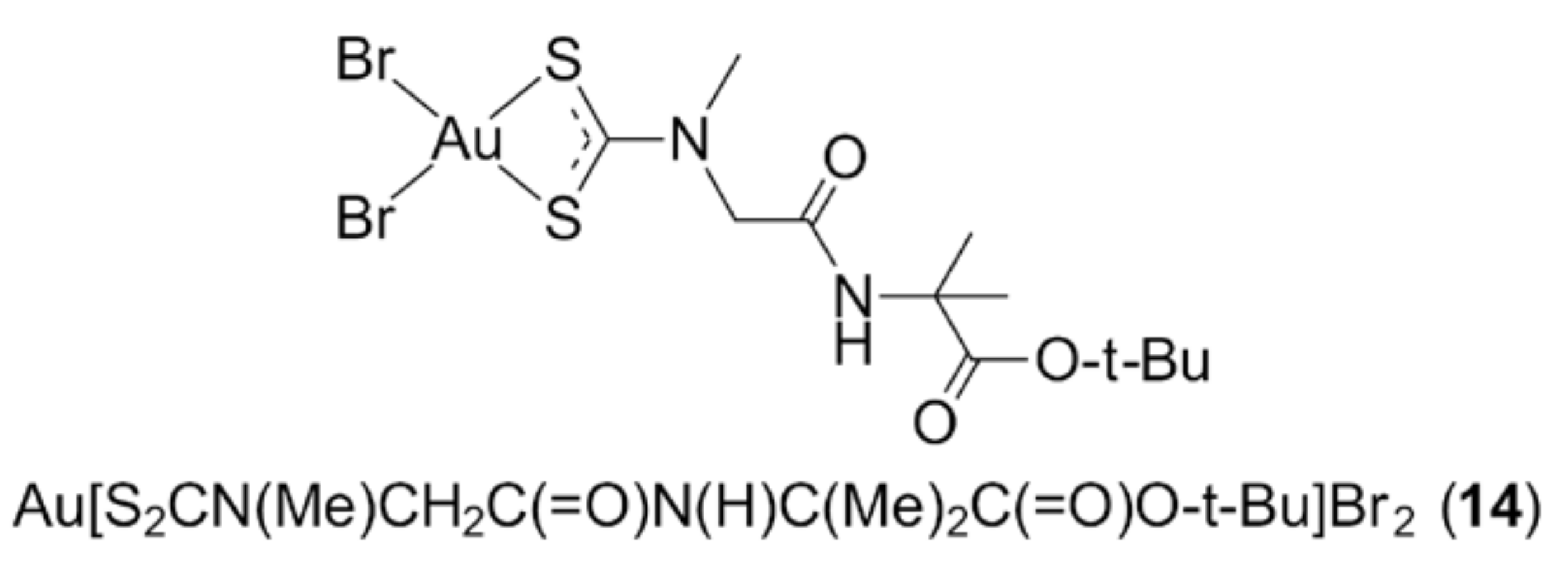
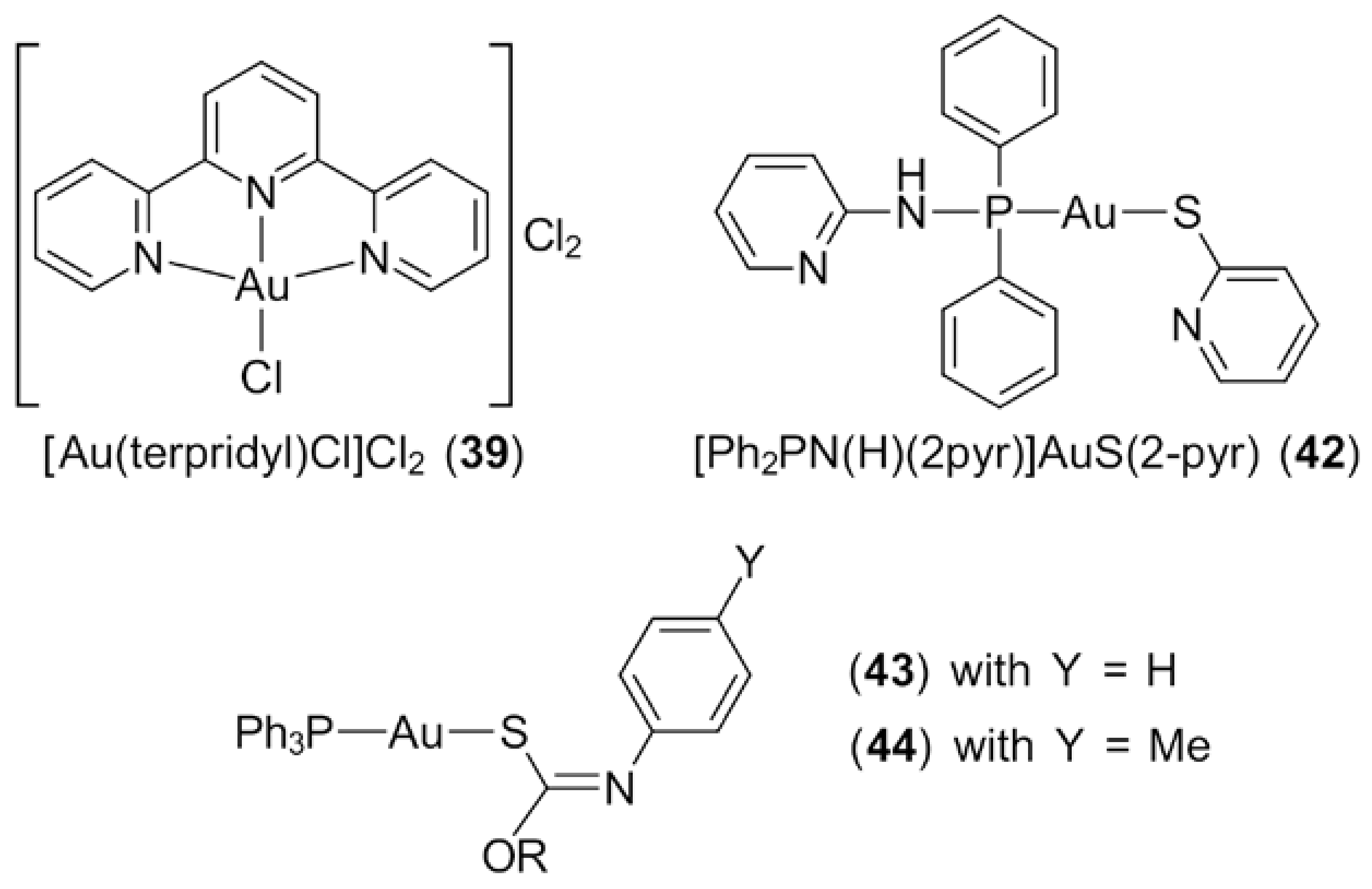
3.1. Phosphanegold(I) Compounds
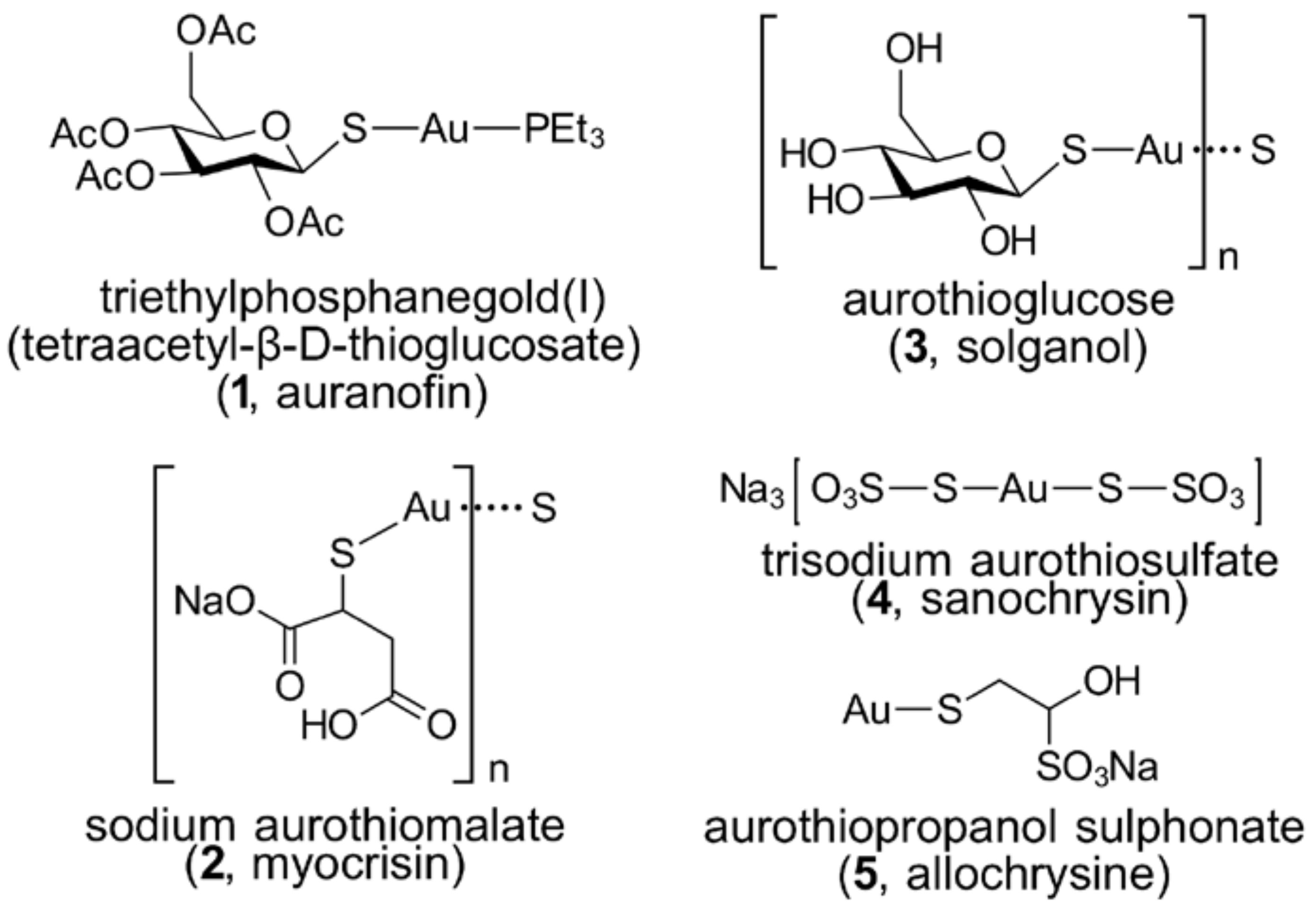
3.2. Gold Thiolates
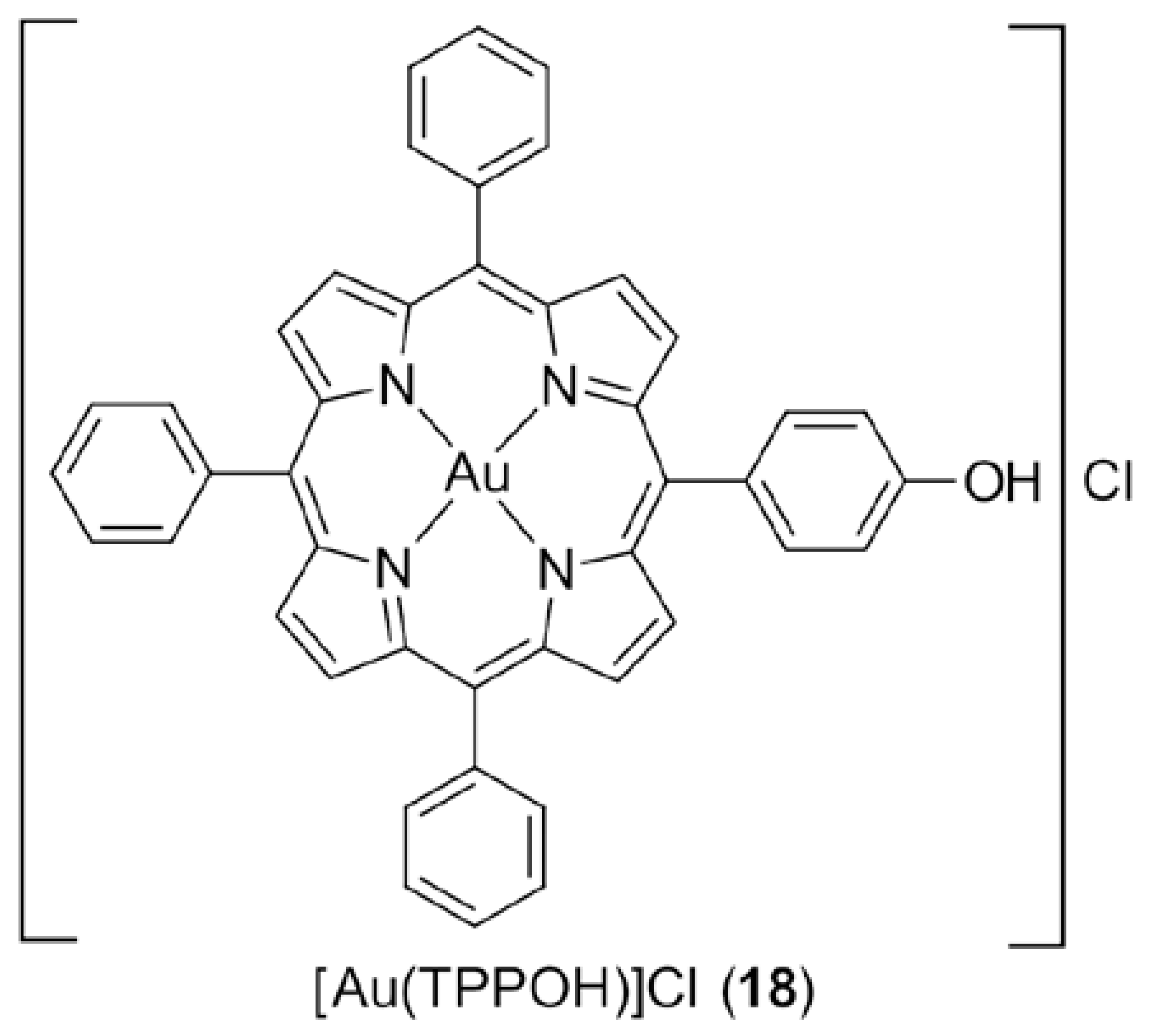
3.3. Gold Porphyrins
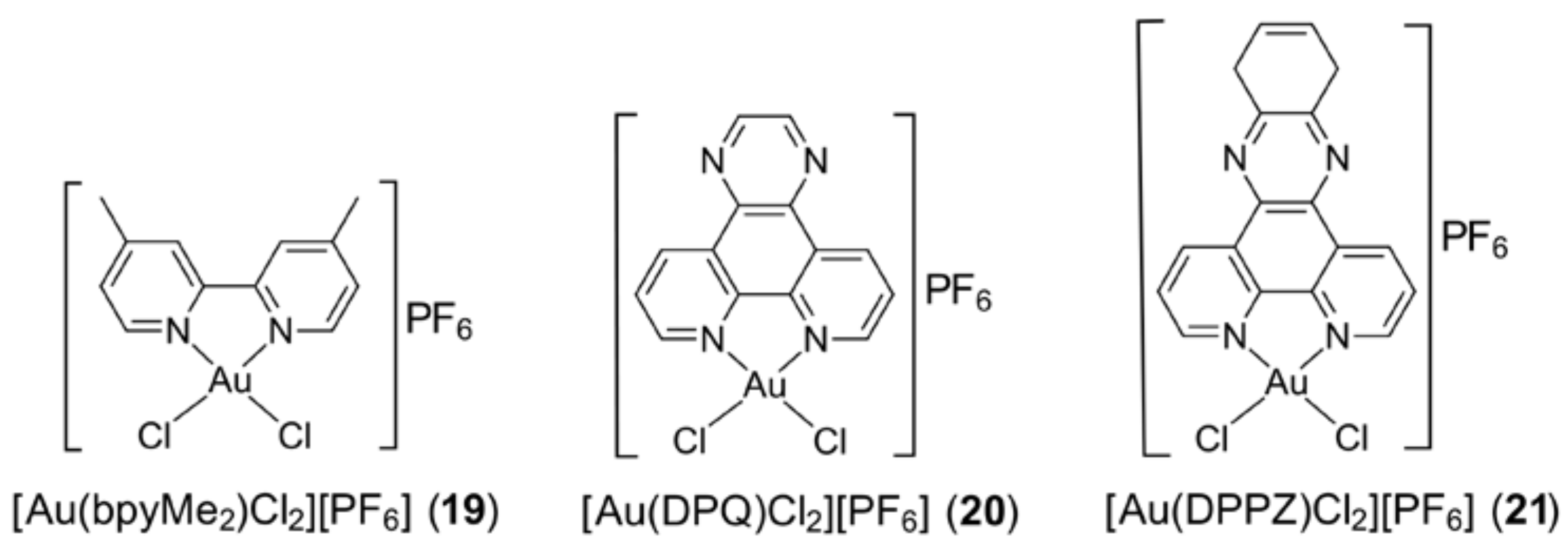
3.4. Gold Compounds with Bipyridyl-Type Ligands
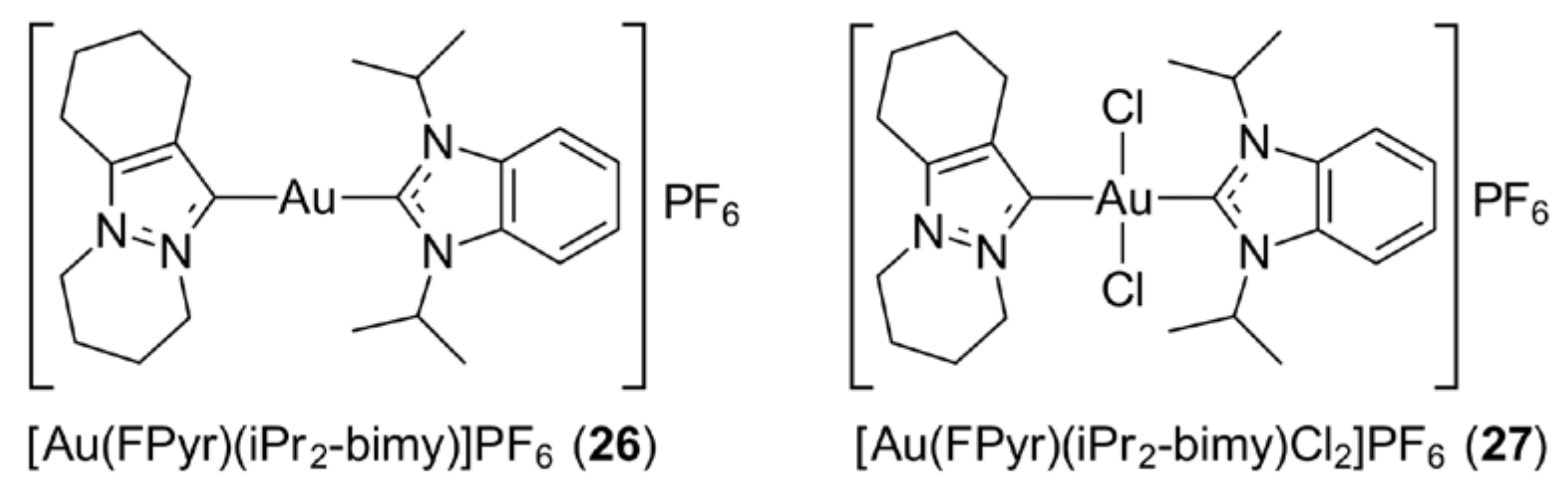
3.5. Gold N-Heterocyclic Carbenes
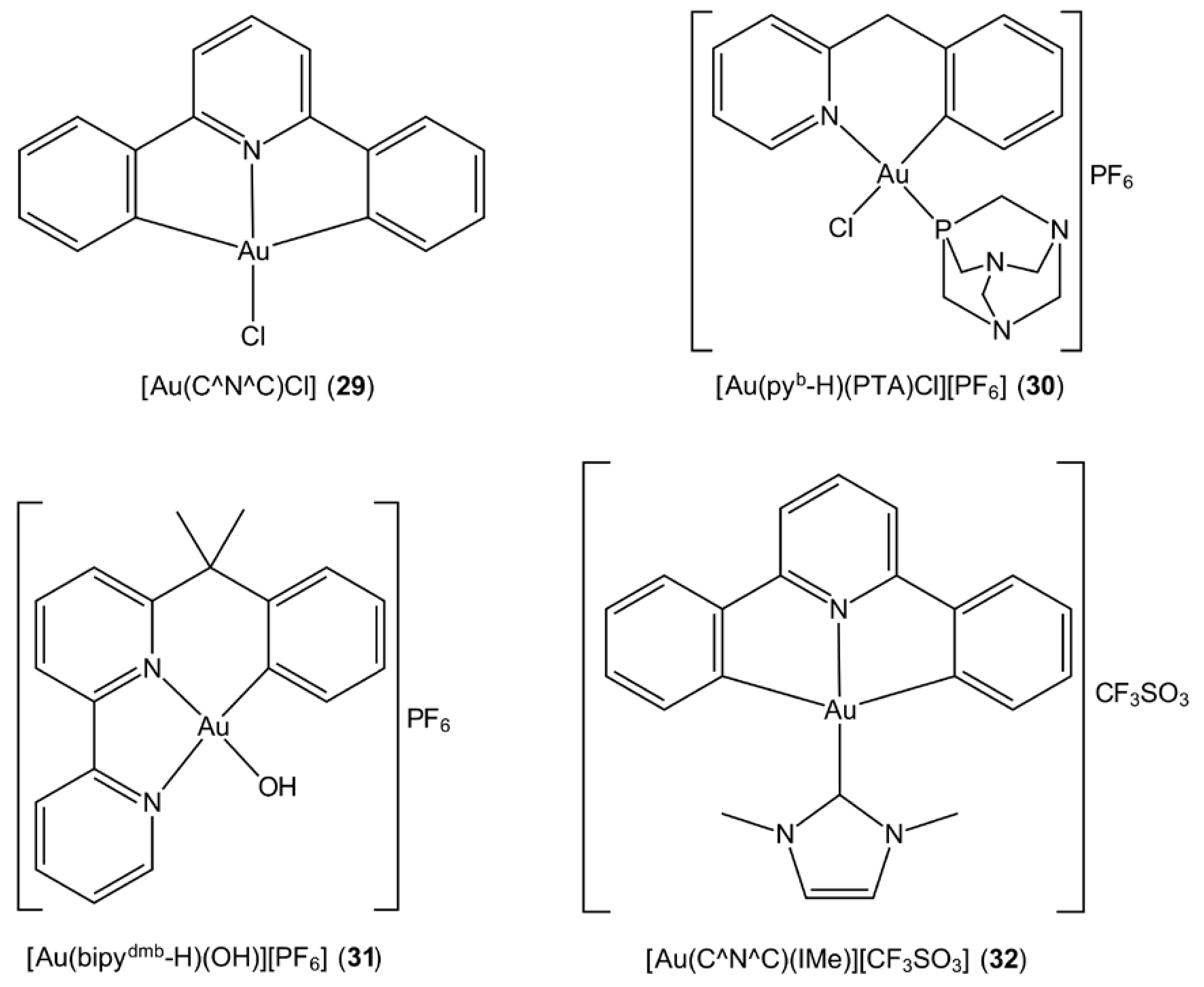
3.6. Cyclometallated Gold Complexes
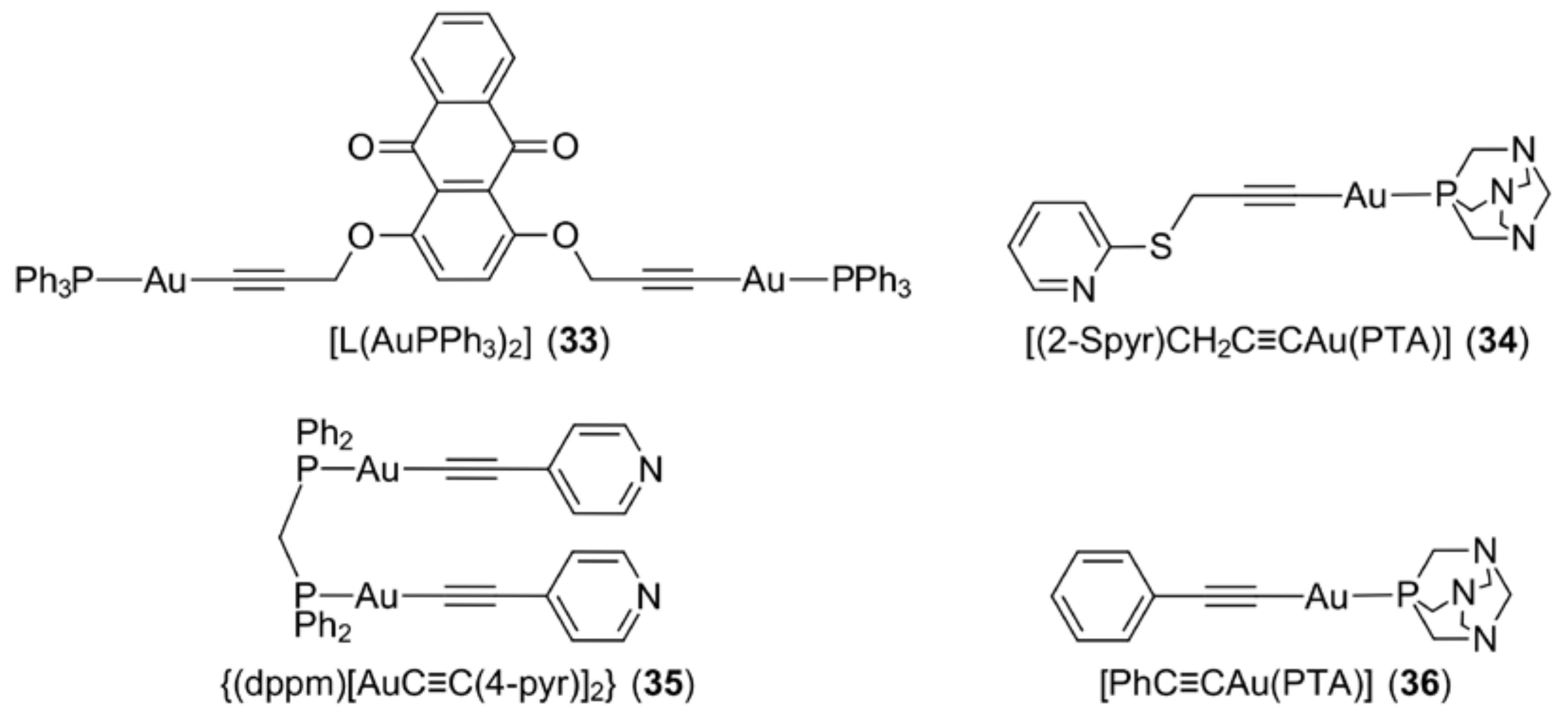
3.7. Gold Alkynyls
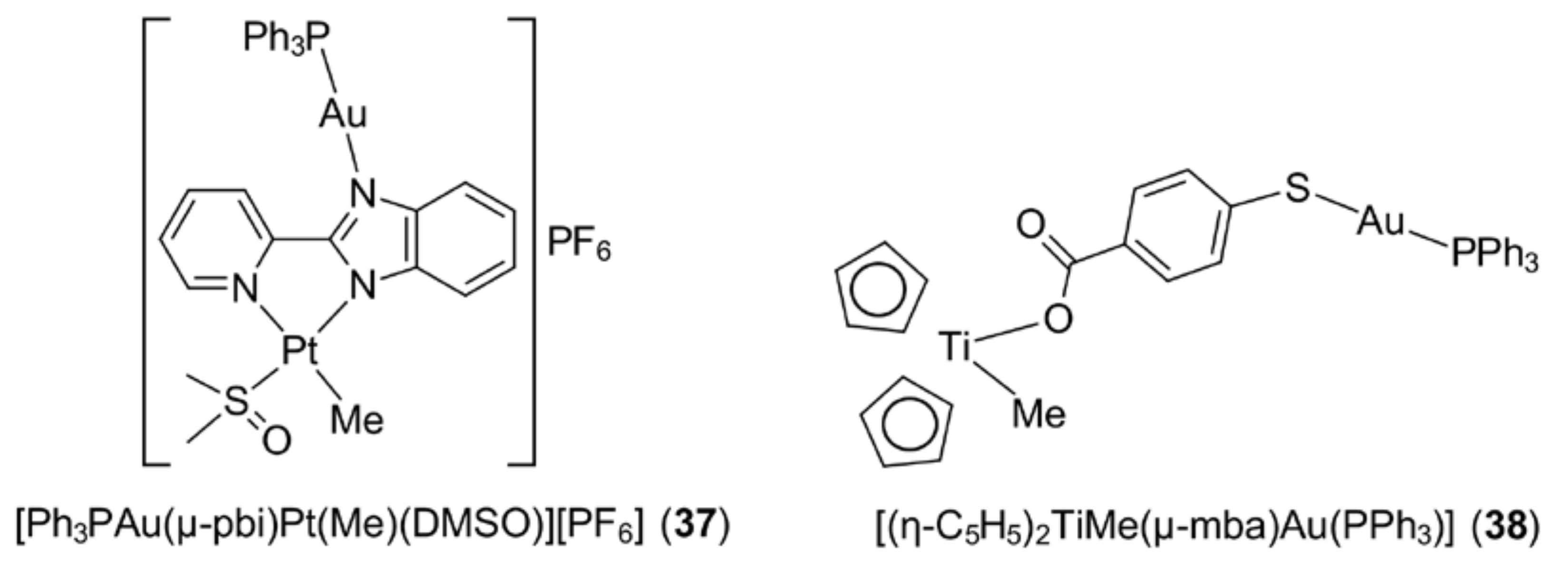
4. Other Approaches
5. Gold Compounds and Their Biological Mechanisms of Action
5.1. Direct DNA Damage
5.2. Inhibition of Topoisomerase
5.3. Mitochondria and the Inhibition of Their Function
5.4. Mitochondrial-Dependent Apoptosis Pathway
5.5. Inhibition of Thioredoxin
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nathan, C.; Ding, A. Nonresolving Inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.R.; Mantovani, A. Cancer-related inflammation: Common themes and therapeutic opportunities. Semin. Cancer Biol. 2012, 22, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Benedek, T.G. The history of gold therapy for tuberculosis. J. Hist. Med. Allied Sci. 2004, 59, 50–89. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble metals in medicine: Latest advances. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Scheffler, H.; You, Y.; Ott, I. Comparative studies on the cytotoxicity, cellular and nuclear uptake of a series of chloro gold(I) phosphine complexes. Polyhedron 2010, 29, 66–69. [Google Scholar] [CrossRef]
- Gielen, M.; Tiekink, E.R.T. Metallotherapeutic Drugs and Metal-Based Diagnostic Agents: The Use of Metals in Medicine; John Wiley & Sons Ltd.: Chichester, UK, 2005; pp. 1–598. ISBN 978-0-470-86403-6. [Google Scholar]
- Bruijnincx, P.C.A.; Sadler, P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gou, Z. Targeting and delivery of platinum-based anticancer drugs. Chem. Soc. Rev. 2013, 42, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, R.; Momtazi, A.A.; Monemi, A.; Sahebkar, A. Curcumin: A potentially powerful tool to reserve cisplatin-induced toxicity. Pharmacol. Res. 2017, 117, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Altoum, A.O.S.; Vanĉo, J.; Křikovová, R.; Trávníček, Z.; Dvořák, Z.; Altaf, M.; Ahmad, S.; Sulaiman, S.; Ahmad, S.; Sulaiman, A.A.A.; et al. Synthesis, structural characterization and cytotoxicity evaluation of platinum(II) complexes of heteroyclic selenones. Polyhedron 2017, 128, 2–8. [Google Scholar] [CrossRef]
- Nobili, S.; Mini, E.; Landini, I.; Gabbiani, C.; Casini, A.; Messori, L. Gold compounds as anticancer agents: Chemistry, cellular pharmacology, and preclinical studies. Med. Res. Rev. 2010, 30, 550–1128. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Hartinger, C.; Gabbiani, C.; Mini, E.; Dyson, P.J.; Keppler, B.K.; Messori, L. Gold(III) compounds as anticancer agents: Relevance of gold-protein interactions for their mechanism of action. J. Inorg. Biochem. 2008, 102, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Fricker, S.P. Medical uses of gold compounds: Past, present and future. Gold Bull. 1996, 29, 53–60. [Google Scholar] [CrossRef]
- Ott, I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar] [CrossRef]
- Berners-Price, S.J.; Filipovska, A. Gold compounds as therapeutic agents for human diseases. Metallomics 2011, 3, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Casini, A. A golden future in medicinal inorganic chemistry: The promise of anticancer gold organometallic compounds. Dalton Trans. 2014, 43, 4209–4219. [Google Scholar] [CrossRef] [PubMed]
- Nardon, C.; Boscutti, G.; Fregona, D. Beyond platinums: Gold complexes as anticancer agents. Anticancer Res. 2014, 34, 487–492. [Google Scholar] [PubMed]
- Lima, J.C.; Rodríguez, L. Phosphine-gold(I) compounds as anticancer agents: General description and mechanisms of action. Anti-Cancer Agents Med. Chem. 2011, 11, 921–928. [Google Scholar] [CrossRef]
- Milacic, V.; Fregona, D.; Dou, Q.P. Gold complexes as prospective metal-based anticancer drugs. Histol. Histopathol. 2008, 23, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kostrova, I. Gold coordination complexes as anticancer agents. Anticancer Agents Med. Chem. 2006, 6, 19–32. [Google Scholar] [CrossRef]
- Chen, D.; Milacic, V.; Frezza, M.; Dou, Q.P. Metal complexes, their cellular targets and potential for cancer therapy. Curr. Pharm. Des. 2009, 15, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Ott, I. Non platinum metal complexes as anti-cancer drugs. Arch. Pharm. 2007, 340, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Alama, A.; Tasso, B.; Novelli, F.; Sparatore, F. Organometallic compounds in oncology: Implications of novel organotins as antitumor agents. Drug Discov. Today 2009, 14, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, L.; Aldinucci, D.; Dou, Q.P.; Fregona, D. Latest insights into the anticancer activity of gold(III)-dithiocarbamato complexes. Anti-Cancer Agents Med. Chem. 2010, 10, 283–292. [Google Scholar] [CrossRef]
- Zou, T.; Lum, C.T.; Lok, C.-N.; Zhang, J.-J.; Che, C.-M. Chemical biology of anticancer gold(III) and gold(I) complexes. Chem. Soc. Rev. 2015, 44, 8786–8801. [Google Scholar] [CrossRef] [PubMed]
- Maia, P.I.S.; Deflon, V.M.; Abram, U. Gold(III) complexes in medicinal chemistry. Future Med. Chem. 2014, 6, 1515–1536. [Google Scholar] [CrossRef] [PubMed]
- Nardon, C.; Pettenuzzo, N.; Fregona, D. Gold complexes for therapeutic purposes: An updated patent review (2010–2015). Curr. Med. Chem. 2016, 23, 3374–3403. [Google Scholar] [CrossRef] [PubMed]
- Elder, D.P.; Holm, R.; Lopez de Diego, H. Use of pharmaceutical salts and cocrystals to address the issue of poor solubility. Int. J. Pharm. 2013, 453, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Tilborg, A.; Norberg, B.; Wouters, J. Pharmaceutical salts and cocrystals involving amino acids: A brief structural overview of the state-of-art. Eur. J. Med. Chem. 2014, 74, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Contel, M. Gold chemistry. Applications and future directions in the life sciences. Angew. Chem. Int. Ed. 2010, 49, 250–251. [Google Scholar] [CrossRef]
