Abstract
Background: Dyslipidaemia promotes atherosclerotic plaque formation. Plaques that are vulnerable to rupture have a higher proportion of inflammatory (M1:CD86) macrophages in their cap. Many plaque macrophages are derived from blood monocytes which have been exposed to elevated blood lipid levels. Here, we explored whether the inflammatory state of monocyte-derived macrophages is associated with blood lipid levels and assessed whether oxidised low-density lipoprotein (oxLDL) directly induces some of the observed changes. Method: Blood was collected from 20 individuals. Lipid profiles were measured, and monocytes differentiated into macrophages. Macrophage inflammatory state was assessed by flow cytometry for phenotypic markers (e.g., CD86 and CD163) and cytokine production: TNF, IL-1β, and IL-6. Furthermore, monocytes were isolated from 6 normo-lipidaemic individuals and cultured with oxLDL, followed by stimulation with LPS/IFNγ and assessment of the cytokine response. Results: The inflammatory phenotype acquired by macrophages (ex vivo) was related to levels of in vivo circulating lipids. Correlations for CD86/CD163 were found with CVD risk markers; most strongly with triglycerides (TG) and TG/HDL-C, but also with cholesterol/HDL-C and ApoB/ApoA1 and inversely with LDL particle size. Functionally, macrophage production of inflammatory cytokines (TNF and IL-1β) correlated with oxLDL levels and inversely with ApoA1. Macrophages differentiated from monocytes cultured with oxLDL produced significantly higher IL-1β but lower IL-10 (in response to LPS/IFNγ), compared to control cells. Conclusions: Monocyte-derived macrophages adopt an inflammatory phenotype relative to the levels of circulating lipid factors that are characteristic of atherogenic dyslipidaemia (such as high TG, TG/HDL-C and low LDL particle size), but not LDL-C.
1. Introduction
While dyslipidaemia is largely asymptomatic, abnormal blood lipid levels—particularly high levels of low-density lipoprotein-cholesterol (LDL-C)—promote the formation of atherosclerotic plaques that underpin cardiovascular disease (CVD) [1]. These plaques develop over years, often decades, through a chronic inflammatory process instigated by LDL retention and oxidation in the vessel wall [2]. Advanced plaques acquire morphologically complex features that make them unstable and thus more prone to rupture and triggering of a clinical event [3]. Such plaques have a higher proportion of inflammatory macrophages (M1:CD86), compared to healing macrophages (M2:CD163), as seen by a higher CD86/CD163 (M1/M2) ratio in thin-capped plaques [4], and a dominance of CD86 over CD163 macrophages in the plaque shoulder region [5]. The phenotype of macrophages in the plaque is thought to be directed by the microenvironment [6] and, indeed, a range of phenotypes relative to the environment are evident, such as M(Hb) macrophages that form in regions of haemorrhage [7].
While there are resident macrophages in the vessel wall, plaque progression relies heavily on recruited monocytes from the circulation [8,9]. Monocytes are exposed to a person’s blood lipids, which may impact their phenotype prior to their entry into the vessel wall. Indeed, we found that CVD patients’ monocytes are more inflammatory than those of healthy controls [10]. Moreover, monocytes from generally healthy individuals expressed many inflammatory markers relative to the levels of specific lipids. TLR2 expression correlated with total cholesterol (Chol) and LDL-C [11], while CD86 was inversely associated with HDL-C [11]. Furthermore, many adhesion molecules were inversely correlated with ApoA1 [12], indicating that monocytes have a greater potential to migrate into the tissues when ApoA1 is low. As an estimated 64% of Australian adults have dyslipidaemia [13], this suggests a prevalent underlying myeloid-cell immunopathology.
To assess whether the immunopathology extends to macrophages, here we conducted an exploratory study to assess whether the phenotype that monocyte-derived macrophages adopt (ex vivo) is also related to an individual’s blood lipid levels. By assessing correlations between macrophage inflammatory maker expression and blood lipid levels, we sought to understand whether the phenotype that macrophages adopt may be primed in the circulation, even before they enter the vessel wall.
We explored this in a cohort considered generally healthy, for we propose that there is an underlying immunopathology associated with dyslipidaemia—even in generally healthy people—that largely goes undetected and untreated. Generally healthy individuals with high ApoB-containing lipoproteins form a population in danger of increased cumulative LDL-C exposure and thus increased plaque burden over time and a high life-time risk of atherosclerotic cardiovascular events [14]. An altered myeloid cell pathology in generally healthy dyslipidaemic individuals could be a contributing factor to development of this high life-time risk.
2. Materials and Methods
2.1. Study Population
This study was approved by the Human Research Ethics Committee Western Sydney Local Health District (2019/PID13966) and conducted in line with ICH-GCP guidelines. The study was conducted on the Westmead Campus on 20 adult participants who responded to our call for generally healthy volunteers. We used the term ‘generally’ healthy because though our participants did not have any known clinical condition that would exclude them from the study, we expected (given the Australian dyslipidaemia rates: 64% of adults [13]) that many of our so-called ‘healthy’ participants would have dyslipidaemia, albeit many unknowingly, as dyslipidaemia is largely asymptomatic. Inclusion criteria: participants needed to be generally healthy adults (over 18 years); though it was permissibleto have dyslipidaemia. The criteria for dyslipidaemia were based on our state health department (NSW Health Pathology) references ranges at the time of the study. Individuals were classed as having dyslipidaemia if they had perturbed levels of one or more of the traditionally measured lipids as follows: cholesterol > 5.5 mmol/L, LDL-C > 3 mmol/L, HDL-C < 1 mmol/L, or triglycerides > 2 mmol/L. Exclusion criteria: documented history of CVD, diabetes or hyperglycaemia (fasting glucose > 5.5 mM), cancer, a chronic inflammatory condition, obesity, current smoker, taking statins, or taking anti-inflammatory medications. If individuals had an infection or acute inflammatory condition (including vaccination) they could still participate, but blood was not collected until at least six weeks later. After written informed consent was provided, general participant health information was collected and blood pressure (for calculation of CVD risk), as well as waist circumference and height were measured (to assess ‘obese’ exclusion criteria). Venous blood samples, taken after an overnight fast ( of at least 12 h ), were also collected.
