The Crosstalk Between Non-Coding RNAs and Lipid Metabolism in Chronic Disease Progression
Abstract
1. Introduction
2. Mechanisms and Dysregulation of Lipid Metabolism in Chronic Disease
2.1. Cardiovascular Diseases
2.2. Obesity and Type 2 Diabetes
2.3. Metabolic Dysfunction–Associated Steatotic Liver Disease (MASLD)
2.4. Neurological Disorders
2.5. Cancer
3. NcRNAs as Regulators of Lipid Metabolism in Chronic Diseases
3.1. MiRNAs
3.2. LncRNAs
3.3. CircRNAs
3.4. Other Subclasses of ncRNAs
4. NcRNA-Lipid Interaction Networks Across Organ Systems
4.1. Liver
4.2. Adipose Tissue
4.3. Brain
4.4. Vasculature
4.5. Kidney
4.6. Cross-Study Comparative Analysis of ncRNA–Lipid Associations Across Organ Systems
5. Therapeutic Targeting Strategies, Biomarker Potential and Delivery Technologies of ncRNAs in Lipid-Related Chronic Diseases
5.1. Therapeutic Targeting Strategies
5.1.1. Antisense Oligonucleotides (ASOs) and antagomiRs
5.1.2. MiRNA Mimics and Inhibitors
5.1.3. Multi-Omics and Gene-Editing Approaches
5.2. Biomarker Potential
5.3. Delivery Technologies
5.3.1. Lipid Nanoparticles (LNPs)
5.3.2. GalNAc-Conjugates
5.3.3. Engineered EVs
5.3.4. Aptamer-Functionalized Systems
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gyamfi, D.; Awuah, E.O.; Owusu, S. Lipid Metabolism: An Overview. Mol. Nutr. Fats 2019, 17–32. [Google Scholar] [CrossRef]
- Bruce, K.D.; Tang, M.; Reigan, P.; Eckel, R.H. Genetic Variants of Lipoprotein Lipase and Regulatory Factors Associated with Alzheimer’s Disease Risk. Int. J. Mol. Sci. 2020, 21, 8338. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Parton, R.G. Not Just Fat: The Structure and Function of the Lipid Droplet. Cold Spring Harb. Perspect. Biol. 2011, 3, a004838. [Google Scholar] [CrossRef] [PubMed]
- Wymann, M.P.; Schneiter, R. Lipid Signalling in Disease. Nat. Rev. Mol. Cell Biol. 2008, 9, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Bidooki, S.H.; Spitzer, L.; Petitpas, A.; Sánchez-Marco, J.; Martínez-Beamonte, R.; Lasheras, R.; Pellerin, V.; Rodríguez-Yoldi, M.J.; Navarro, M.A.; Osada, J. Chitosan Nanoparticles, a Novel Drug Delivery System to Transfer Squalene for Hepatocyte Stress Protection. ACS Omega 2024, 9, 51379–51393. [Google Scholar] [CrossRef]
- Song, R.; Hu, M.; Qin, X.; Qiu, L.; Wang, P.; Zhang, X.; Liu, R.; Wang, X. The Roles of Lipid Metabolism in the Pathogenesis of Chronic Diseases in the Elderly. Nutrients 2023, 15, 3433. [Google Scholar] [CrossRef]
- Siddique, M.M.; Li, Y.; Chaurasia, B.; Kaddai, V.A.; Summers, S.A. Dihydroceramides: From Bit Players to Lead Actors. J. Biol. Chem. 2015, 290, 15371–15379. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Transcriptional Mediators of Lipid Homeostasis. In Proceedings of the Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 2002; Volume 67, pp. 491–498. [Google Scholar]
- Priest, C.; Tontonoz, P. Inter-Organ Cross-Talk in Metabolic Syndrome. Nat. Metab. 2019, 1, 1177–1188. [Google Scholar] [CrossRef]
