Abstract
Occupational asthma (OA) and rhinitis are health problems occurring in facilities employing animals for medical and scientific reasons. We have compared the UK trends (2006–2023) in these outcomes reported to the SWORD scheme with changes in routine and personal air monitoring for the major mouse (Mus m 1) and rat (Rat n 1) allergens. The exposure data contained 1540 and 688 mouse and rat results, respectively, expressed in ng.m−3. The median, 75th and 90th percentiles were used as exposure characteristics, and annually incrementing three-yearly rolling data slices compared exposure and health outcomes by linear regression. The median, P75 and P90 for Mus m 1 all showed annual declines of around 5–6% (p < 0.001), suggesting general improvements in controlling mouse allergen exposure, but without evidence of a decline in rat allergen levels (p > 0.05), although control measures for both species are largely identical. An annual mean decline in OA of 2.9% (p = 0.021) was identified, but without a significant decline in rhinitis (−1.4%; p = 0.21). Over 16 years, reductions in exposure to the predominant rodent species were accompanied by a concomitant but smaller reduction in OA. These data confirm the immediate value of controlling relevant allergen exposure in reducing the incidence of IgE-E mediated OA.
1. Introduction
Rodents are extensively used animal experimental models, and have been known for a long time to shed potent allergens that can cause IgE-mediated sensitisation with subsequent risk of ocular, nasal and respiratory symptoms, including occupational asthma (OA) [1,2]. As such, it is a clear example of protein allergen-mediated short latency occupational respiratory disease. A number of studies have identified risk factors related to levels of exposure to allergens and sensitisation by laboratory animals in the occupational setting, e.g., [3,4,5,6,7,8,9]. Efforts have been made to control exposure in the sector to rodent allergens and thus reduce the level of ill health [10], but there remains current evidence of some levels of ill health in the sector.
The number of UK staff potentially exposed in this sector is around 12,000 and has remained relatively static over the last twenty years. Data shows that exposure to mice is predominant. In 2018 in Great Britain roughly equivalent numbers of animal procedures were carried out for either experimental reasons or for the creation and breeding of genetically modified animals [11]. For the former reason, mice and rats were used in 60% and 9% of procedures, respectively, while for genetic modification reasons 87% and 0.4% of procedures used mice and rats, respectively. Therefore, mice are much more widely used than rats across the medical and scientific sector employing laboratory animals.
HSE’s laboratory has been monitoring airborne exposure to mouse and rat major allergens in largely UK animal facilities since 2005 [5,12]. Such monitoring has not been mandated by the UK regulatory body (Health and Safety Executive) but carried out by occupational hygienists on a commercial basis on behalf of duty holders/managers across the sector. All results from samples submitted to our laboratory and any contextual information on samples, where supplied, are maintained in an electronic database (EMDB) that allows for anonymised, interpretative data to be published and supplied back to submitters in terms of monitoring their control of exposure to allergens. The Health and Occupation Research (THOR) network is operated by the University of Manchester [13], and as part of THOR, the well-established Surveillance of Work-related and Occupational Respiratory Disease (SWORD) reporting scheme collates the incidence of cases of occupational respiratory disease in the UK, including OA and rhinitis, as reported by consultant chest physicians across the UK. It is acknowledged that the statistics produced by SWORD underestimate the true incidence of OA and rhinitis, given the “high bar” nature of the reporting route, but can give valuable information on time trends on occupational respiratory diseases.
While the incidence of health outcomes from laboratory animal allergen has significantly decreased over time, laboratory animal allergy is a relatively simplistic model for short latency health outcomes caused by IgE-mediated mechanisms from exposure to protein allergens. This paper focuses on the temporal relatioaddeddnships between potential exposure to major mouse and rat allergens from our airborne monitoring data and the incidence of OA and rhinitis during the period of 2005 to 2023 collected by the SWORD scheme.