2.2. Biochemical and Lipid Measurements
Blood cell counts were assessed using the DxH 500 Hematology Analyser (Beckman Coulter, Brea, CA, USA). Blood biochemistry: Total cholesterol, LDL-C, HDL-C, triglycerides, ApoB, ApoA1, Lp(a), hs-CRP, glucose, and insulin were measured by the Institute of Clinical Pathology and Medical Research (ICPMR) New South Wales Health Pathology (NSWHP), Westmead. LDL particle size and oxLDL levels were measured from isolated serum by NutriPATH Integrative Pathology Services (Melbourne, Australia). Lp(a) and oxLDL are not routinely measured when testing for dyslipidaemia. In this study, individuals with Lp(a) > 300 mg/L were considered to have dyslipidaemia, based on NSW Health Pathology reference ranges. Normal ranges for oxLDL were not reported in test results.
2.3. Cardiovascular Risk
Cardiovascular risk was calculated using the online Australian cardiovascular disease risk calculator [15]. Participants aged under 30 years were deemed low risk; they were too young for the risk calculator. For three females, blood pressure had not been measured. An estimate of their maximal likely risk was determined by using a systolic blood pressure of 179 mmHg. This value was chosen as it is the highest value prior to hypertensive crisis [16]. There was also one female whose cholesterol was not measured. To achieve an estimate (CVD risk) for her, a Chol/HDL value of 8 was used. This value was chosen as it is the highest Chol/HDL ratio used in Australian CVD risk charts [17]. Note: It was used only for risk calculation, and not for the later correlation analysis.
2.4. Monocyte Isolation and Culture
Human monocytes were isolated directly from K2EDTA anti-coagulated whole blood using the immunomagnetic negative selection method EasySep™ Direct Human Monocyte Isolation Kit (as per manufacturer instructions) with an additional incubation against the magnet to maximise purity. Isolated cells were then cultured in RPMI 1640 Media containing 20% autologous serum at 4–5 × 105 cells/mL at 1 mL/well in a 12-well plate and cultured at 37 °C with 5% CO2. (Autologous serum was isolated by allowing whole blood to clot at room temperature in an SST-II Vacutainer tube, before centrifuging at 200× g and collecting the upper serum layer). After overnight adherence, non-adherent cells were removed by a change of media. Media was changed again on day 3. On day 6 of culture, macrophages were collected either for surface marker analysis, or for cytokine analysis post-LPS stimulation. Lifting of cells off the plate was achieved by incubating the cells with TrypLE™ Express until they detached. While most participants’ samples were used for both surface marker and cytokine assessment, some were used only for one set of analysis due to technical issues, such as not obtaining enough cells. Full reagent details are in Supplementary Table S1.
2.5. Macrophage Surface Marker Assessment
To determine the inflammatory profile of the macrophages, harvested cells were resuspended in PBS at 5 × 104 cells/mL and 50 µL aliquots stained for surface markers. The cells were first incubated with Fc block (10 min) prior to the addition of Brilliant Stain Buffer Plus. Cells were stained (30 min) with LIVE/DEAD fixable Violet Dead Cell stain and fluorescently labelled antibodies as per Supplementary Table S2. Stained cells were washed and fixed with 1% formaldehyde. UltraComp eBeads™ Plus beads were used to prepare compensation controls for the surface marker antibodies. The ArC™ Amine Reactive Compensation Bead Kit was used to prepare a compensation control for the LIVE/DEAD™ Fixable Violet Dead Cell Stain. Data were acquired on the Invitrogen Attune NxT Acoustic Focusing flow cytometer and analysed using FlowJo® version 10.10. The gating strategy is depicted in Supplementary Figure S1.
2.6. Macrophage Cytokine Assessment via Flow Cytometry
To determine the cytokine profile of macrophages cultured in autologous serum, cells (on day 6) were stimulated with 1 µg/mL LPS, followed by 10 µg/mL Brefeldin-A (added after 2 h), and cultured for a total of 6 h at 37 °C and 5% CO2. Macrophages were then harvested and stained with LIVE/DEAD fixable Violet Dead Cell stain and fluorescently labelled with anti-cytokine antibodies listed in Supplementary Table S1. Intracellular cytokines were detected following fixation with 1X FACS™ Lysing Solution and permeabilization by 1X FACS™ Permeabilizing Solution 2. Data were acquired on the Invitrogen Attune NxT Acoustic Focusing flow cytometer and analysed using FlowJo® version 10.10. The gating strategy is depicted in Supplementary Figure S2.
2.7. Macrophage Cytokine Assessment with oxLDL Exposure via LEGENDplex
To assess the impact of oxLDL on macrophage cytokine production, blood was collected from 6 normo-lipidaemic individuals. They were classified as normo-lipidaemic based on having normal lipid levels as outlined in Section 2.1. Monocytes from whole blood (isolated as described above) were cultured in RPMI 1640 with 10% foetal bovine serum (FBS) and 50 ng/mL macrophage colony-stimulating factor (M-CSF) ± 30 μg/mL oxLDL. At day 6, cells were either continued with M-CSF (to give M0 conditions) or the M-CSF was replaced with granulocyte macrophage colony-stimulating factor (GM-CSF is more pro-inflammatory) [18] and LPS (50 ng/mL)/IFNγ (50 ng/mL) for 24 h (to give M1 conditions). Note, oxLDL was retained in the respective wells till day 7. On days 1 and 7 of culture, supernatant was collected for analysis by the LEGENDplex Human Essential Immune Response 13-plex panel for IL-4, IL-2, CXCL10, IL-1β, TNF, CCL2, IL-17A, IL-6, IL-10, IFNγ, IL-12p70, IL-8, and TGF-β1. Samples were handled following manufacturer’s instructions, and data acquired with BD FACS™ Canto II Flow Cytometer and analysed using LEGENDplex Data Analysis Software V8.0.
