Oral Microbiome Diversity in Transfusion-Dependent Thalassemia Using a Metagenomic Approach in Indonesian Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design and Sample Size
2.3. Clinical Examination and Sample Collection
2.4. Metagenomic Analysis
3. Results
3.1. Participant Characteristics
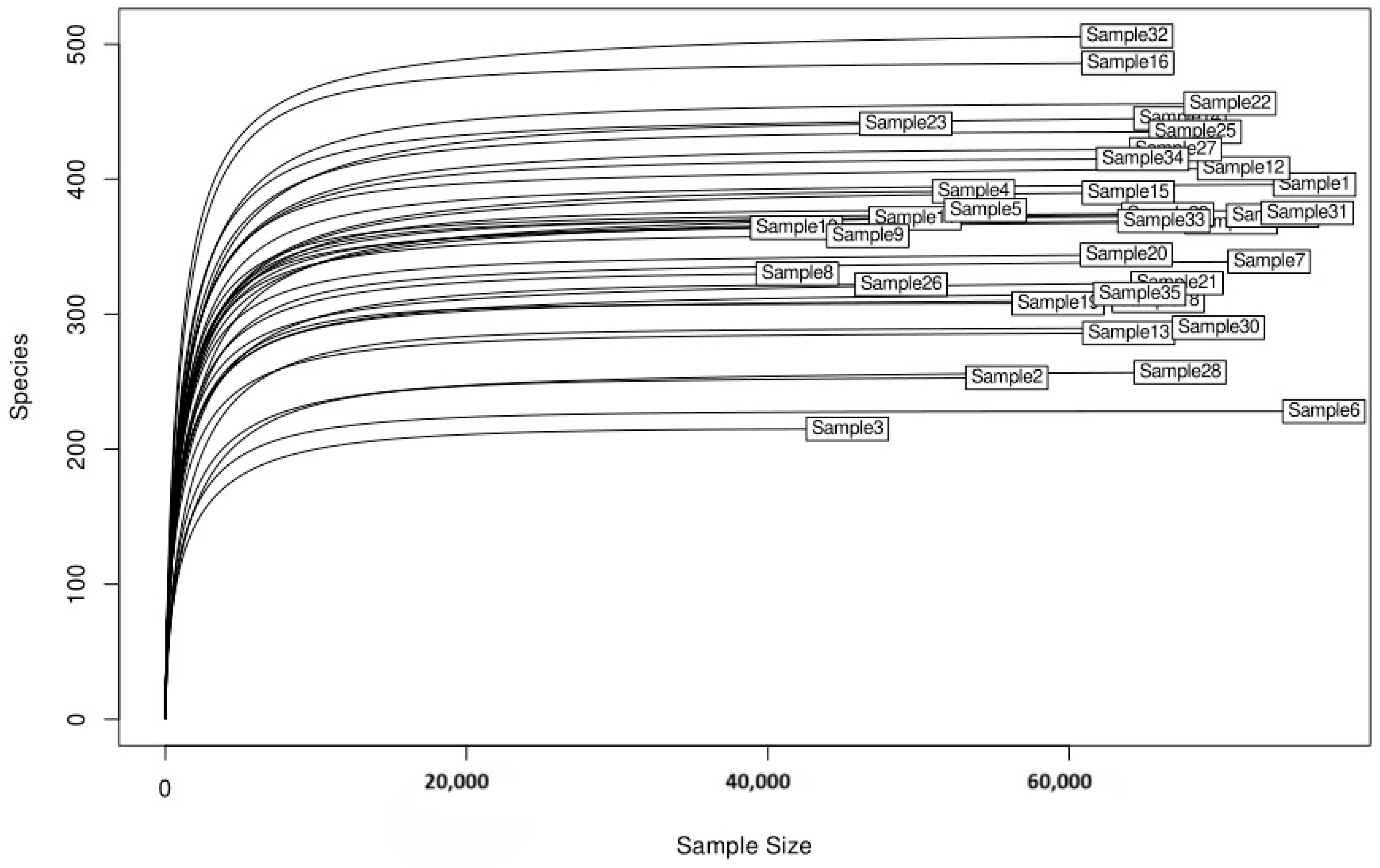
3.2. Alpha Diversity and Rarefaction Analysis
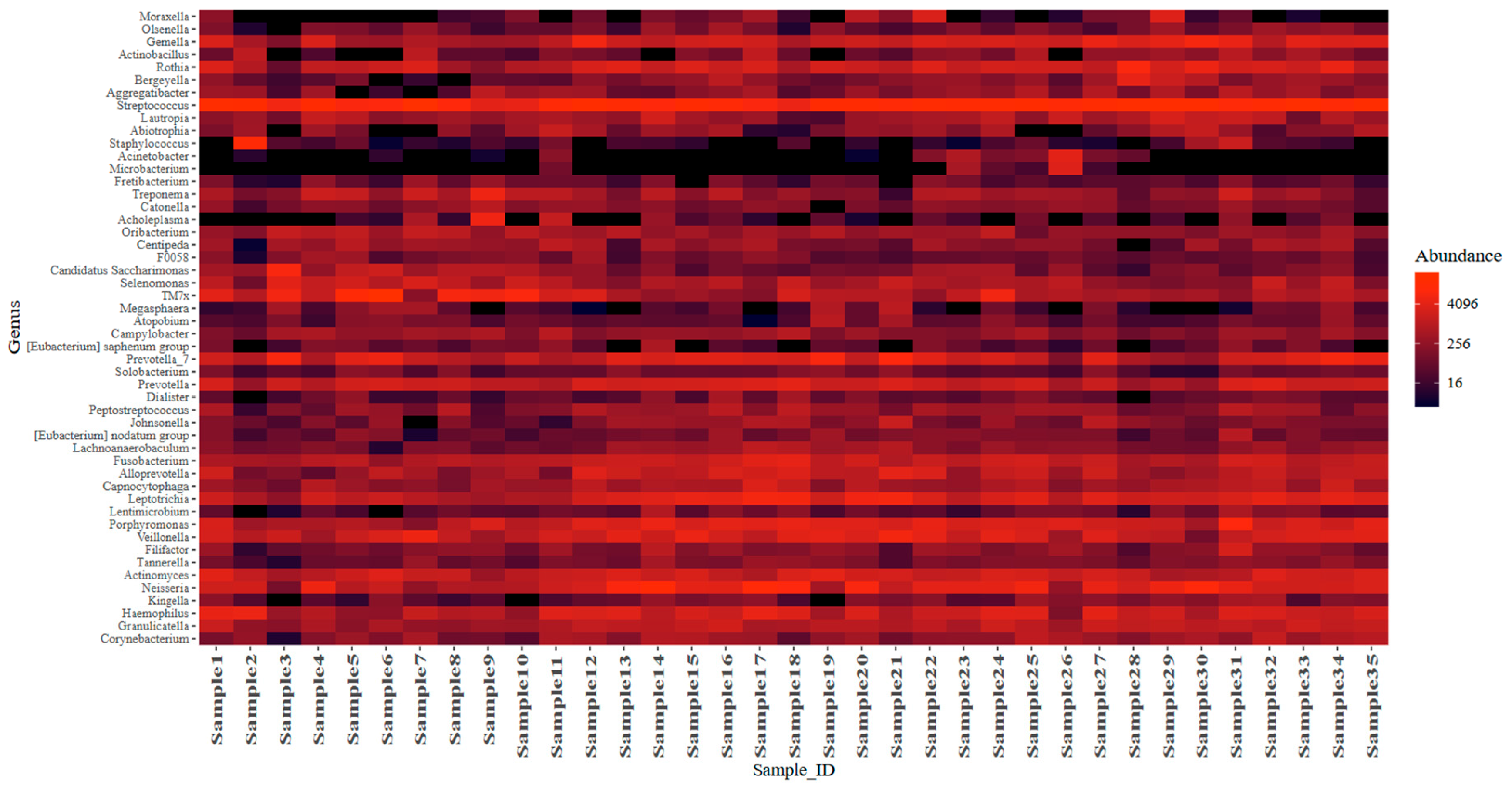
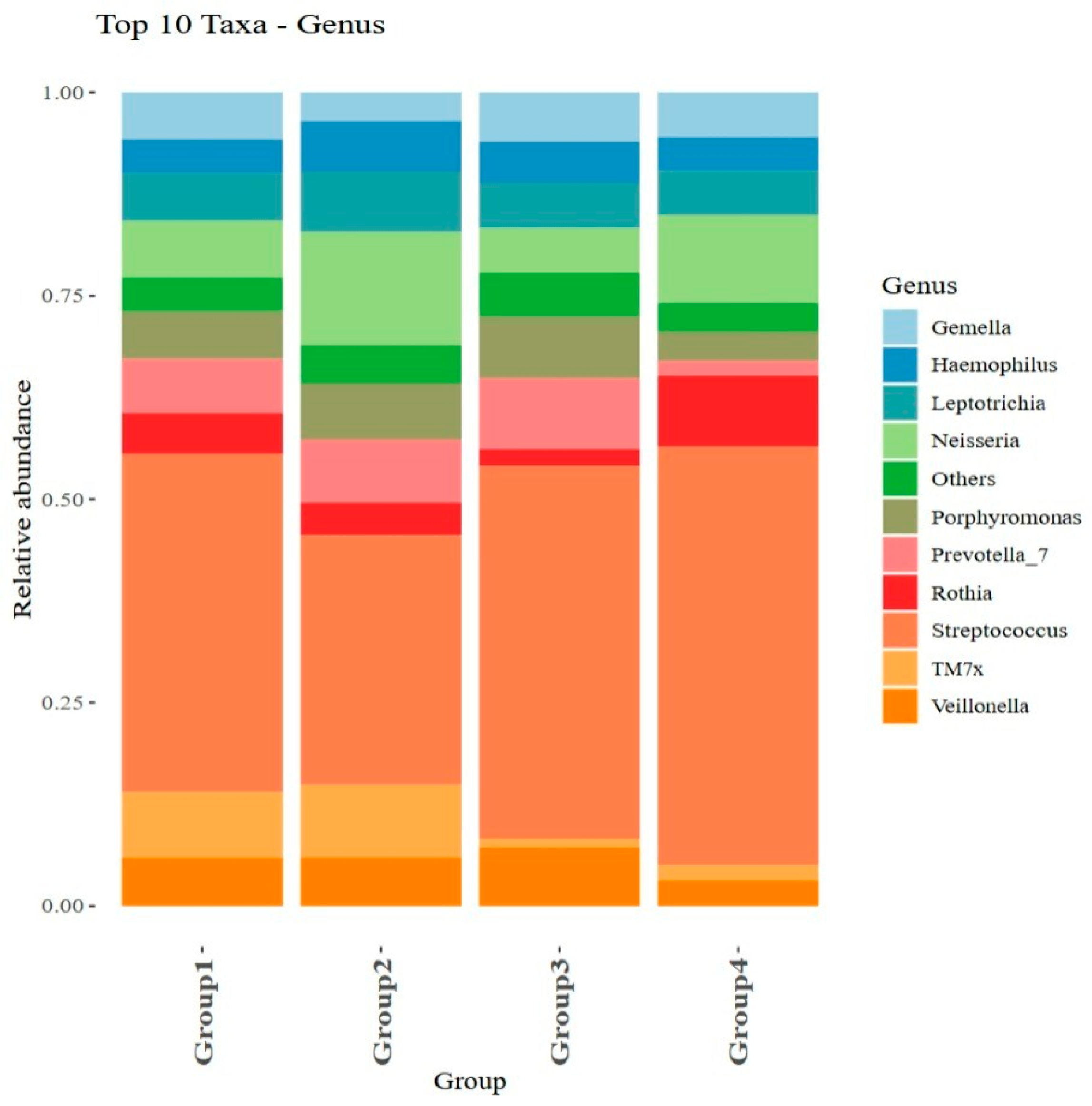
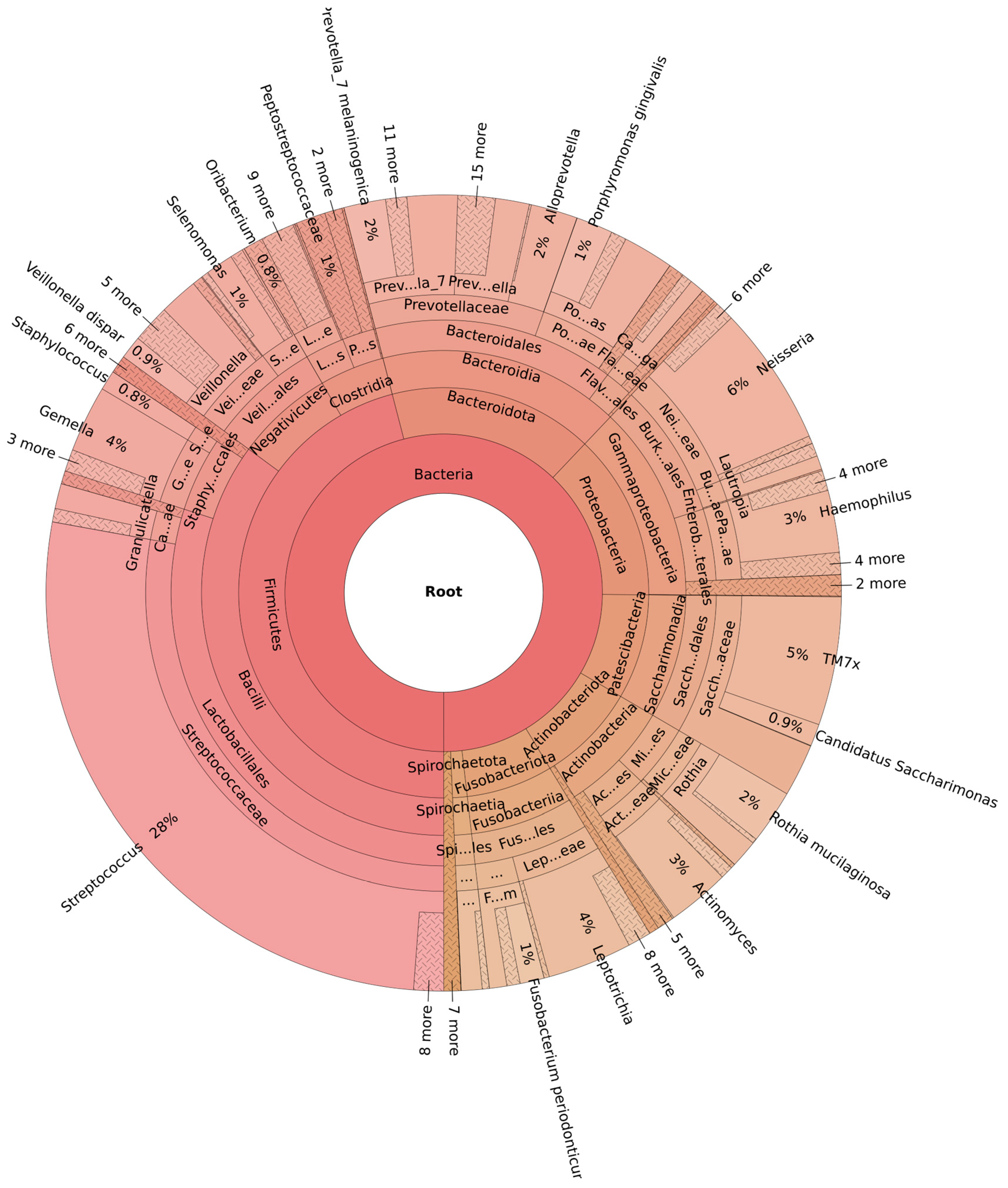
3.3. Microbiome Diversity
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OHI-S | Oral Hygiene Index—Simplified |
| DMFT | Decayed, Missing, and Filled Teeth |
| DNA | Deoxyribonucleic acid |
| rRNA | Ribosomal ribonucleic acid |
| ASVs | Amplicon Sequence Variants |
References
- Angastiniotis, M.; Lobitz, S. Thalassemias: An Overview. Int. J. Neonatal Screen. 2019, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Rujito, L.; Maritska, Z.; Sofro, A.S. β Thalassemia Mutation Flow in Indonesia: A Migration Perspective. Thalass. Rep. 2023, 13, 253–261. [Google Scholar] [CrossRef]
- Tuo, Y.; Li, Y.; Li, Y.; Ma, J.; Yang, X.; Wu, S.; Jin, J.; He, Z. Global, regional, and national burden of thalassemia, 1990–2021: A systematic analysis for the global burden of disease study 2021. eClinicalMedicine 2024, 72, 102619. [Google Scholar] [CrossRef] [PubMed]
