Trichomonas vaginalis in Vaginal Samples from Symptomatic Women in Greece: Assessment of Test Performance and Prevalence Rate, and Comparison with European Prevalence Estimates
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Investigation of T. vaginalis Infection in Symptomatic Greek Women
2.1.1. Study Design
2.1.2. Sampling
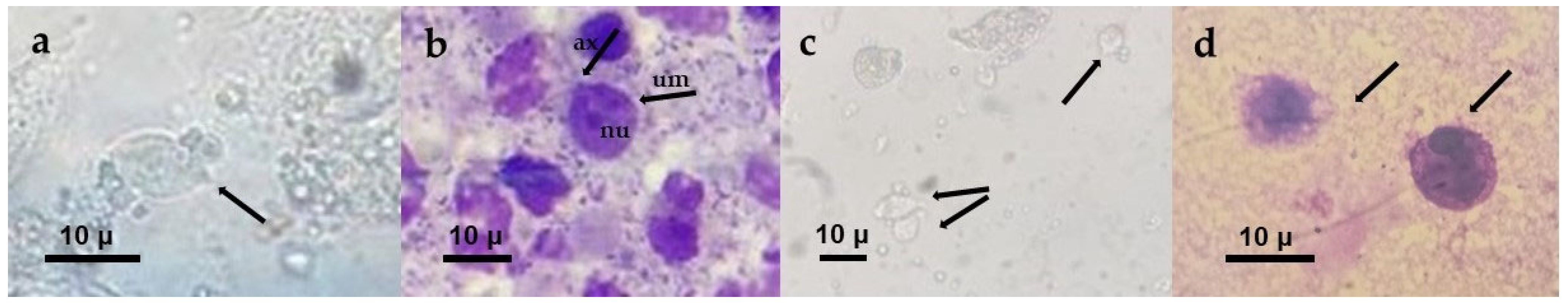
2.1.3. Wet Mount Microscopy
2.1.4. Giemsa-Stained Smear Microscopy
2.1.5. Culture
2.1.6. Commercial NAAT
2.1.7. Test Performance and Prevalence Calculation for T. vaginalis
2.2. Estimation of Pooled T. vaginalis Prevalence in European Symptomatic Women
2.2.1. Study Design
2.2.2. Database Search
2.2.3. Eligibility Criteria
- Peer-reviewed original articles covering countries in the European region;
- Laboratory-based cross-sectional studies on the prevalence of T. vaginalis among women with vaginal symptoms;
- Studies identifying T. vaginalis in vaginal samples;
- Papers published from 1 January 2008 to 31 December 2023;
- Research studies with accessible abstracts and full texts in English;
- Articles with English abstracts, even if their full texts were not in English;
- Studies providing the exact number of study participants and positive cases.
- Editorials;
- Letters;
- Reviews;
- Papers referring to other geographical regions;
- Articles without a specified total sample size and number of positives;
- Surveys using electronic data collection;
- Research studies based on screening or routine testing;
- Laboratory-based studies testing samples other than vaginal ones;
- Prevalence studies on men, children, female sex workers, pregnant women, and asymptomatic women.
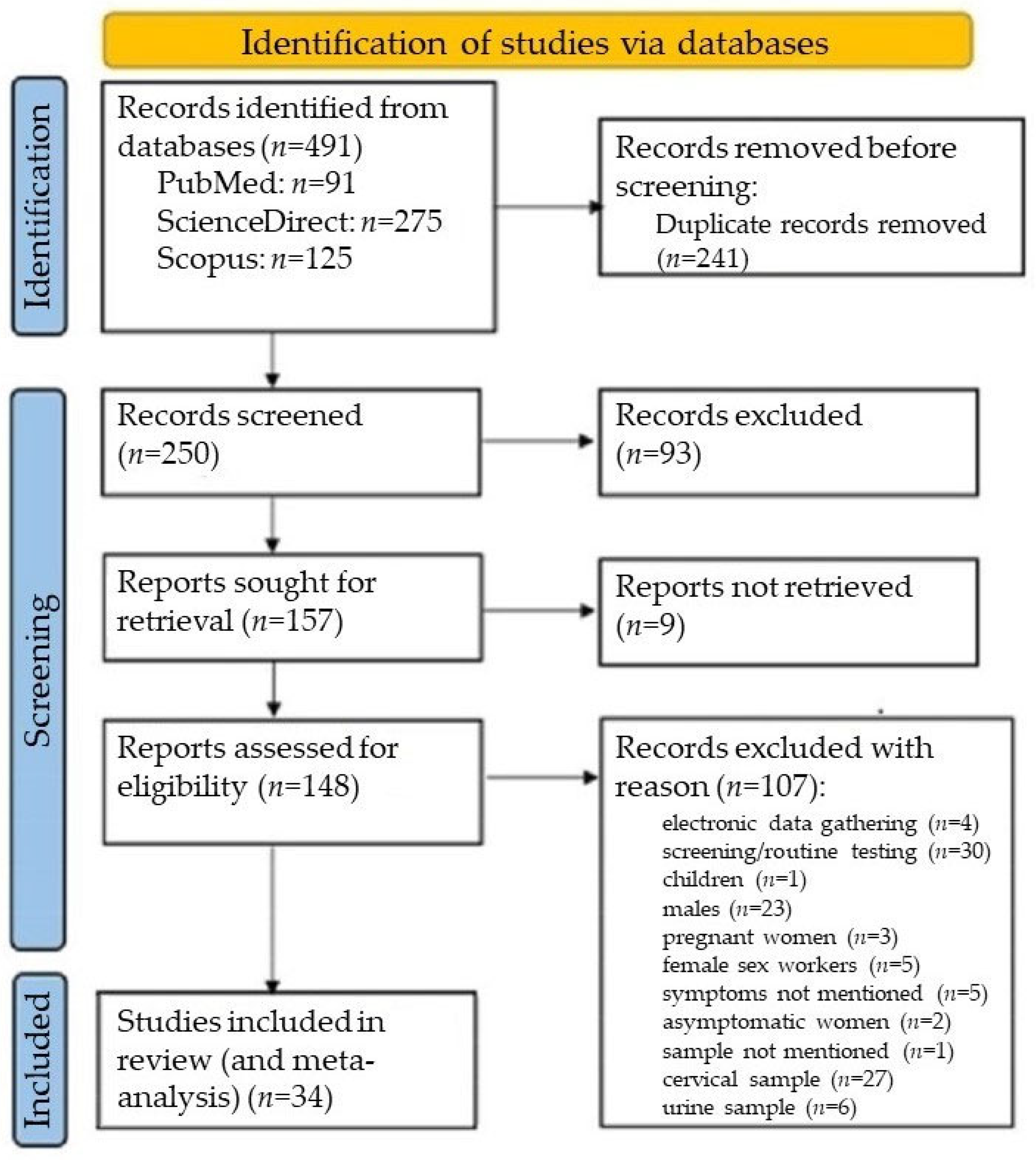
2.2.4. Selection Process
2.2.5. Data Synthesis
2.3. Statistical Analysis
3. Results
3.1. Results from Laboratory Investigation of T. vaginalis Infection in Symptomatic Greek Women
3.1.1. Study Samples
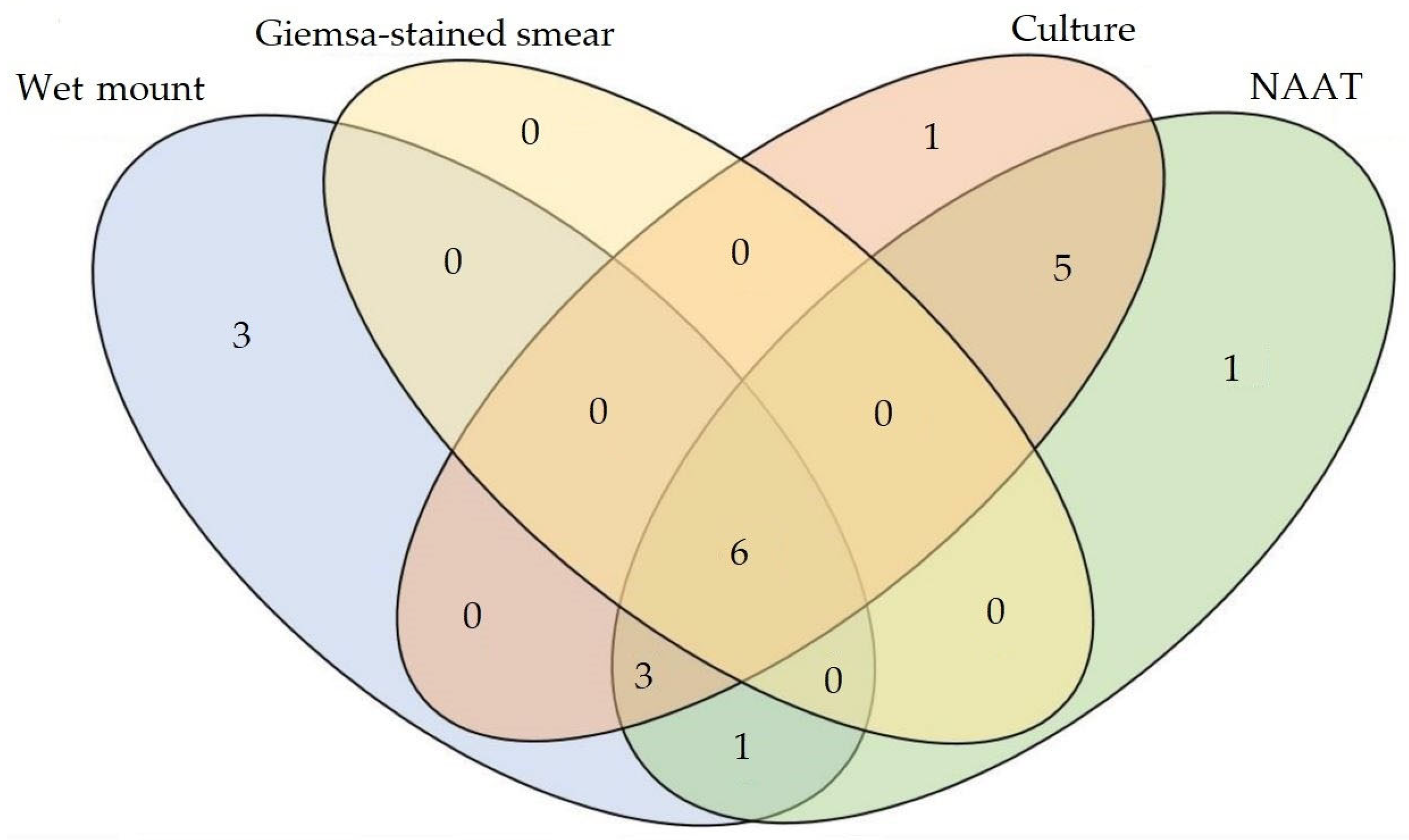
3.1.2. Detection of T. vaginalis in Vaginal Samples
3.1.3. Comparative Test Performance and Prevalence for T. vaginalis
3.2. Results from Systematic Review and Meta-Analyses
3.2.1. Systematic Review
3.2.2. Subgroup Analyses Based on Geographical Location
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shiratori, M.; Patel, A.; Gerhold, R.W.; Sullivan, S.A.; Carlton, J.M. Persistent Trichomonas vaginalis infections and the pseudocyst form. Trends Parasitol. 2023, 39, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Rimache, J.; Inolopú, J.L.; Soncco-Llulluy, F.C.; Medina-Ciprian, L. Comparison of three methods for diagnosing trichomoniasis in female patients with sexual activity attended at a hospital in Peru. J. Parasitol. Res. 2023, 2023, 9528942. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, R.; Sharma, N.; Kanaujia, R.; Malhorta, S.; Chaundry, H.; Rathore, S.; Saini, A.; Bagga, R.; Mewara, A.; Khurana, S.; et al. Prevalence of Trichomonas vaginalis by polymerase chain reaction-based molecular method among symptomatic women from Northern India. Indian J. Sex. Transm. Dis. AIDS 2023, 44, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bola, G.A. Sexually transmitted diseases treatment guidelines, 2025. MMWR Recomm. Rep. 2015, 64, 1–137. [Google Scholar]