2.8. Statistical Analysis
Statistical analyses were completed using GraphPad Prism 10. Firstly, outlier analysis of surface marker expression and cytokine percentage was performed (with ROUT Q = 0.5%), leading to the removal of unusually high data points: one for CD86, two for CD163 and one for CD36. There were no outliers in the cytokine data. Tests for normality were performed (Shapiro–Wilk test) and with CD163 and CD86/CD163 not considered normal, the data for these was logged before analysis. The cytokine percentages were normally distributed. Two-tailed Pearson’s correlations were used to determine associations between macrophage surface markers or cytokine production relative to lipid factors (cholesterol, LDL-C, HDL-C, cholesterol/HDL-C, TG, ApoA1, ApoB, ApoB/ApoA1, Lp(a) TG/HDL-C, Mean LDL particle size, and oxLDL) and age. Correction for multiple comparisons was not performed as, being an exploratory study, we aimed to see which lipid factors most closely correlated with inflammation. p values are reported and a p-value < 0.05 was considered statistically significant.
For the addition of oxLDL to macrophages and assessment of cytokine outcome, LENGENDplex values reported as less than the limit of detection (LOD) were considered undetectable, and those between the LOD and limit of quantitation (LOQ) were considered minimal expression. When this minimal detection led to cytokine data sets having fewer than 4 data points, statistical analysis was not performed. Data points that were greater than the maximal value (for the respective cytokine) were given the maximal value for analysis, with identification of when this occurred mentioned in the results. Cytokine data were then multiplied by their respective normalisation factors to account for varying cell counts in the culture wells. (For each well, images were taken for five fields of view and the mean percentage area determined. This mean was used to calculate a normalisation factor for each sample which was then applied to the raw cytokine concentrations to convert the raw concentrations to the cytokine concentration expected from a 100% cell coverage). An assessment of normality by the Shapiro–Wilk test was performed (for cytokines cultured under each of the 6 conditions) and based on this, parametric/non-parametric analyses were performed accordingly. Analysis was conducted comparing cytokine production at day one for control (C-D1) versus oxLDL-treated cells (Ox-D1) by a two-tailed ratio-paired T-tests or the non-parametric Wilcoxon tests.
For the cytokines produced under all four day 7 conditions: control not stimulated (C-M0), control M1 stimulated (C-M1), oxLDL-treated cells not stimulated (Ox-M0) and oxLDL-treated cells M1 stimulated (Ox-M1), data sets were compared by a repeated measures ANOVA or the non-parametric Friedman test. Except for IL-6 where a Kruskal–Wallis test was performed as the data were not paired due to 2 non-stimulated samples having undetectable IL-6.
As several cytokines were not produced (or had minimal detection) on day 7 under C-M0 and Ox-M0 conditions, comparisons were made between the M1 stimulated samples (C-M1 and Ox-M1) using a two-tailed ratio paired T-test or the non-parametric Wilcoxon matched-pairs signed rank test. The p values of these were then corrected for multiple comparisons using the False Discovery Rate (FDR) approach with the FDR set at 5%. Figure values are representative of the mean value ± SEM.
3. Results
3.1. Characteristics of Study Participants
The cohort consisted of generally healthy males (n = 2) and females (n = 18) with a median age of 45 years (Table 1). Based on traditional risk factors (cholesterol, LDL-C, HDL-C and TG and using NSWHP references ranges), 10 out of 19 (52.6%) participants had dyslipidaemia (traditional lipid data were not obtained for one donor). This increased to 12 (out of 20; 60%) when including perturbations in Lp(a) (8 participants). Five participants had insulin levels ≥9 mIU/L and 3 had elevated hsCRP (>3 but <10 mg/L). No participant’s glucose level was above the normal range. Assessing CVD risk, all females were deemed low risk (including those whose risk was calculated using blood pressure or Chol/HDL-C estimations) and the two males, intermediate risk (<5%, and 5% to <10% estimated 5-year CVD risk).

Table 1.
Participant demographics.
3.2. Macrophage Phenotypic Marker Expression Is Related to Lipid Levels
To assess whether macrophage phenotype may be related to lipid levels, monocyte-derived macrophages were assessed for expression of inflammatory and anti-inflammatory markers as well as lipid uptake molecules. Macrophages from all participants expressed CD86, CD163, and CD36 with considerable differences between individuals (Supplementary Figure S3). CD204 was only expressed by two samples, CD93 by 8 and CD206 by 12. As such, expression of these markers was not used for further analysis. Notably, lipid levels were not an explanation for who expressed these markers.
Exploring the relationships between CD86, CD163, and CD36 with participant lipid levels, no correlations were evident for CD86 or CD163 individually. Multiple correlations were evident for the CD86/CD163 ratio with lipid levels: Chol/HDL-C (r = 0.5117; 95% CI [0.04, 0.80], p = 0.0358), TG (r = 0.6638; 95% CI [0.27, 0.87] p = 0.0037), LDL particle size (r = −0.7031; 95% CI [−0.88, −0.35] p = 0.0011), TG/HDL ratio (r = 0.6161; 95% CI [0.19, 0.85], p = 0.0085) and ApoB/ApoA1 (r = 0.5093; 95% CI [0.04, 0.80], p = 0.0368; Figure 1A). CD36 was found to correlate with TG (r = 0.5828; 95% CI [0.14, 0.83] p = 0.0141), the TG/HDL-C ratio (r = 0.5606,95% CI [0.11, 0.82] p = 0.0192), and inversely with LDL particle size (r = −0.5649,95% CI [−0.82, −0.12] p = 0.0181; Figure 1B). There was no correlation of the macrophage markers with ApoB, oxLDL or Lp(a).
