Abstract
Intervertebral disc disease is the most common disease of the spine in dogs and is a main cause of pain and neurologic dysfunction. This article reviews fundamental aspects of the pathophysiology, clinical presentation, diagnosis, and treatment of disc extrusions. Chondroid metaplasia of the nucleus pulposus is the central mechanism of disc degeneration. The clinical presentation varies considerably, depending on the breed, the location of the disc extrusion, and the degree of neurological damage. Advanced imaging techniques, such as computed tomography and magnetic resonance imaging, have greatly improved diagnosis, with magnetic resonance considered the gold standard. As for treatment, both medical and surgical management are effective options, depending on the degree of neurological damage and the initial response to conservative treatment. This comprehensive analysis underlines the importance of a multidisciplinary approach to optimize the quality of life of patients affected by intervertebral disc disease.
1. Introduction
Intervertebral disc disease (IVDD) is the most common disease of the spinal cord in dogs and is considered one of the main causes of pain and neurological dysfunction in this species. This disease is characterized by the progressive degeneration of the intervertebral discs, crucial structures for shock absorption, flexibility, and stability of the spine [1,2].
Intervertebral disc (IVD) degeneration is characterized by chondroid metaplasia of the nucleus pulposus, a process that occurs in both chondrodystrophic and non-chondrodystrophic dogs. However, in chondrodystrophic canine breeds, the expression of a fibroblast growth factor 4 retrogene (FGF4) on chromosome 12 is associated with a significant acceleration of disc degeneration [3,4]. As a consequence of IVD degeneration, mineralized disc material can extrude into the spinal canal, where it causes compression of the spinal cord and nerve roots. This phenomenon, known as disc extrusion or Hansen type I intervertebral disc herniation, is the most common form of disc herniation in dogs and constitutes one of the main causes of neurological signs in these animals [5,6,7].
Because of the contusion and compression of the spinal cord and nerve roots, disc extrusions can cause pain, ataxia, paresis, or plegia, as well as the loss of sphincter control, depending on the severity of the lesion. The impact of this disease on the animal’s quality of life is significant, and its management requires appropriate intervention to minimize neurological damage and alleviate clinical signs [6,7,8].
The diagnosis of IVDD has improved considerably with the advancement of imaging techniques. Currently, computed tomography (CT) and magnetic resonance imaging (MRI) are the most widely used diagnostic tools, as they allow for the detailed visualization of the spine and disc lesions, facilitating accurate localization and assessment of the degree of compression of the nerve structures. However, definitive diagnosis usually requires a multimodal approach combining clinical findings, neurological evaluation, and imaging studies [9].
The treatment of dogs affected by IVDD can be medical or surgical, depending on several factors, such as the degree of neurological damage or the degree of clinical response of the dog to conservative treatment, among others. The prognosis varies considerably, depending on the degree of neurological damage and the effectiveness of the treatment provided. When the intervention is appropriate, outcomes can be very positive, with complete recovery of neurological function in many cases. However, in dogs with severe injuries, the prognosis for regaining ambulatory function and sphincter control may range from guarded to poor [1,2].
The aim of this review article is to provide a comprehensive analysis of the fundamental aspects of IVDD in dogs, focusing on the pathophysiology, clinical presentation, most effective diagnostic strategies, and current treatments for disc extrusions (Hansen type I herniations). Through this review, we seek to provide a comprehensive and updated view of the disease, based on the most recent scientific literature, in order to improve the knowledge and clinical management of this pathology in veterinary practice.
2. Topic Presentation
2.1. Pathophysiology
The IVD consists of two main components: the nucleus pulposus, a gelatinous center rich in proteoglycans and water, and the annulus fibrosus, a concentric ring of fibrous lamellae composed primarily of collagen that provides structural support. The degeneration of the IVD is defined by chondroid metaplasia of the nucleus pulposus, a process that occurs in both chondrodystrophic and non-chondrodystrophic dogs. Traditionally, two types of metaplasia have been described: chondroid metaplasia, commonly associated with Hansen type I extrusions and early-onset degeneration in chondrodystrophic breeds, and fibroid metaplasia, more typical of Hansen type II protrusions and chronic changes in non-chondrodystrophic dogs. In this review, we focus on chondroid metaplasia due to its central role in the pathogenesis of acute disc extrusion. During the initial stages of this degenerative process, the nucleus pulposus undergoes remarkable cellular changes, in which clusters of notochordal cells are replaced by chondrocytes and their corresponding extracellular matrix. This matrix presents similar characteristics to those of hyaline cartilage and is mainly composed of disorganized collagen fibers. Simultaneously, a decrease in the content of glycosaminoglycans is observed, while the proportion of collagen increases (Figure 1) [10].
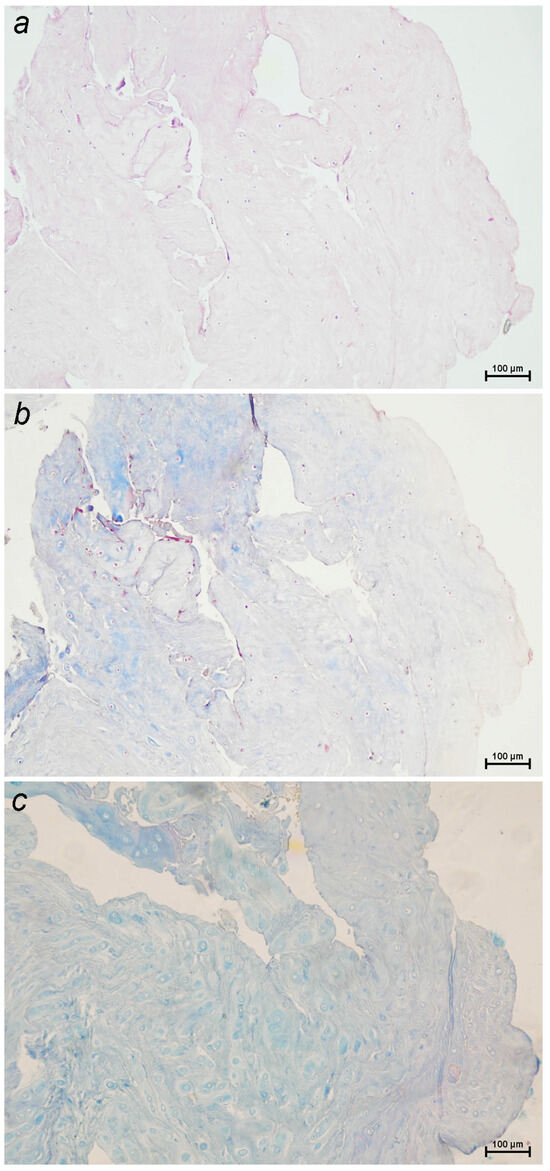
Figure 1.
Sections of a canine intervertebral disc extrusion with a high degree of degeneration stained with: (a) hematoxylin–eosin, highlighting general tissue architecture and cellular detail; (b) Masson’s trichrome, which stains collagen fibers blue and cytoplasm, muscle, keratin, and inflammatory cells red, making it useful for assessing both fibrosis and inflammatory infiltration; (c) Alcian blue–PAS, which stains acidic mucopolysaccharides blue and neutral mucopolysaccharides magenta, aiding in the identification of proteoglycans and the cartilage matrix (10×).
To assess the degree of IVD degeneration, Bergknut et al. (2013) proposed a classification scheme based on postmortem analysis of complete intervertebral segments. Subsequently, Kranenburg et al. (2013) adapted this model for application in surgical biopsies, facilitating its usefulness in the clinical setting [11,12]. Recently, a tool to assess the degree of disc degeneration via T2-weighted imaging using the Pfirrmann classification scheme has been reported [13,14,15].