- Sinka, K. The global burden of sexually transmitted infections. Clin. Dermatol. 2024, 42, 110–118. [Google Scholar] [CrossRef]
- Sherrard, J.; Pitt, R.; Russel Hobbs, K.; Maynard, M.; Cochrane, E.; Wilson, J.; Tipple, C. British Association for Sexual Health and HIV (BASHH) United Kingdom national guideline on the management of Trichomonas vaginalis 2021. Int. J. STD AIDS 2022, 33, 740–750. [Google Scholar] [CrossRef]
- Wayal, S.; Aicken, C.R.H.; Griffiths, C.; Blomquist, P.B.; Hughes, G.; Mercer, C.H. Understanding the burden of bacterial sexually transmitted infections and Trichomonas vaginalis among black Caribbeans in the United Kingdom: Findings from a systematic review. PLoS ONE 2018, 13, e0208315. [Google Scholar] [CrossRef]
- Meites, E.; Gaydos, C.A.; Hobbs, M.M.; Kissinger, P.; Nyirjesy, P.; Schwebke, J.R.; Secor, W.E.; Sobel, J.D.; Workowski, K.A. A review of evidence-based care of symptomatic trichomoniasis and asymptomatic Trichomonas vaginalis infections. Clin. Infect. Dis. 2015, 61 (Suppl. S8), S837–S848. [Google Scholar] [CrossRef]
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; AbuRaddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef]
- World Health Organization. Global Lobal Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections for the Period 2022–2030; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-005377-9. [Google Scholar]
- National Health Organization. Epidemiological and Laboratory Surveillance of Sexually Transmitted Diseases. 2023. Available online: www.eody.gov.gr/smn_dedomena_2023 (accessed on 4 May 2025). (In Greek)
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Hathorn, E.; Ng, A.; Page, M.; Hodson, J.; Gaydos, C.; Ross, J.D. A service evaluation of the Gen-Probe APTIMA nucleic acid amplification test for Trichomonas vaginalis: Should it change whom we screen for infection? Sex Transm Infect. 2015, 91, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Schwebke, J.R.; Gaydos, C.A.; Davis, T.; Marrazzo, J.; Furgerson, D.; Taylor, S.N.; Smith, B.; Bachmann, L.H.; Ackerman, R.; Spurrell, T.; et al. Clinical evaluation of the Cepheid Xpert TV assay for detection of Trichomonas vaginalis with prospectively collected specimens from men and women. J. Clin. Microbiol. 2018, 56, e01091-17. [Google Scholar] [CrossRef]
- Çulha, G.; Günoren, A.; Demir, C.; Hakverdi, A.; Duran, N. Detection of Trichomonas vaginalis in vaginal specimens from women by wet mount, culture and PCR. J. Clin. Anal. Med. 2015, 6, 537–554. [Google Scholar]
