Abstract
Syphilis, which is caused by Treponema pallidum, remains one of the most common congenital infection worldwide and has tremendous consequences for the mother and her developing foetus if left untreated. The complexity of the exposure to this pathogen extends beyond the well-established clinical manifestations, as it can profoundly affect placental histomorphology. This study aimed to compare T. pallidum-exposed placental villi structures with healthy placentae at term to evaluate the histomorphological differences using stereology. In this case-control study conducted at term (38 weeks ± 2 weeks), 78 placentae were collected from the hospital delivery suites, comprising 39 cases (T. pallidum-exposed) and 39 controls (non-exposed), who were gestational age-matched with other potential confounders excluded. Blood samples from the umbilical vein and placental basal plate were tested for syphilis, using rapid diagnostic test (RDT) kits for T. pallidum (TP) antibodies (IgG and IgM) to classify placentae as exposed to T. pallidum (cases) and non-exposed (controls). Tissue sections were prepared and stained with haematoxylin and eosin, and the mean volume densities of syncytial knots, foetal capillaries, syncytial denuded areas, and intervillous spaces were estimated using stereological methods. Statistical analysis was performed to compare the mean values between the case and control groups. Stereological assessment revealed significant differences between the T. pallidum-exposed and non-exposed groups with regard to syncytial knots (p < 0.0001), syncytial denudation (p < 0.0001), and foetal capillaries (p < 0.0001), but no significant difference in the intervillous space was found (p = 0.1592). Therefore, our study shows, for the first time, that the histomorphology of human placental villi appears to be altered by exposure to T. pallidum. It will, therefore, be interesting to determine whether these changes in the placental villi translate into long-term effects on the baby.
1. Introduction
Sexually transmitted illnesses, such as syphilis, remain a significant concern in contemporary society, affecting an estimated 7.1 million individuals in 2020, the majority of whom live in underdeveloped countries [1]. The prevalence of syphilis among pregnant mothers in sub-Saharan Africa is estimated to be 2.7%, placing approximately one million pregnancies at risk each year [2]. Globally, the regional prevalence varies, with estimates of 0.65% in Europe, 0.63% in the Americas, 0.97% in Africa, and 1.27% in Southeast Asia [3], reflecting regional disparities in disease burden and access to maternal healthcare services. Syphilis is caused by Treponema pallidum, and its infectious stages are generally classified as primary, secondary, and early latent syphilis. If untreated during the first four years, there is up to a 70% chance of vertical transmission. The negative outcomes of syphilis during pregnancy can be severe, including foetal death, stillbirth, hydrops fetalis, neonatal and infant mortality, premature birth, low birth weight, active congenital syphilis in the newborn, congenital abnormalities, and long-term consequences (e.g., deafness and neurological disorders such as learning disabilities and epilepsy) [4,5]. Beyond these detrimental effects on pregnancy, syphilis also increases the risk of HIV acquisition and transmission by two to five folds due to the breakdown of mucosal and epithelial barriers caused by syphilitic sores [6].
Although the adverse effects of syphilis on pregnancy outcomes are well-documented [4,5], the precise histomorphological alterations it induces in the placenta remain obscure. Exposure of the placenta to T. pallidum can cause pathological changes in the organ, which may persist until delivery. The pathological changes potentially disrupt the maternoplacental interphase and contribute to unfavourable birth outcomes. Although the placenta preserves important biological evidence of events occurring throughout pregnancy [7,8], most studies on the placenta have mostly focused on themes such as pathogens present in the placenta [9,10,11], vertical transmission [12], and maternal-foetal immunoglobulin transfer [13], with limited attention paid to structural alterations. This gap may stem from the under-prioritisation of placental pathology in routine research, technical barriers to stereological analysis, and limited access to tissue samples in low-resource settings. We, therefore, hypothesised that exposure to T. pallidum induces measurable histomorphological alterations in the placenta, indicative of functional compromise and adaptive responses to intrauterine stress. To test this, we conducted a comparative stereological analysis of placental tissue obtained from pregnancies exposed and unexposed to T. pallidum, focusing on key structural parameters, such as syncytial knots, syncytial denudation, intervillous spaces, and foetal capillaries.