- Van Herpen, N.A.; Schrauwen-Hinderling, V. Lipid Accumulation in Non-Adipose Tissue and Lipotoxicity. Physiol. Behav. 2008, 94, 231–241. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, Y.; Nabavi, S.M.; Sahebkar, A.; Little, P.J.; Xu, S.; Weng, J.; Ge, J. Mechanisms of Oxidized LDL-Mediated Endothelial Dysfunction and Its Consequences for the Development of Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 925923. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Kuschner, C.E.; Kazmi, J.; Mcdevitt, L.; Espin, B.B.; Essaihi, M.; Nishikimi, M.; Becker, L.B.; Kim, J. The Role of Phospholipid Alterations in Mitochondrial and Brain Dysfunction after Cardiac Arrest. Int. J. Mol. Sci. 2024, 25, 4645. [Google Scholar] [CrossRef]
- Bidooki, S.H.; Barranquero, C.; Sánchez-Marco, J.; Martínez-Beamonte, R.; Rodríguez-Yoldi, M.J.; Navarro, M.A.; Fernandes, S.C.M.; Osada, J. TXNDC5 Plays a Crucial Role in Regulating Endoplasmic Reticulum Activity through Different ER Stress Signaling Pathways in Hepatic Cells. Int. J. Mol. Sci. 2024, 25, 7128. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wei, Y.; Pagliassotti, M.J. Saturated Fatty Acids Promote Endoplasmic Reticulum Stress and Liver Injury in Rats with Hepatic Steatosis. Endocrinology 2006, 147, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Zayani, Z.; Matinahmadi, A.; Tavakolpournegari, A.; Bidooki, S.H. Exploring Stressors: Impact on Cellular Organelles and Implications for Cellular Functions. Stresses 2025, 5, 26. [Google Scholar] [CrossRef]
- Glass, C.K.; Olefsky, J.M. Inflammation and Lipid Signaling in the Etiology of Insulin Resistance. Cell Metab. 2012, 15, 635–645. [Google Scholar] [CrossRef]
- Esteller, M. Non-Coding RNAs in Human Disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Gholamzad, A.; Khakpour, N.; Khosroshahi, E.M.; Asadi, S.; Koohpar, Z.K.; Matinahmadi, A.; Jebali, A.; Rashidi, M.; Hashemi, M.; Sadi, F.H. Cancer Stem Cells: The Important Role of CD Markers, Signaling Pathways, and MicroRNAs. Pathol.-Res. Pract. 2024, 256, 155227. [Google Scholar] [CrossRef]
- Hashemi, M.; Daneii, P.; Asadalizadeh, M.; Tabari, K.; Matinahmadi, A.; Bidoki, S.S.; Motlagh, Y.S.M.; Jafari, A.M.; Ghorbani, A.; Dehghanpour, A. Epigenetic Regulation of Hepatocellular Carcinoma Progression: MicroRNAs as Therapeutic, Diagnostic and Prognostic Factors. Int. J. Biochem. Cell Biol. 2024, 170, 106566. [Google Scholar] [CrossRef]
- Salzman, J. Circular RNA Expression: Its Potential Regulation and Function. Trends Genet. 2016, 32, 309–316. [Google Scholar] [CrossRef]
- Wilczynska, A.; Bushell, M. The Complexity of MiRNA-Mediated Repression. Cell Death Differ. 2015, 22, 22–33. [Google Scholar] [CrossRef]
- Moore, K.J.; Rayner, K.J.; Suárez, Y.; Fernández-Hernando, C. The Role of MicroRNAs in Cholesterol Efflux and Hepatic Lipid Metabolism. Annu. Rev. Nutr. 2011, 31, 49–63. [Google Scholar] [CrossRef]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Park, J.G.; Kim, G.; Jang, S.Y.; Lee, Y.R.; Lee, E.; Lee, H.W.; Han, M.-H.; Chun, J.M.; Han, Y.S.; Yoon, J.S. Plasma Long Noncoding RNA LeXis Is a Potential Diagnostic Marker for Non-Alcoholic Steatohepatitis. Life 2020, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Zayani, Z.; Hooshmandi, E.; Borhani-Haghighi, A.; Rahimi, M.; Ostovan, V.R.; Fadakar, N.; Tabrizi, R.; Bayat, M.; Hojati, S.S.; Gharbi, N. Diagnostic Potential of LncRNAs-ANRIL and MIAT in the Blood of Patients with Cerebral Venous Thrombosis. Curr. J. Neurol. 