2. Materials and Methods
Major allergens for mice and rats (Mus m 1 and Rat n 1) have been measured in our laboratory since 2005 by essentially the same immunoassays [5,12]. The recommended air sampling methods, using Millipore FALP02500 filters (Merck Life Sciences, Gillingham, UK) and extraction of these filters have also remained the same over the study period. The antibody-based sandwich assays were specific to the allergens, fully automated to improve precision, and, importantly, were sensitive enough to measure sub ng/mL concentrations on the filters used in air sampling. The detection limits of 0.04 and 0.1 ng of allergen on an air filter equates to 1.3 and 3.3 ng.m3 for Mus m 1 and Rat n 1, respectively, in 15 min air samples, which was sensitive enough to monitor short-term tasks. Quality assurance (QA) samples have been analyzed with all batches of samples, and new batches of QA were run in comparison with old batches prior to their implementation to ensure stability of the methods over time.
Samples were collected by occupational hygienists using a standardized methodology. Many of the air samples submitted to the laboratory were supplied with air volumes and therefore allow the calculation of airborne concentrations of Mus m 1 and Rat n 1 in ng.m−3. Samples supplied were based on either personal sampling in the breathing zone of staff or static/background sampling. This paper focuses on personal air samples as it was considered that such samples reflect the better risk of inhalation exposure to staff, albeit mitigated by respiratory protective equipment worn, whereas static samples were spatially ill-defined with regard to both the sources of exposure and staff.
SWORD data on the number of actual reported cases of OA were utilised; SWORD also contains a diagnostic category termed “other cases,” which on inspection very largely reflects cases of occupational rhinitis. This was also included in the analysis.
Data on the number of animal procedures in Great Britain was supplied in annual reports published by the Home Office e.g., [11,14,15]. The data was presented in terms of the number of procedures undertaken for a species, rather than the actual number of animals used, but was a good estimate on the relative number of mice or rats.
Results were analyzed using MedCalc Statistical Software version 19.2 (MedCalc Software Ltd., Ostend, Belgium; https://www.medcalc.org; 2020). Three yearly running averages of the personal monitoring data and health outcomes were analyzed in an attempt to smooth out any abnormalities in a single year’s results. The characteristics of the exposure data included the median value, the 75th percentile (P75) and 90th percentiles (P90). The P75 and P90 were calculated to reflect changes in higher exposures. The P90 was well established in biological monitoring as a tool to identify high exposures, focus exposure control measures and to establish guidance values [16,17].
Although airborne monitoring for mice and rat allergens commenced in 2005, we have started the analysis from 2006 so that the initial three-yearly period contained more than one hundred results. Regression analyses were performed either using linear regression or a regression model reflecting an exponential decay.
Exposure results that were less than the analytical detection limit were replaced by the (detection limit/1.414) and then corrected for the air sample to give results in ng.m−3. This occurred in around 7% of samples.
3. Results
Regression analyses of exposure data and health outcomes versus time suggested that in no case was regression fitting to an exponential model statistically better than using a simple linear regression model, so all analyses employed linear regression.
Data has been published annually since 2005 by the Home Office on the number of procedures carried out in Great Britain. Figure 1 shows the annual number of procedures per million for mice and rats in Great Britain. Annual procedures on both mice and rats over the period of 2005–2022 were on average 79.8% (standard deviation (sd) 2.45%) of all procedures conducted on living animals, highlighting the predominance of these two rodent species. Across this time period 9.8-fold (Range 4.8–14.7-fold) more procedures were performed on mice compared to rats, identifying mice as the principle experimental animal between these two rodent species.
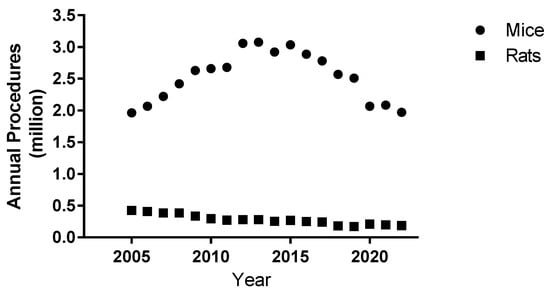
Figure 1.
Annual total number of procedures for mice and rats conducted in Great Britain from 2005 to 2022.
Interestingly, the absolute numbers of rat procedures had been significantly decreasing (p < 0.001) over 2005–2022, whereas mice procedures increased up to 2012 and declined from 2015. A relatively sharp drop in 2020 of around 0.5 million mice procedures probably reflects the influence of COVID-19, but the mice procedure numbers subsequently have not fully recovered to pre-COVID levels.
