Histomorphology of Chorionic Villi of Term Placentae of Mothers Exposed to Retroviral and Hepatitis B Viruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Site and Sampling
2.2. Rapid Diagnostic Test (RDT) for HBV, HCV, and HIV
2.3. Slicing and Processing of Tissues
2.4. Sectioning of Placenta Tissue and Staining
2.5. Stereological Investigations
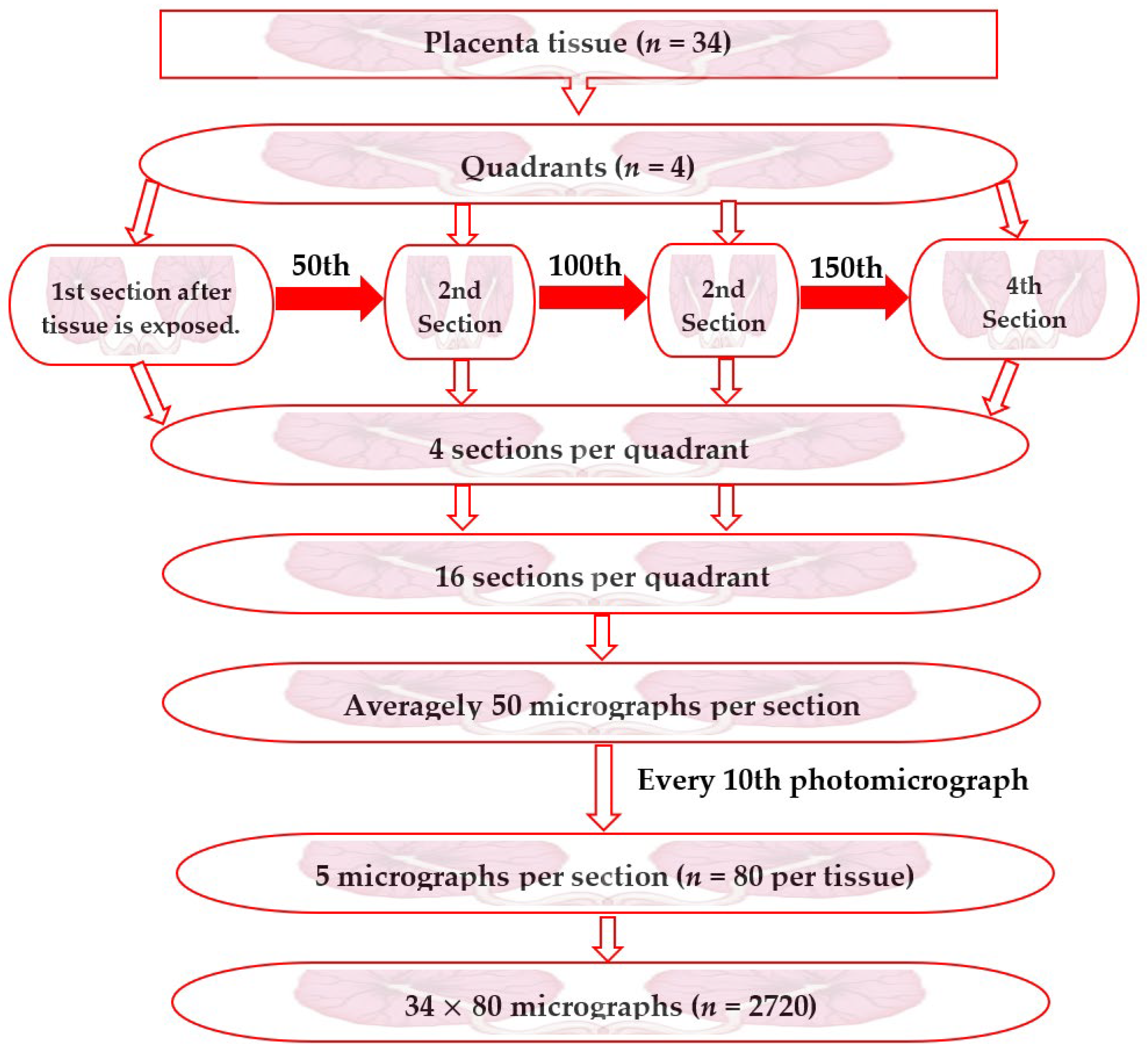
2.5.1. Sampling of Photomicrographs of Placentae Sections
2.5.2. Stereological Examination of Placental Photomicrographs
2.6. Statistical Analysis
2.7. Ethical Consideration
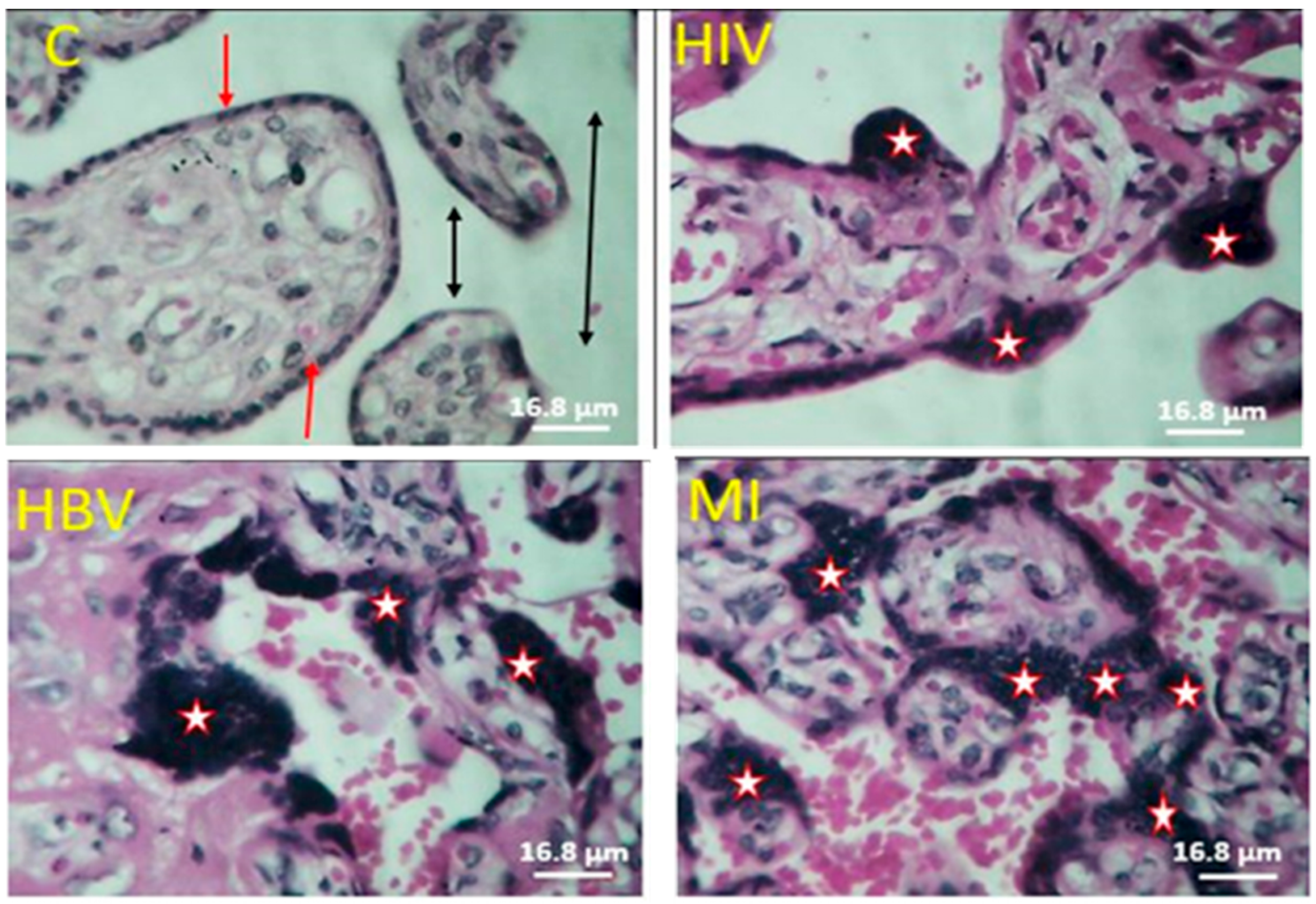
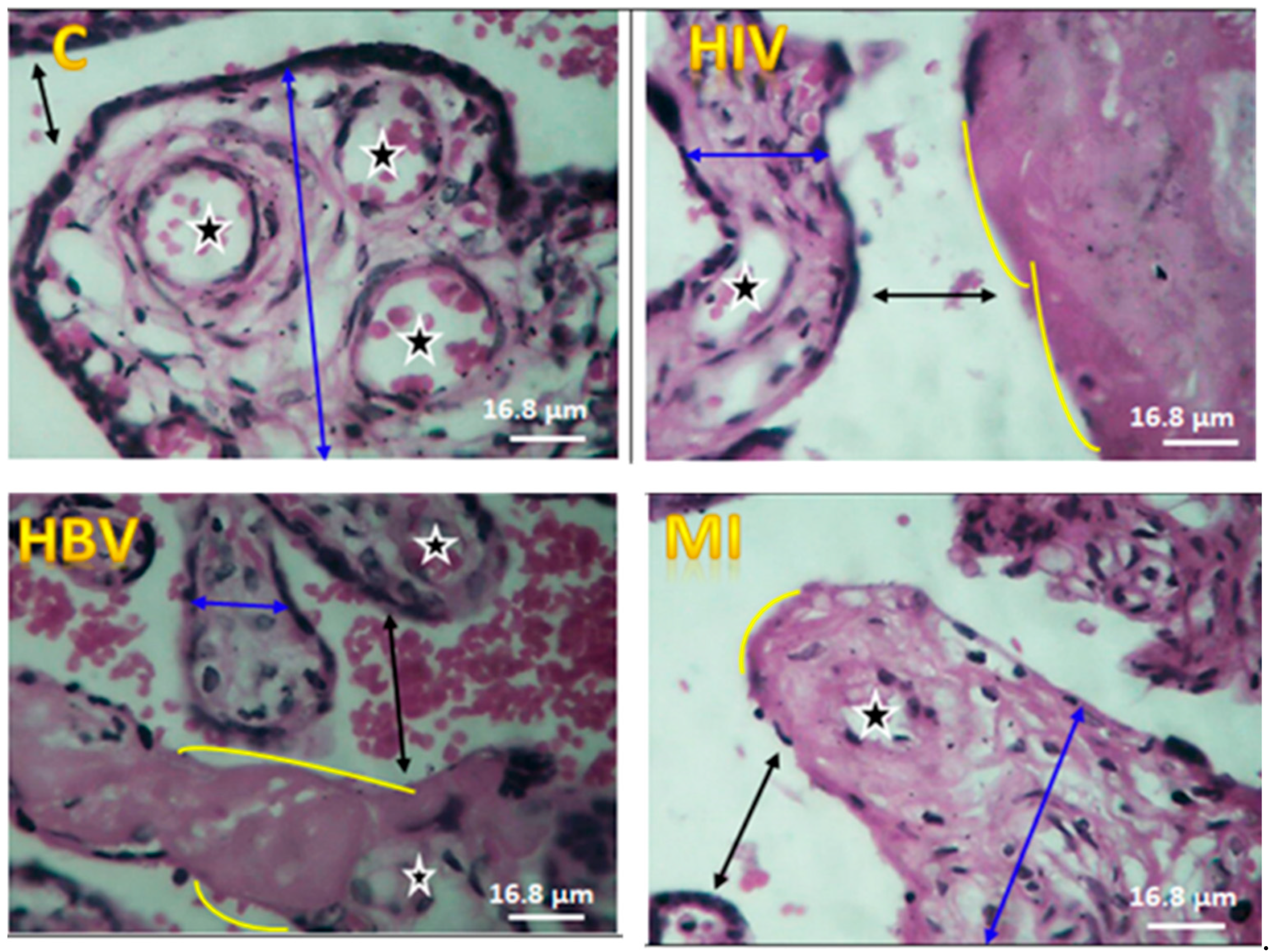
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, M.Y.; Smith, M.A. Infections in Pregnancy. Compr. Toxicol. 2018, 8, 232–249. [Google Scholar] [CrossRef]
- Cardenas, I.; Means, R.E.; Aldo, P.; Koga, K.; Lang, S.M.; Booth, C.; Manzur, A.; Oyarzun, E.; Romero, R.; Mor, G. Viral Infection of the Placenta Leads to Foetal Inflammation and Sensitization to Bacterial Products Predisposing to Preterm Labor. J. Immunol. 2010, 185, 1248–1257. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Hirsch, E. Intrauterine infection and preterm labor. Semin. Fetal Neonatal Med. 2012, 17, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ochs, M.; Schipke, J. A short primer on lung stereology. Respir. Res. 2021, 22, 305. [Google Scholar] [CrossRef] [PubMed]
- Altunkaynak, B.Z.; Onger, M.E.; Altunkaynak, M.E.; Ayranci, E.; Canan, S. A brief introduction to stereology and sampling strategies: Basic concepts of stereology. NeuroQuantology Interdiscip. J. Neurosci. Quantum Phys. 2012, 10, 31. Available online: https://link.gale.com/apps/doc/A485671454/AONE?u=anon~b320218b&sid=googleScholar&xid=53f9f1d5 (accessed on 18 February 2023). [CrossRef]