- Sönmez, C.; Usluca, S. Cases with urogenital discharge: Trichomonas vaginalis rates? Flora J. Infect. Dis. Clin. Microbiol. 2018, 23, 79–83. [Google Scholar] [CrossRef]
- Shone, J.; Winter, A.; Jones, B.L.; Butt, A.; Brawley, D.; Cunningham, C.; Paterson, J.; McAllister, G.; Alexander, C.L. A Scottish multi-centre service evaluation examining the prevalence and diagnosis of Trichomonas vaginalis in symptomatic women attending sexual health clinics. Int. J. STD AIDS 2016, 27, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Radonjic, I.V.; Dzamic, A.M.; Mitrovic, S.M.; Arsenijevic, V.S.A.; Popadic, D.M.; Zec, I.F.K. Diagnosis of Trichomonas vaginalis infection: The sensitivities and specificities of microscopy, culture and PCR assay. Eur. J. Obstet. Gynecol. Repro. Biol. 2006, 126, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Gomes Cardoso, F.; Freitas, M.D.; Tasca, T.; Vargas Rigo, G. From wet mount to nucleic acid amplification techniques: Current diagnostic methods and future perspectives based on patenting of new assays, stains, and diagnostic images for Trichomonas vaginalis detection. Venereology 2024, 3, 35–50. [Google Scholar] [CrossRef]
- Mahmoud, A.; Sherif, N.A.; Abdella, R.; El-genedy, A.R.; Ek Kateb, A.Y.; Askalani, A.N.H. Prevalence of Trichomonas vaginalis infection among Egyptian women using culture and Latex agglutination: Cross-sectional study. BMC Women’s Health 2015, 15, 7. [Google Scholar] [CrossRef]
- Nathan, B.; Appiah, J.; Saunders, P.; Heron, D.; Nichols, T.; Brum, R.; Alexander, S.; Baraitser, P.; Ison, C. Microscopy outperformed in a comparison of five methods for detecting Trichomonas vaginalis in symptomatic women. Int. J. STD AIDS 2014, 26, 251–256. [Google Scholar] [CrossRef]
- Nicholls, J.E.; Turner, K.M.E.; North, P.; Ferguson, R.; May, M.T.; Gough, K.; Macleod, J.; Muir, P.; Horner, P.J. Cross-sectional study to evaluate Trichomonas vaginalis positivity in women tested for Neisseria gonorrhoeae and Chlamydia trachomatis, attending genitourinary medicine and primary care clinics in Bristol, South West England. Sex. Transm. Infect. 2018, 94, 93–99. [Google Scholar] [CrossRef]
- Hobbs, M.M.; Seña, A.C. Modern diagnosis of Trichomonas vaginalis infection. Sex. Transm. Infect. 2013, 89, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Papapetropoulou, M.; Legakis, N.J.; Detorakis, J.; Kalambokas, E.; Lymberopoulou, T. Frequency and epidemiologic associations of different types of vaginitis in symptomatic women in Greece. Eur. J. Clin. Microbiol. 1986, 5, 447–449. [Google Scholar] [CrossRef]
- Vitoratos, N.; Gregoriou, O.; Papadias, C.; Liapis, A.; Zourlas, P.A. Sexually transmittes diseses in women with urethral syndrome. Int. J. Gynecol. Obstet. 1988, 27, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Iavazzo, C.; Vogiatzi, C.; Falagas, M.E. A retrospective analysis of isolates from patients with vaginitis in a private Greek obstetric/gynecological hospital (2003–2006). Med. Sci. Monit. 2008, 14, CR228-31. [Google Scholar] [PubMed]