The numbers of personal air samples analyzed for Mus m 1 and Rat n 1 from 2005 to the end of 2023 were 1540 and 688, respectively. These are out of totals of 5856 Mus m 1 and 2127 Rat n 1 results for both personal and static samples that were expressible as ng.m−3.
Table 1 shows the characteristics of the three-yearly rolling exposure data sets starting in 2006 when more than 100 results began to be accumulated for a three-year period. Obviously, the confidence intervals of the medians are considerably smaller than for the P75 and P90 levels. There are fewer results for rat n 1 to some extent reflecting the usage of the rat in comparison to the mouse.

Table 1.
Number of results, medians (Medn), P75 and P90 for exposure and the average annual number of reported health outcomes to SWORD for three-yearly running data sets.
3.1. Overview of Health Outcomes
One hundred and ninety-four cases related to laboratory animal allergens (LAA) were reported to SWORD for 2005–2023. The majority of diagnoses were reported under the “other” diagnostic category, of which 93% were rhinitis with two reported as anaphylaxis. Therefore this “other” diagnostic category is a good surrogate for rhinitis. Thirty-four percent were reported as asthma. Averages for three-year rolling periods were calculated for OA and rhinitis (Table 1), starting in 2006 for comparability with the exposure data.
The causative animals for reports to SWORD are held in a free text field and may not always be completed by the physician. Table 2 identifies the frequencies over the time period expressed as percentages of all animals causing the health outcomes. The data confirm the predominance of mice and rats as causative species. Mice alone are generally the greatest cause of the health outcomes, but rats alone seem to be a significant cause of respiratory health outcomes given the much smaller numbers utilized, as identified previously. The data also suggests a diagnosis of asthma is accompanied by a diagnosis of rhinitis. The data for health outcomes for mice and rats together may reflect that there is the potential for cross-reactivity between the major allergens for the two species [18].

Table 2.
Percentages of causative animals related to SWORD reports for the period.
3.2. Regression Analyses
3.2.1. Mus m 1 Exposure Data
Simple linear regression analyses of the 3-year rolling periods for median, P75 and P90 by year show that all have statistically significant (p < 0.05) negative slopes.
Median = 5.64 − 0.337 (CI = −0.246 to −0.4250). [Year]; r = 0.91, p < 0.001
P75 = 25.64 − 1.3963 (CI = −0.774 to −2.018). [Year]; r = 0.79, p < 0.001
P90 = 99.67 − 5.085 (CI = −2.55 to −7.62). [Year]; r = 0.75, p = 0.001
When the data is expressed as percentages, a linear regression model suggests that the median has a percentage annual decrease of −5.97% per annum (CI −4.39 to −7.56), while the P75 shows −5.45% (CI −3.02 to −7878) and the P90 shows −5.10% (CI −2.56 to−7.64). Thus, the median, P75 and P90 all show significant decreases over time, but these are not statistically different between each other as descriptors of the data set over the time period. Therefore, there is no evidence that the worst exposure scenarios (greater than P75 or P90) are making the most improvement over time.
3.2.2. Rat n 1 Exposure Data
There are considerably fewer atmospheric personal Rat n 1 results, which is in line with the predominance of mice as the rodent model. The linear regression models for Rat n 1 personal air monitoring data over time are shown below and although the three analyses for the median, P75 and P90 suggested increases over time they failed to show significance (p > 0.05)
Median = 0.519 + 0.03654 (CI = −0.0077 to 0.0808). [Year] r = 0.43, p = 0.098
P75 = 2.42152 + 0.1184 (CI = −0.029 to 0.2657). [Year] r = 0.42, p = 0.107
P90 = 12.6815 + 0.2323 (CI = −0.3362 to 0.8308). [Year] r = 0.22, p = 0.419
3.2.3. Health Outcomes
Linear regression analysis suggested that the OA showed a significant downward trend, in contrast to rhinitis where there was no statistically significant trend (p > 0.05) over time.