2. Materials and Methods
2.1. Study Design and Study Site
This was a comparative case-control study designed to evaluate histomorphological differences between placentae exposed and unexposed to T. pallidum. This study was conducted at the LEKMA Hospital and Weija-Gbawe Municipal Hospital, two geographically distant and distinct hospitals in southern Ghana. The LEKMA Hospital, a district hospital of the Ghana Health Service (GHS), is situated in the Ledzokuku Municipality. The hospital provides a variety of medical services with monthly delivery rates of 250–300. Weija-Gbawe Municipal Hospital, situated in McCarthy Hills, is another district hospital of the GHS that provides various medical services, with an average of 200 monthly deliveries.
2.2. Placenta Sampling and Grouping
Placentae were collected at the various delivery sites using a purposive sampling strategy. All consenting pregnant women who delivered at the study sites during the study period were included. Written informed consent was obtained from each participant using the English, Ga, and Twi languages, after which the placenta was taken after delivery. These placentae were categorised into two groups: cases (T. pallidum-exposed) and controls (T. pallidum non-exposed) after drawing 2–3 mL of blood from the maternal side (basal plate) as well as cord blood from the umbilical vein on the foetal side of the placenta, each into K2EDTA tubes (BD Vacutainer®, Becton, Dickinson & Company (BD), Plymouth, UK) for the Syphilis Antibodies Rapid Test. In addition to gestational age matching, we carefully reviewed patients’ clinical records to reduce the impact of potential confounders. Particular attention was given to comorbidities and exposure to other common pathogens endemic to our region, such as HIV, Hepatitis B and C, Plasmodium spp., Toxoplasma gondii, Salmonella spp., genetic disorders such as sickle cell, and various maternal clinical factors. Only placentae from women exposed solely to T. pallidum IgG and IgM in both the basal plate and cord blood, but none of the others screened for were included as cases, while controls were negative for T. pallidum and all other screened infections.
2.3. Immunoassay for Detection of Treponema pallidum IgG and IgM
A drop of blood derived from the placenta was added to the sample column of the immunoblot test kit (Immunetics Inc., Boston, MA, USA) according to the manufacturer’s instructions. Following the addition of four drops of the lysing buffer, the resultant bands obtained were compared with the control band and classified as either RDT-positive or RDT-negative for T. pallidum antibodies (IgG and IgM).
2.4. Tissue Slicing and Processing
For histological investigations, tissue samples were obtained from the chorionic plate through to the basal plate of the placenta. The sampling was standardised by dividing each placenta into four quadrants. Tissue samples were systematically collected as follows: one from the region adjacent to the umbilical cord insertion, one from the midpoint between the cord insertion and the edge, one from near the placental margin, and one from the peripheral edge. The placenta samples, cubes measuring roughly 2 cm by 2 cm by 5 cm, were cut and placed in a tissue cassette with clear labelling and fixed in 10% buffered formalin, with a pH of 7.24–7.28, until tissue processing was started. A twelve-chambered automated tissue processor (LEICA TP 1020, Wetzlar, Germany) was used to process each cassette containing tissue. Briefly, the tissues were dehydrated through ascending grades of ethanol from 50% to 95%, two changes of 100% ethanol, and cleared in two changes of xylene. The tissues were then impregnated with molten wax at 60 °C and moulded into tissue blocks for microtomy.
2.5. Sectioning of Placenta Tissue Samples
The tissue blocks were trimmed using a Leica microtome (Leica RM 2125RT, Wetzlar, Germany) to determine the complete tissue profile. They were subsequently sectioned at a thickness of 4 μm. Following the addition of 20% H2SO4 to aid in spreading, ribbons formed during the sectioning process were carefully selected and floated in a water bath maintained at 56 °C. From each block, four sections were systematically selected and stained with haematoxylin and eosin (H&E). Specifically, the 1st, 50th, 100th, and 150th sections were selected. These tissue sections were placed on well-labelled frosted glass slides (76 mm × 26 mm × 1 mmP) for H&E staining and for assessment and histomorphometric quantification.