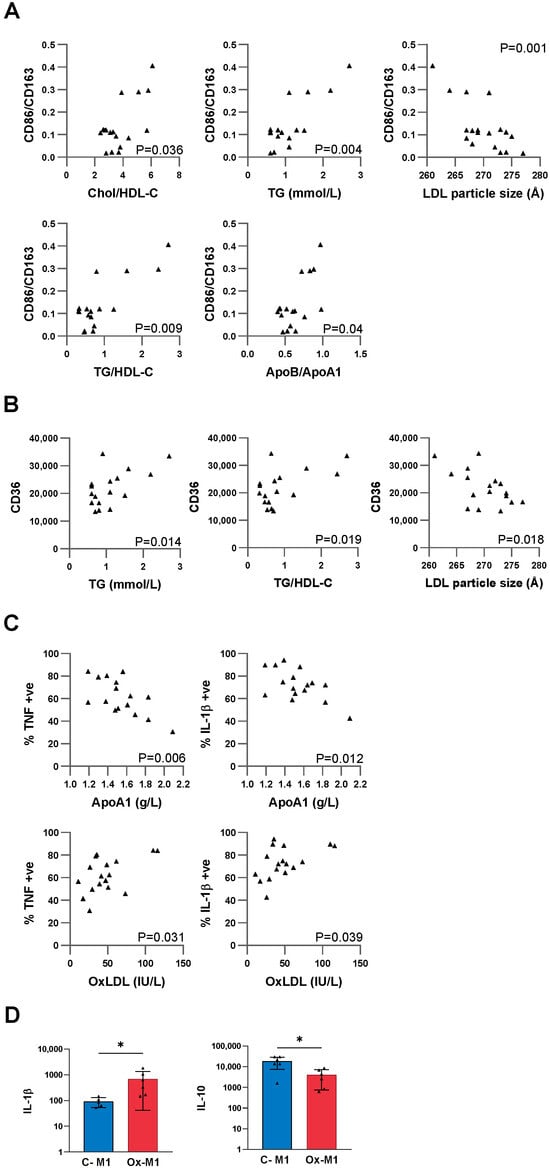
Figure 1.
Macrophage surface marker and cytokine correlations with blood lipid levels. (A,B) Monocytes were differentiated into macrophages by culture in autologous serum for 6 days and then assessed for macrophage surface markers and cytokines (post-LPS stimulation) by flow cytometry. Outlier detection was performed, then data tested for normality and log transformations performed as required. Two-tailed Pearson correlation analysis was performed. (A) Macrophage CD86/CD163 positively correlated with cholesterol/HDL-C, TG, TG/HDL ratio and ApoB/ApoA1 and inversely correlated with LDL particle size, (all n = 17 except LDL particle size, n = 18). (B) CD36 correlated with TG and the TG/HDL-C ratio and inversely with LDL particle size (n = 17). (C) Inverse correlations are seen for ApoA1 with TNF, and IL-1β (n = 16). Positive correlations are observed for oxLDL with TNF and IL-1β (n = 17). (D) Monocytes were cultured in M-CSF ± oxLDL for 7 days with either no stimulation, or replacement of the M-CSF with GM-CSF and LPS/IFNγ for the last 24 h, followed by assessment for cytokines by LEGENDplex. Differences between groups were assessed by two-tailed ratio paired t-tests (or non-parametric Wilcoxon test as appropriate: n = 6). The p values were corrected for multiple comparisons using the False Discovery Rate (FDR) approach with the FDR set at 5%. Bars are representative of the mean value ± SEM. * p < 0.05.
3.3. Macrophage Cytokine Production Is Related to Lipid Levels
To address whether the macrophages were functionally more inflammatory in dyslipidaemia, we assessed cytokine response to LPS stimulation. Without stimulation, macrophages produced minimal levels of cytokines. Upon LPS stimulation, cytokines were produced with considerable differences between individuals (Supplementary Figure S3), which were related to participants’ lipid levels. These included inverse correlations between ApoA1 and both TNF and IL-1β (TNF: r = −0.6539, 95%CI [−0.87,−0.23] p = 0.006; IL-1β: r = −0.6114, 95% CI [−0.85, 0.17], p = 0.0119; Figure 1C), and positive correlations between oxLDL and both TNF and IL-1β (TNF: r = 0.5239, 95% CI [0.06, 0.80] p = 0.0309; IL-1β: r = 0.5033, 95% CI [0.03, 0.79], p = 0.0394; Figure 1C). No correlations were evident for IL-6.
3.4. OxLDL Primes Macrophages to an Inflammatory State
Noting that TNF and IL-1β production correlated with participants’ oxLDL, we then assessed whether this relationship may be causal by adding oxLDL to in vitro cultures. Firstly, we assessed the cytokine response after 1 day (24 h) of oxLDL treatment (Ox-D1) compared to untreated control cells (C-D1). At this time point, most cytokines (IL-4, IL-1β, TNF, IL-17, IL-6, IL-10, IFNγ, IL-12p70, TGFβ) had minimal detection (i.e., not detected, had minimal expression, or were expressed in only a few participant samples). Only IP-10, MCP-1, and IL-8 were detected in all participant samples for both C-D1 and Ox-D1-treated monocytes. There was, however, no significant difference in the level of these cytokines between the two treatments. Notably, two of the values for MCP-1 (in the oxLDL-treated monocytes) were at the maximum LEGENDplex detection value and thus the value for MCP-1 in these samples upon analysis is an underestimation.