OA = 5.613–1.606 (CI = −0.293 to −0.0208). [Year] r = 0.57, p = 0.021
Rhinitis = 10.4415 − 0.147 (CI = –0.3618 to 0.0949). [Year] r = 0.33, p = 0.214
When the data were expressed as percentage changes (Table 3), the decrease in OA as a linear regression model suggested a year-on-year reduction in OA by −2.86% (CI = −0.50 to −5.22; p = 0.021). The percentage decrease for rhinitis was −1.41% (CI −3.725 to 0.091); p = 0.214).

Table 3.
Comparison of the relative slopes of regression analyses per year over the 16-year period for the exposure measured and health outcomes.
There have been concerns that the COVID pandemic influenced reporting to SWORD from 2020 [19], so we undertook an analysis for the health outcome data prior to 2020. The percentage decease in OA across the restricted time period was −3.55% (CI −6.45 to −0.65; p 0.021); while rhinitis gave a non-significant % decrease of −1.61% (CI −4.26 to 1.04; p = 0.21). Thus, the results for the health outcomes for the full 16-year period and for results prior to 2020 were essentially similar.
4. Discussion
This study has two novelties in comparison with many of the other published studies on occupational exposure to LAA and health risks. It is a longitudinal study, whereas most other studies have been cross-sectional in nature, and used physician-diagnosed health endpoints of occupational asthma and rhinitis, rather than self-reporting symptom questionnaires or IgE sensitisation to rodent allergens as health endpoints [3,20,21,22].
There are several potential biases in the data presented. The exposure data is not from a defined longitudinal study population, but from commercial monitoring activity. However, we have reported on the extensive coverage of the collected air monitoring data with regard to the UK sector [5]. This paper reported that approximately one third of samples were derived from each of the Pharma/contract laboratories, research institutes and universities. These ratios have been largely maintained across the period except for COVID during 2020–2021. The exposure data also relates to potential inhalation as it does not take into account the influence of RPE, which is widely worn in this sector.
We have presented the Great Britain data on the usage of mice and rats over the relevant time period (Figure 1). This is largely to highlight the predominance of mice over rats in experimental studies when comparing causative species linked with the health outcomes (Table 2), and also to confirm that large numbers of rodents continue to be used annually over the period of this study. Over time the use of genetically modified mice in disease models has increased in contrast to a general decrease in the use of rodents in toxicological studies. Genetically modified and immunodeficient animals are valuable, important resources for disease models and as such work practices may change to ensure the protection of such animals.
The SWORD data is based on reporting cases identified and reported by UK specialist chest physicians. Therefore, there are likely to be many more cases that never complete this clinical journey, so the SWORD data underestimate the absolute number of cases related to LAA and other respiratory diseases. Around two-fold more cases of rhinitis were reported to SWORD than asthma over the period for laboratory animal allergens. The cases reported to SWORD each year do not necessarily represent new incident cases, as the time between the true incidence of symptoms in an individual and their reporting to SWORD by a consultant respiratory physician is likely to be variable. However, we believe that this data remains valuable in evidencing the relationship between changes in exposure characteristics and the IgE-mediated health outcomes of asthma and rhinitis from exposure to a relatively restricted range of proteinaceous allergens.
We are unaware of any collated information on the changes over time on the prevalence or incidence of new cases of relevant serum IgE or positive skin prick tests in this sector. Such data would be a valuable link between the exposure data and health outcomes. A relatively recent cross sectional UK study of 526 participants suggested prevalence rates for mouse sensitization varied between 0–9.1% according to the unit but considerably lower than historical data suggesting 15% sensitization prevalence [23].
Around 30% of the samples submitted to our labs for mice or rat allergen measurements were personal samples collected in the breathing zone of staff members and represent respiratory risk, albeit mitigated by RPE. Therefore, such measurements have value in the context of an epidemiological analysis of the data. In contrast, static or background samples are not characterized spatially to any staff members or the point source of exposure, so although they may have significant value in the context of each individual sampling exercise, they have limited value in being combined and used epidemiologically.