- Mohamed, A.A. Frontiers in gold chemistry. Inorganics 2015, 3, 370–373. [Google Scholar] [CrossRef]
- Pyykkö, P. Theoretical chemistry of gold. Angew. Chem. Int. Ed. 2004, 43, 4412–4456. [Google Scholar] [CrossRef] [PubMed]
- Schwerdtfeger, P. Relativistic effects in properties of gold. Heteroatom Chem. 2002, 13, 578–584. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Gold derivatives for the treatment of cancer. Crit. Rev. Oncol. Hematol. 2002, 42, 225–248. [Google Scholar] [CrossRef]
- Kean, W.F.; Kean, I.R.L. Clinical pharmacology of gold. Inflammopharmacology 2008, 3, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, I. Treatment of rheumatoid arthritis with cytostatic drugs. Ann. Clin. Res. 1975, 7, 195–201. [Google Scholar] [PubMed]
- Fries, J.F.; Bloch, D.; Spitz, P.; Mitchell, D.M. Cancer in rheumatoid arthritis: A prospective long-term study of mortality. Am. J. Med. 1985, 78, 56–59. [Google Scholar] [CrossRef]
- Ward, J.R. Role of disease-modifying antirheumatic drugs versus cytotoxic agents in the therapy of rheumatoid arthritis. Am. J. Med. 1988, 85, 39–44. [Google Scholar] [CrossRef]
- Maiore, L.; Cinellu, M.A.; Michelucci, E.; Moneti, G.; Nobili, S.; Landini, I.; Mini, E.; Guerri, A.; Gabbiani, C.; Messori, L. Structural and solution chemistry, protein binding and antiproliferative profiles of gold(I)/(III) complexes bearing the saccharinato ligand. J. Inorg. Biochem. 2011, 105, 348–355. [Google Scholar] [CrossRef] [PubMed]
- McKeage, M.J.; Maharaj, L.; Berners-Price, S.J. Mechanisms of cytotoxicity and antitumor activity of gold(I) phosphine complexes: The possible role of mitochondria. Coord. Chem. Rev. 2002, 232, 127–135. [Google Scholar] [CrossRef]
- Elie, B.T.; Levine, C.; Ubarretxena-Belandia, I.; Varela-Ramírez, A.; Aguilera, R.J.; Ovalle, R.; Contel, M. Water-soluble (phosphane)gold(I) complexes—Applications as recyclable catalysts in a three-component coupling reaction and as antimicrobial and anticancer agents. Eur. J. Inorg. Chem. 2009, 23, 3421–3430. [Google Scholar] [CrossRef] [PubMed]
- Hoke, G.D.; Rush, G.F.; Bossard, G.E.; McArdle, J.V.; Jensen, B.D.; Mirabelli, C.K. Mechanism of alterations in isolated rat liver mitochondrial function induced by gold complexes of bidentate phosphines. J. Biol. Chem. 1988, 263, 11203–11210. [Google Scholar] [PubMed]
- Rackham, O.; Nichols, S.J.; Leedman, P.J.; Berners-Price, S.J.; Filipovska, A. A gold(I) phosphine complex selectively induces apoptosis in breast cancer cells: Implications for anticancer therapeutics targeted to mitochondria. Biochem. Pharmacol. 2007, 74, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Wedlock, L.E.; Kilburn, M.R.; Cliff, J.B.; Filgueira, L.; Saunders, M.; Berners-Price, S.J. Visualising gold inside tumour cells following treatment with an antitumour gold(I) complex. Metallomics 2011, 3, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, C.; Kunz, P.C.; Kassack, M.U.; Hamacher, A.; Böhler, P.; Watjen, W.; Ott, I.; Rubbiani, R.; Spingler, B. Gold(I) complexes of water-soluble diphos-type ligands: Synthesis, anticancer activity, apoptosis and thioredoxin reductase inhibition. Dalton Trans. 2011, 40, 9212–9220. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Siu, F.-M.; Kui, S.C.F.; Lok, C.-N.; Che, C.-M. Anticancer gold(I)–phosphine complexes as potent autophagy-inducing agents. Chem. Commun. 2011, 47, 9318–9320. [Google Scholar] [CrossRef] [PubMed]
- Cattaruzza, L.; Fregona, D.; Mongiat, M.; Ronconi, L.; Fassina, A.; Colombatti, A.; Aldinucci, D. Antitumor activity of gold(III)-dithiocarbamato derivatives on prostate cancer cells and xenografts. Int. J. Cancer 2011, 128, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Marzano, C.; Ronconi, L.; Chiara, F.; Giron, M.C.; Faustinelli, I.; Cristofori, P.; Trevisan, A.; Fregona, D. Gold(III)-dithiocarbamato anticancer agents: Activity, toxicology and histopathological studies in rodents. Int. J. Cancer 2011, 129, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Kouodom, M.N.; Ronconi, L.; Celegato, M.; Nardon, C.; Marchiò, L.; Dou, Q.P.; Aldinucci, D.; Formaggio, F.; Fregona, D. Toward the selective delivery of chemotherapeutics into tumor cells by targeting peptide transporters: Tailored gold-based anticancer peptidomimetics. J. Med. Chem. 2012, 55, 2212–2226. [Google Scholar] [CrossRef] [PubMed]
- Nardon, C.; Schmitt, S.M.; Yang, H.; Zuo, J.; Fregona, D.; Dou, Q.P. Gold(III)-dithiocarbamato peptidomimetics in the forefront of the targeted anticancer therapy: Preclinical studies against human breast neoplasia. PLoS ONE 2014, 9, e84248. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.; Isab, A.A.; Vančo, J.; Dvořák, Z.; Trávníček, A.; Stoeckli-Evans, H. Synthesis, characterization and in vitro cytotoxicity of gold(III) dialkyl/diaryldithiocarbamato complexes. RSC Adv. 2015, 5, 81599–81607. [Google Scholar] [CrossRef]
- Shi, Y.; Chu, W.; Wang, Y.; Wang, S.; Du, J.; Zhang, J.; Li, S.; Zhou, G.; Qin, X.; Zhang, C. Synthesis, characterization and cytotoxicity of the Au(III) complexes with cyclic amine-based dithiocarbamate ligands. Inorg. Chem. Commun. 2013, 30, 178–181. [Google Scholar] [CrossRef]
- Altaf, M.; Monim-ul-Mehboob, M.; Isab, A.A.; Dhuna, V.; Bhatia, G.; Dhuna, K.; Altuwaijri, S. The synthesis, spectroscopic characterization and anticancer activity of new mono and binuclear phosphanegold(I) dithiocarbamate complexes. New J. Chem. 2015, 39, 377–385. [Google Scholar] [CrossRef]