2.6. Stereological Studies
2.6.1. Sampling of Photomicrographs of Placenta Sections
Photomicrographs were acquired using a light microscope (Leica Galen III; catalogue number 317506; serial number ZG6JA4) connected to a desktop computer (HP Compaq dx2300 Microtower, HP Inc., Palo Alto, CA, USA) via a digital eyepiece camera (Lenovo Q350 USB PC Camera, Beijing, China). The stage of the microscope was systematically shifted in increments of three graduation units along both the X- and Y-axes to visualise the entire placental section. Images were captured at a ×40 objective magnification using the digital camera. Systematic uniform random sampling was employed for image selection. Each placenta was sectioned into 16 tissue slices, derived from four quadrants with four blocks each (4 blocks × 4 quadrants = 16 sections). From each section, approximately 50 photomicrographs were captured, from which 5 images were selected using systematic uniform random sampling. Every 10th image was chosen, beginning from a randomly selected starting point. This yielded 80 photomicrographs per placenta (16 sections × 5 micrographs).
Across all 78 placentae, a total of 6240 photomicrographs (78 × 80) were systematically selected and analysed for stereological quantification of syncytial knots, syncytial denudated areas, intervillous spaces, and foetal capillaries (Figure 1).
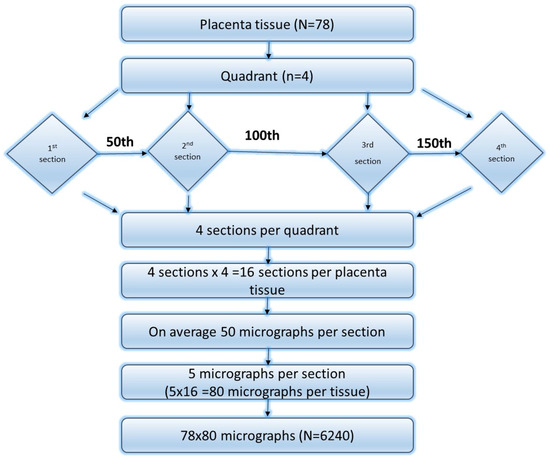
Figure 1.
Flow chart of how placenta tissues were selected for stereology.
2.6.2. Stereological Study of Placental Photomicrographs
Cavalieri’s technique of point counting was utilised in a design-based stereological approach to determine the relative volume densities of several placental structural components (syncytial knots, syncytial denudation, intervillous space, and foetal capillaries). Using Adobe Photoshop CS6 Extended (Trial Version 13.0.1), a stereological grid with regularly spaced points measuring (1 cm × 1 cm) was superimposed on each micrograph of the placental sections. At each grid line intersection on the micrographs, the following parameters were counted: foetal capillaries, villous syncytial knots, areas of syncytial denudation, and intervillous spaces. The relative volume densities were then calculated using the Cavalieri estimator of volume formula, based on the point count data:
where Vv indicates the volume density; ∑P is the sum of all test points encountered; (a/p) is the area per point of the stereological grid; t is the thickness of the section; and M is the linear magnification [12].
Vv = (a/pƩP) × t/M2
2.7. Statistical Analysis
Microsoft Office Excel 2016 (Microsoft® Office Professional 2007, Microsoft Corporation, Redmond, WA, USA) was used to store and organise the data. The primary analytical method was descriptive statistics. Where appropriate, tables and graphical representations were used to summarise the data, including frequencies and modes. Means, standard deviations (SDs), and standard errors of the means (SEMs), at a 95% confidence interval (CI), were computed using the GraphPad Prism software (Version 5). Following the acquisition of volume density data, one-way ANOVA and t-tests were conducted to compare means across the different placental groups. The data were assessed for normality prior to performing ANOVA and t-tests. Statistical significance was determined at a p-value of <0.05.
2.8. Ethical Consideration
Ethical approval was obtained from the Ethics and Protocol Review Committee of the College of Health Sciences, the University of Ghana, with protocol identification number CHS-Et/M4-P.7/2019-2020. Participation in this study was voluntary, and informed consent was obtained from each participant before their inclusion in this study. Approval was also sought from the appropriate hospital authorities.