- Mayhew, T.M.; Sisley, I. Quantitative studies on the villi, trophoblast and intervillous pores of placentae from women with well-controlled diabetes mellitus. Placenta 1998, 19, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Salmani, D.; Purushothaman, S.; Somashekara, S.C.; Gnanagurudasan, E.; Sumangaladevi, K.; Harikishan, R.; Ven-kateshwarareddy, M. Study of structural changes in placenta in pregnancy-induced hypertension. J. Nat. Sci. Biol. Med. 2014, 5, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Burton Graham, J.; Jones, C.J.P. Syncytial knots, sprouts, apoptosis, and trophoblast deportation from the human placenta. Taiwan. J. Obstet. Gynecol. 2009, 48, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J. Deportation of syncytial sprouts from the term human placenta. Placenta 2011, 32, 96–98. [Google Scholar] [CrossRef]
- Sharp, A.N.; Heazell, A.E.P.; Baczyk, D.; Dunk, C.E.; Lacey, H.A.; Jones, C.J.P.; Perkins, J.E.; Kingdom, J.C.P.; Baker, P.N.; Crocker, I.P. Preeclampsia is associated with alterations in the p53-pathway in villous trophoblast. PLoS ONE 2014, 9, e87621. [Google Scholar] [CrossRef]
- Sharp, A.N.; Heazell, A.E.P.; Crocker, I.P.; Mor, G. Placental apoptosis in health and disease. Am. J. Reprod. Immunol. 2010, 64, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.; Gerza, L.; Jones, C.; Sibley, C.; Aplin, J.; Heazell, A. Syncytial nuclear aggregates in normal placenta show increased nuclear condensation, but apoptosis and cytoskeletal redistribution are uncommon. Placenta 2013, 34, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Heazell, A.E.P.; Moll, S.J.; Jones, C.J.P.; Baker, P.N.; Crocker, I.P. Formation of Syncytial Knots is Increased by Hyperoxia, Hypoxia and Reactive Oxygen Species. Placenta 2007, 28, S33–S40. [Google Scholar] [CrossRef] [PubMed]