This metaplasia and chondroid calcification may eventually lead to a type of IVD herniation, known as disc extrusion or Hansen type I herniation, in which the degenerated nucleus pulposus extrudes acutely through a ruptured fibrous ring into the spinal canal [16]. The other types of herniations described in the canine species, which will not be addressed in this review, include the following [5]:
- Hansen type II disc herniation: chronic protrusion of the annulus fibrosus, causing spinal cord compression.
- Acute non-compressive nucleus pulposus extrusion: sudden extrusion of the hydrated nucleus pulposus, without significant spinal cord compression.
- Acute hydrated nucleus pulposus extrusion: extrusion of nucleus pulposus with higher water content, causing spinal cord compression.
- Acute intradural/intramedullary nucleus pulposus extrusion: extrusion of nucleus pulposus material into the spinal cord itself.
- Acute nucleus pulposus extrusion with extensive epidural hemorrhage: extrusion accompanied by significant bleeding in the epidural space.
- Traumatic intervertebral disc extrusion: disc extrusion caused by external trauma rather than degeneration.
In cases where disc material is extruded into the epidural space, it is common to observe an inflammatory response accompanied by hemorrhage. This inflammation is characterized by the predominant infiltration of lymphocytes and macrophages into the extruded disc material. It has been suggested that this inflammatory response is triggered by the exposure of antigenic components of the nucleus pulposus which, upon herniation into the vertebral canal, activate the immune system [17].
However, inflammatory infiltrates have not been identified in histological specimens of complete, non-extruded functional spinal cord units, which explains their exclusion from previous classification schemes. Inflammation is typically observed only in extruded disc material, where exposure of the nucleus pulposus to the epidural space triggers an acute inflammatory response. It has been proposed that acute inflammation could exacerbate clinical signs through extradural tissue swelling generated by inflammatory edema and the resulting compression on the dura mater [12]. Furthermore, in the event that the dura is compromised, inflammatory mediators could directly affect the spinal cord [12].
It is relevant to note that no correlation has been established between the amount of extruded disc material or degree of IVD degeneration and the severity of neurological deficits or prognosis in IVDD. However, a recent study provides some weak evidence suggesting that the total volume of extruded material may influence neurological severity [18]. This suggests that current histological classification systems exhibit limitations in predicting the functional consequences of intervertebral disc herniation, as they do not account for key pathological features such as traumatic injury, neural compression, inflammatory response, necrosis, and hemorrhage. Notably, Figure 2 illustrate clear histological evidence of inflammation, which may play a critical role in the progression and clinical outcome of the disease [11,12].
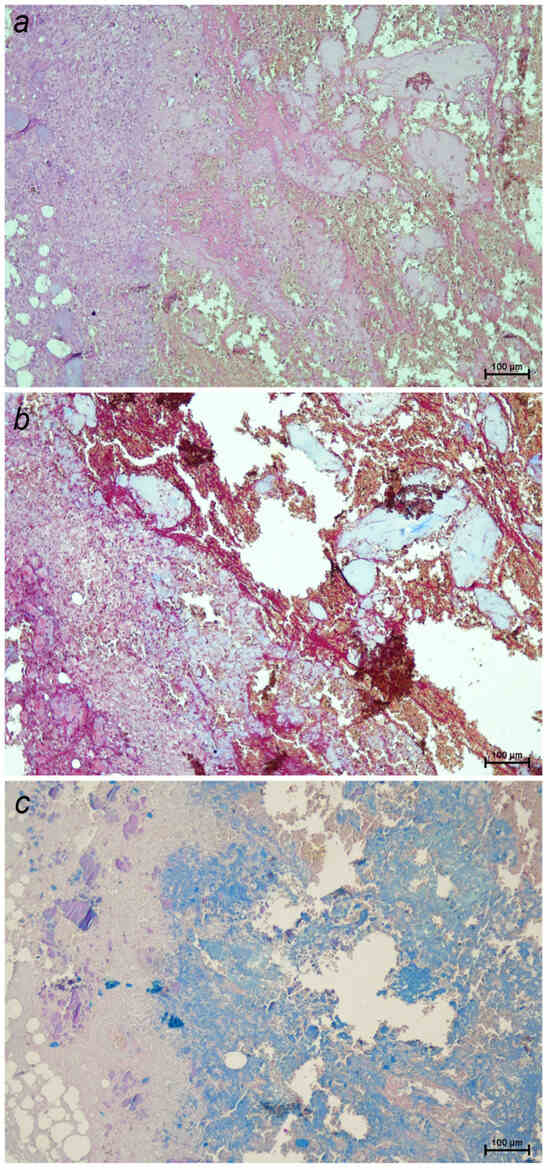
Figure 2.
Sections of a canine intervertebral disc extrusion stained with: (a) hematoxylin–eosin, highlighting general tissue architecture and showing numerous inflammatory cells (blue-purple nuclei); (b) Masson’s trichrome, staining collagen fibers blue and revealing inflammatory infiltrates and necrotic areas in red; (c) Alcian blue–PAS, detecting acidic and neutral mucopolysaccharides in the extracellular matrix (10×). A severe inflammatory reaction, with extensive necrosis and hemorrhage, is observed in more than 75% of the sample.
2.2. Clinical Presentation
The overall prevalence of intervertebral disc herniation in dogs is estimated at 2%, with its clinical presentation varying according to different factors; among the presentations, there is considerable variability, depending on the breed analyzed [19,20]. The most commonly affected chondrodystrophic dog breeds include the dachshund, Pekingese, French bulldog, and beagle, among others. These breeds, particularly young and middle-aged individuals, are at increased risk of developing intervertebral disc extrusions due to their genetic predisposition and the early degeneration of the nucleus pulposus. Although less frequent, this type of herniation can also occur in non-chondrodystrophic dogs. In chondrodystrophic breeds, the incidence of disc herniation peaks between 3 and 7 years of age, while in non-chondrodystrophic breeds, it peaks between 6 and 8 years [1,21].
Several studies have indicated that neutered males and females are at increased risk of developing IVD herniation compared to intact dogs, although the results vary between investigations. However, the risk of disc extrusion does not appear to be associated with factors such as body weight, physical condition, or activity level of the animal [22].
Disc extrusion can occur in any segment of the spine, most commonly between T11-T12 and L2-L3, which varies across studies. Clinical signs depend on the location of the herniation and their severity varies from mild spinal pain without neurological deficits to complete paralysis with loss of nociception (see Supplementary Material). To assess the severity of the neurological injury, the modified Frankel scale is commonly used, which classifies patients from grade 1 (hyperesthesia without neurological deficits) to grade 5 (plegia without nociception) (Table 1). The typical clinical presentation of disc extrusion includes acute-onset myelopathy, with progression of neurological deficits characterized by significant pain [1,23,24].

Table 1.
Modified Frankel score [25].
Cervical disc extrusion occurs in 12.9% to 25.4% of cases of IVD herniation in dogs. Between 15% and 61% of patients with cervical herniation exhibit clinical signs of cervical hyperesthesia, often without further neurologic deficits. This lower incidence of neurological deficits compared with the results for thoracolumbar herniations has traditionally been attributed to the larger diameter of the vertebral canal in the cervical region. However, recent evidence in certain breeds, such as French bulldogs, suggests that the epidural space may not be larger in the cervical than in the thoracolumbar region, indicating that the reasons for more severe deficits with thoracolumbar disk extrusion remain unclear [26]. In cases of cervical herniation, unilateral or bilateral lameness caused by nerve root compression (called root signature) is seen in 15% to 50% of cases. Although rare, serious complications may include profound sensory loss and respiratory distress. The most frequently affected disc spaces in small-breed dogs are C2-C3, while in large breeds, C6-C7 predominate. In general, C5-C6 and C6-C7 are the disc spaces most commonly involved in cervical herniation of the IVD (See Video S2) [27,28,29,30].