We then assessed cytokine production from differentiated macrophages after 7 days of control (untreated) or oxLDL treatment, either without stimulation (C-M0 and Ox-M0) or in response to M1 inducing conditions: (GM-CSF) and cytokines LPS/IFNγ (Ox-M1 and C-M1). As for day 1, basal cytokine production at day 7 in Ox-M0 and C-M0 was minimal for most cytokines (IL-4, IL-1β, TNF, IL-17, IL-10, IL-12p70, TGFβ). MCP-1, IP-10 and IL-8, were detected in all samples, while IL-6 was produced by four participants for both control (C-M0) and oxLDL-treated (Ox-M0) macrophages.
Most cytokines were detected at day 7 after LPS/IFNγ stimulation of control macrophages (C-M1) or those exposed to oxLDL (Ox-M1), with the values for one or more participants hitting the maximum (for C-M1: MCP-1, IP-10, IL-8, IL-6, IL-10 and TNF, and for Ox-M1: MCP-1, IP-10, IL-6). TGFβ was only detected in 4 out of 12 M1 samples (i.e., C-M1 or Ox-M1) and IL-12p70 was produced by 8 M1 samples (i.e., 4 out of 6 participants for both C-M1 and Ox-M1); statistical comparison for IL-12p70 was based on the data from these 4 participants.
As MCP-1, IP-10, IL-8, and IL-6 were found in all four treatment groups, (Ox-M0, C-M0, Ox-M1 and C-M1), differences in expression between the four groups were assessed. There was no significant difference in the cytokines between the oxLDL-treated group and its respective control (Ox-M0 vs. C-M0 or Ox-M1 vs. C-M1). However, for the oxLDL-treated cells, M1 stimulation (Ox-M1) increased IP-10 and IL-6 production compared to the unstimulated M0 (Ox-M0) cells (p = 0.0014 and p = 0.0223, respectively.
For the 7 cytokines that were only consistently produced after M1 stimulation of control and oxLDL-treated cells (i.e., by C-M1 and Ox-M1 but not expressed in C-M0 or Ox-M0), exposure to oxLDL led to an increase in IL-1β (Ox-M1 vs. C-M1: p = 0.0354) and decrease in IL-10 (Ox-M1 vs. C-M1: p = 0.0273: Figure 1D) with these being the corrected p values (accounting for multiple comparisons).
4. Discussion
4.1. Interpretation of Main Findings
Dyslipidaemia often lies undetected, and untreated, in otherwise generally healthy adults. Though asymptomatic, we previously found that monocyte inflammatory changes relate to blood lipid levels [11,12]. Here, we extended this work to assess whether the phenotype adopted by monocyte-derived macrophages is also related to cardiovascular risk factors. Multiple observed associations indicate that macrophages adopt an inflammatory phenotype relative to the level of factors characteristic of an ‘atherogenic dyslipidaemia’ profile (elevated TG and high TG/HDL-C, rather than high LDL-C), with the association with oxLDL likely being a causal effect.
All participants entering our study were generally healthy, yet many were unaware that they had dyslipidaemia. With ~2/3 of Australians having dyslipidaemia [13] it is not surprising that 60% (12/20) of our generally healthy cohort were dyslipidaemic. Notably, 2 of these participants were found to be dyslipidaemic based on Lp(a), which is not routinely measured by standard testing. Unfortunately, the sex balance of our study was skewed heavily towards females; this is because we relied on individuals to respond to study advertising and more females than males responded. We thus could not conduct comparisons between the sexes. Looking at age, there was no association between it and macrophage inflammatory markers.
Macrophage phenotype varied considerably between the participants. The inconsistent detection of CD93, CD204 and CD206 may in part be due to culturing the monocytes in autologous serum as, for example, CD93 was originally identified as an M2 marker in macrophages that were cultured using foetal calf serum with added growth factors and cytokines [19] but we cultured the cells using autologous serum and no growth factors/cytokines. There was no clear lipid-related explanation for which participants’ macrophages these markers could be detected on.
The high levels of CD86/CD163 relative to risk factors, highlights that macrophages likely adopt an inflammatory phenotype in dyslipidaemic individuals. With the strongest positive correlation being with TG and the TG/HDL-C ratio, this suggests that high triglycerides (in vivo) promote monocyte differentiation into M1 macrophage phenotype. This aligns with cell culture studies on THP-1 monocytes where triglycerides promoted the adoption of an M1 phenotype [20]. Furthermore, triglyceride levels (increased after a high fat meal) were associated with pro-inflammatory skewing of monocytes (seen by increased CD11c/CD18) [21]. Our results suggest that triglyceride levels in the blood might contribute to macrophage adoption of an inflammatory phenotype in atherosclerosis. Notably, the two individuals with TG > 2 mmol both had high TG/HDL-C ratio (5.6 and 6.18 when using mg/dL units), insulin levels of 12, and calculated HOMA-IR of 2.67 and 2.78 which suggests they have early insulin resistance [22]. In addition, they also had low HDL-C which flags them as having atherogenic dyslipidaemia [23,24,25]. The heightened macrophage inflammation relative to triglycerides could contribute to chronic inflammation and may explain, in part, the association of triglycerides with residual risk of CVD events [26].
Furthermore, there was a strong inverse relationship between macrophage inflammatory phenotype and LDL particle size. There are several possible reasons, such as the enhanced oxidisability of small, dense LDLs [27], or the fact that LDL particle size inversely correlates with the TG/HDL-C ratio [22]. As high levels of small, dense LDL are also characteristic of atherogenic dyslipidaemia, it further highlights the connection between an atherogenic lipid profile and myeloid cell inflammation.