- Bamfo, J.E.A.K.; Odibo, A.O. Diagnosis and management of foetal growth restriction. J. Pregnancy 2011, 2011, 640715. [Google Scholar] [CrossRef] [PubMed]
- Warrander, L.K.; Batra, G.; Bernatavicius, G.; Greenwood, S.L.; Dutton, P.; Jones, R.L.; Sibley, C.P.; Heazell, A.E.P. Maternal perception of reduced foetal movements is associated with altered placental structure and function. PLoS ONE 2012, 7, e34851. [Google Scholar] [CrossRef] [PubMed]
- Heazell, A. The placenta and adverse pregnancy outcomes—Opening the black box? BMC Pregnancy Childbirth 2015, 15, 14–15. [Google Scholar] [CrossRef]
- Pollock, D.; Ziaian, T.; Pearson, E.; Cooper, M.; Warland, J. Breaking through the silence in antenatal care: Foetal movement and stillbirth education. Women Birth 2020, 33, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Ahenkorah, J.; Tetteh-Quarcoo, P.B.; Nuamah, M.A.; Kwansa–Bentum, B.; Nuamah, H.G.; Hottor, B.; Korankye, E.; Torto, M.; Ntumy, M.; Addai, F.K. The Impact of Plasmodium Infection on Placental Histomorphology: A Stereo-logical Preliminary Study. Infect. Dis. Obstet. Gynecol. 2019, 2019, 2094560. [Google Scholar] [CrossRef]
- Bentley-Lewis, R.; Dawson, D.L.; Wenger, J.B.; Thadhani, R.I.; Roberts, D.J. Placental histomorphometry in gestational diabetes mellitus: The relationship between subsequent type 2 diabetes mellitus and race/ethnicity. Am. J. Clin. Pathol. 2014, 141, 587–592. [Google Scholar] [CrossRef]
- Gheorman, L.; Pleşea, I.E.; Gheorman, V. Histopathological considerations of placenta. Rom. J. Morphol. Embryol. 2012, 53, 329–336. [Google Scholar] [PubMed]