- Piperaki, E.T.; Theodora, M.; Mendris, M.; Barbitsa, L.; Pitiriga, V.; Antsaklis, A.; Tsakris, A. Prevalence of Trichomonas vaginalis infection in women attending a major gynaecological hospital in Greece: A cross-sectional study. J. Clin. Pathol. 2010, 63, 249–253. [Google Scholar] [CrossRef]
- Ioannidis, A.; Papaioannou, P.; Magiorkinis, E.; Magana, M.; Ioannidou, V.; Tzanetou, K.; Burriel, A.R.; Tsironi, M.; Chatzipanagiotou, S. Detecting the diversity of Mycoplasma and Ureaplasma endosymbionts hosted by Trichomonas vaginalis isolates. Front. Microbiol. 2017, 28, 1188. [Google Scholar] [CrossRef]
- Sianou, A.; Galyfos, G.; Moragianni, D.; Baka, S. Prevalence of vaginitis in different age groups among females in Greece. J. Obstet. Gynaecol. 2017, 37, 790–794. [Google Scholar] [CrossRef]
- Florou, Z.; Pantelidi, K.; Fountas, S.; Skoulakis, A.; Messini, C.; Lachanas, V.; Petinaki, E. High prevalence of sexually transmitted infections (STIs) in asymptomatic Greek women. Arch. Clin. Microbiol. 2018, 9, 78. [Google Scholar]
- Parthenis, C.; Panagopoulos, P.; Margari, N.; Kottaridi, C.; Spathis, A.; Pouliakis, A.; Konstantoudakis, S.; Chrelias, G.; Chrelias, C.; Papantoniou, N.; et al. The association between sexually transmitted infections, human papillomavirus, and cervical cytology abnormalities among women in Greece. Int. J. Infect. Dis. 2018, 73, 72–77. [Google Scholar] [CrossRef]
- von Hippel, P.T. The hererogeneity statistic I2 can be biases in small meta-analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef]
- Augusteijn, H.E.M.; van Aert, R.C.; van Assen, M.A.L.M. The effect of publication bias on the Q test and assessment of heterogeneity. Psychol. Methods 2010, 24, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Spineli, L.M.; Pandis, N. Exploring heterogeneity in meta-analysis: Subgroup analysis. Part 1. Am. J. Orthod. Dentofacial Orthop. 2020, 158, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Özarmagan, G.; Bingham, J.S. Sexually transmitted infections in Turkey. Int. J. STD AIDS 2001, 12, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Polat, F.; Kaya Şenol, D. Examining the correlation between sexual and reproductive health stigmatization level and gender perception: A case of a university in Turkey—A descriptive cross-sectional study. Sao Paulo Med. J. 2022, 141, 146–153. [Google Scholar] [CrossRef]
- Kalavrezou, N. Gender and access to healthcare in Greece. Soc. Cohes. Dev. 2009, 4, 205–216. [Google Scholar] [CrossRef]