- Wahidiyat, P.A.; Sari, T.T.; Rahmartani, L.D.; Iskandar, S.D.; Pratanata, A.M.; Yapiy, I.; Setianingsih, I.; Atmakusuma, T.D.; Lubis, A.M. Thalassemia in Indonesia. Hemoglobin 2022, 46, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Rujito, L.; Wardana, T.; Mulyanto, J.; Nainggolan, I.M.; Sasongko, T.H. Profiling circulating microRNA and regulatory pathways in transfusion-dependent thalassemia and thalassemia trait compared to healthy controls: A preliminary study. ExRNA 2024, 6, 0011. [Google Scholar] [CrossRef]
- Shadlinskaya, R.V. Evaluation of Oral Hygiene and Dental Caries Status in Patients with Beta Thalassemia. World Med. Biol. 2020, 16, 141. [Google Scholar] [CrossRef]
- Jaddoa, M.F.; Qasim, A.A. Assessment of the correlation between the salivary flow rate and dental caries experience among children with β-thalassemia major. Indian J. Forensic Med. Toxicol. 2020, 14, 310–315. [Google Scholar]
- Trirattanapa, K.; Tantiworawit, A.; Suparan, K.; Sriwichaiin, S.; Kerdpoo, S.; Punnachet, T.; Hantrakun, N.; Hantrakool, S.; Piriyakhuntorn, P.; Rattanathammethee, T.; et al. Alterations of Gut Microbiota Related with Status of Iron-Overload in Thalassemia Patients: A Cross-Sectional Pilot Study. Blood 2023, 142, 5257. [Google Scholar] [CrossRef]
- Alyousef, Y.M.; Alonaizan, F.A.; Alsulaiman, A.A.; Aldarwish, M.I.; Alali, A.A.; Almasood, N.N.; Vatte, C.; Cyrus, C.; Habara, A.H.; Koeleman, B.P. Oral microbiota analyses of Saudi sickle cell anemics with dental caries. Int. Dent. J. 2023, 73, 144–150. [Google Scholar] [CrossRef]