2024, 23, 117. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The Biogenesis, Biology and Characterization of Circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Le Lay, S.; Scherer, P.E. Exploring Adipose Tissue-Derived Extracellular Vesicles in Inter-Organ Crosstalk: Implications for Metabolic Regulation and Adipose Tissue Function. Cell Rep. 2025, 44, 115732. [Google Scholar] [CrossRef]
- Grillone, K.; Caridà, G.; Luciano, F.; Cordua, A.; Di Martino, M.T.; Tagliaferri, P.; Tassone, P. A Systematic Review of Non-Coding RNA Therapeutics in Early Clinical Trials: A New Perspective against Cancer. J. Transl. Med. 2024, 22, 731. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA Therapeutics: Towards a New Era for the Management of Cancer and Other Diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Q.; Meng, Y.; Zhao, T.; Mu, C.; Fu, C.; Deng, C.; Feng, J.; Du, S.; Liu, W. Saturated Fatty Acids Increase LPI to Reduce FUNDC1 Dimerization and Stability and Mitochondrial Function. EMBO Rep. 2023, 24, e54731. [Google Scholar] [CrossRef]
- Jimenez, A.G.; Winward, J.D.; Walsh, K.E.; Champagne, A.M. Effects of Membrane Fatty Acid Composition on Cellular Metabolism and Oxidative Stress in Dermal Fibroblasts from Small and Large Breed Dogs. J. Exp. Biol. 2020, 223, jeb221804. [Google Scholar] [CrossRef]
- Niki, E. Lipid Peroxidation: Physiological Levels and Dual Biological Effects. Free Radic. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J. Oxy Radicals, Lipid Peroxidation and DNA Damage. Toxicology 2002, 181, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, L.; Rong, S.; Qu, H.; Zhang, Y.; Chang, D.; Pan, H.; Wang, W. Relation between Gastric Cancer and Protein Oxidation, DNA Damage, and Lipid Peroxidation. Oxid. Med. Cell Longev. 2013, 2013, 543760. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Shaw, J.L.; Haigis, M.C.; Greka, A. Lipid Metabolism in Sickness and in Health: Emerging Regulators of Lipotoxicity. Mol. Cell 2021, 81, 3708–3730. [Google Scholar] [CrossRef]
- Gofman, J.W.; Lindgren, F.; Elliott, H.; Mantz, W.; Hewitt, J.; Strisower, B.; Herring, V.; Lyon, T.P. The Role of Lipids and Lipoproteins in Atherosclerosis. Science 1950, 111, 166–186. [Google Scholar] [CrossRef]
- Kang, N.; Ji, Z.; Li, Y.; Gao, J.; Wu, X.; Zhang, X.; Duan, Q.; Zhu, C.; Xu, Y.; Wen, L. Metabolite-derived Damage-associated Molecular Patterns in Immunological Diseases. FEBS J. 2024, 291, 2051–2067. [Google Scholar] [CrossRef]
- Sun, T.; Chen, M.; Shen, H.; PingYin; Fan, L.; Chen, X.; Wu, J.; Xu, Z.; Zhang, J. Predictive Value of LDL/HDL Ratio in Coronary Atherosclerotic Heart Disease. BMC Cardiovasc. Disord. 2022, 22, 273. [Google Scholar] [CrossRef]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic Insights into the Oxidized Low-density Lipoprotein-induced Atherosclerosis. Oxid. Med. Cell Longev. 2020, 2020, 5245308. [Google Scholar] [CrossRef]
- Berberich, A.J.; Hegele, R.A. A Modern Approach to Dyslipidemia. Endocr. Rev. 2022, 43, 611–653. [Google Scholar] [CrossRef]