- Garcia, P.J.; Espinosa Miranda, A.; Gupta, S.; Garland, S.M.; Escobar, M.E.; Fortenberry, J.D.; The International Union Against Sexually Transmitted Infections. The role of sexually transmitted infections (STI) prevention and control programs in reducing gender, sexual and STI-related stigma. eClinicalMedicine 2021, 33, 100764. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Progress Towards Reaching the Sustainable Development Goals Related to HIV, Viral Hepatitis, Sexually Transmitted Infections and Tuberculosis in the EU/EEA: 2024 Progress Report; ECDC: Stockholm, Sweden, 2025. [Google Scholar]
- Jacobsson, S.; Boiko, I.; Golparian, D.; Blondeel, K.; Kiarie, J.; Toskin, I.; Peeling, R.W.; Unemo, M. WHO laboratory validation of Xpert® CT/NG and Xpert® TV on the GeneXpert system verifies high performances. APMIS 2018, 126, 907–912. [Google Scholar] [CrossRef]
- Sönmez Tamer, G.; Dündar, D.; Çalışkan, Ş.; Doğer, E. Comparison of direct microscopy and in–vitro cultures in detection of Trichomonas vaginalis. Turk. Bull. Hyg. Exp. Biol. 2008, 65, 75–80. [Google Scholar]
- Sönmez Tamer, G.; Keçeli Ozcan, S.; Yücesoy, G.; Gacar, G. The relation between trichomoniasis and contraceptive methods. Turk. Parazitol. Derg. 2009, 33, 266–269. (In Turkish) [Google Scholar]
- Tibaldi, C.; Cappello, N.; Latino, M.A.; Masuelli, G.; Marini, S.; Benedetto, C. Vaginal and endocervical microorganisms in symptomatic and asymptomatic non-pregnant females: Risk factors and rates of occurrence. Clin. Microbiol. Infect. 2009, 15, 670–679. [Google Scholar] [CrossRef]
- Akdemir, C.; Keskin, N.; Çoksüer, H. A survey of prevalence of Trichomonas vaginalis in cases with vaginal discharge in Kütahya by classic miscroscopy and DNA hybridization. Türk. Hij. Den. Biyol. Derg. 2010, 67, 161–166. [Google Scholar][Green Version]
- Casari, E.; Ferrario, A.; Morenghi, E.; Montanelli, A. Gardnerella, Trichomonas vaginalis, Candida, Chlamydia trachomatis, Mycoplasma hominis and Ureaplasma urealyticum in the genital discharge of symptomatic fertile and asymptomatic infertile women. New Microbiol. 2010, 33, 69–76. [Google Scholar] [PubMed][Green Version]
- Keleştemur, N.; Kaplan, M. The prevalence of T. vaginalıs in women with vaginitis in Elazığ. Med. Sci. 2010, 5, 1–5. [Google Scholar][Green Version]
- Çetinkaya, Ü.; Yazar, S.; Serin, S.; Hamamci, B.; Kuk, S. Trichomonas vaginalis positivity according to type of vaginal discharge in women. Turk. Klin. Tip Bilim. Derg. 2011, 31, 1094–1099. [Google Scholar] [CrossRef]
- Değerli, S.; Şalk, S.; Malatyalı, E. Incidence in Sivas of Trichomonas vaginalis in patients with vaginitis. Turk. Parazitol. Derg. 2011, 35, 145–147. (In Turkish) [Google Scholar] [CrossRef]
- Polat, E.; Sirekbasan, S.; Yıldırım, Z.; Bağdatlı, Y.; Çepni, I.; Çift, T.; Baltalı, N.D. Comparing the occurrence of Trichomonas vaginalis infections today to ten years ago among women prostitutes and gynecology and obstetrics patients in Istanbul. Turk. Parazitol. Derg. 2011, 35, 68–71. (In Turkish) [Google Scholar] [CrossRef]