The rate of decline for Mus m 1 allergen is indistinguishable whether based on the median value or the P75 and/or P90 in identifying higher exposures. This suggests a general improvement in exposure to Mus m 1 rather than the higher exposures being particularly curtailed by duty holders. In contrast, the Rat n 1 results show no statistical evidence of a decline in exposure and, while non-significant, appears to show a trend of increasing over time. We have highlighted this phenomenon of divergence between mouse and rat exposure [5] and it is difficult to explain, given that exposure control measures for rats are identical to those that can be employed for mice in animal facilities. It should be noted that during the period of this study the uptake of individually ventilated cages (IVCs) has become much more widely utilised in the UK. Using IVCs to contain rodents has been noted as significantly reducing allergen exposure [24,25] and can be used for both mice and rats. The number of results for rat n 1 in the dataset somewhat overestimates the species compared with the data on the actual number of procedures reported for the two rodent species.
The lack of a decline in exposure to rat allergen over the period, together with the SWORD data suggesting that rats are the sole causative species of a significant proportion of the health outcomes outweighing their relative utilisation, is troubling. The identified cross-reactivity between rat and mouse allergens [18] may complicate the issue in terms of “causative species”. It may be that removing someone from occupational exposure to either mice or rats because of their sensitization to that species and allowing them to continue to work with the other rodent species may not prevent allergic symptoms or the pathway to occupational asthma as an outcome.
The relationship between mice exposure (the predominant rodent species utilized) and the health outcome of asthma suggests a relationship over the 16-year time period where the Mus m 1 exposure reduced at a linear rate of a little less than 6% per annum while associated asthma decreased concomitantly by a lesser amount of almost 3% per annum. There is no evidence that the decrease in mouse allergen is statistically significantly reducing the incidence of rhinitis over the period. Both asthma and rhinitis are related to IgE sensitisation. Asthma presentation relates to a period of continuing allergen exposure subsequent to sensitisation; rhinitis will only present in sensitised individuals above some triggering level of exposure to the relevant allergen. Thus, the levels of allergens over the 16-year period appear high enough to cause rhinitis, while the decrease in the predominant mice allergen in those sensitised was enough to impact significantly on the incidence of OA. Occupational rhinitis may be thought to be a significantly less deleterious health outcome than asthma. However, in this context the rhinitis is likely to be continuous, related to work and serious enough to be diagnosed by a consultant. It is likely such rhinitis will significantly impact on a sufferer working in the sector.
Laboratory allergen exposure is a simple but real-life model of a high molecular weight (protein allergen) IgE-mediated mechanism causing short-latency respiratory outcomes. Exposure is to a relatively narrow range of allergens in a limited number of animal species. Exposure monitoring has been relatively widely used and, importantly, by measuring major allergens rather than a surrogate such as dust levels. However, the data presented here may suggest more generally that reduction in predominant allergen(s) where there is a mechanism of protein-induced, IgE mediated health outcomes may be associated with some reduction in OA with no obvious evidence of a time lag. Therefore, the control of occupational exposure to protein allergens can bring some immediate impact on reducing the risk of a significant health outcome.
Exposure to LAA is now a relatively low cause of occupational asthma in the UK, due to implemented exposure control measures within a small, generally well-managed sector. Occupational asthma related to IgE sensitisation has been reported in the fish/shellfish processing industry [26,27]. More significant causes of occupational asthma are in the bakery sector and where isocyanates are used [19,28,29,30,31]. Unlike laboratory animal allergy, there are a large number of allergenic proteins in flour that bakers handle and there has been a debate whether isocyanate OA is fully IgE mediated [32].
However, we suggest that the LAA data presented strengthens the argument that ongoing allergen exposure control for proteinaceous allergens is paramount and should have an immediate effect on reducing levels of IgE-mediated occupational asthma. The UK data for animal usage in scientific and medical research suggests that significant use of rodents will continue, particularly related to genetically modified and immunodeficient animals as disease models.
Author Contributions
Conceptualization, H.M.; methodology, H.M., L.B., and K.J.; SWORD data curation, L.B.; EMDB data curation, K.J.; writing—original draft preparation, H.M.; writing—review and editing, K.J., L.B. All authors have read and agreed to publication of the proof-read version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This publication and the work it describes were in part funded by the Health and Safety Executive (HSE). Its contents, including any opinions and/or conclusions expressed, are those of the authors alone and do not necessarily reflect HSE policy. H.M. and K.J. are employees of the HSE.