Thoracolumbar extrusions account for 66% to 87% of cases of IVD herniation in dogs. The most commonly affected disc spaces in chondrodystrophic breeds are T12-T13 and T13-L1, while in non-chondrodystrophic breeds, T13-L1 and L1-L2 predominate [1]. These herniations can generate a wide range of clinical signs, from mild hyperesthesias to paraplegia, with or without profound sensory loss (See Video S1). Lesions at the nerve root level may also occur in the lumbosacral region and usually present with lameness. In some acute cases, dogs with intervertebral disc herniation may present spinal shock, a temporary loss or reduction of spinal reflexes below the injury site, lasting hours to days due to disruption of the descending pathways. They may also show Schiff–Sherrington posture, characterized by rigid forelimbs caused by the loss of inhibitory interneurons in the thoracolumbar spinal cord. Neither sign predicts long-term prognosis. Progressive myelomalacia (PMM) is the most severe complication that can occur in dogs with thoracolumbar disc extrusions. Its incidence is between 10–17.5% in paraplegic dogs with absence of nociception and can reach 33% in French bulldogs. In this breed, this complication is more frequent in the caudal lumbar location. Although it is a clinical diagnosis in many cases, it is important to recognize its characteristics on MRI, which will be discussed later [1,2,31,32,33]. As clinical presentation varies significantly between breeds [34,35], studying each breed separately provides more meaningful insights into the disease.
2.3. Diagnosis
The diagnosis of IVDD has undergone significant advances in recent years, positioning CT and MRI as the most accurate tools. These modalities have proven superior to traditional techniques such as plain radiographs and myelography [36,37].
Plain radiographs (Figure 3) are a commonly used tool in the veterinary clinic, although they show notable limitations in the evaluation of disc extrusions. Radiographic findings associated with extrusions include reduction of the IVD space, narrowing of the facet joints, opacity of the intervertebral foramen, presence of mineralized disc material in the vertebral canal, and vacuum phenomena. Although radiographs achieve an accuracy of between 51% and 94.7% in the identification of the herniated disc space, they do not provide sufficient information on the extent of extrusion, lateralization, nor the degree of spinal cord compression. Therefore, they are not suitable as an exclusive diagnostic tool, and their usefulness is limited to ruling out other spinal diseases [38].
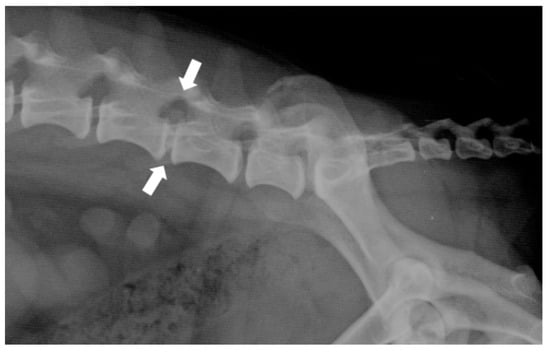
Figure 3.
Right-lateral radiograph of the lumbosacral spine of a dog with a surgically confirmed disc extrusion at L5-L6 (white arrow). Note the reduction of the intervertebral disc space and the presence of mineralized disc material in the vertebral canal.
Myelography is a technique that has been displaced by advanced imaging tests. This technique can be combined with CT. The following criteria have been proposed for the diagnosis of disc extrusions: thinning and deviation of the contrast columns, discontinuity or mild to severe thinning of the contrast columns, diffuse thinning beyond the limits of the affected disc, and asymmetric distribution of contrast cranially or caudal to the injured disc. The diagnostic sensitivity of myelography varies between 53% and 97%. However, its accuracy in determining the lateralization of the extrusion is lower compared to that of other advanced techniques and in patients with severe spinal cord inflammation, contrast columns may not be correctly visualized, making surgical planning difficult. In addition, it carries associated risks, such as temporary neurological deterioration and post-myelographic seizures, especially in large dogs or those receiving high volumes of intrathecal contrast [39,40].
Before myelography is performed, cerebrospinal fluid (CSF) is usually collected for analysis. In dogs with disc extrusions, pleocytosis (mixed or neutrophilic) has been detected in 51% of cases, and is more prevalent in thoracolumbar (61%) compared to cervical (23%) lesions. The presence of pleocytosis was associated with greater spinal cord damage in cases of thoracolumbar IVDD. Also, a higher percentage of macrophages was observed in dogs without profound sensibility that failed to recover the ability to walk. Protein concentration was increased more frequently in cervical extrusions (60%) than in thoracolumbar extrusions (16%). On the other hand, in dogs examined more than 7 days after the onset of clinical signs, lymphocytes predominated, suggesting the presence of inflammatory changes secondary to chronic spinal cord injury [41].
CT (Figure 4) is a rapid and lower-cost diagnostic tool compared to MRI. It offers a sensitivity of 81% to 100%, particularly in chondrodystrophic dogs with mineralized discs. Typical features of disc extrusion seen on CT include hyperattenuating material within the vertebral canal, loss of epidural fat, and distortion of the spinal cord. CT allows for distinguishing between acute and chronic mineralized disc material based on differences in radiodensity and morphology: acute extrusions typically appear as well-defined, dense, and homogeneous structures, while chronic material tends to be fragmented, irregular, or mixed with fibrous tissue. However, CT is reported to be less accurate in older dogs (>5 years) due to age-related disc mineralization that may occur without clinical signs, as well as in small dogs (<7 kg) because of lower spatial resolution and anatomical limitations that reduce contrast and image clarity; moreover, it does not provide details on the severity of intramedullary lesions, limiting its prognostic utility [38,42].
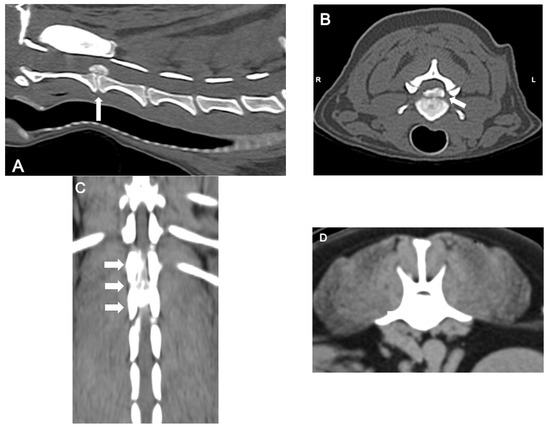
Figure 4.
Computed tomographic (CT) images showing several examples of the appearance of mineralized intervertebral disc extrusion in the vertebral canal. (A) Sagittal reconstructed non-contrast CT image showing a hyperattenuating mass suggestive of extruded disc material into the vertebral canal between C2-3 (arrow). (B) Transverse non-contrast CT image of the same patient showing a large hyperattenuating mass extending into the vertebral canal at the intervertebral disc C2-3 with a mild lateralization to the left (arrow). (C) Dorsal reconstructed post-contrast CT image showing an extensive heterogeneously hyperattenuating material (compared to the spinal cord), suggestive of an extruded degenerated disc mixed with epidural hemorrhage, compressing the spinal cord along the vertebral bodies of T13 and L1. (D) Transverse non-contrast CT image showing a large hyperattenuating mass disc extrusion occupying most of the vertebral canal at the intervertebral disc L1-L2.
