Abstract
The endometrial cavity was considered sterile until the second half of the 20th century. Through modern technological advances and the sequencing of the bacterial 16S rRNA gene, it was proven that the area possesses its own unique microbiome, which can be categorised into two types, Lactobacillus-dominant (LD, with a Lactobacillus spp. abundance percentage greater than 90%) and non-Lactobacillus-dominant (non-LD, with a Lactobacillus spp. abundance percentage smaller than 90%), with other species like Bifidobacterium, Gardnerella, Prevotella, and Streptococcus also being prominent. The aim of this study was to investigate the possible correlation of the endometrial microbiome to female infertility, through the identification and appraisal of studies published in the databases PubMed, Web of Science, and Scopus. Moreover, 12 studies met the research criteria, including the analysis of endometrial fluid or tissue samples from infertile women through PCR, culturomics-based, or NGS methods. According to most of these studies, a eubiotic LD-type microbiome seems to be best for maximising endometrial receptivity and pregnancy chances, whereas a dysbiotic non-LD-type microbiome, with increased α-diversity and a higher number of pathogens, has a harmful effect. There were few studies that presented contradictory results without, however, a satisfactory explanation. Thus, more time and a greater number of studies are required to clarify contradictions and achieve more certain results.
1. Introduction
1.1. The Microbiome
Humans have co-evolved with trillions of microorganisms that colonise the human body and create a complex, adaptive and dynamic ecosystem, which is fully attuned to the constantly changing physiology of the host [1]. The human microbiome is defined as the trillions of commensal microbial cells hosted by every human—primarily bacteria, but also archaea, viruses, bacteriophages and fungi—and their genomes [2,3]. There has been some confusion in the definition of the human microbiome, due to the very subtle difference in the terms “microbiota” (the microbial taxa associated with humans) and “microbiome” (the catalogue of these microbes and their genes), though in most cases, the two terms are used interchangeably [2]. A microbiome can be found in every niche of the human body that has been examined [3], even in organs that were traditionally considered sterile, such as the lungs [4] and the stomach [5], and the largest microbiome in terms of the number of microorganisms it contains is found in the intestinal tract and, more specifically, mainly in the large intestine [2]. In contrast to the host’s genome, which remains relatively stable, the microbiome and its genome are, as previously mentioned, dynamic, and undergo changes during a human’s development due to environmental factors, such as their diet, use of antibiotics, way of birth, past infections, etc., but also as a response to disease [6]. The recent advances in DNA sampling and sequencing techniques have given answers to a number of questions regarding the composition of the microbiome of every niche of the human body, the formation of microbial communities of various degrees of complexity, their correlation to the microbiomes of other species [7], and, most importantly, their correlation to different diseases [6]. Dysbiosis in a microbial community can be described as a disturbance in the balance of the community’s ecology which causes or intensifies a health problem. Thus, dysbiosis in the human microbiome has been associated with a plethora of diseases and conditions, such as irritable bowel syndrome, diabetes, allergies, asthma, and even cancer [1]. More specifically, in the case of reproduction and fertility-related conditions, like endometriosis, studies have shown that the gastrointestinal, neuroendocrine and immune system interact and play an important part. The gut microbiome has been shown to influence the progression of endometriosis, with the endometriotic microbiome consisting of the genera Prevotella, Bautia, and Bifidobacterium [8]. A dysbiotic gut microbiome has also been shown to affect the pathological process of polycystic ovary syndrome (PCOS), with lower α- and β-diversities and an increased abundance of Bacteroides, Parabacteroides, and Clostridium [9]. The characterisation of the human microbiome and the factors that affect the composition and evolution of the microorganisms that it is composed of was the main goal of the Human Microbiome Project (HMP) as a logical extension of the Human Genome Project (HGP) [10]. The HMP, which started in 2007 and was set up by the National Health Institutes of the U.S.A., has produced a total of 2.3 Tb of metagenomic data about the 16S ribosomal RNA (16S rRNA). The use of the 16S rRNA gene in the identification and taxonomy of bacteria began in the 1980s by C. Woese, as it was proven that it makes for an excellent molecular chronograph. It is defined by a great degree of conservation, which stems from the importance of the gene as a crucial element of cell function, and very few other genes are as conserved as this [11]. It is clear that big projects like the HMP and its European equivalent, MetaHIT, already offer a deeper understanding of the biology and the clinical importance of the human microbiome and its genome [12].