Data Availability Statement
The EMDB contains both personal and commercially sensitive information and is therefore not accessible. Information from the SWORD database is widely published by the University of Manchester and HSE. Researchers may contact the SWORD coordinator (LB) for specific enquiries.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SWORD | Surveillance of Work-related and Occupational Respiratory Disease |
| THOR | The Health and Occupation Research Network |
| EMDB | Environmental Monitoring Database |
| HSE | Health and Safety Executive |
| LAA | Laboratory animal allergens |
| RPE | Respiratory protective equipment |
References
- Newman Taylor, A.; Gordon, S. Laboratory Animal and Insect Allergy. In Asthma in the Workplace; Bernstein, I., Chan-Yeung, M., Malo, J.-L., Bernstein, D., Eds.; Marcel Dekker: New York, NY, USA, 1993; pp. 399–414. [Google Scholar]
- Wood, R. Laboratory animal allergens. ILAR J. 2001, 42, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Jeal, H.; Jones, M. Allergy to rodents: An update. Clin. Exp. Allergy 2010, 40, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Schofield, S.; Jeal, H.; Cullinan, P. Respiratory protective equipment reduces occurrence of sensitization to laboratory animals. Occup. Med. 2014, 642, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Mason, H.; Jones, K. Airborne exposure to laboratory animal allergens: 2005–2022. AIMS Allergy Immunol. 2024, 8, 18–33. [Google Scholar] [CrossRef]
- Filon, F.; Drusian, A.; Mauro, M.; Negro, C. Laboratory animal allergy reduction from 2001 to 2016: An intervention study. Respir. Med. 2018, 136, 71–76. [Google Scholar] [CrossRef]
- Renström, A.; Karlsson, A.; Malmberg, P.; Larsson, P.; van Hage-Hamsten, M. Working with male rodents may increase risk of allergy to laboratory animals. Allergy 2001, 56, 964–970. [Google Scholar] [CrossRef]
- Schweitzer, I.; Smith, E.; Harrison, D.; Myers, D.; Eggleston, P.; Stockwell, J.; Paigen, B.; Smith, A. Reducing Exposure to Laboratory Animal Allergens. Comp. Med. 2003, 53, 487492. [Google Scholar] [CrossRef]
- Zahradnik, E.; Raulf, M. Allergens in laboratory animal facilities. Allergologie 2016, 39, 86–95. [Google Scholar] [CrossRef]
- Feary, J.; Schofield, S.; Canizales, J.; Fitzgerald, B.; Potts, J.; Jones, M.; Cullinan, P. Laboratory animal allergy is preventable in modern research facilities. Eur. Respir. J. 2019, 53, 1900171. [Google Scholar] [CrossRef]
- Home Office. Annual Statistics of Scientific Procedures on Living Animals, Great Britain, 2018; Home Office: London, UK, 2018; pp. 1–32.
- Mason, H.; Willerton, L. Airborne exposure to laboratory animal allergens. AIMS Allergy Immunol. 2017, 1, 78–88. [Google Scholar] [CrossRef]
- Iskandar, I.; Carder, M.; Barradas, A.; Byrne, L.; Gittins, M.; Seed, M.; van Tongeren, M. Time Trends in the Incidence of Work-Related Ill-Health in the UK, 1996–2019: Estimation from THOR Surveillance Data; The University of Manchester: Manchester, UK, 2020. [Google Scholar]
- Home Office. Annual Statistics of Scientific Procedures on Living Animals Great Britain 2020; Home Office: London, UK, 2020; pp. 1–30. Available online: https://www.gov.uk/government/statistics/statistics-of-scientific-procedures-on-living-animals-great-britain-2020 (accessed on 3 March 2025).
- Home Office. Annual Statistics of Scientific Procedures on Living Animals Great Britain 2014; Home Office: London, UK, 2015; pp. 1–62.