MRI (Figure 5) is currently considered the gold standard for the diagnosis of IVDD in both veterinary and human medicine. With a diagnostic sensitivity of over 98.5%, it provides detailed information on spinal cord compression and intramedullary lesions [41]. Characteristic findings include extradural compression of the spinal cord, visible as loss of the hyperintense signal of the nucleus pulposus on T2-weighted images, and the presence of a hypointense mass (extruded nucleus pulposus) on both T1- and T2-weighted images. The degree of spinal cord compression can be classified (on both CT and MRI) as mild (<25%), moderate (25–50%), or severe (>50%), according to the percentage reduction in spinal cord diameter. In addition, certain parameters observed on MRI images have been associated with unfavorable prognoses. Intramedullary T2 hyperintensity (more than six times the sagittal L2 length), T2 hypointensity, and CSF signal attenuation on HASTE/T2 * sequences have been variably associated with worse locomotor outcome and the development of PMM [43]. Despite its many advantages, MRI has certain limitations, such as high cost, long acquisition times, and lower availability compared to other imaging modalities [38,43].
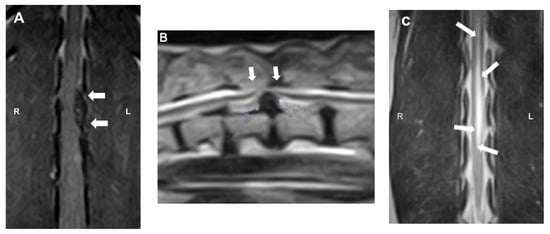
Figure 5.
Magnetic resonance imaging (MRI) images: (A) dorsal T1W image showing the hypointense compressive material (arrows) at L2-3; (B) sagittal T2W image showing a severe ventral extradural compression in the ventral aspect of the vertebral canal at the intervertebral T13-L1 (arrows); (C) dorsal T2W image showing ill-defined intramedullary hyperintensity consistent with myelomalacia centered over L1–4 (arrows).
CT is considered a first-line option in dogs with acute disc extrusion and degenerated discs because of its speed and affordability. It is particularly useful in young to middle-aged chondrodystrophic dogs. On the other hand, MRI is preferable in complex cases, as it identifies intramedullary lesions and concomitant pathologies. In conclusion, the selection of the imaging modality should be based on the clinical characteristics of the patient, the availability of the equipment, and the specific diagnostic objectives [44,45].
2.4. Treatment
Disc extrusions can be managed medically or surgically, with specific variations depending on the location of the herniation (cervical or thoracolumbar) [46]. The particularities of each location will be discussed below.
- Thoracolumbar disc extrusions
Medical treatment includes restriction of physical activity and analgesia. Surgical treatment consists mainly of decompression of the spinal cord by hemilaminectomy, with or without fenestration of the intervertebral disc. Recurrence rates following medical management have been reported to range from 15% to 66%, although the available data are limited and variable in quality. While some studies suggest lower recurrence rates in dogs undergoing hemilaminectomy combined with fenestration, especially when multiple discs are fenestrated, no controlled studies have directly compared surgical and medical management in terms of long-term outcome or recurrence [47].
Ambulatory dogs can be successfully treated conservatively, although the risk of recurrence must be considered. Surgical decompression is indicated in cases of progressive neurologic signs, lack of improvement, or persistent pain despite adequate medical management [45,48].
For nonambulatory paraparetic or paraplegic dogs with profound sensibility, medical management can be effective; however, success rates, recovery, and reduction of recurrences are generally superior with surgery, so the latter is recommended. In paraplegic patients with the absence of profound sensibility, the success of medical management is low, and an increased incidence of PMM is observed, justifying surgical treatment [45,49].
The main components of medical management include activity restriction (at least 4 weeks) to promote healing of the annulus fibrosus, pain management, treatment of urinary incontinence, and prevention of pressure ulcers. During this period, it is recommended to keep the animal in a confined area, except for rehabilitation exercises or physiological needs [47].
Regarding medical treatment, the use of corticosteroids in the acute phase remains controversial. While they are not routinely recommended, some clinicians may opt for their use in specific cases based on individual patient needs and clinical judgment. In the chronic phase, a short course of anti-inflammatory doses may be useful in some cases. NSAIDs are commonly recommended for 5–7 days, when there are no contraindications. Persistent pain beyond this period may indicate the need for reevaluation and potential surgical management. Medications for pain management include NSAIDs; gabapentin or pregabalin (for neuropathic pain); and muscle relaxants, such as diazepam or methocarbamol. In cases of severe pain requiring opioids, hospitalization is suggested until patient stabilization is achieved [45].
Brief hospitalization (1–2 days) is recommended in medically treated dogs with progressive signs, allowing for close monitoring for neurologic deterioration. In situations where surgical treatment is not feasible, medical management can be considered for any degree of injury, except in cases with clinical evidence of PMM [45,50]. In a recent publication, it has been reported that a high proportion of dogs (96% and 48% with and without profound sensibility, respectively) treated conservatively regained ambulation after conservative management [51].
In the surgical management of thoracolumbar extrusions, the most common techniques include hemilaminectomy; mini-hemilaminectomy/pediculectomy; dorsal laminectomy; intervertebral disc fenestration, with or without concurrent laminectomy; and laminectomy with durotomy. In recent years, various minimally invasive techniques have also been reported. Hemilaminectomy and mini-hemilaminectomy, with or without concurrent fenestration, are usually the preferred surgical approaches because of their efficacy in accessing and removing disc material [45,52,53].
The literature supports the early performance of surgical decompression in dogs with significant neurological deficits but does not establish a fixed schedule for its urgency. In this context, it is important to emphasize that surgery should not be withheld in dogs that have been paralyzed for a prolonged period, as recovery of ambulation is possible, even in those without profound sensibility and with paralysis of more than one week of evolution [45,47,54,55,56,57,58].
Prophylactic fenestration consists of the removal of disc material in situ to prevent future extrusions. Originally, this technique was used to treat disc extrusions without the need for laminectomy; however, it is now more frequently performed at the site of extrusion, along with decompression, to reduce the risk of further extrusion through the ruptured annulus fibrosus in the early postoperative period [59].
Fenestration of the herniated disc space at the time of surgical decompression is commonly performed to reduce the likelihood of recurrence at the affected site, although this practice largely depends on clinician preference and experience. It can also be performed on adjacent discs, typically between T11 and L4, as a preventive measure. In predisposed breeds, such as dachshunds and French bulldogs, fenestration is recommended, even in non-mineralized discs [45,60].
The decision to fenestrate should take into account factors such as the patient’s clinical status and surgical time. Multi-site fenestration has a low complication rate when performed by an experienced surgeon. Complications, although infrequent, are usually mild and do not affect the patient’s mobility or quality of life. These may include minor hemorrhage, localized inflammation, or transient pain. However, routine fenestration is not recommended for discs caudal to L4–L5 due to increased technical difficulty, proximity to critical neurovascular structures, and a higher risk of complications such as nerve injury. Fenestration is also not suggested at T10-T11 or cranially above due to the low incidence of extrusions at these locations [61,62].
Following surgery, confinement and activity restriction for at least 4 weeks are essential components of postoperative care, although these restrictions should be combined with rehabilitative exercises. The recommended analgesic protocol includes opioids for 24–48 h postoperatively (or longer, if necessary), a fentanyl patch for 3–5 days, NSAIDs for 7 days, and gabapentin or pregabalin to manage neuropathic pain. However, the use of a fentanyl patch at home carries significant risks, including accidental exposure to humans or other animals, and should only be prescribed when proper monitoring and safety precautions can be ensured. Additionally, interventions such as erector spinae muscle blockade, epidural morphine, and pulsed electromagnetic field therapy have been shown to be effective in reducing intraoperative and postoperative pain [45].