1.2. The Endometrial Microbiome
The data that originally described the healthy microbiome of the female reproductive system were derived from studies performed exclusively on the vagina and how its microbiome changes throughout the reproductive years of a woman’s life and during the menstrual cycle [13]. As a result, the endometrial cavity was considered to be a sterile field until the second half of the 20th century [14], and the prevailing opinion at the time was that it was protected from chemical and mechanical trauma and the invasion of microorganisms by the cervical mucus plug [15]. However, recent studies on the area, and on the upper reproductive tract in general, prove that the endometrium possesses its own unique microbiome, which has greater species diversity than that of the lower reproductive tract [16]. The results of studies on women of reproductive age who underwent hysterectomy led to the categorisation of the endometrial microbiome into two main types, according to their microbial composition: Lactobacillus-dominant (LD, with a Lactobacillus spp. abundance percentage greater than 90%) and non-Lactobacillus-dominant (non-LD, with a Lactobacillus spp. abundance percentage smaller than 90%). Other species prominent in the samples include Bifidobacterium, Gardnerella, Prevotella, and Streptococcus [14]. Further analysis and organisation of the aforementioned species in communities showed a negative correlation of Lactobacillus with Gardnerella, Bifidobacterium, and Atopobium and a positive correlation with the commensal genera Clostridium and Streptomyces [14]. The endometrial microbiome, as shown by the analyses of samples from women before the start of in vitro fertilisation (IVF), plays an important role in the reproductive process, as women with an LD-type microbiome had higher chances of a successful implantation, full-term pregnancy and live birth, while women with an abundance of other genera and a decreased presence of Lactobacillus in their microbiome were significantly less likely to achieve pregnancy and more likely to suffer a miscarriage [17]. Moreover, it has been proven that the presence of a non-LD-type microbiome is also associated with pathological conditions and diseases of the endometrium, such as endometriosis [18] and chronic endometritis [19]. Nonetheless, an agreement on the microbial composition of the healthy microbiome, or the existence of a core microbiome, has not yet been reached [18].
1.3. Analysis of the Endometrial Microbiome
All data published until now concerning the analysis of the endometrial microbiome, and mainly the bacteria found in it, have been extracted using one of two method types: culture-based or sequencing-based methods. Culture-based methods, which are the ones traditionally used, include cultivation of the samples in appropriate conditions and identification of the bacteria found, either by the substances they produce or via characterisation of conserved genes in their 16S rRNA. However, analyses of the vaginal microbiome have shown that several microorganisms are impossible to cultivate, and unsuccessful cultivation, in many cases, fails to fully reveal the diversity of the microorganisms present in the sample [20]. For these reasons, methods based on sequencing the 16S rRNA gene are increasingly being used lately. This gene’s sequence is approximately 1550 base pairs (bp) long, consisting of conserved and variable regions, and the many intraspecific polymorphisms present are sufficient for identifying and classifying strains all the way to the sub-species level [11]. The most common sequencing methods are next-generation sequencing (NGS) or high-throughput sequencing methods, which allow the sequencing of large DNA or RNA molecules, up to 30,000–50,000 bp long, in real time. NGS methods are based on using an engineered DNA polymerase attached to the DNA to be sequenced at the bottom of a well, which incorporates nucleotides, labelled with different phosphor-linked fluorophores for differential detection, to the growing chain. When a nucleotide is incorporated into the growing chain, light is emitted, which enables the identification of the nucleotide due to its colour, and then the fluorophore is released, allowing for the incorporation of the next nucleotide. The whole process occurs simultaneously in up to one million wells on a single microchip, producing sequences of a total length of 10,000–15,000 bp (equivalent to a data volume of up to 7.6 Gb) [21]. In the case of the 16S rRNA gene in microbiome analyses, universal primers are used, which are complementary to the conserved region at the beginning of the gene and also to either the region at approximately 540 bp or the end of the gene, whereas the variable regions in between [11], mainly the V4 hypervariable region [22,23], are used for bacterial differentiation. In recent years, there has also been an increase in the use of fourth-generation sequencing methods, which include the identification of the individual nucleotides of a single-strand DNA molecule as it passes through a small-diameter nanopore, according to the different electrical signal each one produces. This way, the production of even larger data volumes is possible, utilising less time and space [21,24]. However, in the case of the endometrium, which is characterised by a microbiome of relatively low biomass and a high risk of DNA from contamination being present, an appropriate protocol, which combines both high reliability and low cost, has not yet been designed [24].
2. Methods and Materials
2.1. Information Sources and Search Strategy
This systematic review was conducted according to the PRISMA guidelines (preferred reporting items for systematic reviews and meta-analyses. The literature search about the endometrial microbiome and infertility was performed using the databases PubMed, Scopus, and Web of Science, with the last search date being October 2023. The search strategy was accordingly modified for each database, taking into consideration alternate spellings, synonyms for the keywords used, and changes in terminology over the years, and MeSH (medical subject headings) terms were also used where possible, in order to increase the specificity of the search. Furthermore, the references of the relevant studies were hand-searched to ensure a more thorough coverage of the topic.
The search strategy formed for the PubMed database was as follows: (“Microbiota” [MeSH terms] OR microbiot* [All fields] OR microbiom* [All fields]) AND (“Endometrium” [MeSH terms] OR endometrial [All fields] OR endometrium [All fields]) AND (“Infertility” [MeSH terms] OR infertil* [All fields] OR fertil* [All fields] OR steril* [All fields]). The number of retrieved results was 177.