- Benirschke, K.; Burton, G.J.; Baergen, R.N. Pathology of the Human Placenta, 6th ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–941. [Google Scholar] [CrossRef]
- Baurakiades, E.; Martins, A.P.C.; Moreschi, N.V.; Souza, C.D.A.; Abujamra, K.; Saito, A.O.; Mecatti, M.C.; Santos, M.G.; Pimentel, C.R.; Silva, L.L.G.; et al. Histomorphometric and immunohistochemical analysis of infectious agents, T-cell subpopulations and inflammatory adhesion molecules in placentas from HIV-seropositive pregnant women. Diagn. Pathol. 2011, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Miller, R.K. Receptor-mediated uptake and transport of macromolecules in the human placenta. Int. J. Dev. Biol. 2010, 54, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, T.M.; Bowles, C.; Yücel, F. Villous trophoblast growth in pregnancy at high altitude. J. Anat. 2002, 200, 533. [Google Scholar] [CrossRef]
- Huppertz, B.; Kingdom, J.; Caniggia, I.; Desoye, G.; Black, S.; Korr, H.; Kaufmann, P. Hypoxia Favours Necrotic Versus Apoptotic Shedding of Placental Syncytiotrophoblast into the Maternal Circulation. Placenta 2003, 24, 181–190. Available online: http://linkinghub.elsevier.com/retrieve/pii/S0143400402909033 (accessed on 5 March 2023). [CrossRef]
- Caniggia, I.; Mostachfi, H.; Winter, J.; Gassmann, M.; Lye, S.J.; Kuliszewski, M.; Post, M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFβ3. J. Clin. Investig. 2000, 105, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Kosanke, G.; Kadyrov, M.; Korr, H.; Kaufmann, P. Maternal anemia results in increased proliferation in human placental villi. Placenta 1998, 19, 339–357. [Google Scholar] [CrossRef]