- Rujito, L.; Basalamah, M.; Mulatsih, S.; Sofro, A.S.M. Molecular Scanning of β-Thalassemia in the Southern Region of Central Java, Indonesia; a Step Towards a Local Prevention Program. Hemoglobin 2015, 39, 330–333. [Google Scholar]
- Dewi, S.R.P.; Septhimoranie, S.; Muchzal, S. Correlation of saliva characteristics and caries in beta-thalassemia major patients. Maj. Kedokt. Gigi Indones. 2021, 6, 100. [Google Scholar] [CrossRef]
- Adiningrat, A.; Kusmaharani, H.A.; Utami, S.; Astuti, N.R. Evaluation of International Caries Detection and Assessment System (ICDAS)-related Caries Severity among Caries Risk Groups in Pendul District: An Observational Study. J. Int. Soc. Prev. Community Dent 2020, 10, 498–503. Available online: https://journals.lww.com/jpcd/fulltext/2020/10040/evaluation_of_international_caries_detection_and.17.aspx (accessed on 27 June 2025). [CrossRef]
- Moussa, C.; Savard, G.; Estrade, L.; Bourgi, R.; Kharouf, N.; Denis, F.; Daou, M.H. Dental Health in Children with Congenital Heart Defects: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 7022. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.-C.; Wei, S.-M.; Luo, X.-T.; Yang, Q.-Q.; Wong, K.-H.; Cheung, P.C.K.; Zhang, B.-B. How probiotics, prebiotics, synbiotics, and postbiotics prevent dental caries: An oral microbiota perspective. Npj Biofilms Microbiomes 2024, 10, 14. [Google Scholar] [CrossRef]
- Anne Marie, U.; Murererehe, J.; Rehman, M.; Chittilla, M.; Uwambaye, P.; Razzaque, M.S. Oral manifestations of iron imbalance. Front. Nutr. 2023, 10, 1272902. [Google Scholar] [CrossRef]