- Keleştemur, N.; Kaplan, M.; Özdemir, E.; Erensoy, A. The frequency of Trichomonas vaginalis, Gardnerella vaginalis and Candida spp. among infertile men and women with vaginitis. Kafkas Unv. Vet. Fak. Derg. 2012, 18, 47–52. [Google Scholar]
- Keşli, R.; Pektaş, B.; Ozdemir, M.; Günenc, O.; Coşkun, E.; Baykan, M.; Baysal, B. Microscopic examination of vaginal discharge specimens for Trichomonas vaginalis and other micro-organisms in 18–45 age group women. Turk. Parazitol. Derg. 2012, 36, 182–184. (In Turkish) [Google Scholar] [CrossRef]
- Jahic, M.; Mulavdic, M.; Nurkic, J.; Jahic, E.; Nurkic, M. Clinical characteristics of aerobic vaginitis and its association to vaginal candidiasis, trichomonas vaginitis and bacterial vaginosis. Med. Arch. 2013, 67, 428–430. [Google Scholar] [CrossRef]
- Aycan-Kaya, Ö.; Silfeler, D.B.; Kurt, R.K.; Gözükarad, I.; Yengil, E.; Bayramoǧlu, N. Investigation of the presence of Trichomonas vaginalis in infertile Turkish women. Asian Biomed. 2015, 9, 659–663. [Google Scholar] [CrossRef]
- Pellrud, H.; Golparian, D.; Nilsson, C.S.; Falk, M.; Fredlund, H.; Unemo, M. Trichomonas vaginalis infections are rare among young patients attending an STI clinic in Sweden. Acta Derm. Venereol. 2015, 95, 343–344. [Google Scholar] [CrossRef] [PubMed]
- Salfa, M.C.; Suligoi, B.; Italian STI Laboratory-based surveillance working group. prevalence of Chlamydia trachomatis, Trichomonas vaginalis and Neisseria gonorrhoeae based on data collected by a network of clinical microbiology laboratories, in Italy. Adv. Exp. Med. Biol. 2016, 901, 47–57. [Google Scholar] [PubMed]
- Carrillo-Ávila, J.A.; Serrano-García, M.L.; Fernández-Parra, J.; Sorlózano-Puerto, A.; Navarro-Marí, J.M.; Stensvold, C.R.; Gutiérrez-Fernández, J. Prevalence and genetic diversity of Trichomonas vaginalis in the general population of Granada and co-infections with Gardnerella vaginalis and Candida species. J. Med. Microbiol. 2017, 66, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Korycińska, J.; Dzika, E.; Waśniewski, T.; Lepczyńska, M.; Kubiak, K. The prevalence of Trichomonas vaginalis infections in the population of Warmińsko-Mazurskie voivodeship (North-Eastern Poland). Przegl. Epidemiol. 2017, 71, 547–554. [Google Scholar]
- Akyıldız, F.; Özçelik, S.; Özpınar, N.; Karakuş, S. Comparison of three different culture methods in the diagnosis and investigation of frequency of Trichomonas vaginalis in women with the pre–diagnosis of vaginitis. Turk. Bull. Hyg. Exp. Biol. 2018, 75, 43–52. [Google Scholar] [CrossRef]
- Sonmez, C.; Usluca, S.; Hakki Usluca, I.; Kalipci, I.; Sezen, F.; Resat Atalay, C.; Kilic, S. Evaluation of symptomatic patients with resistant discharge. Acta Dermatovenerol. Croat. 2018, 26, 1–7. [Google Scholar]
- Dogan, N.; Gitmez, F. Investigation of Trichomonas vaginalis frequency by different methods in women in Eskişehir province and evaluation of its relation with various social variables. Osman. J. Med. 2019, 41, 46–57. [Google Scholar] [CrossRef]