- Fox, H. Effect of hypoxia on trophoblast in organ culture. A morphologic and autoradiographic study. Am. J. Obstet. Gynecol. 1970, 107, 1058–1064. [Google Scholar] [CrossRef]
- Alsat, E.; Wyplosz, P.; Malassiné, A.; Guibourdenche, J.; Porquet, D.; Nessmann, C.; Evain-Brion, D. Hypoxia impairs cell fusion and differentiation process in human cytotrophoblast, in vitro. J. Cell. Physiol. 1996, 168, 346–353. [Google Scholar] [CrossRef]
- Aliota, M.T.; Caine, E.A.; Walker, E.C.; Larkin, K.E.; Camacho, E.; Osorio, J.E. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl. Trop. Dis. 2016, 10, e0004682. [Google Scholar] [CrossRef]
- Seferovic, M.D.; Turley, M.; Valentine, G.C.; Rac, M.; Castro, E.C.C.; Major, A.M.; Sanchez, B.; Eppes, C.; Sanz-Cortes, M.; Dunn, J.; et al. Clinical importance of placental testing among suspected cases of congenital zika syndrome. Int. J. Mol. Sci. 2019, 20, 712. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Redline, R.W. Villous capillary lesions of the placenta: Distinctions between chorangioma, chorangiomatosis, and chorangiosis. Hum. Pathol. 2000, 31, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Dureau, Z.J.; Rogers, B.B. Placental pathology. Diagn. Histopathol. 2019, 25, 341–349. [Google Scholar] [CrossRef]
- Akbarzadeh-Jahromi, M.; Soleimani, N.; Mohammadzadeh, S. Multiple chorangioma following long-term secondary infertility: A rare case report and review of pathologic differential diagnosis. Int. Med. Case Rep. J. 2019, 12, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Kingdom, J.C.K.P. Oxygen and placental vascular development. Hypoxia Next Millenn. 1999, 474, 259–275. [Google Scholar]