One of the most challenging consequences of thoracolumbar IVDD is the loss of voluntary urination, as well as incontinence. Paraplegic dogs cannot urinate voluntarily, and although they may regain this ability over time, bladder emptying is often incomplete for several weeks, increasing the risk of urinary tract infections and bladder overdistension. Even dogs that recover nociception and motor function may experience residual incontinence or detrusor–sphincter dyssynergia. Management should begin with an assessment of bladder tone and function, as patients may present with either an atonic bladder or increased sphincter resistance. Alpha-adrenergic antagonists (e.g., prazosin) can be used to relax the internal urethral sphincter, and skeletal muscle relaxants (e.g., diazepam) may be added to target the external sphincter, if needed. Regular bladder emptying is essential and can be achieved by manual expression, intermittent catheterization, or short-term use of indwelling catheters, in selected cases. Bladder management protocols should include regular monitoring of residual urine volume and signs of urinary tract infection. Educating dog owners regarding hygiene, handling techniques, and early recognition of complications is a critical component of long-term care [47,63].
PMM is a clinical syndrome characterized by progressive necrosis, ischemia, and hemorrhage of the spinal cord that expands cranially and caudally from the initial injury site. It develops within 24 h to 14 days after an acute injury associated with a thoracolumbar spinal cord extrusion. In paraplegic dogs without profound sensibility, its prevalence ranges from 10% to 33%. Although French bulldogs seem to be more prone to this condition, previous studies did not control for factors that might influence its incidence [64].
Although histopathologic examination is the gold standard for the diagnosis of PMM, the presumptive diagnosis of PMM includes the combination of clinical findings such as ascending paralysis, loss of spinal reflexes, cranial migration of the cutaneous truncal reflex, hypoventilation, Horner’s syndrome, diffuse pain, and thermodysregulation. A combination of clinical findings can support a high level of suspicion for PMM and when combined with imaging findings (discussed previously) and serum biomarkers (GFAP and pNfH), may be even more suggestive of the condition. It has been reported that focal or extensive hemilaminectomy and durotomy may decrease the risk of PMM development in dogs presenting as paraplegic, without profound sensibility [65,66]. However, other publications have shown that durotomy is ineffective in improving functional outcome for severe acute thoracolumbar spinal cord injury in dogs [67].
Although several prognostic markers have been investigated, the absence of nociception is the main unfavorable factor encountered in daily clinical practice. Dogs with nociception have a good prognosis, especially if treated surgically. In contrast, the prognosis is guarded for those without nociception, with recovery rates after surgery ranging from 0% to 76%. In general, the prognosis is poor if no recovery of sensibility is observed within the first 2 to 4 weeks postoperatively [68,69,70,71,72,73].
- Cervical disc extrusions
The medical treatment for cervical extrusions is similar to that for thoracolumbar extrusions. Surgical treatment is usually recommended in dogs presenting with severe cervical pain, neurologic deficits, recurrence or deterioration of clinical signs after medical treatment, or in patients with a chronic history of the condition. Surgical techniques include ventral fenestration, hemilaminectomy, dorsal laminectomy, and ventral slot. The latter is considered a safe and effective procedure, as it allows access to the ventrally located extruded nucleus pulposus. Performing multiple ventral slots does not affect the prognosis, and recurrence of clinical signs such as cervical hyperesthesia or tetraparesis is documented in only 0% to 17% of cases (Figure 6). Postoperative management does not differ with respect to thoracolumbar extrusions [1,74].
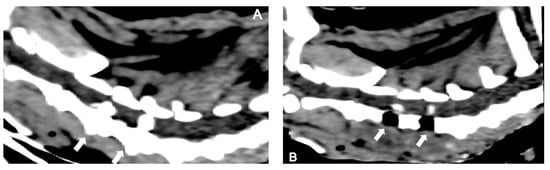
Figure 6.
Sagittal reconstructed non-contrast CT images of a dog presenting with two simultaneous cervical disc herniations (C3-4 and C4-5) (arrows): (A) pre-surgical and (B) post-surgical.
Other therapies reported for the treatment of disc extrusions include acupuncture and various modalities, with limited or inconclusive evidence. While acupuncture has shown some potential benefits, it is not currently recommended as a substitute for surgical management. Basic physical rehabilitation exercises, however, are widely recommended as a complementary treatment. Further controlled studies are necessary to establish the efficacy of other therapies, such as low-level laser therapy, chondroitinase ABC, 4-aminopyridine, and cell transplantation [75,76,77].
3. Conclusions
IVDD in dogs is one of the most common conditions encountered in veterinary practice, given its prevalence and significant impact on patient quality of life. This review article addresses the most recent advances in the pathophysiology, clinical presentation, diagnosis, and treatment of disc extrusions.
Intervertebral disc degeneration, marked by chondroid metaplasia of the nucleus pulposus, is central to the development of IVDD. The clinical presentation varies considerably, depending on the breed, the location of the disc extrusion, and the degree of neurological damage, underscoring the need for individualized approaches in the diagnosis and treatment of each case.
Advanced imaging techniques, such as CT and MRI, have greatly improved diagnostic accuracy, allowing for the detailed evaluation of disc extrusions and their neurological consequences. MRI is established as the gold standard, although the choice of diagnostic modality should be tailored to the available resources and clinical needs of each patient.
As for treatment, both medical and surgical management have their place in the treatment of IVDD, depending on the degree of neurological damage and the initial response to conservative treatment. Surgical techniques, such as hemilaminectomy, fenestration, and ventral slot, offer favorable results in patients with severe neurological deficits, while medical management may be sufficient in less severe cases.
This comprehensive analysis underscores the importance of a multidisciplinary approach in the management of IVDD in dogs in order to improve the clinical outcomes and quality of life of affected patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pets2030026/s1, Video S1; Video S2.