3. Results
Based on the previously mentioned criteria, a total of 78 placentae qualified for inclusion in this study, comprising 39 T. pallidum-exposed pregnant women (cases) and 39 non-exposed pregnant women (controls) who delivered at full term (38 ± 2 weeks). Statistically significant differences in the mean volume densities of syncytial knots, syncytial denudation, and foetal capillaries were observed between the placenta groups (Table 1 and Figure 2). The volume densities of syncytial knots (p < 0.0001), the denudation of the syncytium (p < 0.0001), and foetal capillaries (p < 0.0001) were significantly different in the cases compared with the controls at the 5% significance level (Table 1).

Table 1.
Volume density (Vd) of placental parameters among case and control placenta groups.
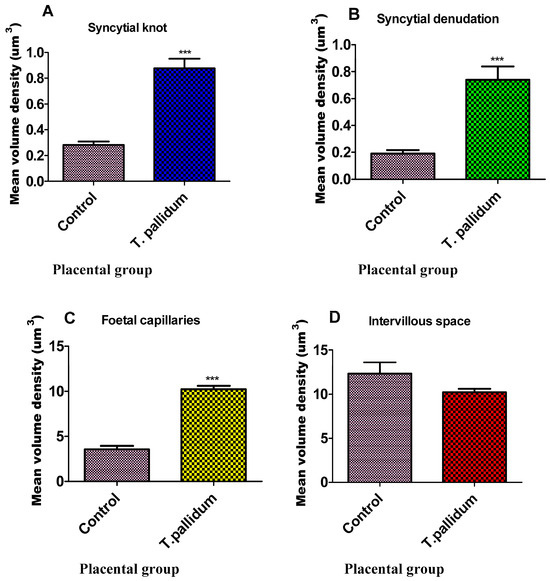
Figure 2.
Bar graph of mean volume density of placental parameters. Each column represents the mean ± standard error of the mean (S.E.M.). Statistical comparison was done using an unpaired t-test, with *** denoting p < 0.001 relative to matched pairs. (A)—syncytial knot, (B)—syncytial denudation, (C)—foetal capillaries, and (D)—intervillous space.
Conversely, the volume density of the intervillous space (p = 0.1592) was lower in the exposed placentae than in the non-exposed placentae, although the difference was not statistically significant at the 5% chance of error commission (Table 1).
Histomorphological assessment of placentae exposed to T. pallidum revealed several significant changes. There was a reduction in intervillous space (Figure 3A). Additionally, a significant increase in the number of syncytial knots was observed (Figure 3B). T. pallidum-exposed placentae also showed a notable increase in the volume density of villous syncytial denuded areas (Figure 3C). Furthermore, there was a significant increase in the number of foetal capillaries in the exposed cases compared with the controls (Figure 3D).
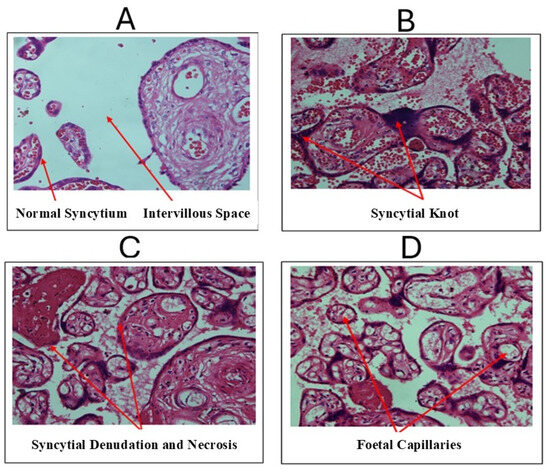
Figure 3.
Micrographs from histomorphological assessment of T. pallidum-infected placentae. (A) Control placenta with red arrows indicating the normal syncytium and intervillous space. (B) T. pallidum-exposed placentae with red arrows indicating syncytial knots. (C) T. pallidum-exposed placentae with red arrow (right) indicating syncytial denudation with fibrinoid necrosis (left arrow). (D) T. pallidum-exposed placentae with red arrows indicating foetal capillaries.