- Rainey, A.; Mayhew, T. Volumes and Numbers of Intervillous Pores and Villous Domains in Placentas Associated with Intrauterine Growth Restriction and/or Pre-eclampsia. Placenta 2010, 31, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Dellschaft, N.S.; Hutchinson, G.; Shah, S.; Jones, N.W.; Bradley, C.; Leach, L.; Platt, C.; Bowtell, R.; Gowland, P.A. The haemodynamics of the human placenta in utero. PLoS Biol. 2020, 18, e3000676. [Google Scholar] [CrossRef] [PubMed]
- Karimu, A.L.; Burton, G.J. Star volume estimates of the intervillous clefts in the human placenta: How changes in umbilical arterial pressure might influence the maternal placental circulation. J. Dev. Physiol. 1993, 19, 137–142. [Google Scholar] [PubMed]
- Mayhew, T.; Wadrop, E. Placental morphogenesis and the star volumes of villous trees and intervillous pores. Placenta 1994, 15, 209–217. [Google Scholar] [CrossRef]
- Mayhew, T.; Brotherton, L.; Holliday, E.; Orme, G.; Bush, P. Fibrin-type fibrinoid in placentae from pregnancies associated with maternal smoking: Association with villous trophoblast and impact on intervillous porosity. Placenta 2003, 24, 501–509. [Google Scholar] [CrossRef]
- Mayhew, T.M. A stereological perspective on placental morphology in normal and complicated pregnancies. J. Anat. 2009, 215, 77–90. [Google Scholar] [CrossRef]
- Plitman Mayo, R. Advances in Human Placental Biomechanics. Comput. Struct. Biotechnol. J. 2018, 16, 298–306. [Google Scholar] [CrossRef]

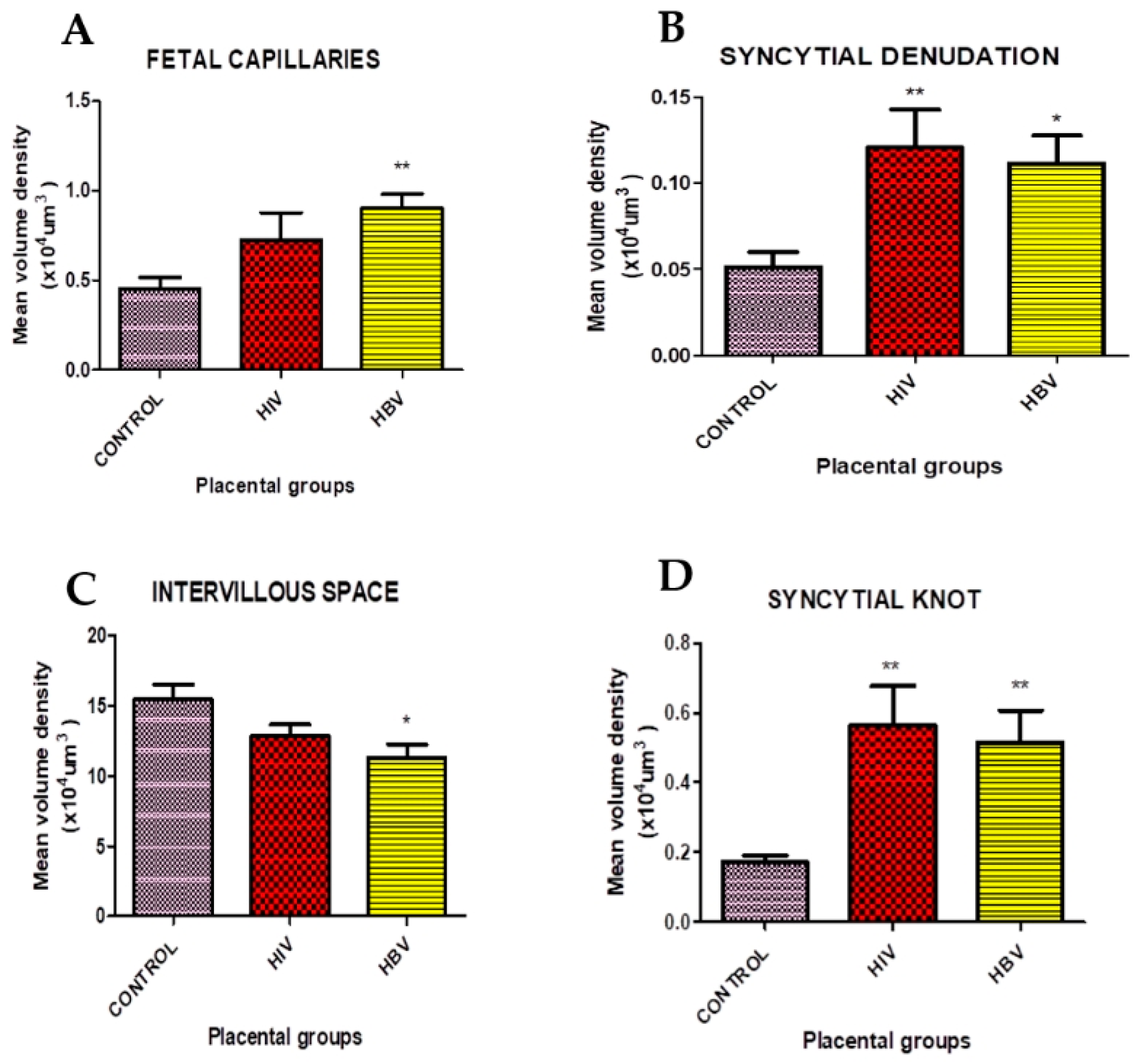
| Placental Groups | Placental Parameters | |||
|---|---|---|---|---|
| Syncytial Knots | Intervillous Space | Syncytial Denudation | Foetal Capillaries | |
| Control (n = 14) | 0.171 ± 0.018 | 15.450 ± 1.075 | 0.051 ± 0.00 | 0.451 ± 0.064 |