- Jamaludin, N.S.; Goh, Z.-J.; Cheah, Y.K.; Ang, K.-P.; Sim, J.H.; Khoo, C.H.; Fairuz, Z.A.; Halim, S.N.B.H.; Ng, S.W.; Seng, H.L.; et al. Phosphanegold(I) dithiocarbamates, R3PAu[SC(=S)N(iPr)CH2CH2OH] for R = Ph, Cy and Et: Role of phosphane-bound R substituents upon in vitro cytotoxicity against MCF-7R breast cancer cells and cell death pathways. Eur. J. Med. Chem. 2014, 67, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Keter, F.K.; Guzei, I.A.; Nell, M.; Zyl, W.E.V.; Darkwa, J. Phosphinogold(I) dithiocarbamate complexes: Effect of the nature of phosphine ligand on anticancer properties. Inorg. Chem. 2014, 53, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.I.; Ooi, K.K.; Akim, A.M.; Ang, K.P.; Fairuz, Z.A.; Halim, S.N.B.H.; Ng, S.W.; Seng, H.-L.; Tiekink, E.R.T. The influence of R substituents in triphenylphosphinegold(I) carbonimidothioates, Ph3PAu[SC(OR)=NPh] (R = Me, Et and iPr), upon in vitro cytotoxicity against the HT-29 colon cancer cell line and upon apoptotic pathways. J. Inorg. Biochem. 2013, 127, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Vergara, E.; Cerrada, E.; Clavel, C.; Casini, A.; Laguna, M. Thiolato gold(I) complexes containing water-soluble phosphane ligands: A characterization of their chemical and biological properties. Dalton Trans. 2011, 40, 10927–10935. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, E.; Gascón, S.; Atrián-Blasco, E.; Rodriguez-Yoldi, M.J.; Cerrada, E.; Laguna, M. Gold(I) complexes with alkylated PTA (1,3,5-triaza-7-phosphaadamantane) phosphanes as anticancer metallodrugs. Eur. J. Med. Chem. 2014, 79, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Lok, C.-N.; Bierla, K.; Che, C.-M. Gold(I) complex of N,N′-disubstituted cyclic thiourea with in vitro and in vivo anticancer properties—Potent tight-binding inhibition of thioredoxin reductase. Chem. Commun. 2010, 46, 7691–7693. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Liu, Y.; Lai, Y.-T.; Tong, K.-C.; Fung, Y.-M.; Lok, C.-N.; Che, C.-M. Anticancer gold(III) porphyrins target mitochondrial chaperone Hsp60. Angew. Chem. Int. Ed. 2016, 55, 1387–1391. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Q.-Y.; Sun, R.W.-Y.; Che, C.-M.; Chiu, J.-F. Cellular pharmacological properties of gold(III) porphyrin 1a, a potential anticancer drug lead. Eur. J. Pharmacol. 2007, 554, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Q.-Y.; Che, C.-M.; Tsao, S.W.; Sun, R.W.-Y.; Chiu, J.-F. Modulation of gold(III) porphyrin 1a-induced apoptosis by mitogen-activated protein kinase signaling pathways. Biochem. Pharmacol. 2008, 75, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- To, Y.F.; Sun, R.W.-Y.; Chen, Y.; Chan, V.S.-F.; Yu, W.-Y.; Tam, P.K.-H.; Che, C.-M.; Lin, C.-L.S. Gold(III) porphyrin complex is more potent than cisplatin in inhibiting growth of nasopharyngeal carcinoma in vitro and in vivo. Int. J. Cancer 2009, 124, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Lum, C.T.; Li, X.; Sun, R.W.-Y.; Li, X.-P.; Peng, Y.; He, M.-L.; Kung, H.F.; Che, C.-M.; Lin, M.C.M. Gold(III) porphyrin 1a inhibited nasopharyngeal carcinoma metastasis in vivo and inhibited cell migration and invasion in vitro. Cancer Lett. 2010, 294, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lum, C.T.; Sun, R.W.-Y.; Zou, T.; Che, C.-M. Gold(III) complexes inhibit growth of cisplatin resistant ovarian cancer in association with upregulation of proapoptotic PMS2 gene. Chem. Sci. 2014, 5, 1579–1584. [Google Scholar] [CrossRef]
- Che, C.M.; Sun, R.W.; Yu, W.Y.; Ko, C.B.; Zhu, N.; Sun, H. Gold(III) porphyrins as a new class of anticancer drugs: Cytotoxicity, DNA binding and induction of apoptosis in human cervix epitheloid cancer cells. Chem. Commun. 2003, 14, 1718–1719. [Google Scholar] [CrossRef]
- Lum, C.T.; Yang, Z.F.; Li, H.Y.; Sun, R.W.; Fan, S.T.; Poon, R.T.; Lin, M.C.; Che, C.M.; Kung, H.F. Gold(III) compound is a novel chemo-cytotoxic agent for hepatocellular carcinoma (HCC). Int. J. Cancer 2006, 118, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Sun, R.W.; Lin, M.C.; Cui, J.T.; Zou, B.; Gu, Q.; Kung, H.F.; Che, C.M.; Wong, B.C. Gold(III) porphyrin complexes induce apoptosis and cell cycle arrest and inhibit tumor growth in colon cancer. Cancer 2009, 115, 4456–4469. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xie, Y.; Sun, R.W.-Y.; Liu, Q.; Young, J.; Yu, W.-Y.; Che, C.-M.; Tam, P.K.; Ren, Y. Inhibition of Akt sensitises neuroblastoma cells to gold(III) porphyrin 1a, a novel antitumour drug induced apoptosis and growth inhibition. Br. J. Cancer 2009, 101, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.W.-Y.; Li, C.K.-L.; Ma, D.-L.; Yan, J.J.; Lok, C.-N.; Leung, C.-H.; Zhu, N.; Che, C.-M. Stable anticancer gold(III)–porphyrin complexes: Effects of porphyrin structure. Chem. Eur. J. 2010, 16, 3097–3113. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Chen, T.; You, Y.; Hu, H.; Zheng, W.; Kwong, W.-L.; Zou, T.; Che, C.-M. A Cancer-targeted nanosystem for delivery of gold(III) complexes: Enhanced selectivity and apoptosis-inducing efficacy of a gold(III) porphyrin complex. Angew. Chem. Int. Ed. 2014, 53, 12532–12536. [Google Scholar] [CrossRef]
- Casini, A.; Diawara, M.C.; Scopelliti, R.; Zakeeruddin, S.M.; Grätzel, M.; Dyson, P.J. Synthesis, characterisation and biological properties of gold(III) compounds with modified bipyridine and bipyridylamine ligands. Dalton Trans. 2010, 39, 2239–2245. [Google Scholar] [CrossRef] [PubMed]
- Palanichamy, K.; Sreejayan, N.; Ontko, A.C. Overcoming cisplatin resistance using gold(III) mimics: Anticancer activity of novel gold(III) polypyridyl complexes. J. Inorg. Biochem. 2012, 106, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, C.D.; Olsen, P.M.; Elix, C.; Peng, S.; Wang, D.; Chen, Z.; Shin, D.M.; Hardcastle, K.I.; MacBeth, C.E.; Eichler, J.F. Antitumor properties of five-coordinate gold(III) complexes bearing substituted polypyridyl ligands. J. Inorg. Biochem. 2013, 128, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Cinellu, M.A.; Minghetti, G.; Gabbiani, C.; Coronnello, M.; Mini, E.; Messori, L. Structural and solution chemistry, antiproliferative effects, and DNA and protein binding properties of a series of dinuclear gold(III) compounds with bipyridyl ligands. J. Med. Chem. 2006, 49, 5524–5531. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.; Monti, D.M.; Amoresano, A.; Pontillo, N.; Petruk, G.; Pane, F.; Cinellu, M.A.; Merlino, A. Gold-based drug encapsulation within a ferritin nanocage: X-ray structure and biological evaluation as a potential anticancer agent of the Auoxo3-loaded protein. Chem. Commun. 2016, 52, 9518–9521. [Google Scholar] [CrossRef] [PubMed]