4. Discussion
The findings of this study show various forms of histomorphological changes in placentae exposed to T. pallidum, particularly in relation to syncytial knots, intervillous spaces, syncytial denudation, and foetal capillaries. There was a significant increase in the formation of syncytial knots in placentae exposed to T. pallidum. Syncytial knot (aggregates of syncytial nuclei at the surface of terminal villi) formation reflects placental maturity [14,15] and is considered a normal event in the placenta. However, syncytial knots are not simply predictable end-stage features of placental ageing; their excessive presence is considered pathological, especially when observed in abundance, especially at term. An increased number of syncytial knots may result from the apoptotic death of the syncytiotrophoblast, leading to oxidative stress—an imbalance in the production and clearance of reactive oxygen in tissues—which damages cells. This results in hypoxia [16,17], impairing the transfer of substances from the mother to the foetus. Similar to findings reported by Salmani et al. [18], a significant increase in syncytial knots within placental villi can lead to disruptions in hormonal regulation critical for placental function, potentially contributing to impaired blood flow in the placenta. Elevated syncytial knot formation is often linked to conditions of uteroplacental malperfusion and is considered a key feature in histological placental examination, as noted by Loukeris et al. [15]. The increased formation of syncytial knots has been linked to complications during pregnancy, including preeclampsia (PE) and foetal growth restriction (FGR), when compared with pregnancies without such abnormalities [17,19].
Our study revealed that the exposed placentae had a significant increase in the volume density of villous syncytial denuded areas. Syncytial denudation, defined as areas of the villous surface where the syncytiotrophoblast is either thinned or absent, is a well-recognised pathological entity and is distinguishable from physiological thinning. The observation in our study may suggest changes in villous maturation, potentially caused by placental exposure to T. pallidum. This finding suggests a potential breach in the structural integrity of the placenta-blood barrier as a result of exposure to T. pallidum. Maternal immune cells, such as immunoglobulin G (IgG), can easily cross the barrier [20] and provide immunity to the foetus. However, in instances where immunoglobulin M (IgM) crosses the placenta-blood barrier [17], it is likely that the barrier’s defence has been compromised, allowing higher-molecular-weight immunoglobulins such as IgM to enter the cord blood. Since the foetal-maternal barrier has been breached due to syncytial denudation, blood from the intervillous space may reach the foetal capillaries more easily, facilitating the transmission of blood-borne pathogens from mother to foetus [21,22].
The observation of a significant increase in the volume density of foetal capillaries in placentae exposed to T. pallidum, compared with the controls in this study, implies placental stress in the exposed group. The foetal capillaries are very close to the maternal blood in the intervillous space [23] and may increase in number under disease conditions [24,25], potentially leading to hypoxic changes. Stafford et al. [5] reported that hypoxia may stimulate foetal capillary growth and proliferation. However, chorangiosis (a marked increase in the number of vascular channels in the non-infarcted area of the placenta or proliferation of capillaries in terminal chorionic villi) is considered to be a marker for hypoxia and poor clinical outcome in response to placental-foetal hypoxia [26,27,28]. This may be an adaptive mechanism by which the placenta compensates for reduced nutrient and gas exchange, which could be due to disease conditions, as might have occurred in this study.
Although the reduction in intervillous space observed in the T. pallidum-exposed placentae was not statistically significant, the slight decrease is consistent with the findings of Ernst et al. [29], who reported a similar reduction in cases of intrauterine growth restriction (IUGR). This slight decrease in intervillous space might also be attributed to villous congestion or excessive proliferation, potentially leading to hypoxic events that increase the risk of foetal demise or preterm labour, as suggested by Ravishankar and Redline [30].
Although larger intervillous spaces typically facilitate maternal-foetal blood flow [31], the T. pallidum-exposed placentae in this study showed a slight reduction in intervillous space, suggesting mildly reduced perfusion, which may contribute to impaired oxygen and nutrient delivery [32,33].