- Paquette, M.; Carrié, A.; Bernard, S.; Cariou, B.; Hegele, R.A.; Genest, J.; Trinder, M.; Brunham, L.R.; Béliard, S.; Baass, A. Effect of the LDL Receptor Mutation Type on Incident Major Adverse Cardiovascular Events in Familial Hypercholesterolaemia. Eur. J. Prev. Cardiol. 2022, 29, 2125–2131. [Google Scholar] [CrossRef]
- Besler, K.J.; Blanchard, V.; Francis, G.A. Lysosomal Acid Lipase Deficiency: A Rare Inherited Dyslipidemia but Potential Ubiquitous Factor in the Development of Atherosclerosis and Fatty Liver Disease. Front. Genet. 2022, 13, 1013266. [Google Scholar] [CrossRef]
- Rehman, W.U.; Yarkoni, M.; Ilyas, M.A.; Athar, F.; Javaid, M.; Ehsan, M.; Khalid, M.T.; Pasha, A.; Selma, A.B.; Yarkoni, A. Cholesteryl Ester Transfer Protein Inhibitors and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. J. Cardiovasc. Dev. Dis. 2024, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Abuobeid, R.; Herrera-Marcos, L.V.; Arnal, C.; Bidooki, S.H.; Sánchez-Marco, J.; Lasheras, R.; Surra, J.C.; Rodríguez-Yoldi, M.J.; Martínez-Beamonte, R.; Osada, J. Differentially Expressed Genes in Response to a Squalene-Supplemented Diet Are Accurate Discriminants of Porcine Non-Alcoholic Steatohepatitis. Int. J. Mol. Sci. 2023, 24, 12552. [Google Scholar] [CrossRef] [PubMed]
- Stoekenbroek, R.M.; Hartgers, M.L.; Rutte, R.; de Wijer, D.D.; Stroes, E.S.G.; Hovingh, G.K. PCSK9 Inhibitors in Clinical Practice: Delivering on the Promise? Atherosclerosis 2018, 270, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kiani, P.; Khodadadi, E.S.; Nikdasti, A.; Yarahmadi, S.; Gheibi, M.; Yousefi, Z.; Ehtiati, S.; Yahyazadeh, S.; Shafiee, S.M.; Taghizadeh, M. Autophagy and the Peroxisome Proliferator-Activated Receptor Signaling Pathway: A Molecular Ballet in Lipid Metabolism and Homeostasis. Mol. Cell Biochem. 2025, 480, 3477–3499. [Google Scholar] [CrossRef]
- Coutinho-Wolino, K.S.; Almeida, P.P.; Mafra, D.; Stockler-Pinto, M.B. Bioactive Compounds Modulating Toll-like 4 Receptor (TLR4)-Mediated Inflammation: Pathways Involved and Future Perspectives. Nutr. Res. 2022, 107, 96–116. [Google Scholar] [CrossRef]
- Tong, Y.; Xu, S.; Huang, L.; Chen, C. Obesity and Insulin Resistance: Pathophysiology and Treatment. Drug Discov. Today 2022, 27, 822–830. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose Tissue and Insulin Resistance in Obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Chait, A.; Den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 522637. [Google Scholar] [CrossRef]
- Bou Matar, D.; Zhra, M.; Nassar, W.K.; Altemyatt, H.; Naureen, A.; Abotouk, N.; Elahi, M.A.; Aljada, A. Adipose Tissue Dysfunction Disrupts Metabolic Homeostasis: Mechanisms Linking Fat Dysregulation to Disease. Front. Endocrinol. 2025, 16, 1592683. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Youm, Y.-H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 Inflammasome Instigates Obesity-Induced Inflammation and Insulin Resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Chen, B.; Li, T.; Wu, Y.; Song, L.; Wang, Y.; Bian, Y.; Qiu, Y.; Yang, Z. Lipotoxicity: A New Perspective in Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2025, 18, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Bidooki, S.H.; Navarro, M.A.; Fernandes, S.C.M.; Osada, J. Thioredoxin Domain Containing 5 (TXNDC5): Friend or Foe? Curr. Issues Mol. Biol. 2024, 46, 3134–3163. [Google Scholar] [CrossRef] [PubMed]