For the Scopus database, the search strategy was formed as follows: (endometrium OR endometrial) (Title, Abstract, Keywords) AND (microbiot* OR microbiom*) (Title, Abstract, Keywords) AND (infertil* OR fertil* OR steril*) (Title, Abstract, Keywords). The number of retrieved results was 261.
Finally, for the Web of Science database, the search strategy was formed as follows: (endometrium OR endometrial) (Topic) AND (microbiot* OR microbiom*) (Topic) AND (infertil* OR fertil* OR steril*) (Topic). The number of retrieved results was 230.
2.2. Results Screening and Eligibility Criteria
First, the retrieved results were imported into the reference management software EndNote Online Classic (Clarivate Analytics (former Thomson Reuters), Philadelphia, U.S.A.), where duplicate results were identified and deleted. Then, the relevant results were screened by their titles and abstracts, and the full texts of all eligible studies were retrieved, as well as those of articles where eligibility could not be decided based solely on their title and abstract. The final stage of the results management was picking the studies that met all the eligibility criteria after studying their full texts when necessary. The eligibility criteria set for this review were as follows:
- Only articles of studies;
- Publication date: 2020 or later;
- Language: English;
- Subjects: humans;
- Analysis of the endometrial microbiome either exclusively or in combination with the microbiome of other parts of the reproductive tract;
- Correlation to infertility and/or the outcome of IVF treatment.
Exclusionary criteria, corresponding to the aforementioned eligibility criteria, were:
- Reviews, systematic reviews, books or chapters of books and other types of text;
- A publication date of 2019 or earlier;
- Language of the article other than English;
- Animal species as subjects of the studies;
- Analysis and focus on the microbiome of other organs and systems of the human body besides that of the female reproductive tract;
- Correlation of the microbiome to pathological conditions or diseases of the female reproductive system (e.g., endometriosis, endometritis, etc.).
2.3. Data Extraction
The data extraction from each study was conducted using the Microsoft Excel program. The relevant data extracted were:
- Author(s);
- Publication date;
- Country where the study was conducted;
- Aim of the study;
- Basic demographic data;
- Sample types;
- Analysis method;
- Basic data and results from the analyses;
- Correlation to infertility and/or IVF treatment outcome.
3. Results
3.1. Search Results
The search strategy described previously retrieved a total of 668 results. After combining the results from all databases and deleting duplicates, there were 338 unique results left, which were then screened for their relativity to the search criteria. Then, the irrelevant results were also removed, and the full texts of the 258 remaining articles were retrieved, which were then further assessed for eligibility according to the criteria described in Section 2.2. Finally, 12 studies’ articles were chosen to be included in this review, which contained all the necessary data about the endometrial microbiome analysis on infertile women and its potential correlation to pregnancy achievement or the outcome of IVF treatment, and all of the relevant data were extracted for further studying. The full selection and retrieval process is shown in Figure 1.
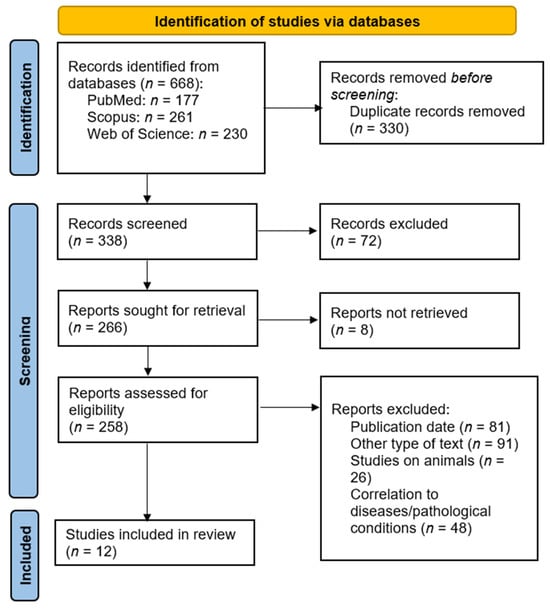
Figure 1.
PRISMA flow diagram.
3.2. Publication Characteristics
From 2020 until October 2023, 12 studies have been published about endometrial microbiome analysis on infertile women and its correlation to pregnancy achievement and/or the outcome of IVF treatment and were therefore included in this review. Five of them (41.67%) were published in 2023, four (33.33%) in 2022, two (16.67%) in 2021, and only one (98.33%) in 2020. With regard to the country where each study was conducted, three studies each (25%) were conducted in Japan and Italy, two (16.67%) in Spain, and one each (8.33%) in Turkey, Russia, China and the U.S.A. The main goal of these studies was the analysis and study of the endometrial microbiome, either exclusively or along with the vaginal and/or cervical microbiome, in women with infertility, and mainly in cases with repeated implantation failure (RIF). In some studies, a comparison to the microbiome of healthy (fertile) women was performed, with the ultimate goal always being the investigation of its microbial composition and its potential correlation to pregnancy achievement and the outcome of IVF treatment or embryo implantation in cases where these were performed. The basic publication characteristics of each study are shown in Table 1.