- Galassi, R.; Burini, A.; Ricci, S.; Pellei, M.; Rigobello, M.P.; Citta, A.; Dolmella, A.; Gandin, V.; Marzano, C. Synthesis and characterization of azolate gold(I) phosphane complexes as thioredoxin reductase inhibiting antitumor agents. Dalton Trans. 2012, 41, 5307–5318. [Google Scholar] [CrossRef] [PubMed]
- Serratrice, M.; Cinellu, M.A.; Maiore, L.; Pilo, M.; Zucca, A.; Gabbiani, C.; Guerri, A.; Landini, I.; Nobili, S.; Mini, E.; et al. Synthesis, structural characterization, solution behavior, and in vitro antiproliferative properties of a series of gold complexes with 2-(2′-pyridyl)benzimidazole as ligand: Comparisons of gold(III) versus gold(I) and mononuclear versus binuclear derivatives. Inorg. Chem. 2012, 51, 3161–3171. [Google Scholar] [CrossRef] [PubMed]
- Sivaram, H.; Tan, J.; Huynh, H.V. Syntheses, characterizations, and a preliminary comparative cytotoxicity study of gold(I) and gold(III) complexes bearing benzimidazole- and pyrazole-derived N-heterocyclic carbenes. Organometallics 2012, 31, 5875–5883. [Google Scholar] [CrossRef]
- Rana, B.K.; Nandy, A.; Bertolasi, V.; Bielawski, C.W.; Saha, K.D.; Dinda, J. Novel gold(I)− and gold(III)−N-heterocyclic carbene complexes: Synthesis and evaluation of their anticancer properties. Organometallics 2014, 33, 2544–2548. [Google Scholar] [CrossRef]
- Dinda, J.; Samanta, T.; Nandy, A.; Saha, K.D.; Seth, S.K.; Chattopadhyay, S.K.; Bielawski, C.W. N-heterocyclic carbene supported Au(I) and Au(III) complexes: A comparison of cytotoxicities. New J. Chem. 2014, 38, 1218–1224. [Google Scholar] [CrossRef]
- Nandy, A.; Samanta, T.; Mallick, S.; Mitra, P.; Seth, S.K.; Saha, K.D.; Al-Deyab, S.S.; Dinda, J. Synthesis of gold(III) ← gold(I)–NHC through disproportionation: The role of gold(I)–NHC in the induction of apoptosis in HepG2 cells. New J. Chem. 2016, 40, 6289–6298. [Google Scholar] [CrossRef]
- Rubbiani, R.; Schuh, E.; Meyer, A.; Lemke, J.; Wimberg, J.; Metzler-Nolte, N.; Meyer, F.; Mohr, F.; Ott, I. TrxR inhibition and antiproliferative activities of structurally diverse gold N-heterocyclic carbene complexes. MedChemComm 2013, 4, 942–948. [Google Scholar] [CrossRef]
- Schmidt, C.; Karge, B.; Misgeld, R.; Prokop, A.; Franke, R.; Brönstrup, M.; Ott, I. Gold(I) NHC complexes: Antiproliferative activity, cellular uptake, inhibition of mammalian and bacterial thioredoxin reductases, and gram-positive directed antibacterial effects. Chem. Eur. J. 2017, 23, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Messori, L.; Marchetti, L.; Massai, L.; Scaletti, F.; Guerri, A.; Landini, I.; Nobili, S.; Perrone, G.; Mini, E.; Leoni, P.; et al. Chemistry and biology of two novel gold(I) carbene complexes as prospective anticancer agents. Inorg. Chem. 2014, 53, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Oehninger, L.; Rubbiani, R.; Ott, I. N-Heterocyclic carbene metal complexes in medicinal chemistry. Dalton Trans. 2013, 42, 3269–3284. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, X.; Wang, W.; Zhang, R.; Deng, L. Metal-N-heterocyclic carbene complexes as anti-tumor agents. Curr. Med. Chem. 2014, 21, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Aher, S.B.; Muskawar, P.N.; Thenmozhi, K.; Bhagat, P.R. Recent developments of metal N-heterocyclic carbenes as anticancer agents. Eur. J. Med. Chem. 2014, 81, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Gautier, A.; Cisnetti, F. Advances in metal-carbene complexes as potent anti-cancer agents. Metallomics 2012, 4, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Dominelli, B.; Correia, J.D.G.; Kühn, F.E. Medicinal applications of gold(I/III)-based complexes bearing N-heterocyclic carbene and phosphine ligands. J. Organomet. Chem. 2018, 866, 153–164. [Google Scholar] [CrossRef]
- Li, C.K.-L.; Sun, R.W.-Y.; Kui, S.C.-F.; Zhu, N.; Che, C.-M. Anticancer cyclometalated [AuIIIm(C^N^C)mL]n+ compounds: Synthesis and cytotoxic properties. Chem. Eur. J. 2006, 12, 5253–5266. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.W.-Y.; Lok, C.-N.; Fong, T.T.-H.; Li, C.K.-L.; Yang, Z.F.; Zou, T.; Siu, A.F.-M.; Che, C.-M. A dinuclear cyclometalated gold(III)–phosphine complex targeting thioredoxin reductase inhibits hepatocellular carcinoma in vivo. Chem. Sci. 2013, 4, 1979–1988. [Google Scholar] [CrossRef]
- Bertrand, B.; Spreckelmeyer, S.; Bodio, E.; Cocco, F.; Picquet, M.; Richard, P.; Le Gendre, P.; Orvig, C.; Cinellu, M.A.; Casini, A. Exploring the potential of gold(III) cyclometallated compounds as cytotoxic agents: Variations on the C^N theme. Dalton Trans. 2015, 44, 11911–11918. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.M.; Gerner, C.; Keppler, B.K.; Cinellu, M.A.; Casini, A. Mass spectrometry uncovers molecular reactivities of coordination and organometallic gold(III) drug candidates in competitive experiments that correlate with their biological effects. Inorg. Chem. 2016, 55, 4248–4259. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Chow, A.L.-F.; Leung, C.-H.; Sun, R.W.-Y.; Ma, D.-L.; Che, C.-M. Cyclometalated gold(III) complexes with N-heterocyclic carbene ligands as topoisomerase I poisons. Chem. Commun. 2010, 46, 3893–3895. [Google Scholar] [CrossRef] [PubMed]
- Balasingham, R.G.; Williams, C.F.; Mottram, H.J.; Coogan, M.P.; Pope, S.J.A. Gold(I) complexes derived from alkynyloxy-substituted anthraquinones: Syntheses, luminescence, preliminary cytotoxicity, and cell imaging studies. Organometallics 2012, 31, 5835–5843. [Google Scholar] [CrossRef]
- García-Moreno, E.; Tomás, A.; Atrián-Blasco, E.; Gascón, S.; Romanos, E.; Rodriguez-Yoldi, M.J.; Cerrada, E.; Laguna, M. In vitro and in vivo evaluation of organometallic gold(I) derivatives as anticancer agents. Dalton Trans. 2016, 45, 2462–2475. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Gutiérrez, A.; Ott, I.; Rodríguez, L. Phosphine-bridged dinuclear gold(I) alkynyl complexes: Thioredoxin reductase inhibition and cytotoxicity. Inorg. Chim. Acta 2013, 398, 72–76. [Google Scholar] [CrossRef]