The association of higher CD86/CD163 with Chol/HDL-C indicates that macrophages likely adopt an increased inflammatory state with increased cardiovascular risk, as Chol/HDL-C is used, for example, in Australian cardiovascular risk charts [17]. As we previously found that CD86/CD163 is associated with plaque instability [4] then our finding here offers a mechanistic link, that when Chol/HDL-C is high, (monocyte-derived) macrophages are skewed to an inflammatory phenotype, which in turn promotes plaque instability. Akin to our findings in monocytes [11,12], the degree to which the macrophages become inflammatory is likely regulated by HDL and ApoA1 levels. Indeed, in mice, HDL infusion shifts plaque macrophages to a less inflammatory state [28] and HDL has been shown in culture to increase macrophage expression of anti-inflammatory (M2) markers [29].
That macrophage CD36 correlated with triglyceride levels is consistent with studies on monocytes [30]. The increased CD36 may in turn enhance signalling by oxLDL, as CD36 is a receptor for oxLDL, which mediates metabolic and inflammatory cellular changes [31]. OxLDL is known to reprogram (train) myeloid cells, leading to increased cytokine production upon restimulation with another agent, such as LPS [32]. In essence, stimulation with LPS unveiled the presence of trained immunity. In line with this, we found that macrophage production of TNF and IL-1β (post-LPS stimulation) was positively correlated with oxLDL. Their production was also negatively associated with ApoA1 levels. As ApoA1 directly binds, and neutralises LPS [33] (which was used to stimulate cytokine production), this may be the mechanisms behind this finding. Combined with the surface marker findings, dyslipidaemia may promote monocyte and macrophage skewing to an inflammatory (M1) form (as seen by increased CD86/CD163), which is further primed by oxLDL to give stronger inflammatory responses in the plaque.
That oxLDL may indeed be training macrophages to be inflammatory is seen by the elevated IL-1β and decreased IL-10 response to LPS/IFNγ in macrophages that were exposed to oxLDL. However, it is important to note that myeloid cell training models usually use in vitro short-term exposure (usually 24 h) of the first stimulus [34]—in this case, oxLDL. Extended exposure to oxLDL throughout the culture was used here, as we aimed to more closely mimic clinical conditions, where the length of exposure to oxLDL would be chronic, or variable at least.
4.2. Study Limitations
There are several limitations to our study. Firstly, the cohort size meant the study was largely exploratory. However, the observed associations provide direction for future research to confirm these findings. Furthermore, the large bias towards females in our cohort limits the translatability of our results to males. In addition, assessment of monocyte-derived macrophages was only possible by ex vivo culture of primary monocytes. While the cells were cultured in autologous serum, the culture system remains a proxy of the in vivo biological condition which is far more complex and includes many other confounding factors that may influence macrophage phenotype.
4.3. Summary
Our findings, combined with our previous monocyte studies in this area [10,11,12], are revealing that there is an underlying immunopathology associated with dyslipidaemia, even in generally healthy younger adults. Notably, these changes are not primarily related to LDL-C levels. Rather, here, we found that monocyte-derived macrophages adopt a more inflammatory phenotype relative to the level of lipid factors typical of atherogenic dyslipidaemia. This is consistent with clinical studies that find that elevated remnant cholesterol (which is rich in triglycerides and elevated in atherogenic dyslipidaemia) is associated with a higher C-reactive protein, whereas LDL-C levels are not [35]. Moreover, we found that higher blood oxLDL levels (which are also found in atherogenic dyslipidaemia) were associated with (monocyte-derived) macrophage priming, such that the cells produced elevated inflammatory cytokines when stimulated with LPS.
It is important to highlight that the macrophage alterations found occur in people who, having a clinically perceived low short-term CVD risk, are unlikely to be pharmacologically treated for dyslipidaemia. The recognition that prolonged, cumulative LDL exposure leads to increased plaque burden [14], as well as the more recent findings that early intermittent dyslipidaemia promotes atherosclerosis [36,37] has led to calls for dyslipidaemia to be addressed earlier. Our findings that (monocyte-derived) macrophages are inflammatory in generally healthy individuals relative to factors associated with atherogenic dyslipidaemia (TG, TG/HDL-C and low LDL particle size), independent of age, calls for early attention to be paid to atherogenic dyslipidaemia, particularly as the inflammatory changes begin even before these factors reach levels at which atherogenic dyslipidaemia would be diagnosed. This is particularly important as myeloid cells play key roles beyond CVD. Indeed, individuals with dyslipidaemia have poorer clinical outcomes in multiple, highly prevalent, burdensome conditions. For example, high triglycerides or low HDL levels play a role in diabetes [38] and infection [39,40]. As such, understanding of the immunopathology associated with atherogenic dyslipidaemia is needed to enable early, personalised treatment that would prevent the potentially dire clinical outcomes that individuals with prolonged dyslipidaemia face.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/lipidology2040018/s1. Figure S1: Gating strategy for macrophage surface markers; Figure S2: Gating strategy for macrophage cytokine production; Figure S3: Inflammatory state of participant macrophages; Table S1: Materials. Table S2: Flow Cytometry Antibodies/reagents.
Author Contributions
Conceptualization, H.J.M.; methodology, C.D.M. and S.N.; formal analysis, C.D.M., L.D.Q. and H.J.M.; investigation, C.D.M. and S.D.; resources, R.B.; writing—original draft preparation C.D.M., H.J.M. and L.D.Q.; writing—review and editing, all authors; visualisation, H.J.M. and L.D.Q.; supervision, H.J.M., H.W. and R.B.; project administration, R.B.; funding acquisition, S.C.H.L., H.J.M. and H.W. Data interpretation C.D.M., H.J.M., and S.C.H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This project and publication were funded by NSWHP-ICPMR Westmead Private Practice Fund.