Table 1.
Basic publication characteristics of the studies included.
3.3. Population Characteristics
The studies in this review included a total of 1229 infertile patients, and three of them also included a total of 65 control patients (not facing fertility problems). All studies excluded patients with secondary infertility, which could be attributed to pathological causes such as endometriosis, polyps and other masses and lesions in the endometrial cavity, underlying infections, etc. The age range of the patients was between 18 and 50 years, and no studies presented any further demographic data. In every study, the patients were characterised either as infertile, meaning unable to achieve clinical pregnancy after 12 consecutive months of natural efforts, or as infertile with RIF, meaning infertile with a history of at least three failed IVF attempts and good-quality embryo transfers. Moreover, the studies can also be split into those where IVF or embryo transfer was performed as part of them and those that only included microbiome analysis. In total, five studies were conducted on infertile patients with a simultaneous IVF attempt, including 549 patients and 26 controls, while four studies were conducted on patients with RIF with a simultaneous IVF attempt, including 387 patients and 18 controls. One study was conducted with a simultaneous IVF attempt on patients with and without RIF. With regard to the studies not including an IVF attempt, one of them was conducted on a total of 100 infertile patients and one on a total of 145 patients with RIF. The basic population characteristics of each study are shown in Table 2.

Table 2.
Basic population data of the studies.
3.4. Sample Types and Analysis Methods
The main type of sample used for microbiome analysis was endometrial fluid, which was used in eight studies (66.67%). In three studies (25%), an endometrial tissue sample was taken after a biopsy, and in one study (8.33%), samples from both endometrial fluid and tissue were used. There were some studies that also used vaginal and/or cervical samples for analysis and comparison, but for the purposes of this review, only the endometrial samples were taken into consideration. A graph with the percentage distribution of the sample types used in the included studies is shown in Figure 2.
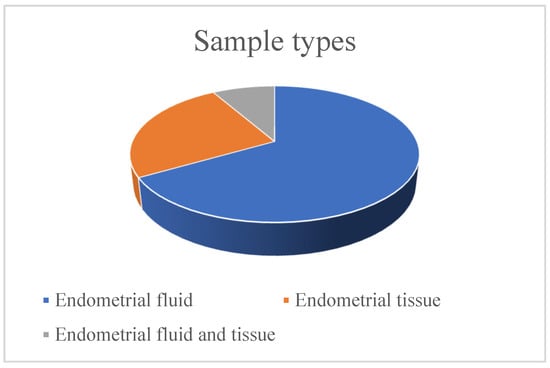
Figure 2.
Sample types used in the studies.
Regarding the methods used for DNA analysis after its extraction, the majority of the studies, and more specifically nine of them (75%), used NGS methods for sequencing the 16S rRNA gene. Real-time PCR, wide-spectrum PCR, and MALDI-TOF (matrix-assisted laser desorption/ionisation—time of flight) mass spectrometry after culture were used in one study each. A graph with the percentage distribution of the DNA analysis methods used in the included studies is shown in Figure 3.
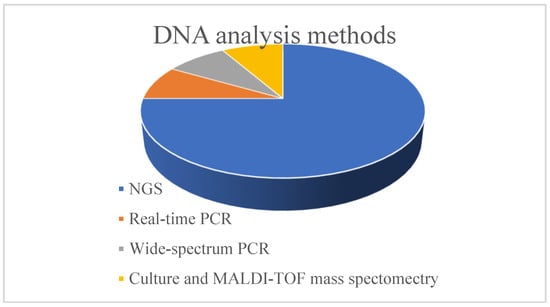
Figure 3.
DNA analysis methods used in the studies.
3.5. Data and Results from the DNA Analysis
According to the data from the analysis of the microbial DNA extracted from the samples, a non-LD-type microbiome was found in most women with infertility or RIF, with low percentages of Lactobacillus and a higher abundance of pathogenic bacterial genera, primarily Prevotella, Gardnerella, Atopobium, Pseudomonas, Streptococcus and Staphylococcus. In general, the main trend in patients with infertility problems seems to be the presence of a dysbiotic microbiome, with looser and disorganised connections among microbial species in its communities, while it also seems that the β-diversity, meaning the composition of the microbial communities of the endometrium, plays a more important role in endometrial receptivity and pregnancy rates rather than the α-diversity, meaning the number of species in the communities, as the α-diversity did not significantly differ between infertile patients and the controls. Regarding the outcome of IVF attempts, in the studies where they were performed, in most cases, there were successful pregnancies in women with an LD-type microbiome, with a higher Lactobacillus abundance and low percentages of pathogens. However, this is not absolute because, in some cases, it was proven that the abundance of Lactobacillus was not as important for pregnancy achievement as it did not differ greatly between patients who got pregnant and those who did not, but rather, the presence of pathogens was of greater importance, which seems to decrease the chances of a successful IVF attempt and pregnancy. Moreover, it is worth noting that in one study, the high abundance of species other than Lactobacillus seemed beneficial, as there were higher pregnancy rates in women with this microbiome type, while another study presented a higher number of pregnancies in women with a complete absence of Lactobacillus and a higher IVF failure rate in women with an LD-type microbiome. The data from the DNA analysis and the basic results of each study are shown in Table 3.