- Kale, G.; Nelakurthi, V.M.; Paul, P. Exploring the Impact of Blood Disorders on Dental Caries. Cureus 2023, 15, e47159. [Google Scholar] [CrossRef]
- Helmi, N.; Bashir, M.; Shireen, A.; Ahmed, I.M. Thalassemia review: Features, dental considerations and management. Electron. Physician 2017, 9, 4003–4008. [Google Scholar] [CrossRef]
- Akbarnejad, A.A.; Mahjoub, S.; Tamaddoni, A.; Masrour-Roudsari, J.; Seyedmajidi, S.A.; Ghasempour, M. Salivary Oxidative Stress, Total Protein, Iron and pH in Children with β-Thalassemia Major and their Correlation with Dental Caries. J. Dent. 2022, 23, 266–271. [Google Scholar]
- Spatafora, G.; Li, Y.; He, X.; Cowan, A.; Tanner, A.C.R. The Evolving Microbiome of Dental Caries. Microorganisms 2024, 12, 121. [Google Scholar] [CrossRef]
- Bloch, S.; Hager-Mair, F.F.; Andrukhov, O.; Schäffer, C. Oral streptococci: Modulators of health and disease. Front. Cell Infect. Microbiol. 2024, 14, 1357631. [Google Scholar] [CrossRef]
- Dieckow, S.; Szafrański, S.P.; Grischke, J.; Qu, T.; Doll-Nikutta, K.; Steglich, M.; Yang, I.; Häussler, S.; Stiesch, M. Structure and composition of early biofilms formed on dental implants are complex, diverse, subject-specific and dynamic. Npj Biofilms Microbiomes 2024, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral Microbiota: Community Composition, Influencing Factors, Pathogenesis, and Interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontol 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Fadel, H.T.; Zolaly, M.A.; Alharbi, M.O.; Qarah, L.A.; Alrehili, M.S.; Alamri, A.D.; Tarawah, A.M. Oral Health Profiles and Related Quality of Life in Thalassemia Children in Relation to Iron Overload: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 9444. [Google Scholar] [CrossRef]
- Abbasi, F.; Tabesh, A.; Yavari, A.; Makaremi, R.; Bizhani, O.; Mahmood, M. Evaluation and relation of oral health-related quality of life and oral health status in Thalassemia Major patients, a cross-sectional study. BMC Oral Health 2023, 23, 493. [Google Scholar] [CrossRef]
- Farmakis, D.; Porter, J.; Taher, A.; Cappellini, M.D.; Angastiniotis, M.; Eleftheriou, A.; Alassaf, A.; Angelucci, E.; Aydinok, Y.; Rayan, R.B.-F.; et al. 2021 Thalassaemia International Federation Guidelines for the Management of Transfusion-dependent Thalassemia. HemaSphere 2022, 6, e732. [Google Scholar] [CrossRef]
- Al-Zain, A.O.; Fakhry, L.M.; Tallab, R.A.; Natto, Z.S. Attitude, Practice, and Knowledge Regarding Fluoridated Toothpaste, Brushing, and Rinse Usage Among Residents of Jeddah City in Saudi Arabia. Patient Prefer Adherence 2023, 17, 23–39. [Google Scholar] [CrossRef]
- Špiljak, B.; Ozretić, P.; Andabak Rogulj, A.; Brzak, B.L.; Brailo, V.; Škerlj, M.; Juras, D.V. Oral Microbiome Research in Biopsy Samples of Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma and Its Challenges. Appl. Sci. 2024, 14, 11405. [Google Scholar] [CrossRef]

| Variable | Number |
|---|---|