- Aon, M.A.; Bhatt, N.; Cortassa, S.C. Mitochondrial and Cellular Mechanisms for Managing Lipid Excess. Front. Physiol. 2014, 5, 282. [Google Scholar] [CrossRef] [PubMed]
- Bidooki, S.H.; Quero, J.; Sánchez-Marco, J.; Herrero-Continente, T.; Marmol, I.; Lasheras, R.; Sebastian, V.; Arruebo, M.; Osada, J.; Rodriguez-Yoldi, M.J. Squalene in Nanoparticles Improves Antiproliferative Effect on Human Colon Carcinoma Cells Through Apoptosis by Disturbances in Redox Balance. Int. J. Mol. Sci. 2024, 25, 13048. [Google Scholar] [CrossRef]
- Vasselli, J.R.; Scarpace, P.J.; Harris, R.B.S.; Banks, W.A. Dietary Components in the Development of Leptin Resistance. Adv. Nutr. 2013, 4, 164–175. [Google Scholar] [CrossRef]
- Luo, L.; Liu, M. Adiponectin: Friend or Foe in Obesity and Inflammation. Med. Rev. 2022, 2, 349–362. [Google Scholar] [CrossRef]
- Guo, X.; Yin, X.; Liu, Z.; Wang, J. Non-Alcoholic Fatty Liver Disease (NAFLD) Pathogenesis and Natural Products for Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 15489. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, S.; Pan, B.; Xie, W.; Meng, J. Ectopic and Visceral Fat Deposition in Aging, Obesity, and Idiopathic Pulmonary Fibrosis: An Interconnected Role. Lipids Health Dis. 2023, 22, 201. [Google Scholar] [CrossRef]
- Bidooki, S.H.; Alejo, T.; Sánchez-Marco, J.; Martínez-Beamonte, R.; Abuobeid, R.; Burillo, J.C.; Lasheras, R.; Sebastian, V.; Rodríguez-Yoldi, M.J.; Arruebo, M. Squalene Loaded Nanoparticles Effectively Protect Hepatic AML12 Cell Lines against Oxidative and Endoplasmic Reticulum Stress in a TXNDC5-Dependent Way. Antioxidants 2022, 11, 581. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Porthan, K.; Ahlholm, N.; Rosqvist, F.; Dufour, S.; Zhang, X.-M.; Lehtimäki, T.E.; Seppänen, W.; Orho-Melander, M.; Hodson, L. The PNPLA3 I148M Variant Increases Ketogenesis and Decreases Hepatic de Novo Lipogenesis and Mitochondrial Function in Humans. Cell Metab. 2023, 35, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Caon, E.; Martins, M.; Hodgetts, H.; Blanken, L.; Vilia, M.G.; Levi, A.; Thanapirom, K.; Al-Akkad, W.; Abu-Hanna, J.; Baselli, G. Exploring the Impact of the PNPLA3 I148M Variant on Primary Human Hepatic Stellate Cells Using 3D Extracellular Matrix Models. J. Hepatol. 2024, 80, 941–956. [Google Scholar] [CrossRef] [PubMed]
- Bidooki, S.H.; Sánchez-Marco, J.; Martínez-Beamonte, R.; Herrero-Continente, T.; Navarro, M.A.; Rodríguez-Yoldi, M.J.; Osada, J. Endoplasmic Reticulum Protein TXNDC5 Interacts with PRDX6 and HSPA9 to Regulate Glutathione Metabolism and Lipid Peroxidation in the Hepatic AML12 Cell Line. Int. J. Mol. Sci. 2023, 24, 17131. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, O. Hypoxic Signaling and Cholesterol Lipotoxicity in Fatty Liver Disease Progression. Oxid. Med. Cell Longev. 2018, 2018, 2548154. [Google Scholar] [CrossRef]