Table 3.
Analysis data and studies’ results.
4. Discussion
4.1. Population Characteristics and Demographics
With regards to the age of the patients recruited for the studies, it is only mentioned in half of the studies. The patients cover almost the entire range of the reproductive ages (18–50 years old), with the majority of them, however, being 25 to 40 years old. As the studies investigate the correlation of the microbiome of the endometrium and, in some cases, of other organs of the reproductive system to female infertility, we can safely deduce that the rest of the patients whose age is not included in the studies, are also of reproductive age. Other demographic data are also omitted, apart from the countries where the studies were conducted, which also constitute the patients’ countries of origin. Regarding these, half of the studies were conducted in Asian countries and the rest in European countries (and more specifically, in countries of the European south), apart from one which was conducted in the U.S.A. It is obvious that the number of countries is quite limited, which could potentially lead to misleading results. For this reason, it is suggested that subsequent studies on the subject include patients from a larger number of countries, especially African, American and Oceanian countries, which have not so far been represented. This way, the results can be more representative, and there can be further investigation on whether the country of origin and the lifestyle and standard of living there plays a role in the composition of the microbiome and infertility. Finally, the population sample sizes were quite small, with the average being 103 patients per study and the median being 73 patients per study, which is mainly due to the complexity of the process and the time required to choose and recruit the appropriate patients and perform all the analyses. Nonetheless, studies with a significantly larger sample size, to the extent that this is feasible, would lead to safer and more complete results and would help us decipher if any unusual or unexpected results are really statistically important or just the result of chance.
4.2. Sample Types
The main sample type used in the studies was fluid from the endometrial cavity, which, in most cases, was taken using a double-lumen catheter [23,26,28,30,31,32]. With this device, the internal catheter easily passes through the external one without making contact with the vaginal or cervical epithelium, and the fluid is drawn through the syringe on the other end [30]. In only two cases, the sample was taken using a cell-collecting brush, specifically a Tao Brush (Cook Medical, Madrid, Spain) in one [27] and a Yuino Brush (Asuka Pharmaceuticals, Tokyo, Japan) in the other [29]. In the studies which used tissue from a biopsy as the preferred sample type, this was taken using a Pipelle-type device (Laboratoire CDD, Paris, France). This device is a flexible polypropylene tube with an external diameter of 3.1 mm and an internal diameter of 2.6 mm. By removing an internal piston, negative pressure is created, and the tissue is aspirated in the cannula [35]. All the aforementioned modern techniques are significantly less invasive than traditional ones, causing minimal pain and discomfort to the patients and eliminating the need for anaesthesia. Moreover, through the simultaneous ultrasound guidance performed by experienced technicians, the sample is taken quickly from the exact anatomical spot [30].
Any unusual or unexpected results did not occur only in studies using one or the other sample type, and thus it cannot be safely said that either sample type is more or less reliable than the other. The most important point during the sample collection process is avoiding contamination from the lower reproductive tract organs. For this reason, the vagina and cervix should be washed out with plenty of saline solution prior to the sample collection and then carefully dried using a sterile gauze or cotton pieces [29,34]. Also, extra precaution should be taken during the insertion and removal of the catheter or brush into and out of the endometrial cavity to avoid contact with the vaginal walls [36]. Lastly, proper storage of the samples until further analysis is also crucial, and it should be carried out using sterile containers and appropriate buffer solutions. The samples should then be stored either in the refrigerator (4 °C) or the freezer (−80 °C), according to how much time there will be between the collection and analysis stages.
4.3. Sample Analysis Methods
The three types of methods used in the studies are, as previously mentioned, culturomics-based methods, which include the mass culture of microorganisms and identification using MALDI-TOF mass spectrometry, PCR-based methods and NGS methods. First, MALDI-TOF mass spectrometry allows for the identification of microorganisms by species-specific peptide and protein mass profiles. This method can identify microorganisms up to the species level, with a high level of accuracy and reliability even in samples with a very low microorganism biomass. However, it is a method with a high workload as it requires cultivation and incubation of the microorganisms from the samples, which subsequently increases the total time and cost of the process. Furthermore, the methods that make use of the various different forms of PCR achieve detection, identification and quantification of the microbial species in the samples, in a quick, highly accurate and effective way. Their main disadvantage, however, is the fact that they require knowledge of the sequences of interest of the microorganisms under investigation in order for the appropriate primer sequences to be designed. Lastly, NGS methods, along with the evolution of bioinformatics, allow for DNA sequencing straight from the sample, even if only a small amount is available, produce a high data volume in real time and only require a little amount of space.