- Vergara, E.; Cerrada, E.; Casini, A.; Zava, O.; Laguna, M.; Dyson, P.J. Antiproliferative activity of gold(I) alkyne complexes containing water-soluble phosphane ligands. Organometallics 2010, 29, 2956–2603. [Google Scholar] [CrossRef]
- Sánchez-de-Diego, C.; Mármol, I.; Pérez, R.; Gascón, S.; Rodriguez-Yoldi, M.J.; Cerrada, E. The anticancer effect related to disturbances in redox balance on Caco-2 cells caused by an alkynyl gold(I) complex. J. Inorg. Biochem. 2017, 166, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Serratrice, M.; Maiore, L.; Zucca, A.; Stoccoro, S.; Landini, I.; Mini, E.; Massai, L.; Ferraro, G.; Merlino, A.; Messori, L.; et al. Cytotoxic properties of a new organometallic platinum(II) complex and its gold(I) heterobimetallic derivatives. Dalton Trans. 2016, 45, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Massai, L.; Fernández-Gallardo, J.; Guerri, A.; Arcangeli, A.; Pillozzi, S.; Contel, M.; Messori, L. Design, synthesis and characterisation of new chimeric ruthenium(II)–gold(I) complexes as improved cytotoxic agents. Dalton Trans. 2015, 44, 11067–11076. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gallardo, J.; Elie, B.T.; Sanaú, M.; Contel, M. Versatile synthesis of cationic N-heterocyclic carbene–gold(I) complexes containing a second ancillary ligand. Design of heterobimetallic ruthenium–gold anticancer agents. Chem. Commun. 2016, 52, 3155–3158. [Google Scholar] [CrossRef] [PubMed]
- Mui, Y.F.; Fernández-Gallardo, J.; Elie, B.T.; Gubran, A.; Maluenda, I.; Sanaú, M.; Navarro, O.; Contel, M. Titanocene−gold complexes containing N-heterocyclic carbene ligands inhibit growth of prostate, renal, and colon cancers in vitro. Organometallics 2016, 35, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gallardo, J.; Elie, B.T.; Sulzmaier, F.J.; Sanaú, M.; Ramos, J.W.; Contel, M. Organometallic titanocene−gold compounds as potential chemotherapeutics in renal cancer. Study of their protein kinase inhibitory properties. Organometallics 2014, 33, 6669–6681. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Bertrand, B.; Eymin, B.J.; Comte, V.; Harvey, J.A.; Richard, P.; Groessl, M.; Zava, O.; Amrouche, H.; Harvey, P.D.; et al. Multinuclear cytotoxic metallodrugs: Physicochemical characterization and biological properties of novel heteronuclear gold-titanium complexes. Inorg. Chem. 2011, 50, 9472–9480. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gallardo, J.; Elie, B.T.; Sadhukha, T.; Prabha, S.; Sanaú, M.; Rotenberg, S.A.; Ramos, J.W.; Contel, M. Heterometallic titanium–gold complexes inhibit renal cancer cells in vitro and in vivo. Chem. Sci. 2015, 6, 5269–5283. [Google Scholar] [CrossRef] [PubMed]
- Dykmana, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Akrami, M.; Balalaie, S.; Hosseinkhani, S.; Alipour, M.; Salehi, F.; Bahador, A.; Haririan, I. Tuning the anticancer activity of a novel pro-apoptotic peptide using gold nanoparticle platforms. Sci. Rep. 2016, 6, 31030. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, X.; Hu, J.; Shao, N.; Wang, F.; Zhang, Q.; Xiao, J.; Cheng, Y. Glutathione-triggered “off−on” release of anticancer drugs from dendrimer-encapsulated gold nanoparticles. J. Am. Chem. Soc. 2013, 135, 9805–9810. [Google Scholar] [CrossRef] [PubMed]
- Bachelet, M. Design of pH-responsive gold nanoparticles in oncology. Mater. Sci. Technol. 2016, 32, 794–804. [Google Scholar] [CrossRef]
- Adnan, N.N.M.; Cheng, Y.Y.; Ong, N.M.N.; Kamaruddin, T.T.; Rozlan, E.; Schmidt, T.W.; Duong, H.T.T.; Boyer, C. Effect of gold nanoparticle shapes for phototherapy and drug delivery. Polym. Chem. 2016, 7, 2888–2903. [Google Scholar] [CrossRef]
- Bao, H.; Zhang, Q.; Xu, H.; Yan, Z. Effects of nanoparticle size on antitumor activity of 10-hydroxycamptothecin-conjugated gold nanoparticles: In vitro and in vivo studies. Int. J. Nanomed. 2016, 11, 929–940. [Google Scholar] [CrossRef]
- Li, T.; Li, F.; Xiang, W.; Yi, Y.; Chen, Y.; Cheng, L.; Liu, Z.; Xu, H. Selenium-containing amphiphiles reduced and stabilized gold nanoparticles: Kill cancer cells via reactive oxygen species. ACS Appl. Mater. Interfaces 2016, 8, 22106–22112. [Google Scholar] [CrossRef] [PubMed]
- Bhabak, K.P.; Bhuyan, B.J.; Mugesh, G. Bioinorganic and medicinal chemistry: Aspects of gold(I)-protein complexes. Dalton Trans. 2011, 40, 2099–2111. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.T.; Zhang, J.J.; Cao, B.; Tong, K.C.; Lok, C.N.; Che, C.M. Deubiquitinases as anticancer targets of gold complexes. Isr. J. Chem. 2016, 56, 825–833. [Google Scholar] [CrossRef]
- Walther, W.; Dada, O.; O’Beirne, C.; Ott, I.; Sanchez-Sanz, G.; Schmidt, C.; Werner, C.; Zhu, X.M.; Tacke, M. In vitro and in vivo investigation into carbine gold chloride and thioglucoside anticancer drug candidates NHC-AuCl and NHC-AuSR. Lett. Drug Des. Discov. 2017, 14, 125–134. [Google Scholar] [CrossRef]
- Bindoli, A.; Rigobelli, M.P.; Guido, S.; Gabbiani, C.; Casini, A.; Luigi, M. Thioredoxin reductase: A target for gold compounds acting as potential anticancer drugs. Coord. Chem. Rev. 2009, 253, 1692–1707. [Google Scholar] [CrossRef]
- Chaves, J.D.S.; Neumann, F.; Francisco, T.M.; Corrêa, C.C.; Lopes, M.T.P.; Silva, H.; Fontes, A.P.S.; Almeida, M.V. Synthesis and cytotoxic activity of gold(I) complexes containing phosphines and 3-benzyl-1,3-thiozolidine-2-thione or 5-phenyl-1,3,4-oxadiazole-2-thione as ligands. Inorg. Chim. Acta 2014, 414, 85–90. [Google Scholar] [CrossRef]