| Age (years) | 12–18 |
| DMFT (caries index) | |
| Very high | 31 (88.57%) |
| High | 4 (11.43%) |
| OHIS (oral hygiene) | |
| Poor | 22 (62.86%) |
| Medium | 13 (37.14%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Hellenic Society for Microbiology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siswandari, W.; Arini, D.N.; Taqwim, A.; Ardinas, S.P.; Anjarwati, D.U.; Rujito, L. Oral Microbiome Diversity in Transfusion-Dependent Thalassemia Using a Metagenomic Approach in Indonesian Communities. Acta Microbiol. Hell. 2025, 70, 28. https://doi.org/10.3390/amh70030028
Siswandari W, Arini DN, Taqwim A, Ardinas SP, Anjarwati DU, Rujito L. Oral Microbiome Diversity in Transfusion-Dependent Thalassemia Using a Metagenomic Approach in Indonesian Communities. Acta Microbiologica Hellenica. 2025; 70(3):28. https://doi.org/10.3390/amh70030028
Chicago/Turabian StyleSiswandari, Wahyu, Dyahayu Nisa Arini, Ali Taqwim, Shinta Prima Ardinas, Dwi Utami Anjarwati, and Lantip Rujito. 2025. "Oral Microbiome Diversity in Transfusion-Dependent Thalassemia Using a Metagenomic Approach in Indonesian Communities" Acta Microbiologica Hellenica 70, no. 3: 28. https://doi.org/10.3390/amh70030028
APA StyleSiswandari, W., Arini, D. N., Taqwim, A., Ardinas, S. P., Anjarwati, D. U., & Rujito, L. (2025). Oral Microbiome Diversity in Transfusion-Dependent Thalassemia Using a Metagenomic Approach in Indonesian Communities. Acta Microbiologica Hellenica, 70(3), 28. https://doi.org/10.3390/amh70030028