From these, it becomes clear that modern molecular methods are the most advantageous, defined by their high degree of sensitivity, specificity and reliability. However, the endometrial cavity is a niche of the human body whose microbiome is only recently starting to get more thoroughly explored. Thus, a universal protocol for sample analysis has not yet been created, and therefore it is up to each researcher to choose the desired methods, according to the available funds and materials and the specific needs and expected results of each study.
4.4. Analysis Results and Correlation of the Microbiome to Infertility
In accordance with most of the earlier studies conducted on the topic, the majority of the studies included in this review present a eubiotic endometrial microbiome, with a percentage of species of the genus Lactobacillus greater than 90% and very few or no other species of pathogenic bacteria present, as the most beneficial for increasing the chances of a successful IVF attempt and pregnancy. The genus Lactobacillus was positively correlated to the commensal bacteria and negatively correlated to pathogenic ones, and the microbial networks of patients who achieved a successful pregnancy were found to be denser and with more and denser microbial relations compared to those of patients with RIF. According to two of the studies, the mechanisms through which the eubiotic microbiome of the vagina and the endometrium alike affects endometrial receptivity and chances of a successful embryo implantation are most possibly immunological, as the immune tolerance of some cells participating in the immune response (e.g., T-regulatory lymph cells) potentially affects implantation. When bacteria invade the endometrium and stimulate the pattern recognition receptors (PRRs) of the epithelial cells, these cells secrete cytokines that affect the local lymph cell population. Bacteria of the genus Lactobacillus prevent pathogens from entering by acting on the PRRs of the mucosal cells to regulate the immune response necessary for embryo implantation [17,29]. Furthermore, bacteria of the genus Lactobacillus secrete lactic acid, thus creating an acidic environment that inhibits pathogen growth [31].
On the other hand, a dysbiotic microbiome, defined by a decrease in the percentage of Lactobacillus and other commensal bacteria and an increase in pathogen levels, has negative effects in IVF outcome. Almost all studies presented similar results regarding the composition of the dysbiotic microbiome, with the most common genera appearing as biomarkers of RIF being Gardnerella, Prevotella, Megasphaera, Atopobium, Streptococcus and Staphylococcus, with most of these coexisting in the microbiome. As the different bacteria interact and the dense community formed by the Lactobacillus species and other commensal bacteria is necessary for the stability of the local “ecosystem” in the endometrium, the presence of pathogens, which also interact with each other, plays an important role in the disorganisation of this “ecosystem” in patients with RIF. However, it is not clear whether the presence of a non-LD-type microbiome facilitates the entrance of pathogens in the endometrium or if the entrance of pathogens is what causes the decrease in the Lactobacillus percentage [34]. In general, it seems that the increase in α- diversity in a dysbiotic, non-LD-type microbiome has harmful effects on endometrial receptivity and the chances of a successful pregnancy, as the lack of a dominant species potentially facilitates colonisation by many bacterial species, especially pathogens, and creates an adverse environment related to infertility [32].
Nevertheless, there were studies that presented contradictory results. One of these showed that the microbiome of patients with lower levels of Lactobacillus was not as highly correlated to endometrial receptivity compared to patients with a complete lack of microbiomes, which may indicate that it is the quantity rather than the percentage ratio of Lactobacillus that affects endometrial receptivity [25]. Another study also agrees with these results, in which the microbiome of all patients with a successful pregnancy was found completely lacking the Lactobacillus species, with high levels of Lactobacillus found in non-pregnant women [33]. In a third study, the Lactobacillus abundance did not significantly differ between control patients and ones with RIF, with the successful pregnancy rates also being similar between patients with a Lactobacillus percentage greater and smaller than 90% [23]. Finally, one study showed a higher α- diversity in the microbiome of pregnant patients than non-pregnant ones, which led the researchers to assume that more diverse microbiomes are more beneficial for endometrial receptivity than a non-LD-type one [30]. Therefore, it seems that in some cases, the absence of Lactobacillus and the presence of more diverse microbiomes does not necessarily lead to dysbiosis, while higher Lactobacillus levels could even prove to be detrimental to pregnancy chances. However, these studies are only a small percentage of the total, and as none of them provide a satisfactory explanation, a larger number of studies are required in order to prove if these results are indeed statistically important or just the result of chance.
5. Conclusions
This review investigated the composition of the endometrial microbiome and its potential correlation to female infertility and the outcome of IVF treatment based on the most recent studies on the topic. The strength of this study is that it examines a very interesting topic that only recently started getting more attention from researchers, and since female infertility is a problem many people are struggling with, we feel that this study would be valuable to researchers and practitioners working in the field of human reproduction. According to the majority of the studies included in this systematic review, as well as most of the earlier ones, a eubiotic LD-type microbiome seems to be best for maximising endometrial receptivity and the chances of a successful pregnancy, whereas a dysbiotic non-LD-type microbiome, with increased α- diversity and a higher number of pathogens present, has a harmful effect. On the other hand, there were few studies that presented contradictory results, without, however, a satisfactory explanation. Thus, also taking into consideration the fact that studies on the endometrial microbiome are still in the early stages, there is only a small number of them, from few countries and with small population sizes, and it is clear that more time and a larger number of studies are needed in order to decipher contradictions and produce more certain results.