- Lin, Y.X.; Gao, Y.J.; Wang, Y.; Qiao, Z.Y.; Fan, G.; Qiao, S.L.; Zhang, R.X.; Wang, L.; Wang, H. pH-sensitive polymeric nanoparticles with gold(I) compounds payloads synergistically induce cancer cell death through modulation of autophagy. Mol. Pharm. 2015, 12, 2869–2878. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, F.; Parveen, S.; Afzal, M.; Shahid, M. Synthesis, characterization, biological studies (DNA binding, cleavage, antibacterial and toposisomerase I) and molecular docking of copper(II) benzimidazole complexes. J. Photochem. Photobiol. B 2012, 114, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.S.; Yuan, W.B.; Wang, H.Y.; Zhang, Q.; Liu, M.; Yu, K.B. Synthesis, crystal structure and interaction with DNA and HAS of (N,N′-dibenzylethane-1,2-diammine) transition metal complexes. J. Inorg. Biochem. 2008, 102, 2026–2034. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K.K.; Yeo, C.I.; Theventhiran, M.; Ang, K.P.; Abdah, M.A.; Cheah, Y.K.; Seng, H.L.; Tiekink, E.R.T. G2/M cell cycle arrest on HT-29 cancer cells and toxicity assessment of triphenylphosphanegold(I) carbonimidothioates, Ph3PAu[SC(OR)=NPh], R = Me, Et, and iPr, during zebrafish development. J. Inorg. Biochem. 2017, 166, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yan, C.; Gan, T.; Chen, Z.; Lu, X.; Duerksen-Hughes, P.J.; Zhu, X.; Yang, J. Nuclear proteasome analysis of cisplatin-treated HeLa cells. Mutat. Res. 2010, 691, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.I.; Hussien, H.M. Cisplatin-induced renal toxicity via tumor necrosis factor-α, interleukin 6, tumor suppressor p53, DNA damage, xanthine oxidase, histological changes, oxidative stress and nitric oxide in rats: Protective effect of -ginseng. Food Chem. Toxicol. 2015, 78, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.N.; Bhatt, B.S.; Dosi, P.A. Synthesis and evaluation of gold(III) complexes as efficient DNA binders and cytotoxic agents. Spectrochim. Acta A 2013, 110, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.; Monim-Ul-Mehboob, M.; Kawde, A.N.; Corona, G.; Larcher, R.; Ogasawara, M.; Casagrande, N.; Celegato, M.; Borghese, C.; Siddik, Z.H.; et al. New bipyridine gold(III) dithiocarbamate-containing complexes exerted a potent anticancer activity against cisplatin-resistant cancer cells independent of p53 status. Oncotarget 2017, 8, 490–505. [Google Scholar] [CrossRef] [PubMed]
- Gabbiani, C.; Casini, A.; Messori, L. Gold(III) compounds as anticancer drugs. Gold Bull. 2017, 40, 73–81. [Google Scholar] [CrossRef]
- Shi, P.; Jiang, Q.; Zhao, Y.; Zhang, Y.; Lin, J.; Lin, L.; Ding, J.; Guo, Z. DNA binding properties of novel cytotoxic gold(III) complexes of terpyridine ligands: The impact of steric and electrostatic effects. J. Biol. Inorg. Chem. 2006, 11, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Sarioglu, O.F.; Tekiner-Gursacli, R.; Ozdemir, A.; Tekinay, T. Comparison of Au(III) and Ga(III) ions binding to calf thymus DNA: Spectroscopic characterization and thermal analysis. Biol. Trace Elem. Res. 2014, 160, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, L.; Marzano, C.; Zanello, P.; Corsini, M.; Miolo, G.; Macca, C.; Trevisan, A.; Fregona, D. Gold(III) dithiocarbamate derivatives for the treatment of cancer: Solution chemistry, DNA binding and hemolytic properties. J. Med. Chem. 2006, 49, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Gandin, V.; Fernandes, A.P.; Rigobello, M.P.; Dani, B.; Sorrentino, F.; Tisato, F.; Bjornstedt, M.; Bindolli, A.; Sturaro, A.; Rella, R.; et al. Cancer cell death induced by phosphinegold(I) compounds targeting thioredoxin reductase. Biochem. Pharmacol. 2010, 79, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Marcon, G.; Messori, L.; Orioli, P.; Cinellu, M.A.; Minghetti, G. Reactions of gold(III) complexes with serum albumin. Eur. J. Biochem. 2003, 270, 4655–4661. [Google Scholar] [CrossRef] [PubMed]
- Maiore, L.; Cinellu, M.A.; Nobili, S.; Landini, I.; Mini, E.; Gabbiani, C.; Messori, L. Gold(III) complexes with 2-substituted pyridine as experimental anticancer agents: Solution behavior, reactions with model proteins, antiproliferative properties. J. Inorg. Biochem. 2012, 108, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.Z.; Zhou, L.X. Insights into the mechanism of binding of the gold(III) dithiocarbamate derivatives to cysteine or DNA purine bases. Struct. Chem. 2016, 27, 651–662. [Google Scholar] [CrossRef]
- Messori, L.; Marcon, G.; Innocenti, A.; Gallori, E.; Franchi, M.; Orioli, P. Molecular recognition of metal complexes by DNA: A comparative study of the interactions of the parent complexes [PtCl(TERPY)Cl] and [AuCl(TERPY)Cl2 with double stranded DNA. Bioinorg. Chem. Appl. 2005, 3, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Tiekink, E.R.T. Anti-cancer potential of gold complexes. Inflammopharmacology 2008, 16, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Ortego, L.; Cardoso, F.; Martins, S.; Fillat, M.F.; Laguna, A.; Meireles, M.; Villacampa, M.D.; Gimeno, M.C. Strong inhibition of thioredoxin reductase by highly cytotoxic gold(I) complexes. DNA binding studies. J. Inorg. Biochem. 2014, 130, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K.K.; Ang, K.P.; Abdah, M.A.; Cheah, Y.K.; Yeo, C.I.; Siti Nadiah, A.H.; Seng, H.L.; Tiekink, E.R.T. Phosphanegold(I) thiolates, Ph3PAu[SC(O-R)=NC6H4Me-4] for R = Me, Et and iPr, induce apoptosis, cell cycle arrest and inhibit cell invasion of HT-29 colon cancer cells through modulation of the nuclear factor κB activation pathway and ubiquitination. J. Inorg. Biochem. 2015, 20, 855–873. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hadwiger, L.A. Nonhost resistance: Reactive oxygen species (ROS) signal causes DNA damage prior to the induction of PR genes and disease resistance in pea tissue. Physiol. Mol. Plant Pathol. 2017, 98, 18–24. [Google Scholar] [CrossRef]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Selvi, S.K.; Vinoth, A.; Varadharajan, T.; Weng, C.F.; Padma, V.V. Neferine augments therapeutic efficacy of cisplatin through ROS-mediated non-canonical autophagy in human lung adenocarcinoma (A549 cells). Food Chem. Toxicol. 2017, 103, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.K.; Gwak, J.; Song, I.S.; Park, H.S.; Oh, S. Induction of apoptosis in colon cancer cells by novel topoisomerase I inhibitor TopIn. Biochem. Biophys. Res. Commun. 2011, 409, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; Languino, L.R.; Raskett, C.M.; Mercurio, A.M.; Dohi, T.; Altieri, D.C. IAP regulation of metastasis. Cancer Cell 2010, 17, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.L.; Perego, P.; Zumino, F. Targeting topoisomerase I: Molecular mechanisms and cellular determinants of response to topoisomerase I inhibitors. Biochem. Pharmacol. 