5. Limitation
Although this study did not include postnatal follow-up of infants, the observed placental changes may have important clinical implications for neonatal health and immunity. Future studies should investigate whether these histomorphological alterations translate into measurable outcomes in the children.
6. Conclusions
Histomorphological assessment of placentae exposed to T. pallidum revealed several significant differences compared with non-exposed placentae with regard to syncytial knots, the volume density of villous syncytial denuded areas, and the number of foetal capillaries, but no significant difference in the intervillous space. These subtle but significant alterations indicate compromised placental function, accelerated placental ageing or stress, and potential vulnerability to damage, necessitating a compensatory increase in foetal capillaries to maintain adequate foetal oxygenation and nutrient supply. Overall, our study has shown, for the first time, that the histomorphology of human placental villi appears to be altered by exposure to T. pallidum. These findings also highlight the need to strengthen existing antenatal screening programmes, ensure timely diagnosis, and closely monitor pregnancies exposed to T. pallidum in order to investigate whether these histomorphological changes in the placental villi may contribute to long-term effects on the baby.
Author Contributions
Conceptualisation, P.B.T.-Q., J.A. and K.K.A.-O.; data curation, P.B.T.-Q., J.A., S.O.-N., J.T., K.K.A.-O., B.A.H. and N.T.K.D.D.; formal analysis, P.B.T.-Q., J.A., S.O.-N., J.T., F.C.N.K., E.S.D. and E.A.; investigation, P.B.T.-Q., J.T., J.A., and N.T.K.D.D.; methodology, P.B.T.-Q., E.A., J.A., N.K.-K.K., B.A.-B. and J.T.; project administration, P.B.T.-Q., and J.A.; resources, P.B.T.-Q., E.A., J.A., K.K.A.-O., B.A.H. and E.S.D.; supervision, P.B.T.-Q., E.A.U. and J.A.; validation, P.B.T.-Q., E.A.U., F.C.N.K. and J.A.; visualisation, P.B.T.-Q., J.A., E.A., E.A.U., N.K.-K.K., F.C.N.K., N.T.K.D.D., B.A.H., P.O.A. and K.K.A.-O.; writing—original draft, P.B.T.-Q., J.A., S.O.-N., J.T., F.C.N.K., N.T.K.D.D., E.S.D., E.A., P.O.A., B.A.-B. and K.K.A.-O.; and writing—review and editing, P.B.T.-Q., E.A., S.O.-N., N.K.-K.K., B.A.-B., J.T., F.C.N.K., N.T.K.D.D., J.A., B.A.H., E.A.U., P.O.A., B.A.-B. and K.K.A.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval for the study was obtained (approval date: 1 June 2020) from the Ethical and Protocol Review Committee of the College of Health Sciences, University of Ghana (CHS-Et/M4-P.7/2019-2020), with permissions also granted by Weija-Gbawe Municipal Hospital and LEKMA Hospital. All procedures involving human participants were conducted in accordance with the ethical standards of the institutional and national research committees and adhered to the principles of the Declaration of Helsinki, as revised in 2013 [34].
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding authors, via patborket2002@yahoo.com, pbtetteh-quarcoo@ug.edu.gh, or kadutwum-ofosu@ug.edu.gh.
Acknowledgments
We would like to acknowledge the staff of LEKMA Hospital and Weija-Gbawe Municipal Hospital of the Greater Accra region for their immense support for this project. Special thanks to Mercy and Gabriel Nuamah, Samuel Mensah, and Forgive Kuvor for training the sample collectors and Dorothea, Seth, Alexander, Sarah, Eric, Benjamin, Etornam, and Cynthia for helping in the sample collection. We also acknowledge Bright Gabriel Dzotofe for his assistance in the counting using stereology.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Annex 1: Key Data at a Glance. In Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2019; World Health Organization: Geneva, Switzerland, 2019; Available online: https://pesquisa.bvsalud.org/portal/resource/pt/who-326037 (accessed on 19 June 2025).