- Xu, G.-X.; Wei, S.; Yu, C.; Zhao, S.-Q.; Yang, W.-J.; Feng, Y.-H.; Pan, C.; Yang, K.-X.; Ma, Y. Activation of Kupffer Cells in NAFLD and NASH: Mechanisms and Therapeutic Interventions. Front. Cell Dev. Biol. 2023, 11, 1199519. [Google Scholar] [CrossRef]
- Lei, Y.-M.; Yan, R.; Gao, Y.-D.; Yang, H.-J.; Bi, H.-Y.; Duan, Y.-Q. Cholesterol Crystals Activate NLRP3 Inflammasomes and Promote Gallstone Formation by Increasing Mucin Secretion. Biotech. Histochem. 2022, 97, 546–553. [Google Scholar] [CrossRef]
- Dixon, L.J.; Barnes, M.; Tang, H.; Pritchard, M.T.; Nagy, L.E. Kupffer cells in the liver. Compr. Physiol. 2013, 3, 785–797. [Google Scholar] [CrossRef]
- Mesa-Herrera, F.; Taoro-González, L.; Valdés-Baizabal, C.; Diaz, M.; Marín, R. Lipid and Lipid Raft Alteration in Aging and Neurodegenerative Diseases: A Window for the Development of New Biomarkers. Int. J. Mol. Sci. 2019, 20, 3810. [Google Scholar] [CrossRef]
- Kanekiyo, T.; Xu, H.; Bu, G. ApoE and Aβ in Alzheimer’s Disease: Accidental Encounters or Partners? Neuron 2014, 81, 740–754. [Google Scholar] [CrossRef]
- Jazvinšćak Jembrek, M.; Hof, P.R.; Šimić, G. Ceramides in Alzheimer’s Disease: Key Mediators of Neuronal Apoptosis Induced by Oxidative Stress and Aβ Accumulation. Oxid. Med. Cell Longev. 2015, 2015, 346783. [Google Scholar] [CrossRef]
- Rushworth, J.V.; Hooper, N.M. Lipid Rafts: Linking Alzheimer′ s Amyloid-β Production, Aggregation, and Toxicity at Neuronal Membranes. Int. J. Alzheimers Dis. 2011, 2011, 603052. [Google Scholar] [CrossRef] [PubMed]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Ma, H.; Yang, Y.; Liao, Y.; Lin, C.; Zheng, J.; Yu, M.; Lan, J. Microglia in Alzheimer’s Disease: Pathogenesis, Mechanisms, and Therapeutic Potentials. Front. Aging Neurosci. 2023, 15, 1201982. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M.M. The Role of Oxidative Stress in Parkinson’s Disease. J. Parkinsons Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef]
- Alkhalifa, A.E.; Alkhalifa, O.; Durdanovic, I.; Ibrahim, D.R.; Maragkou, S. Oxidative Stress and Mitochondrial Dysfunction in Alzheimer’s Disease: Insights into Pathophysiology and Treatment. J. Dement. Alzheimer’s Dis. 2025, 2, 17. [Google Scholar] [CrossRef]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y. Lipid Metabolism in Cancer Progression and Therapeutic Strategies. MedComm 2021, 2, 27–59. [Google Scholar] [CrossRef]
- Latasa, M.-J.; Moon, Y.S.; Kim, K.-H.; Sul, H.S. Nutritional Regulation of the Fatty Acid Synthase Promoter in Vivo: Sterol Regulatory Element Binding Protein Functions through an Upstream Region Containing a Sterol Regulatory Element. Proc. Natl. Acad. Sci. USA 2000, 97, 10619–10624. [Google Scholar]
- Tracz-Gaszewska, Z.; Sowka, A.; Dobrzyn, P. Stearoyl-CoA Desaturase 1 Inhibition Impairs Triacylglycerol Accumulation and Lipid Droplet Formation in Colorectal Cancer Cells. J. Cell Physiol. 2023, 238, 2888–2903. [Google Scholar] [CrossRef]
- Bensaad, K.; Favaro, E.; Lewis, C.A.; Peck, B.; Lord, S.; Collins, J.M.; Pinnick, K.E.; Wigfield, S.; Buffa, F.M.; Li, J.-L. Fatty Acid Uptake and Lipid Storage Induced by HIF-1α Contribute to Cell Growth and Survival after Hypoxia-Reoxygenation. Cell Rep. 2014, 9, 349–365. [Google Scholar] [CrossRef]