2008, 10, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Dartier, J.; Lemaitre, E.; Chourpa, I.; Goupille, C.; Servais, S.; Chevalier, S.; Maheo, K.; Dumas, J.F. ATP-dependent activity and mitochondrial localization of drug efflux pumps in doxorubicin-resistant breast cancer cells. Biochim. Biophys. Acta 2017, 1861, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Rigobello, M.P.; Scutari, G.; Boscolo, R.; Bindoli, A. Induction of mitochondrial permeability transition by auranofin, a gold(I) phosphine derivative. Br. J. Pharmacol. 2002, 136, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.G.; Mohamed, M.F.; Abdelhamid, A.O.; Mohamed, M.S. A novel adamantine thiadiazole derivative induces mitochondria-mediated apoptosis in lung carcinoma cell line. Bioinorg. Med. Chem. 2017, 25, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Barnard, P.J.; Berners-Price, S.J. Targeting the mitochondrial cell death pathways with gold compounds. Coord. Chem. Rev. 2007, 251, 1889–1902. [Google Scholar] [CrossRef]
- Garcia, A.; Machado, R.C.; Grazul, R.M.; Lopes, M.T.P.; Correa, C.C.; Dos Santos, H.F.; de Almeida, M.V.; Silva, H. Novel antitumor adamantine-azole gold(I) complexes as potential inhibitors of thioredoxin reductase. J. Biol. Inorg. Chem. 2016, 21, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Gromer, S.; Urig, S.; Becker, K. The thioredoxin system: From science to clinic. Med. Res. Rev. 2004, 24, 40–89. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.J. Focus on mammalian thioredoxin reductases: Important selenoproteins with versatile functions. Biochim. Biophys. Acta 2009, 1790, 495–526. [Google Scholar] [CrossRef] [PubMed]
- Vanco, J.; Galikova, J.; Hoosek, J.; Dvorak, Z.; Parakova, L.; Travnicek, Z. Gold(I) complexes of 9-deazahypoxanthine as selective antitumor and anti-inflammatory agents. PLoS ONE 2014, 9, e109901. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Rana, N.K.; Singh, P.; Dubey, P.; Pandey, D.S.; Koch, B. p53 dependent apoptosis and cell cycle delay induced by heteroleptic complexes in human cervical cancer cells. Biomed. Pharmacother. 2017, 88, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Kroemer, G. Pharmacological manipulation of cell death: Clinical applications in sight? J. Clin. Investig. 2005, 25, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jiang, H.; Ma, N.; Wang, Y. Phosphorylated-p38 mitogen activated kinase expression is associated with clinical factors in invasive breast cancer. Springerplus 2016, 5, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, I.S. The role of p38 MAPK activation in auranofin-induced apoptosis of human promyelocytic leukemia HL-60 cells. Br. J. Pharmacol. 2015, 146, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Gillissen, B.; Richter, A.; Preissner, R.; Schulze-Osthoff, K.; Essmann, F.; Daniel, P.T. Bax/Bak-independent mitochondrial depolarization and reactive oxygen species induction by sorafenib overcome resistance to apoptosis in renal cell carcinoma. J. Biol. Chem. 2017, 292, 6478–6492. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Jiang, H.; Zhu, X.; Liu, X.; Li, J. Ginsenoside Rg3 promotes cytotoxicity of Paclitaxel through inhibiting NF-κB signaling and regulating Bax/Bcl-2 expression on triple-negative breast cancer. Biomed. Pharmacother. 2017, 89, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Sun, K.; Wang, Y.; Dong, S.; Wang, C.; Liu, L.X.; Wu, Y.H. Role of Cyt-C/caspases-9,3, Bax/Bcl-2 and the FAS death receptor pathway in apoptosis induced by zinc oxide nanoparticles in human aortic endothelial cells and the protective effect by alpha-lipoic acid. Chem. Biol. Interact. 2016, 258, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Reshi, L.; Wang, H.; Hui, C.; Su, Y.; Hong, J. Anti-apoptotic genes Bcl-2 and Bcl-xL overexpression can block iridovirus serine/threonine kinase-induced Bax/mitochondria-mediated cell death in GF-1 cells. Fish Shellfish Immunol. 2017, 61, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.L.; Ruhayel, R.A.; Barnard, P.J.; Baker, M.V.; Berners-Price, S.J.; Filipovska, A. Mitochondria-targeted chemotherapeutics: The rational design of gold(I) N-heterocyclic carbene complexes that are selectively toxic to cancer cells and target protein selenols in preference to thiols. J. Am. Chem. Soc. 2008, 130, 12570–12571. [Google Scholar] [CrossRef] [PubMed]
- Urig, S.; Becker, K. On the potential of thioredoxin reductase inhibitors for cancer therapy. Semin. Cancer Biol. 2006, 16, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Powis, G.; Kirkpatrick, D.L. Thioredoxin signaling as a target for cancer therapy. Curr. Opin. Pharmacol. 2007, 7, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Powis, G.; Montfort, W.R. Properties and biological activities of thioredoxin. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 261–295. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, H.B.; Piao, C.; Kang, M.Y.; Park, S.; Kim, S.W.; Lee, J. P32R2 regulates thioredoxin reductase activity through interaction with TrxR2. Biochem. Biophys. Res. Commun. 2016, 482, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Nagakannan, P.; Iqbal, M.A.; Yeung, A.; Thliveris, J.A.; Rastegar, M.; Ghavami, S.; Eftekharpour, E. Perturbation of redox balance after thioredoxin reductase deficiency interrupts autophagy-lysosomal degradation pathway and enhances cell death in nutritionally stressed SH-SY5Y cells. Free Radic. Biol. Med. 2017, 101, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kobayashi, M.; Sakurada, K.; Imamura, M.; Moriuchi, T.; Hosokawa, M. Possible roles of an adult T-cell leukemia (ATL)-derived factor/thioredoxin in the drug resistance of ATL to Adriamycin. Blood 1997, 89, 2480–2487. [Google Scholar] [CrossRef] [PubMed]