Institutional Review Board Statement
This study was approved (1 April 2020) by the Human Research Ethics Committee Western Sydney Local Health District (2019/PID13966) and conducted in line with ICH-GCP guidelines which align with the principles outlined in the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors acknowledge the Statistical Consulting Service provided by the Sydney Informatics Hub, a Core Research Facility of the University of Sydney and the guidance provided by statisticians from the Western Sydney Local Health District. Thank you to Leo Pasalic, Haematology, NSWHP for flow cytometry access. LEGENDplex was performed at the Westmead Scientific Platforms, which are supported by the Westmead Research Hub, the Cancer Institute New South Wales, the National Health and Medical Research Council and the Ian Potter Foundation.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ApoA1 | Apolipoprotein A1 |
| ApoB | Apolipoprotein B |
| Chol | Cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| Lp(a) | Lipoprotein(a) |
| oxLDL | Oxidised low-density lipoprotein |
| TG | Triglyceride |
| hs-CRP | High-sensitivity C-reactive protein |
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| TNF | Tumour necrosis factor |
| C-D1 | Control cells after 1 day culture |
| Ox-D1 | Cells cultured with oxLDL for 1 day |
| C-M0 | Control cells cultured for 7 days with no stimulation |
| Ox-M0 | Cells cultured with oxLDL for 7 days with no subsequent stimulation |
| C-M1 | Control cells cultured for 7 days and subsequent stimulation with M1 cytokines LPS/IFNγ |
| Ox-M1 | Cells cultured with oxLDL for 7 days and subsequent stimulation with M1 cytokines LPS/IFNγ |
| LOD | Limit of detection |
| LOQ | Limit of quantitation |
| FDR | False discovery rate |
| NSWHP | New South Wales Health Pathology |
References
- Boren, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Williams, K.J.; Boren, J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation 2007, 116, 1832–1844. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef]
- Medbury, H.J.; James, V.; Ngo, J.; Hitos, K.; Wang, Y.; Harris, D.C.; Fletcher, J.P. Differing association of macrophage subsets with atherosclerotic plaque stability. Int. Angiol. 2013, 32, 74–84. [Google Scholar]
- Stoger, J.L.; Gijbels, M.J.; van der Velden, S.; Manca, M.; van der Loos, C.M.; Biessen, E.A.; Daemen, M.J.; Lutgens, E.; de Winther, M.P. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 2012, 225, 461–468. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Guo, L.; Sakamoto, A.; Torii, S.; Sato, Y.; Cornelissen, A.; Kuntz, S.; Paek, K.H.; Fernandez, R.; Fuller, D.; et al. Diversity of macrophage phenotypes and responses in atherosclerosis. Cell. Mol. Life Sci. 2020, 77, 1919–1932. [Google Scholar] [CrossRef]
- Finn, A.V.; Nakano, M.; Polavarapu, R.; Karmali, V.; Saeed, O.; Zhao, X.; Yazdani, S.; Otsuka, F.; Davis, T.; Habib, A.; et al. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J. Am. Coll. Cardiol. 2012, 59, 166–177. [Google Scholar] [CrossRef]
- Williams, J.W.; Zaitsev, K.; Kim, K.W.; Ivanov, S.; Saunders, B.T.; Schrank, P.R.; Kim, K.; Elvington, A.; Kim, S.H.; Tucker, C.G.; et al. Limited proliferation capacity of aortic intima resident macrophages requires monocyte recruitment for atherosclerotic plaque progression. Nat. Immunol. 2020, 21, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Libby, P.; Aikawa, E.; Alcaide, P.; Luscinskas, F.W.; Weissleder, R.; Pittet, M.J. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 2007, 117, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.; Cassorla, G.; Pertsoulis, N.; Patel, V.; Vicaretti, M.; Marmash, N.; Hitos, K.; Fletcher, J.P.; Medbury, H.J. Human classical monocytes display unbalanced M1/M2 phenotype with increased atherosclerotic risk and presence of disease. Int. Angiol. 2017, 36, 145–155. [Google Scholar] [CrossRef]
- Patel, V.K.; Williams, H.; Li, S.C.H.; Fletcher, J.P.; Medbury, H.J. Monocyte inflammatory profile is specific for individuals and associated with altered blood lipid levels. Atherosclerosis 2017, 263, 15–23. [Google Scholar] [CrossRef]
- Patel, V.K.; Williams, H.; Li, S.C.H.; Fletcher, J.P.; Medbury, H.J. Monocyte Subset Recruitment Marker Profile Is Inversely Associated With Blood ApoA1 Levels. Front. Immunol. 2021, 12, 616305. [Google Scholar] [CrossRef]
- Nichols, M.; Peterson, K.; Herbert, J.; Alston, L.; Allender, S. Australian Heart Disease Statistics 2015; National Heart Foundation of Australia: Melbourne, Australia, 2016. [Google Scholar]
- Ference, B.A.; Braunwald, E.; Catapano, A.L. The LDL cumulative exposure hypothesis: Evidence and practical applications. Nat. Rev. Cardiol. 2024, 21, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Australian CVD Risk Calculator. Available online: https://www.cvdcheck.org.au/calculator (accessed on 5 February 2025).