- Jarc, E.; Petan, T. Lipid Droplets and the Management of Cellular Stress. Yale J. Biol. Med. 2019, 92, 435. [Google Scholar]
- Sánchez-Marco, J.; Bidooki, S.H.; Abuobeid, R.; Barranquero, C.; Herrero-Continente, T.; Arnal, C.; Martínez-Beamonte, R.; Lasheras, R.; Surra, J.C.; Navarro, M.A. Thioredoxin Domain Containing 5 Is Involved in the Hepatic Storage of Squalene into Lipid Droplets in a Sex-Specific Way. J. Nutr. Biochem. 2024, 124, 109503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Morris, R.J.; Bode, A.M.; Zhang, T. Prostaglandin Pathways: Opportunities for Cancer Prevention and Therapy. Cancer Res. 2022, 82, 949–965. [Google Scholar] [CrossRef] [PubMed]
- Rolin, J.; Maghazachi, A.A. Effects of Lysophospholipids on Tumor Microenvironment. Cancer Microenviron. 2011, 4, 393–403. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Z.; Xie, M.; Ding, F.; Zheng, X.; Sun, S.; Du, J. Exploring Tumor-Associated Macrophages in Glioblastoma: From Diversity to Therapy. NPJ Precis. Oncol. 2025, 9, 126. [Google Scholar] [CrossRef]
- Balaban, S.; Shearer, R.F.; Lee, L.S.; van Geldermalsen, M.; Schreuder, M.; Shtein, H.C.; Cairns, R.; Thomas, K.C.; Fazakerley, D.J.; Grewal, T. Adipocyte Lipolysis Links Obesity to Breast Cancer Growth: Adipocyte-Derived Fatty Acids Drive Breast Cancer Cell Proliferation and Migration. Cancer Metab. 2017, 5, 1. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Ding, K. Roles of Exosomes in Cancer Chemotherapy Resistance, Progression, Metastasis and Immunity, and Their Clinical Applications. Int. J. Oncol. 2021, 59, 44. [Google Scholar] [CrossRef]
- Jin, H.-R.; Wang, J.; Wang, Z.-J.; Xi, M.-J.; Xia, B.-H.; Deng, K.; Yang, J.-L. Lipid Metabolic Reprogramming in Tumor Microenvironment: From Mechanisms to Therapeutics. J. Hematol. Oncol. 2023, 16, 103. [Google Scholar] [CrossRef]
- Peschansky, V.J.; Wahlestedt, C. Non-Coding RNAs as Direct and Indirect Modulators of Epigenetic Regulation. Epigenetics 2014, 9, 3–12. [Google Scholar] [CrossRef]
- Mirzaei, S.; Paskeh, M.D.A.; Entezari, M.; Bidooki, S.H.; Ghaleh, V.J.; Rezaei, S.; Hejazi, E.S.; Kakavand, A.; Behroozaghdam, M.; Movafagh, A. SiRNA and Targeted Delivery Systems in Breast Cancer Therapy. Clin. Transl. Oncol. 2023, 25, 1167–1188. [Google Scholar] [CrossRef]
- Kaikkonen, M.U.; Lam, M.T.Y.; Glass, C.K. Non-Coding RNAs as Regulators of Gene Expression and Epigenetics. Cardiovasc. Res. 2011, 90, 430–440. [Google Scholar] [CrossRef]
- Liu, C.-X.; Chen, L.-L. Circular RNAs: Characterization, Cellular Roles, and Applications. Cell 2022, 185, 2016–2034. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Weitz, S.H.; Gong, M.; Barr, I.; Weiss, S.; Guo, F. Processing of MicroRNA Primary Transcripts Requires Heme in Mammalian Cells. Proc. Natl. Acad. Sci. USA 2014, 111, 1861–1866. [Google Scholar] [CrossRef]
- Tavakolpournegari, A.; Hashemi, M.; Karizi, S.Z.; Ahmadi, A.M.; Bidooki, S.H.; Banaei, G. Expression Patterns of MiR181a and MiR30d in Patients with Breast Cancer. Iran. J. Public. Health 2022, 51, 1594. [Google Scholar] [CrossRef]
- Santhekadur, P.K.; Kumar, D.P. RISC Assembly and Post-Transcriptional Gene Regulation in Hepatocellular Carcinoma. Genes. Dis. 2020, 7, 199–204. [Google Scholar] [CrossRef]