- Hussen, S.; Tadesse, B.T. Prevalence of Syphilis among Pregnant Women in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Smolak, A.; Rowley, J.; Nagelkerke, N.; Kassebaum, N.J.; Chico, R.M.; Korenromp, E.L.; Abu-Raddad, L.J. Trends and predictors of syphilis prevalence in the general population: Global pooled analyses of 1103 prevalence measures including 136 million syphilis tests. Clin. Infect. Dis. 2018, 66, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Gulersen, M.; Lenchner, E.; Eliner, Y.; Grunebaum, A.; Johnson, L.; Chervenak, F.A.; Bornstein, E. Risk factors and adverse outcomes associated with syphilis infection during pregnancy. Am. J. Obstet. Gynecol. MFM 2023, 5, 100957. [Google Scholar] [CrossRef] [PubMed]
- Stafford, I.A.; Workowski, K.A.; Bachmann, L.H. Syphilis Complicating Pregnancy and Congenital Syphilis. N. Engl. J. Med. 2024, 390, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Sadeep, M.S.; Sobhanakumari, K. Syphilis in pregnancy. J. Ski. Sex. Transm. Dis. 2023, 5, 6–13. [Google Scholar] [CrossRef]
- Jaiman, S. Gross Examination of the Placenta and Its Importance in Evaluating an Unexplained Intrauterine Fetal Demise. J. Fetal Med. 2015, 2, 113–120. [Google Scholar] [CrossRef][Green Version]
- Mumuni, M.; Adutwum-Ofosu, K.K.; Arko-Boham, B.; Hottor, B.A.; Koney, N.K.K.; Adu-Bonsaffoh, K.; Oppong, S.A.; Appiah, P.O.; Ahenkorah, J. Histomorphology of placentae of women with sickle cell disease during pregnancy—A case control study. PLoS ONE 2025, 20, e0319011. [Google Scholar] [CrossRef]
- Cohee, L.M.; Kalilani-Phiri, L.; Mawindo, P.; Joshi, S.; Adams, M.; Kenefic, L.; Jacob, C.G.; Taylor, T.E.; Laufer, M.K. Parasite dynamics in the peripheral blood and the placenta during pregnancy-associated malaria infection. Malar. J. 2016, 15, 483. [Google Scholar] [CrossRef]
- Shukla, G.; Verma, I.; Sharma, L. Effect of Salmonella enteric serovar Typhimurium in pregnant mice: A biochemical and histopathological study. Gastroenterol. Res. 2012, 5, 103. [Google Scholar] [CrossRef][Green Version]
- Umbers, A.J.; Aitken, E.H.; Rogerson, S.J. Malaria in pregnancy: Small babies, big problem. Trends Parasitol. 2011, 27, 168–175. [Google Scholar] [CrossRef]
- Rac, M.W.; Revell, P.A.; Eppes, C.S. Syphilis during pregnancy: A preventable threat to maternal-fetal health. Am. J. Obstet. Gynecol. 2017, 216, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Heidari, Z.; Sakhavar, N.; Mahmoudzadeh-Sagheb, H.; Ezazi-Bojnourdi, T. Stereological analysis of human placenta in cases of placenta previa in comparison with normally implanted controls. J. Reprod. Infertil. 2015, 16, 90. [Google Scholar] [PubMed]
- Mayhew, T.M. Turnover of human villous trophoblast in normal pregnancy: What do we know and what do we need to know? Placenta 2014, 35, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Loukeris, K.; Sela, R.; Baergen, R.N. Syncytial Knots as a Reflection of Placental Maturity: Reference Values for 20 to 40 Weeks’ Gestational Age. Pediatr. Dev. Pathol. 2010, 13, 305–309. [Google Scholar] [CrossRef]
- Fogarty, N.M.; Ferguson-Smith, A.C.; Burton, G.J. Syncytial knots (Tenney-Parker changes) in the human placenta: Evidence of loss of transcriptional activity and oxidative damage. Am. J. Pathol. 2013, 183, 144–152. [Google Scholar] [CrossRef]
- Heazell, A.E.P.; Moll, S.J.; Jones, C.J.P.; Baker, P.N.; Crocker, I.P. Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta 2007, 28, S33–S40. [Google Scholar] [CrossRef]