Author Contributions
Conceptualization, I.G.Á., J.M.V.G. and L.E.L.; Writing, I.G.Á., Review and editing, I.G.Á., J.M.V.G. and L.E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brisson, B.A. Intervertebral disc disease in dogs. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 829–858. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.F.; Levine, J.; Kerwin, S.; Cole, R. Canine thoracolumbar invertebral disk disease: Diagnosis, prognosis, and treatment. Compendium 2009, 31, E3. [Google Scholar]
- Brown, E.A.; Dickinson, P.J.; Mansour, T.; Sturges, B.K.; Aguilar, M.; Young, A.E.; Korff, C.; Lind, J.; Ettinger, C.L.; Varon, S.; et al. FGF4 retrogene on CFA12 is responsible for chondrodystrophy and intervertebral disc disease in dogs. Proc. Natl. Acad. Sci. USA 2017, 114, 11476–11481. [Google Scholar] [CrossRef]
- Dickinson, P.J.; Bannasch, D.L. Current Understanding of the Genetics of Intervertebral Disc Degeneration. Front. Vet. Sci. 2020, 7, 431. [Google Scholar] [CrossRef]
- Fenn, J.; Olby, N.J.; Canine Spinal Cord Injury Consortium (CANSORT-SCI). Classification of Intervertebral Disc Disease. Front. Vet. Sci. 2020, 7, 579025. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.J. A pathologic-anatomical interpretation of disc degeneration in dogs. Acta Orthop. Scand. 1951, 20, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.J. A pathologic-anatomical study on disc degeneration in dog, with special reference to the so-called enchondrosis intervertebralis. Acta Orthop. Scand. Suppl. 1952, 11, 1–117. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N.D.; Levine, J.M.; Olby, N.J.; Stein, V.M. Intervertebral disk degeneration in dogs: Consequences, diagnosis, treatment, and future directions. J. Vet. Intern. Med. 2013, 27, 1318–1333. [Google Scholar] [CrossRef]
- da Costa, R.C.; Samii, V.F. Advanced imaging of the spine in small animals. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 765–790. [Google Scholar] [CrossRef]
- Bergknut, N.; Smolders, L.A.; Grinwis, G.C.M.; Hagman, R.; Lagerstedt, A.-S.; Hazewinkel, H.A.W.; Tryfonidou, M.A.; Meij, B.P. Intervertebral disc degeneration in the dog. Part 1: Anatomy and physiology of the intervertebral disc and characteristics of intervertebral disc degeneration. Vet. J. 2013, 195, 282–291. [Google Scholar] [CrossRef]
- Bergknut, N.; Meij, B.P.; Hagman, R.; de Nies, K.S.; Rutges, J.P.; Smolders, L.A.; Creemers, L.B.; Lagerstedt, A.S.; Hazewinkel, H.a.W.; Grinwis, G.C.M. Intervertebral disc disease in dogs—Part 1: A new histological grading scheme for classification of intervertebral disc degeneration in dogs. Vet. J. 2013, 195, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Kranenburg, H.-J.C.; Grinwis, G.C.M.; Bergknut, N.; Gahrmann, N.; Voorhout, G.; Hazewinkel, H.A.W.; Meij, B.P. Intervertebral disc disease in dogs—Part 2: Comparison of clinical, magnetic resonance imaging, and histological findings in 74 surgically treated dogs. Vet. J. 2013, 195, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, F.; Galbusera, F.; Beukers, M.; Jonas, R.; Tao, Y.; Fusellier, M.; Tryfonidou, M.A.; Neidlinger-Wilke, C.; Kienle, A.; Wilke, H.-J. Automatic grading of intervertebral disc degeneration in lumbar dog spines. JOR Spine 2024, 7, e1326. [Google Scholar] [CrossRef]
- Bergknut, N.; Auriemma, E.; Wijsman, S.; Voorhout, G.; Hagman, R.; Lagerstedt, A.-S.; Hazewinkel, H.A.W.; Meij, B.P. Evaluation of intervertebral disk degeneration in chondrodystrophic and nonchondrodystrophic dogs by use of Pfirrmann grading of images obtained with low-field magnetic resonance imaging. Am. J. Vet. Res. 2011, 72, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Harder, L.; Ludwig, D.; Galindo-Zamora, V.; Wefstaedt, P.; Nolte, I. [Classification of canine intervertebral disc degeneration using high-field magnetic resonance imaging and computed tomography]. Tierarztl. Praxis. Ausg. K Kleintiere/Heimtiere 2014, 42, 374–382. [Google Scholar] [CrossRef]
- Smolders, L.A.; Bergknut, N.; Grinwis, G.C.M.; Hagman, R.; Lagerstedt, A.-S.; Hazewinkel, H.A.W.; Tryfonidou, M.A.; Meij, B.P. Intervertebral disc degeneration in the dog. Part 2: Chondrodystrophic and non-chondrodystrophic breeds. Vet. J. 2013, 195, 292–299. [Google Scholar] [CrossRef]
- Willems, N.; Tellegen, A.R.; Bergknut, N.; Creemers, L.B.; Wolfswinkel, J.; Freudigmann, C.; Benz, K.; Grinwis, G.C.M.; Tryfonidou, M.A.; Meij, B.P. Inflammatory profiles in canine intervertebral disc degeneration. BMC Vet. Res. 2016, 12, 10. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Nishida, H.; Tanaka, H.; Kitamura, M.; Akiyoshi, H.; Nakayama, M. The volume of extruded materials is correlated with neurologic severity in small-breed dogs with type I thoracolumbar intervertebral disk herniation. J. Am. Vet. Med. Assoc. 2023, 261, 348–352. [Google Scholar] [CrossRef]
- Pilkington, E.J.; De Decker, S.; Skovola, E.; Cloquell Miro, A.; Gutierrez Quintana, R.; Faller, K.M.E.; Aguilera Padros, A.; Goncalves, R. Prevalence, clinical presentation, and etiology of myelopathies in 224 juvenile dogs. J. Vet. Intern. Med. 2024, 38, 1598–1607. [Google Scholar] [CrossRef]
- Rossi, G.; Stachel, A.; Lynch, A.M.; Olby, N.J. Intervertebral disc disease and aortic thromboembolism are the most common causes of acute paralysis in dogs and cats presenting to an emergency clinic. Vet. Rec. 2020, 187, e81. [Google Scholar] [CrossRef]
- Hansen, T.; Smolders, L.A.; Tryfonidou, M.A.; Meij, B.P.; Vernooij, J.C.M.; Bergknut, N.; Grinwis, G.C.M. The Myth of Fibroid Degeneration in the Canine Intervertebral Disc: A Histopathological Comparison of Intervertebral Disc Degeneration in Chondrodystrophic and Nonchondrodystrophic Dogs. Vet. Pathol. 2017, 54, 945–952. [Google Scholar] [CrossRef]
- Doeven, L.; Cardy, T.; Crawford, A.H. Investigation of neutering status and age of neutering in female Dachshunds with thoracolumbar intervertebral disc extrusion. J. Small Anim. Pract. 2024, 65, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Suiter, E.; Grapes, N.; Martin-Garcia, L.; De Decker, S.; Gutierrez-Quintana, R.; Wessmann, A. MRI and clinical findings in 133 dogs with recurrent deficits following intervertebral disc extrusion surgery. Vet. Rec. 2023, 193, e2992. [Google Scholar] [CrossRef]
- Mateo, I.; Lorenzo, V.; Foradada, L.; Muñoz, A. Clinical, Pathologic, and Magnetic Resonance Imaging Characteristics of Canine Disc Extrusion Accompanied by Epidural Hemorrhage or Inflammation. Vet. Radiol. Ultrasound 2011, 52, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.J.; Levine, J.M.; Budke, C.M.; Kerwin, S.C.; Au, J.; Vinayak, A.; Hettlich, B.F.; Slater, M.R. Description and repeatability of a newly developed spinal cord injury scale for dogs. Prev. Vet. Med. 2009, 89, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Genain, M.A.; Khan, S.; Gauton, J.R.; Freeman, P. The spinal cord-to-vertebral canal area ratio measured with computed tomography is lower in the thoracolumbar than the cervical region in French Bulldogs. J. Am. Vet. Med. Assoc. 2022, 261, 1–4. [Google Scholar] [CrossRef]