- Raninga, P.V.; Di Trapani, G.; Vuckovic, S.; Tonissen, K.F. Cross-talk between two antioxidants, thioredoxin reductase and heme oxygenase-1, and therapeutic implications for multiple myeloma. Redox Biol. 2016, 8, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Andricopulo, A.D.; Akoachere, M.B.; Krogh, R.; Nickel, C.; McLeish, M.J.; Kenyon, G.L.; Arscott, L.D.; Williams, C.H., Jr.; Davioud-Charvet, E.; Becker, K. Specific inhibitors of Plasmodium falciparum thioredoxin reductase as potential antimalarial agents. Bioorg. Med. Chem. Lett. 2006, 16, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Jortzik, E.; Becker, K. Thioredoxin and glutathione systems in Plasmodium falciparum. Int. J. Med. Microbiol. 2012, 302, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, A.S.; Filipovska, A.; Berners-Price, S.J.; Koutsantonis, G.A.; Skelton, B.W.; White, A.H. Gold(I) chloride adducts of 1,3-bis(di-2-pyridylphosphino)propane: Synthesis, structural studies and antitumour activity. Dalton Trans. 2007, 21, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, P.B.; Edes, K.; Nelson, C.C.; Parsawar, K.; Fitzpatrick, F.A.; Moos, P.J. Thioredoxin reductase is required for the inactivation of tumor suppressor p53 and for apoptosis induced by endogenous electrophiles. Carcinogenesis 2006, 27, 2538–2549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xu, Z.H.; Yuan, J.; Zhao, Y.X.; Qiao, Z.Y.; Gao, Y.J.; Yu, G.A.; Li, J.Y.; Wang, H. Synthesis and molecular recognition studies on small molecule inhibitors for thioredoxin reductase. J. Med. Chem. 2014, 57, 8132–8139. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.J.; Holmgren, A. The thioredoxin system in cancer. Semin. Cancer Biol. 2006, 16, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Marzano, C.; Gandin, V.; Folda, A.; Scurari, G.; Bindoli, A.; Rigobello, M.P. Inhibition of thioredoxin reductase by auranofin induces apoptosis in cisplatin-resistant human ovarian cancer cells. Free Radic. Biol. Med. 2007, 42, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Pennington, J.D.; Jacobs, K.M.; Sun, L.; Bar-Sela, G.; Mishra, M.; Gius, D. Thioredoxin and thioredoxin reductase as redox-sensitive molecular targets for cancer therapy. Curr. Pharm. Des. 2007, 13, 3368–3377. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.M.; Jones, D.P. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta 2008, 1780, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Lu, J.; Holmgren, A. Thioredoxin reductase is irreversibly modified by curcumin. J. Biol. Chem. 2005, 280, 25284–25290. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; de Almeida, A.; van der Burgt, E.P.M.; Picquet, M.; Citta, A.; Folda, A.; Rigobello, M.P.; Le Gendre, P.; Casini, A. New gold(I) organometallic compounds with biological activity in cancer cells. Eur. J. Inorg. Chem. 2014, 27, 4532–4536. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Shimizu, S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 2002, 12, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.S.; Ooi, K.K.; Ang, K.P.; Abdah, M.A.; Cheah, Y.K.; Halim, S.N.B.A.; Seng, H.L.; Tiekink, E.R.T. Molecular mechanisms of apoptosis and cell selectivity of zinc dithiocarbamates functionalized with hydroxyethyl substituents. J. Inorg. Biochem. 2015, 150, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Ishak, D.H.A.; Ooi, K.K.; Ang, K.P.; Abdah, M.A.; Cheah, Y.K.; Nordin, N.; Halim, S.N.B.A.; Seng, H.L.; Tiekink, E.R.T. A bismuth diethyldithiocarbamate compound promotes apoptosis in HepG2 carcinoma, cell cycle arrest and inhibits cell invasion through modulation of the NF-κB activation pathway. J. Inorg. Biochem. 2014, 130, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Gromer, S.; Arscott, L.D.; Williams, C.H.; Schirmer, R.H.; Becker, K. Human placenta thioredoxin reductase: Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J. Biol. Chem. 1998, 273, 20096–20101. [Google Scholar] [CrossRef] [PubMed]
- Rigobello, M.P.; Messori, L.; Marcon, G.; Cinellu, M.A.; Bragadin, M.; Folda, A.; Sutari, G.; Bindoli, A. Gold complexes inhibit mitochondrial thioredoxin reductase: Consequences on mitochondrial functions. J. Inorg. Biochem. 2004, 98, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Coronnello, M.; Mini, E.; Caciagli, B.; Cinellu, M.A.; Bindoli, A.; Gabbiani, C.; Messori, L. Mechanisms of cytotoxicity of selected organogold(III) compounds. J. Med. Chem. 2005, 48, 6761–6765. [Google Scholar] [CrossRef] [PubMed]
- Saggioro, D.; Rigobello, M.P.; Paloschi, L.; Folda, A.; Moggach, S.A.; Parsons, S.; Ronconi, L.; Fregona, D.; Bindoli, A. Gold(III)-dithiocarbamato complexes induce cancer cell death triggered by thioredoxin redox system inhibition and activation of ERK pathway. Chem. Biol. 2007, 14, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, X.; Luo, G.; Shen, G.; Li, K.; Ren, G.; Pan, Y.; Wang, X.; Fan, D. Thioredoxin-like protein 2b facilitates colon cancer cell proliferation and inhibits apoptosis via NF-κB pathway. Cancer Lett. 2015, 363, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.R.; Cappabianca, L.; DeSantis, G.; Di Lanni, N.; Ruggeri, P.; Merolle, S.; Tonissen, K.F.; Gulino, A. Thioredoxin stimulates MMP-9 expression, de-regulates the MMP-9/TIMP-1 equilibrium and promotes MMP-9 dependent invasion in human MDA-MB-231 breast cancer cells. FEBS Lett. 2011, 585, 3328–3336. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; McGrath, K.L.; Di Trapani, G.; Charoentong, P.; Shah, F.; King, M.M.; Clarke, F.M.; Tonissen, K.F. The thioredoxin system in breast cancer cell invasion and migration. Redox Biol. 2016, 8, 68–78. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, C.I.; Ooi, K.K.; Tiekink, E.R.T. Gold-Based Medicine: A Paradigm Shift in Anti-Cancer Therapy? Molecules 2018, 23, 1410. https://doi.org/10.3390/molecules23061410
Yeo CI, Ooi KK, Tiekink ERT. Gold-Based Medicine: A Paradigm Shift in Anti-Cancer Therapy? Molecules. 2018; 23(6):1410. https://doi.org/10.3390/molecules23061410
Chicago/Turabian StyleYeo, Chien Ing, Kah Kooi Ooi, and Edward R. T. Tiekink. 2018. "Gold-Based Medicine: A Paradigm Shift in Anti-Cancer Therapy?" Molecules 23, no. 6: 1410. https://doi.org/10.3390/molecules23061410
APA StyleYeo, C. I., Ooi, K. K., & Tiekink, E. R. T. (2018). Gold-Based Medicine: A Paradigm Shift in Anti-Cancer Therapy? Molecules, 23(6), 1410. https://doi.org/10.3390/molecules23061410