Author Contributions
Conceptualization, P.F. and G.G.; methodology, G.G.; software, P.F.; validation, P.F., M.E., D.C. and G.G.; formal analysis, P.F.; investigation, P.F. and G.G.; resources, P.F. and G.G.; data curation, P.F.; writing- original draft preparation, P.F.; writing-review and editing, P.F., M.E., D.C. and G.G.; visualisation, P.F., M.E., D.C. and G.G.; supervision, M.E., D.C. and G.G.; project administration, M.E., D.C. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Manos, J. The human microbiome in disease and pathology. APMIS 2022, 130, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P.; Erb-Downward, J.R.; Martinez, F.J.; Huffnagle, G.B. The Microbiome and the Respiratory Tract. Annu. Rev. Physiol. 2016, 78, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Bessède, E.; Mégraud, F. Microbiota and gastric cancer. Semin. Cancer Biol. 2022, 86, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Amon, P.; Sanderson, I. What is the microbiome? Arch. Dis. Child. Educ. Pract. Ed. 2017, 102, 258–261. [Google Scholar] [CrossRef]
- Davenport, E.R.; Sanders, J.G.; Song, S.J.; Amato, K.R.; Clark, A.G.; Knight, R. The human microbiome in evolution. BMC Biol. 2017, 15, 127. [Google Scholar] [CrossRef]
- Iavarone, I.; Greco, P.F.; La Verde, M.; Morlando, M.; Torella, M.; de Franciscis, P.; Ronsini, C. Correlations between Gut Microbial Composition, Pathophysiological and Surgical Aspects in Endometriosis: A Review of the Literature. Medicina 2023, 59, 347. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, R.; Huang, Y.; Zhou, F.; Li, F.; Liu, Z.; Geng, Y.; Dong, H.; Ma, W.; Song, K.; et al. Present and Future: Crosstalks Between Polycystic Ovary Syndrome and Gut Metabolites Relating to Gut Microbiota. Front. Endocrinol. 2022, 13, 933110. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Clarridge, J.E. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 2004, 17, 840–862. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.M.; Scott, R.T. Endometrial microbiome. Curr. Opin. Obstet. Gynecol. 2017, 29, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Toson, B.; Simon, C.; Moreno, I. The endometrial microbiome and its impact on human conception. Int. J. Mol. Sci. 2022, 23, 485. [Google Scholar] [CrossRef]
- Peric, A.; Weiss, J.; Vulliemoz, N.; Baud, D.; Stojanov, M. Bacterial colonization of the female upper genital tract. Int. J. Mol. Sci. 2019, 20, 3405. [Google Scholar] [CrossRef] [PubMed]
- Canha-Gouveia, A.; Pérez-Prieto, I.; Rodríguez, C.M.; Escamez, T.; Leonés-Baños, I.; Salas-Espejo, E.; Prieto-Sánchez, M.T.; Sánchez-Ferrer, M.L.; Coy, P.; Altmäe, S. The female upper reproductive tract harbors endogenous microbial profiles. Front. Endocrinol. 2023, 14, 1096050. [Google Scholar] [CrossRef]
- Moreno, I.; Garcia-Grau, I.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Bahçeci, M.; Barrionuevo, M.J.; Taguchi, S.; Puente, E.; Dimattina, M.; Lim, M.W.; et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome 2022, 10, 1. [Google Scholar] [CrossRef]
- Medina-Bastidas, D.; Camacho-Arroyo, I.; García-Gómez, E. Current findings in endometrial microbiome: Impact on uterine diseases. Reproduction 2022, 163, R81–R96. [Google Scholar] [CrossRef]
- Punzón-Jiménez, P.; Labarta, E. The impact of the female genital tract microbiome in women health and reproduction: A review. J. Assist. Reprod. Genet. 2021, 38, 2519–2541. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.M.; Werner, M.D.; Juneau, C.R.; Tao, X.; Landis, J.; Zhan, Y.; Treff, N.R.; Scott, R.T. Endometrial microbiome at the time of embryo transfer: Next-generation sequencing of the 16S ribosomal subunit. J. Assist. Reprod. Genet. 2016, 33, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Slatko, B.E.; Gardner, A.F.; Ausubel, F.M. Overview of Next-Generation Sequencing Technologies. Curr. Protoc. Mol. Biol. 2018, 122, e59. [Google Scholar] [CrossRef]
- Kitaya, K.; Nagai, Y.; Arai, W.; Sakuraba, Y.; Ishikawa, T. Characterization of microbiota in endometrial fluid and vaginal secretions in infertile women with repeated implantation failure. Mediat. Inflamm. 2019, 2019, 4893437. [Google Scholar] [CrossRef]