- Cocker, J.; Jones, K. Biological Monitoring Without Limits. Ann. Work Expo. Health 2017, 61, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Cocker, J.; Cain, J.; Baldwin, P.; McNally, K.; Jones, K. A Survey of Occupational Exposure to 4,4methylene-bis (2-chloroaniline) (MbOCA) in the UK. Ann. Occup. Hyg. 2009, 53, 499–507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jeal, H.; Harris, J.; Draper, A.; Newman Taylor, A.; Cullinan, P.; Jones, M. Dual sensitization to rat and mouse urinary allergens reflects cross-reactive molecules rather than atopy. Allergy 2009, 64, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Health and Safety Executive. Work-Related Asthma Statistics, 2024; HSE: Bootle, UK, 2024. Available online: https://www.hse.gov.uk/statistics/assets/docs/asthma.pdf (accessed on 26 September 2025).
- Kruize, H.; Post, W.; Heederik, D.; Martens, B.; Hollander, A.; van der Beek, E. Respiratory allergy in laboratory animal workers: A retrospective cohort study using pre-employment screening data. Occup. Environ. Med. 1997, 54, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Feary, J.; Fitzgerald, B.; Banya, W.; Jones, M.; Cullinan, P.; Schofield, S. Cross-sectional survey of sensitisation to mouse allergens in contemporary laboratory animal workers: The SPIRAL study. Allergy 2016, 71, 15. [Google Scholar]
- Muzembo, B.; Eitoku, M.; Inaoka, Y.; Oogiku, M.; Kawakubo, M.; Tai, R.; Takechi, M.; Hirabayashi, K.; Yoshida, N.; Ngatu, N.; et al. Prevalence of Occupational Allergy in Medical Researchers Exposed to Laboratory Animals. Ind. Health 2014, 52, 256–261. [Google Scholar] [CrossRef][Green Version]
- Feary, J.; Fitzgerald, B.; Schofield, S.; Jones, M.; Cullinan, P. Sensitisation to mouse allergens in contemporary laboratory animal workers: The Spiral study. Eur. Respir. J. 2016, 48, PA392. [Google Scholar] [CrossRef]
- Canizales, J.; Jones, M.; Semple, S.; Feary, J.; Cullinan, P. To determine mus m 1 personal exposure in laboratory animal workers where mice are housed in open cages and individually ventilated cages. Thorax 2015, 70 (Suppl. 3), A106. [Google Scholar] [CrossRef]
- Renstrom, A.; Bjoring, G.; Hoglund, A. Evaluation of individually ventilated cage systems for laboratory rodents: Occupational health aspects. Lab. Anim. 2001, 35, 42–50. [Google Scholar] [CrossRef]
- Mason, H.; Carder, M.; Money, A.-M.; Evans, G.; Seed, M.; Agius, R.; van Tongeren, M. Occupational Asthma and Its Causation in the UK Seafood Processing Industry. Ann. Work Expo. Health 2020, 64, 817–825. [Google Scholar] [CrossRef]
- Jeebhay, M.; Robins, T.; Miller, M.; Bateman, E.; Smuts, M.; Baatjies, R.; Lopata, A. Occupational Allergy and Asthma among Salt Water Fish Processing Workers. Am. J. Ind. Med. 2008, 51, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Rother, D.; Schlüter, U. Occupational Exposure to Diisocyanates in the European Union. Ann. Work Expo. Health 2021, 65, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, D.; Pechter, E.; Fitzsimmons, K.; Lumia, M.; Stephens, A.; Davis, L.; Flattery, J.; Weinberg, J.; Harrison, R.; Reilly, M.; et al. Isocyanates and Work-Related Asthma: Findings from California, Massachusetts, Michigan, and New Jersey, 1993–2008. Am. J. Ind. Med. 2015, 58, 1138–1149. [Google Scholar] [CrossRef]
- Patouchas, D.; Sampsonas, F.; Papantrinopoulou, D.; Tsoukalas, P.; Karkoulias, K.; Spiropoulos, K. Determinants of specific sensitization in flour allergens in workers in bakeries with use of skin prick tests. Eur. Rev. Med. Pharmacol. Sci. 2009, 13, 407–411. [Google Scholar]
- Baur, X.; Posch, A. Characterized allergens causing bakers’ asthma. Allergy 1998, 53, 562–566. [Google Scholar] [CrossRef]
- Wisnewski, A.; Jones, M. Pro/Con Debate: Is Occupational Asthma Induced by Isocyanates an IgE-Mediated Disease? Clin. Exp. Allergy 2010, 40, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).