- Aikawa, T.; Miyazaki, Y.; Kihara, S.; Muyama, H.; Nishimura, M. Cervical intervertebral disc disease in 307 small-breed dogs (2000-2021): Breed-characteristic features and disc-associated vertebral instability. Aust. Vet. J. 2024, 102, 274–281. [Google Scholar] [CrossRef]
- Bersan, E.; McConnell, F.; Trevail, R.; Behr, S.; De Decker, S.; Volk, H.A.; Smith, P.M.; Gonçalves, R. Cervical intervertebral foraminal disc extrusion in dogs: Clinical presentation, MRI characteristics and outcome after medical management. Vet. Rec. 2015, 176, 597. [Google Scholar] [CrossRef]
- Olender, M.; Couturier, J.; Gatel, L.; Cauvin, E. Cervical jerks as a sign of cervical pain or myelopathy in dogs. J. Am. Vet. Med. Assoc. 2023, 261, 510–516. [Google Scholar] [CrossRef]
- Schachar, J.; Bocage, A.; Nelson, N.C.; Early, P.J.; Mariani, C.L.; Olby, N.J.; Muñana, K.R. Clinical and imaging findings in dogs with nerve root signature associated with cervical intervertebral disc herniation. J. Vet. Intern. Med. 2024, 38, 1111–1119. [Google Scholar] [CrossRef]
- Crawford, A.H.; De Decker, S. Clinical presentation and outcome of dogs treated medically or surgically for thoracolumbar intervertebral disc protrusion. Vet. Rec. 2017, 180, 569. [Google Scholar] [CrossRef] [PubMed]
- Alcoverro, E.; Schofield, I.; Spinillo, S.; Tauro, A.; Ruggeri, M.; Lowrie, M.; Gomes, S.A. Thoracolumbar hydrated nucleus pulposus extrusion and intervertebral disc extrusion in dogs: Comparison of clinical presentation and magnetic resonance imaging findings. Vet. J. 2024, 306, 106178. [Google Scholar] [CrossRef]
- Silva, S.; Guevar, J.; José-López, R.; De Decker, S.; Brocal, J.; de la Fuente, C.; Durand, A.; Forterre, F.; Olby, N.; Gutierrez-Quintana, R. Clinical signs, MRI findings and long-term outcomes of foraminal and far lateral thoracolumbar intervertebral disc herniations in dogs. Vet. Rec. 2022, 190, e1529. [Google Scholar] [CrossRef]
- Aikawa, T.; Shibata, M.; Asano, M.; Hara, Y.; Tagawa, M.; Orima, H. A comparison of thoracolumbar intervertebral disc extrusion in French Bulldogs and Dachshunds and association with congenital vertebral anomalies. Vet. Surg. 2014, 43, 301–307. [Google Scholar] [CrossRef]
- Poli, F.; Calistri, M.; Meucci, V.; DI Gennaro, G.; Baroni, M. Prevalence, clinical features, and outcome of intervertebral disc extrusion associated with extensive epidural hemorrhage in a population of French Bulldogs compared to Dachshunds. J. Vet. Med. Sci. 2022, 84, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Parry, A.T.; Harris, A.; Upjohn, M.M.; Chandler, K.; Lamb, C.R. Does choice of imaging modality affect outcome in dogs with thoracolumbar spinal conditions? J. Small Anim. Pract. 2010, 51, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Harder, L.K. [Diagnostic imaging of changes of the canine intervertebral disc]. Tierarztl. Praxis. Ausg. K Kleintiere/Heimtiere 2016, 44, 359–371. [Google Scholar] [CrossRef]
- da Costa, R.C.; De Decker, S.; Lewis, M.J.; Volk, H.; Canine Spinal Cord Injury Consortium (CANSORT-SCI). Diagnostic Imaging in Intervertebral Disc Disease. Front. Vet. Sci. 2020, 7, 588338. [Google Scholar] [CrossRef]
- Robertson, I.; Thrall, D.E. Imaging dogs with suspected disc herniation: Pros and cons of myelography, computed tomography, and magnetic resonance. Vet. Radiol. Ultrasound 2011, 52 (Suppl. 1), S81–S84. [Google Scholar] [CrossRef]
- Dennison, S.E.; Drees, R.; Rylander, H.; Yandell, B.S.; Milovancev, M.; Pettigrew, R.; Schwarz, T. Evaluation of different computed tomography techniques and myelography for the diagnosis of acute canine myelopathy. Vet. Radiol. Ultrasound 2010, 51, 254–258. [Google Scholar] [CrossRef]
- Srugo, I.; Aroch, I.; Christopher, M.M.; Chai, O.; Goralnik, L.; Bdolah-Abram, T.; Shamir, M.H. Association of cerebrospinal fluid analysis findings with clinical signs and outcome in acute nonambulatory thoracolumbar disc disease in dogs. J. Vet. Intern. Med. 2011, 25, 846–855. [Google Scholar] [CrossRef]
- Emery, L.; Hecht, S.; Sun, X. Investigation of parameters predicting the need for diagnostic imaging beyond computed tomography in the evaluation of dogs with thoracolumbar myelopathy: Retrospective evaluation of 555 dogs. Vet. Radiol. Ultrasound 2018, 59, 147–154. [Google Scholar] [CrossRef]
- Noyes, J.A.; Thomovsky, S.A.; Chen, A.V.; Owen, T.J.; Fransson, B.A.; Carbonneau, K.J.; Matthew, S.M. Magnetic resonance imaging versus computed tomography to plan hemilaminectomies in chondrodystrophic dogs with intervertebral disc extrusion. Vet. Surg. 2017, 46, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.J.; Young, B.D.; Griffin, J.F.; Fosgate, G.T.; Levine, J.M. Comparison between noncontrast computed tomography and magnetic resonance imaging for detection and characterization of thoracolumbar myelopathy caused by intervertebral disk herniation in dogs. Vet. Radiol. Ultrasound 2014, 55, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Olby, N.J.; Moore, S.A.; Brisson, B.; Fenn, J.; Flegel, T.; Kortz, G.; Lewis, M.; Tipold, A. ACVIM consensus statement on diagnosis and management of acute canine thoracolumbar intervertebral disc extrusion. J. Vet. Intern. Med. 2022, 36, 1570–1596. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; Early, P.J.; Hettlich, B.F. Practice patterns in the management of acute intervertebral disc herniation in dogs. J. Small Anim. Pract. 2016, 57, 409–415. [Google Scholar] [CrossRef]
- Moore, S.A.; Tipold, A.; Olby, N.J.; Stein, V.; Granger, N.; Canine Spinal Cord Injury Consortium (CANSORT SCI). Current Approaches to the Management of Acute Thoracolumbar Disc Extrusion in Dogs. Front. Vet. Sci. 2020, 7, 610. [Google Scholar] [CrossRef]
- Langerhuus, L.; Miles, J. Proportion recovery and times to ambulation for non-ambulatory dogs with thoracolumbar disc extrusions treated with hemilaminectomy or conservative treatment: A systematic review and meta-analysis of case-series studies. Vet. J. 2017, 220, 7–16. [Google Scholar] [CrossRef]
- Lewis, M.J.; Jeffery, N.D.; Olby, N.J.; Canine Spinal Cord Injury Consortium (CANSORT-SCI). Ambulation in Dogs With Absent Pain Perception After Acute Thoracolumbar Spinal Cord Injury. Front. Vet. Sci. 2020, 7, 560. [Google Scholar] [CrossRef]
- Klesty, A.; Forterre, F.; Bolln, G. [Outcome of intervertebral disk disease surgery depending on dog breed, location and experience of the surgeon: 1113 cases]. Tierarztl. Praxis. Ausg. K Kleintiere/Heimtiere 2019, 47, 233–241. [Google Scholar] [CrossRef]
- Khan, S.; Jeffery, N.D.; Freeman, P. Recovery of ambulation in small, nonbrachycephalic dogs after conservative management of acute thoracolumbar disk extrusion. J. Vet. Intern. Med. 2024, 38, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Skytte, D.; Schmökel, H. Relationship of preoperative neurologic score with intervals to regaining micturition and ambulation following surgical treatment of thoracolumbar disk herniation in dogs. J. Am. Vet. Med. Assoc. 2018, 253, 196–200. [Google Scholar] [CrossRef]