- Nijhuis, R.H.T.; Duinsbergen, R.G.; Pol, A.; Godschalk, P.C.R. Prevalence of Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium and Trichomonas vaginalis including relevant resistance-associated mutations in a single center in the Netherlands. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 591–595. [Google Scholar] [CrossRef]
- Türkmen Albayrak, H.; Albayrak, A.M.; Bakır, A.; Şahin, İ. Comparison of vulvovaginal infection diagnostic methods and effects of predisposing factors vulvovaginal infections. J. DU Health Sci. Inst. 2020, 10, 52–57. [Google Scholar]
- Yazısız, H.; Koyuncu Özyurt, Ö.; Öztürk Eryiğit, F.; Özhak, B.; Öngüt, G.; Özekinci, M.; Dönmez, L.; Çolak, D.; Gümüş, S.; Öğünç, D. Evaluation of microscopic examination, culture and polymerase chain reaction tests in the diagnosis of Trichomonas vaginalis infection. Mikrobiyol. Bul. 2020, 54, 135–143. (In Turkish) [Google Scholar] [CrossRef]
- Ertabaklar, H.; Malatyali, E.; Özün Özbay, E.P.; Yildiz, İ.; Sinecen, M.; Ertuğ, S.; Bozdoğan, B.; Güçlü, Ö. Microsatellite-Based Genotyping, analysis of population structure, presence of Trichomonas vaginalis Virus (TVV) and Mycoplasma hominis in T. vaginalis isolates from Southwest of Turkey. Iran J. Parasitol. 2021, 16, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Opolskiene, G.; Bumbuliene, Z.; Kiveryte, S.; Bartkeviciute, A.; Ramasauskaite, D.; Bartkeviciene, D. The use of vaginal wet smear: Can we predict Mycoplasmas/Ureaplasmas? Arch. Gynecol. Obstet. 2021, 304, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, E.; Mertens, K.; van Lisdonk, N.; Houwen, C.; Thai, K.T.D. Epidemiology of Mycoplasma genitalium and Trichomonas vaginalis in the primary health care setting in the Netherlands. Epidemiol. Infect. 2023, 5, 151.e79. [Google Scholar] [CrossRef] [PubMed]
| Se a | Sp a | |||
|---|---|---|---|---|
| Method | % | (95% CI) | % | (95% CI) |
| Wet mount | 60 | 32.3–83.7 | 99 | 97.4–99.7 |
| Giemsa stain | 40 | 16.3–67.7 | 99.2 | 97.8–99.8 |
| NAAT b | 93.3 | 68.1–99.8 | 95.5 | 98.1–99.9 |
| Se a | Sp a | |||
|---|---|---|---|---|
| Method | % | (95% CI) | % | (95% CI) |
| Wet mount | 62.5 | 35.4–84.8 | 99.2 | 97.7–99.8 |
| Giemsa stain | 37.5 | 15.2–64.6 | 100 | 99.0–100 |
| Culture | 85.5 | 61.6–98.5 | 99.7 | 98.6–99.9 |
| Se a | Sp a | PPV a | NPV a | |||||
|---|---|---|---|---|---|---|---|---|
| Method | % | (95% CI) | % | (95% CI) | % | (95% CI) | (%) | (95% CI) |
| Wet mount | 58.8 | 32.9–81.6 | 99.2 | 97.7–99.8 | 76.9 | 50.2–91.7 | 98.2 | 96.8–99.0 |
| Giemsa stain | 35.3 | 14.2–61.7 | 100 | 99.0–100 | 100 | 54.1–100 | 97.2 | 95.1–98.6 |
| Culture | 88.2 | 63.6–98.5 | 100 | 99.0–100 | 100 | 78.2–100 | 99.5 | 98.1–99.9 |
| NAAT b | 94.1 | 71.3–99.9 | 100 | 99.0–100 | 100 | 79.4–100 | 99.7 | 98.3–99.9 |