- Salmani, D.; Purushothaman, S.; Somashekara, S.C.; Gnanagurudasan, E.; Sumangaladevi, K.; Harikishan, R.; Venkateshwarareddy, M. Study of structural changes in placenta in pregnancy-induced hypertension. J. Nat. Sci. Biol. Med. 2014, 5, 352. [Google Scholar] [CrossRef]
- Calvert, S.J.; Jones, C.J.P.; Sibley, C.P.; Aplin, J.D.; Heazell, A.E.P. Analysis of syncytial nuclear aggregates in preeclampsia shows increased sectioning artefacts and decreased inter-villous bridges compared to healthy placentas. Placenta 2013, 34, 1251–1254. [Google Scholar] [CrossRef]
- Palmeira, P.; Quinello, C.; Silveira-Lessa, A.L.; Zago, C.A.; Carneiro-Sampaio, M. IgG Placental Transfer in Healthy and Pathological Pregnancies. Clin. Dev. Immunol. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Xi, G.; Leke, R.G.; Thuita, L.W.; Zhou, A.; Leke, R.J.; Mbu, R.; Taylor, D.W. Congenital Exposure to Plasmodium falciparum Antigens: Prevalence and Antigenic Specificity of In Utero-Produced Antimalarial Immunoglobulin M Antibodies. Infect. Immun. 2003, 71, 1242–1246. [Google Scholar] [CrossRef]
- Callaway, P.C.; Farrington, L.A.; Feeney, M.E. Malaria and early life immunity: Competence in context. Front. Immunol. 2021, 12, 634749. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, N.Y.; Shehata, M.H.; Gadallah, H.N.; Sayed, W.M.; Othman, A.A. Morphological and ultrastructural changes in the placenta of the diabetic pregnant Egyptian women. Acta Histochem. 2018, 120, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Sankar, K.D.; Bhanu, P.S.; Ramalingam, K.; Kiran, S.; Ramakrishna, B.A. Histomorphological and morphometrical changes of placental terminal villi of normotensive and preeclamptic mothers. Anat. Cell Biol. 2013, 46, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Miller, R.K. Receptor-mediated uptake and transport of macromolecules in the human placenta. Int. J. Dev. Biol. 2010, 54, 367–375. [Google Scholar] [CrossRef]
- Akbarzadeh-Jahromi, M.; Soleimani, N.; Mohammadzadeh, S. Multiple Chorangioma Following Long-Term Secondary Infertility: A Rare Case Report and Review of Pathologic Differential Diagnosis. Int. Med. Case Rep. J. 2019, 12, 383–387. [Google Scholar] [CrossRef]
- Suzuki, K.; Itoh, H.; Kimura, S.; Sugihara, K.; Yaguchi, C.; Kobayashi, Y.; Hirai, K.; Takeuchi, K.; Sugimura, M.; Kanayama, N. Chorangiosis and placental oxygenation. Congenit. Anom. 2009, 49, 71–76. [Google Scholar] [CrossRef]
- Ernst, L.M. Maternal vascular malperfusion of the placental bed. APMIS 2018, 126, 551–560. [Google Scholar] [CrossRef]
- Ravishankar, S.; Redline, R.W. The placenta. Handb. Clin. Neurol. 2019, 162, 57–66. [Google Scholar]
- Rainey, A.; Mayhew, T.M. Volumes and numbers of intervillous pores and villous domains in placentas associated with intrauterine growth restriction and/or pre-eclampsia. Placenta 2010, 31, 602–606. [Google Scholar] [CrossRef]
- Schneider, H. Oxygenation of the placental–fetal unit in humans. Respir. Physiol. Neurobiol. 2011, 178, 51–58. [Google Scholar] [CrossRef]
- Perazzolo, S.; Lewis, R.M.; Sengers, B.G. Modelling the effect of intervillous flow on solute transfer based on 3D imaging of the human placental microstructure. Placenta 2017, 60, 21–27. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Hellenic Society for Microbiology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).