| HIV (n = 10) | 0.562 ± 0.115 ** | 12.830 ± 0.826 | 0.121 ± 0.022 ** | 0.725 ± 0.152 |
| HBV (n = 10) | 0.516 ± 0.090 ** | 11.32 ± 0.952 * | 0.111 ± 0.016 * | 0.902 ± 0.078 ** |
| p value | 0.001 | 0.022 | 0.004 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahenkorah, J.; Opoku-Nyarko, S.; Adutwum-Ofosu, K.K.; Hottor, B.; Twasam, J.; Afutu, E.; Nyadroh, C.; Kotey, F.C.N.; Donkor, E.S.; Dayie, N.T.K.D.; et al. Histomorphology of Chorionic Villi of Term Placentae of Mothers Exposed to Retroviral and Hepatitis B Viruses. Acta Microbiol. Hell. 2024, 69, 29-40. https://doi.org/10.3390/amh69010005
Ahenkorah J, Opoku-Nyarko S, Adutwum-Ofosu KK, Hottor B, Twasam J, Afutu E, Nyadroh C, Kotey FCN, Donkor ES, Dayie NTKD, et al. Histomorphology of Chorionic Villi of Term Placentae of Mothers Exposed to Retroviral and Hepatitis B Viruses. Acta Microbiologica Hellenica. 2024; 69(1):29-40. https://doi.org/10.3390/amh69010005
Chicago/Turabian StyleAhenkorah, John, Stephen Opoku-Nyarko, Kevin Kofi Adutwum-Ofosu, Bismarck Hottor, Joana Twasam, Emmanuel Afutu, Clement Nyadroh, Fleischer C. N. Kotey, Eric S. Donkor, Nicholas T. K. D. Dayie, and et al. 2024. "Histomorphology of Chorionic Villi of Term Placentae of Mothers Exposed to Retroviral and Hepatitis B Viruses" Acta Microbiologica Hellenica 69, no. 1: 29-40. https://doi.org/10.3390/amh69010005
APA StyleAhenkorah, J., Opoku-Nyarko, S., Adutwum-Ofosu, K. K., Hottor, B., Twasam, J., Afutu, E., Nyadroh, C., Kotey, F. C. N., Donkor, E. S., Dayie, N. T. K. D., Tette, E. M. A., & Tetteh-Quarcoo, P. B. (2024). Histomorphology of Chorionic Villi of Term Placentae of Mothers Exposed to Retroviral and Hepatitis B Viruses. Acta Microbiologica Hellenica, 69(1), 29-40. https://doi.org/10.3390/amh69010005