- Takahashi, F.; Honnami, A.; Toki, M.; Dosaka, A.; Fujita, Y.; Hara, Y.; Yamaguchi, S. Effect of durotomy in dogs with thoracolumbar disc herniation and without deep pain perception in the hind limbs. Vet. Surg. 2020, 49, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.J.A.; Correia, J.H.D.; Jaggy, A. Thoracolumbar disc disease in 71 paraplegic dogs: Influence of rate of onset and duration of clinical signs on treatment results. J. Small Anim. Pract. 2002, 43, 158–163. [Google Scholar] [CrossRef]
- Immekeppel, A.; Rupp, S.; Demierre, S.; Rentmeister, K.; Meyer-Lindenberg, A.; Goessmann, J.; Bali, M.S.; Schmidli-Davies, F.; Forterre, F. Investigation of timing of surgery and other factors possibly influencing outcome in dogs with acute thoracolumbar disc extrusion: A retrospective study of 1501 cases. Acta Vet. Scand. 2021, 63, 30. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Liebel, F.X.; Fadda, A.; Lazzerini, K.; Harcourt-Brown, T. Same-day surgery may reduce the risk of losing pain perception in dogs with thoracolumbar disc extrusion. J. Small Anim. Pract. 2020, 61, 442–448. [Google Scholar] [CrossRef]
- Upchurch, D.A.; Renberg, W.C.; Turner, H.S.; McLellan, J.G. Effect of Duration and Onset of Clinical Signs on Short-Term Outcome of Dogs with Hansen Type I Thoracolumbar Intervertebral Disc Extrusion. Vet. Comp. Orthop. Traumatol. 2020, 33, 161–166. [Google Scholar] [CrossRef]
- Coates, J.R. Surgical considerations of thoracolumbar intervertebral disk disease. Vet. Clin. N. Am. Small Anim. Pract. 2000, 30, 77–100. [Google Scholar] [CrossRef]
- Jeffery, N.D.; Freeman, P.M. The Role of Fenestration in Management of Type I Thoracolumbar Disk Degeneration. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 187–200. [Google Scholar] [CrossRef]
- Aikawa, T.; Fujita, H.; Shibata, M.; Takahashi, T. Recurrent thoracolumbar intervertebral disc extrusion after hemilaminectomy and concomitant prophylactic fenestration in 662 chondrodystrophic dogs. Vet. Surg. 2012, 41, 381–390. [Google Scholar] [CrossRef]
- Aikawa, T.; Fujita, H.; Kanazono, S.; Shibata, M.; Yoshigae, Y. Long-term neurologic outcome of hemilaminectomy and disk fenestration for treatment of dogs with thoracolumbar intervertebral disk herniation: 831 cases (2000–2007). J. Am. Vet. Med. Assoc. 2012, 241, 1617–1626. [Google Scholar] [CrossRef]
- Brisson, B.A.; Holmberg, D.L.; Parent, J.; Sears, W.C.; Wick, S.E. Comparison of the effect of single-site and multiple-site disk fenestration on the rate of recurrence of thoracolumbar intervertebral disk herniation in dogs. J. Am. Vet. Med. Assoc. 2011, 238, 1593–1600. [Google Scholar] [CrossRef]
- Granger, N.; Blenkinsop, H. Bladder and bowel management in dogs with spinal cord injury. Front. Vet. Sci. 2020, 7, 583342. [Google Scholar] [CrossRef]
- Balducci, F.; Canal, S.; Contiero, B.; Bernardini, M. Prevalence and Risk Factors for Presumptive Ascending/Descending Myelomalacia in Dogs after Thoracolumbar Intervertebral Disk Herniation. J. Vet. Intern. Med. 2017, 31, 498–504. [Google Scholar] [CrossRef]
- Castel, A.; Olby, N.J.; Ru, H.; Mariani, C.L.; Muñana, K.R.; Early, P.J. Risk factors associated with progressive myelomalacia in dogs with complete sensorimotor loss following intervertebral disc extrusion: A retrospective case-control study. BMC Vet. Res. 2019, 15, 433. [Google Scholar] [CrossRef]
- Castel, A.; Olby, N.J.; Mariani, C.L.; Muñana, K.R.; Early, P.J. Clinical Characteristics of Dogs with Progressive Myelomalacia Following Acute Intervertebral Disc Extrusion. J. Vet. Intern. Med. 2017, 31, 1782–1789. [Google Scholar] [CrossRef]
- Jeffery, N.D.; Rossmeisl, J.H.; Harcourt-Brown, T.R.; Granger, N.; Ito, D.; Foss, K.; Chase, D. Randomized Controlled Trial of Durotomy as an Adjunct to Routine Decompressive Surgery for Dogs With Severe Acute Spinal Cord Injury. Neurotrauma Rep. 2024, 5, 128–138. [Google Scholar] [CrossRef]
- Jeffery, N.D.; Barker, A.K.; Hu, H.Z.; Alcott, C.J.; Kraus, K.H.; Scanlin, E.M.; Granger, N.; Levine, J.M. Factors associated with recovery from paraplegia in dogs with loss of pain perception in the pelvic limbs following intervertebral disk herniation. J. Am. Vet. Med. Assoc. 2016, 248, 386–394. [Google Scholar] [CrossRef]
- Mayhew, P.D.; McLear, R.C.; Ziemer, L.S.; Culp, W.T.N.; Russell, K.N.; Shofer, F.S.; Kapatkin, A.S.; Smith, G.K. Risk factors for recurrence of clinical signs associated with thoracolumbar intervertebral disk herniation in dogs: 229 cases (1994–2000). J. Am. Vet. Med. Assoc. 2004, 225, 1231–1236. [Google Scholar] [CrossRef]
- Olby, N.J.; da Costa, R.C.; Levine, J.M.; Stein, V.M.; Canine Spinal Cord Injury Consortium (CANSORT SCI). Prognostic Factors in Canine Acute Intervertebral Disc Disease. Front. Vet. Sci. 2020, 7, 596059. [Google Scholar] [CrossRef]
- Svensson, G.; Simonsson, U.S.H.; Danielsson, F.; Schwarz, T. Residual Spinal Cord Compression Following Hemilaminectomy and Mini-Hemilaminectomy in Dogs: A Prospective Randomized Study. Front. Vet. Sci. 2017, 4, 42. [Google Scholar] [CrossRef]
- Wang-Leandro, A.; Siedenburg, J.S.; Hobert, M.K.; Dziallas, P.; Rohn, K.; Stein, V.M.; Tipold, A. Comparison of Preoperative Quantitative Magnetic Resonance Imaging and Clinical Assessment of Deep Pain Perception as Prognostic Tools for Early Recovery of Motor Function in Paraplegic Dogs with Intervertebral Disk Herniations. J. Vet. Intern. Med. 2017, 31, 842–848. [Google Scholar] [CrossRef]
- Woelfel, C.W.; Robertson, J.B.; Mariani, C.L.; Muñana, K.R.; Early, P.J.; Olby, N.J. Outcomes and prognostic indicators in 59 paraplegic medium to large breed dogs with extensive epidural hemorrhage secondary to thoracolumbar disc extrusion. Vet. Surg. 2021, 50, 527–536. [Google Scholar] [CrossRef]
- Guo, S.; Lu, D.; Pfeiffer, S.; Pfeiffer, D.U. Non-ambulatory dogs with cervical intervertebral disc herniation: Single versus multiple ventral slot decompression. Aust. Vet. J. 2020, 98, 148–155. [Google Scholar] [CrossRef]
- Lewis, M.J.; Granger, N.; Jeffery, N.D.; Canine Spinal Cord Injury Consortium (CANSORT-SCI). Emerging and Adjunctive Therapies for Spinal Cord Injury Following Acute Canine Intervertebral Disc Herniation. Front. Vet. Sci. 2020, 7, 579933. [Google Scholar] [CrossRef]
- Prager, J.; Fenn, J.; Plested, M.; Escauriaza, L.; van der Merwe, T.; King, B.; Chari, D.; Wong, L.-F.; Granger, N. Transplantation of encapsulated autologous olfactory ensheathing cell populations expressing chondroitinase for spinal cord injury: A safety and feasibility study in companion dogs. J. Tissue Eng. Regen. Med. 2022, 16, 788–798. [Google Scholar] [CrossRef]
- Hodgson, M.M.; Bevan, J.M.; Evans, R.B.; Johnson, T.I. Influence of in-house rehabilitation on the postoperative outcome of dogs with intervertebral disk herniation. Vet. Surg. 2017, 46, 566–573. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).