| Europe | Included Studies | Estimated Prevalence of T. vaginalis Infection | ||||||
|---|---|---|---|---|---|---|---|---|
| Geograhic Location | Countries a | Number | Sample Size | (%) | (95% CI) | Q | d.f. | p |
| Northwestern Europe | Lt, Nl, Pl, Se, UK | 8 | 13,911 | 2.1 | 1.1–3.1 | 9.83 | 1 | <0.001 |
| Southern Europe | Ba, Es, Gr, It, Tr | 26 | 87,301 | 5.7 | 3.7–7.6 | |||
| Türkiye | Tr | 18 | 4153 | 6.7 | 4.0–9.3 | 10.1 | 1 | <0.001 |
| Northwestern Europe | Lt, Nl, Pl, Se, UK | 8 | 13,911 | 2.1 | 1.1–3.1 | |||
| Türkiye | Tr | 18 | 4153 | 6.7 | 4.0–9.3 | 5.57 | 1 | <0.001 |
| Southern Europe (rest) | Ba, Es, Gr, It | 8 | 83,148 | 3.2 | 1.3–5.1 | |||
| Greece | Gr | 3 | 9387 | 4.5 | 1.6–7.3 | 2.37 | 1 | 0.12 |
| Northwestern Europe | Lt, Nl, Pl, Se, UK | 8 | 13,911 | 2.1 | 1.1–3.1 | |||
| Greece | Gr | 3 | 9387 | 4.5 | 1.6–7.3 | 3.92 | 1 | 0.05 |
| Southern Europe (rest) | Ba, Es, It | 5 | 73,761 | 1.5 | 0.8–2.2 | |||
| Greece | Gr | 3 | 9387 | 4.5 | 1.6–7.3 | 1.22 | 1 | 0.27 |
| Türkiye | Tr | 18 | 4153 | 6.7 | 4.0–9.3 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Hellenic Society for Microbiology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoukalas, L.; Vassalos, C.M.; Gkitsakis, N.; Gkotzamani, P.; Gkoumalatsou, E.; Bakalianou, K.; Palla, E.; Baka, S.; Skanavis, C.; Vassalou, E. Trichomonas vaginalis in Vaginal Samples from Symptomatic Women in Greece: Assessment of Test Performance and Prevalence Rate, and Comparison with European Prevalence Estimates. Acta Microbiol. Hell. 2025, 70, 29. https://doi.org/10.3390/amh70030029
Tsoukalas L, Vassalos CM, Gkitsakis N, Gkotzamani P, Gkoumalatsou E, Bakalianou K, Palla E, Baka S, Skanavis C, Vassalou E. Trichomonas vaginalis in Vaginal Samples from Symptomatic Women in Greece: Assessment of Test Performance and Prevalence Rate, and Comparison with European Prevalence Estimates. Acta Microbiologica Hellenica. 2025; 70(3):29. https://doi.org/10.3390/amh70030029
Chicago/Turabian StyleTsoukalas, Lazaros, Constantine M. Vassalos, Nikos Gkitsakis, Panagiota Gkotzamani, Eleni Gkoumalatsou, Konstantia Bakalianou, Eleftheria Palla, Stavroula Baka, Constantina Skanavis, and Evdokia Vassalou. 2025. "Trichomonas vaginalis in Vaginal Samples from Symptomatic Women in Greece: Assessment of Test Performance and Prevalence Rate, and Comparison with European Prevalence Estimates" Acta Microbiologica Hellenica 70, no. 3: 29. https://doi.org/10.3390/amh70030029
APA StyleTsoukalas, L., Vassalos, C. M., Gkitsakis, N., Gkotzamani, P., Gkoumalatsou, E., Bakalianou, K., Palla, E., Baka, S., Skanavis, C., & Vassalou, E. (2025). Trichomonas vaginalis in Vaginal Samples from Symptomatic Women in Greece: Assessment of Test Performance and Prevalence Rate, and Comparison with European Prevalence Estimates. Acta Microbiologica Hellenica, 70(3), 29. https://doi.org/10.3390/amh70030029