- Ichiyama, T.; Kuroda, K.; Nagai, Y.; Urushiyama, D.; Ohno, M.; Yamaguchi, T.; Nagayoshi, M.; Sakuraba, Y.; Yamasaki, F.; Hata, K.; et al. Analysis of vaginal and endometrial microbiota communities in infertile women with a history of repeated implantation failure. Reprod. Med. Biol. 2021, 20, 334–344. [Google Scholar] [CrossRef]
- Oberle, A.; Urban, L.; Falch-Leis, S.; Ennemoser, C.; Nagai, Y.; Ashikawa, K.; Ulm, P.A.; Hengstschläger, M.; Feichtinger, M. 16S rRNA long-read nanopore sequencing is feasible and reliable for endometrial microbiome analysis. Reprod. Biomed. Online 2021, 42, 1097–1107. [Google Scholar] [CrossRef]
- Fujii, S.; Oguchi, T. Age-and endometrial microbiota-related delay in development of endometrial receptivity. Reprod. Med. Biol. 2023, 22, e12523. [Google Scholar] [CrossRef]
- Sezer, O.; Soyer Çalışkan, C.; Celik, S.; Kilic, S.S.; Kuruoglu, T.; Unluguzel Ustun, G.; Yurtcu, N. Assessment of vaginal and endometrial microbiota by real-time PCR in women with unexplained infertility. J. Obstet. Gynaecol. Res. 2022, 48, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Lozano, F.M.; Lledó, B.; Morales, R.; Cascales, A.; Hortal, M.; Bernabeu, A.; Bernabeu, R. Characterization of the Endometrial Microbiome in Patients with Recurrent Implantation Failure. Microorganisms 2023, 11, 741. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Mladova, E.; Dobychina, A.; Kirillova, K.; Shichanina, A.; Anokhin, D.; Scherbakova, L.; Samokhodskaya, L.; Panina, O. Comparison of microbial profiles and viral status along the vagina-cervix-endometrium continuum of infertile patients. Syst. Biol. Reprod. Med. 2023, 69, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, M.; Mekaru, K.; Tanaka, S.E.; Arai, W.; Ashikawa, K.; Sakuraba, Y.; Nakamura, R.; Oishi, S.; Akamine, K.; Aoki, Y. Endometrial and vaginal microbiomes influence assisted reproductive technology outcomes. J. Bras. Reprod. Assist. 2023, 27, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Reschini, M.; Benaglia, L.; Ceriotti, F.; Borroni, R.; Ferrari, S.; Castiglioni, M.; Guarneri, D.; Porcaro, L.; Vigano’, P.; Somigliana, E.; et al. Endometrial microbiome: Sampling, assessment, and possible impact on embryo implantation. Sci. Rep. 2022, 12, 8467. [Google Scholar] [CrossRef] [PubMed]
- Cariati, F.; Carotenuto, C.; Bagnulo, F.; Pacella, D.; Marrone, V.; Paolillo, R.; Catania, M.R.; Di Girolamo, R.; Conforti, A.; Strina, I.; et al. Endometrial microbiota profile in in-vitro fertilization (IVF) patients by culturomics-based analysis. Front. Endocrinol. 2023, 14, 1204729. [Google Scholar] [CrossRef] [PubMed]
- Diaz-martínez, M.D.C.; Bernabeu, A.; Lledó, B.; Carratalá-munuera, C.; Quesada, J.A.; Lozano, F.M.; Ruiz, V.; Morales, R.; Llácer, J.; Ten, J.; et al. Impact of the vaginal and endometrial microbiome pattern on assisted reproduction outcomes. J. Clin. Med. 2021, 10, 4063. [Google Scholar] [CrossRef] [PubMed]
- Riganelli, L.; Iebba, V.; Piccioni, M.; Illuminati, I.; Bonfiglio, G.; Neroni, B.; Calvo, L.; Gagliardi, A.; Levrero, M.; Merlino, L.; et al. Structural Variations of Vaginal and Endometrial Microbiota: Hints on Female Infertility. Front. Cell. Infect. Microbiol. 2020, 10, 350. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, X.; Chen, P.; Wang, Y.; Li, W.; Huang, R. The endometrial microbiota profile influenced pregnancy outcomes in patients with repeated implantation failure: A retrospective study. J. Reprod. Immunol. 2023, 155, 103782. [Google Scholar] [CrossRef] [PubMed]
- Terzic, M.M.; Aimagambetova, G.; Terzic, S.; Norton, M.; Bapayeva, G.; Garzon, S. Current role of Pipelle endometrial sampling in early diagnosis of endometrial cancer. Transl. Cancer Res. 2020, 9, 7716–7724. [Google Scholar] [CrossRef]
- Vitale, S.G.; Ferrari, F.; Ciebiera, M.; Zgliczyńska, M.; Rapisarda, A.M.C.; Vecchio, G.M.; Pino, A.; Angelico, G.; Knafel, A.; Riemma, G.; et al. The role of genital tract microbiome in fertility: A systematic review. Int. J. Mol. Sci. 2022, 23, 180. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).