- Marik, P.E.; Varon, J. Hypertensive crises: Challenges and management. Chest 2007, 131, 1949–1962. [Google Scholar] [CrossRef] [PubMed]
- National Vascular Disease Prevention Alliance. Absolute Cardiovascular Disease Management. In Quick Reference Guide for Health Professionals; National Stroke Foundation: Melbourne, Australia, 2012; ISBN 978-0-9805933-9-6. [Google Scholar]
- Ushach, I.; Zlotnik, A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J. Leukoc. Biol. 2016, 100, 481–489. [Google Scholar] [CrossRef]
- Beyer, M.; Mallmann, M.R.; Xue, J.; Staratschek-Jox, A.; Vorholt, D.; Krebs, W.; Sommer, D.; Sander, J.; Mertens, C.; Nino-Castro, A.; et al. High-resolution transcriptome of human macrophages. PLoS ONE 2012, 7, e45466. [Google Scholar] [CrossRef]
- Kim, H.-K.; Kim, S.H.; Kang, Y.W.; Kim, B.; Rhee, K.-J.; Kim, Y.S. Triglyceride Regulates the Expression of M1 and M2 Macrophage-specific Markers in THP-1 Monocytes. Biomed. Sci. Lett. 2016, 22, 220–226. [Google Scholar] [CrossRef]
- Gower, R.M.; Wu, H.; Foster, G.A.; Devaraj, S.; Jialal, I.; Ballantyne, C.M.; Knowlton, A.A.; Simon, S.I. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arter. Thromb. Vasc. Biol. 2011, 31, 160–166. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Reaven, G.; Abbasi, F.; Lamendola, C.; Saad, M.; Waters, D.; Simon, J.; Krauss, R.M. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am. J. Cardiol. 2005, 96, 399–404. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (US). Expert Panel on Detection, & Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar]
- Bosomworth, N.J. Approach to identifying and managing atherogenic dyslipidemia: A metabolic consequence of obesity and diabetes. Can. Fam. Physician 2013, 59, 1169–1180. [Google Scholar]
- Paublini, H.; Lopez Gonzalez, A.A.; Busquets-Cortes, C.; Tomas-Gil, P.; Riutord-Sbert, P.; Ramirez-Manent, J.I. Relationship between Atherogenic Dyslipidaemia and Lipid Triad and Scales That Assess Insulin Resistance. Nutrients 2023, 15, 2105. [Google Scholar] [CrossRef]
- Hoogeveen, R.C.; Ballantyne, C.M. Residual Cardiovascular Risk at Low LDL: Remnants, Lipoprotein(a), and Inflammation. Clin. Chem. 2021, 67, 143–153. [Google Scholar] [CrossRef]
- Ohmura, H.; Mokuno, H.; Sawano, M.; Hatsumi, C.; Mitsugi, Y.; Watanabe, Y.; Daida, H.; Yamaguchi, H. Lipid compositional differences of small, dense low-density lipoprotein particle influence its oxidative susceptibility: Possible implication of increased risk of coronary artery disease in subjects with phenotype B. Metabolism 2002, 51, 1081–1087. [Google Scholar] [CrossRef]
- Fotakis, P.; Kothari, V.; Thomas, D.G.; Westerterp, M.; Molusky, M.M.; Altin, E.; Abramowicz, S.; Wang, N.; He, Y.; Heinecke, J.W.; et al. Anti-Inflammatory Effects of HDL (High-Density Lipoprotein) in Macrophages Predominate Over Proinflammatory Effects in Atherosclerotic Plaques. Arter. Thromb. Vasc. Biol. 2019, 39, e253–e272. [Google Scholar] [CrossRef]
- Sanson, M.; Distel, E.; Fisher, E.A. HDL induces the expression of the M2 macrophage markers arginase 1 and Fizz-1 in a STAT6-dependent process. PLoS ONE 2013, 8, e74676. [Google Scholar] [CrossRef]
- Lian, Z.; Perrard, X.D.; Antony, A.K.; Peng, X.; Xu, L.; Ni, J.; Zhang, B.; O’Brien, V.; Saeed, A.; Jia, X.; et al. Dietary Effects on Monocyte Phenotypes in Subjects With Hypertriglyceridemia and Metabolic Syndrome. JACC Basic. Transl. Sci. 2023, 8, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, M.; Huang, W.; Chen, W.; Zhao, Y.; Schulte, M.L.; Volberding, P.; Gerbec, Z.; Zimmermann, M.T.; Zeighami, A.; et al. Mitochondrial Metabolic Reprogramming by CD36 Signaling Drives Macrophage Inflammatory Responses. Circ. Res. 2019, 125, 1087–1102. [Google Scholar] [CrossRef]
- Bekkering, S.; Quintin, J.; Joosten, L.A.; van der Meer, J.W.; Netea, M.G.; Riksen, N.P. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arter. Thromb. Vasc. Biol. 2014, 34, 1731–1738. [Google Scholar] [CrossRef]
- Ma, J.; Liao, X.L.; Lou, B.; Wu, M.P. Role of apolipoprotein A-I in protecting against endotoxin toxicity. Acta Biochim. Biophys. Sin. 2004, 36, 419–424. [Google Scholar] [CrossRef]
- Bekkering, S.; Blok, B.A.; Joosten, L.A.; Riksen, N.P.; van Crevel, R.; Netea, M.G. In Vitro Experimental Model of Trained Innate Immunity in Human Primary Monocytes. Clin. Vaccine Immunol. 2016, 23, 926–933. [Google Scholar] [CrossRef]
- Varbo, A.; Benn, M.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 2013, 128, 1298–1309. [Google Scholar] [CrossRef]
- Lavillegrand, J.R.; Al-Rifai, R.; Thietart, S.; Guyon, T.; Vandestienne, M.; Cohen, R.; Duval, V.; Zhong, X.; Yen, D.; Ozturk, M.; et al. Alternating high-fat diet enhances atherosclerosis by neutrophil reprogramming. Nature 2024, 634, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, M.; Zhao, X.; Lim, H.Y.; Magnussen, C.G.; Ang, O.; Suffee, N.; Schrank, P.R.; Ong, W.S.; Tsiantoulas, D.; Sommer, F.; et al. Early intermittent hyperlipidaemia alters tissue macrophages to fuel atherosclerosis. Nature 2024, 634, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ma, W.; Chen, D.; Wang, C.; Gao, Y.; Ran, X. Association of high-density lipoprotein cholesterol and wound healing in patients with diabetic foot ulcers. Chin. Med. J. 2022, 135, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Morin, E.E.; Guo, L.; Schwendeman, A.; Li, X.A. HDL in sepsis—Risk factor and therapeutic approach. Front. Pharmacol. 2015, 6, 244. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.; Yin, Y.; Chen, W.; Li, X. Association of dyslipidemia with the severity and mortality of coronavirus disease 2019 (COVID-19): A meta-analysis. Virol. J. 2021, 18, 157. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).