- Wu, Y.; Song, Y.; Xiong, Y.; Wang, X.; Xu, K.; Han, B.; Bai, Y.; Li, L.; Zhang, Y.; Zhou, L. MicroRNA-21 (Mir-21) Promotes Cell Growth and Invasion by Repressing Tumor Suppressor PTEN in Colorectal Cancer. Cell. Physiol. Biochem. 2017, 43, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, J.-X.; Shao, Z.-Q. MiR-21 Targets and Inhibits Tumor Suppressor Gene PTEN to Promote Prostate Cancer Cell Proliferation and Invasion: An Experimental Study. Asian Pac. J. Trop. Med. 2017, 10, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Zhao, F.; Liu, Q.; Jiang, K.E.; Yang, G. MicroRNA-21 (MiR-21) Represses Tumor Suppressor PTEN and Promotes Growth and Invasion in Non-Small Cell Lung Cancer (NSCLC). Clin. Chim. Acta 2010, 411, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Z.; O’Loughlin, E.; Wang, L.; Fan, X.; Lai, E.C.; Yi, R. MicroRNA-205 Controls Neonatal Expansion of Skin Stem Cells by Modulating the PI (3) K Pathway. Nat. Cell Biol. 2013, 15, 1153–1163. [Google Scholar] [CrossRef]
- Huang, C.; Azizi, P.; Vazirzadeh, M.; Aghaei-Zarch, S.M.; Aghaei-Zarch, F.; Ghanavi, J.; Farnia, P. Non-Coding RNAs/DNMT3B Axis in Human Cancers: From Pathogenesis to Clinical Significance. J. Transl. Med. 2023, 21, 621. [Google Scholar] [CrossRef]
- Saviana, M.; Le, P.; Micalo, L.; Del Valle-Morales, D.; Romano, G.; Acunzo, M.; Li, H.; Nana-Sinkam, P. Crosstalk between MiRNAs and DNA Methylation in Cancer. Genes 2023, 14, 1075. [Google Scholar] [CrossRef]
- Aryal, B.; Singh, A.K.; Rotllan, N.; Price, N.; Fernández-Hernando, C. MicroRNAs and Lipid Metabolism. Curr. Opin. Lipidol. 2017, 28, 273–280. [Google Scholar] [CrossRef]
- You, Z.; Wu, F.; Zheng, Y.; Yang, H.; Ye, J.; Cai, H.; Luo, C.; Liu, Y.; Ke, Y.; Xu, X. MiR-139-5p Activates Ferroptosis by Inhibiting the Expression of HMG-CoA Reductase to Inhibit the Progression of Glioma. Cell Death Discov. 2025, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yeung, A.W.K.; Atanasov, A.G. A Review: Molecular Mechanism of Regulation of ABCA1 Expression. Curr. Protein Pept. Sci. 2022, 23, 170–191. [Google Scholar] [CrossRef] [PubMed]
- Niculae, A.M.; Dobre, M.; Herlea, V.; Vasilescu, F.; Ceafalan, L.C.; Trandafir, B.; Milanesi, E.; Hinescu, M.E. Lipid Handling Protein Gene Expression in Colorectal Cancer: CD36 and Targeting MiRNAs. Life 2022, 12, 2127. [Google Scholar] [CrossRef] [PubMed]
- Sundrani, D.P.; Karkhanis, A.R.; Joshi, S.R. Peroxisome Proliferator-Activated Receptors (PPAR), Fatty Acids and MicroRNAs: Implications in Women Delivering Low Birth Weight Babies. Syst. Biol. Reprod. Med. 2021, 67, 24–41. [Google Scholar] [CrossRef]
- Salman, M.; Kamel, M.A.; El-Nabi, S.E.H.; Ismail, A.H.A.; Ullah, S.; Al-Ghamdi, A.; Hathout, H.M.R.; El-Garawani, I.M. The Regulation of HBP1, SIRT1, and SREBP-1c Genes and the Related MicroRNAs in Non-Alcoholic Fatty Liver Rats: The Association with the Folic Acid Anti-Steatosis. PLoS ONE 2022, 17, e0265455. [Google Scholar] [CrossRef]
- Ru, L.; Wang, X.; Niu, J. The MiR-23–27–24 Cluster: An Emerging Target in NAFLD Pathogenesis. Acta Pharmacol. Sin. 2022, 43, 1167–1179. [Google Scholar] [CrossRef]
- Holvoet, P. Non-Coding RNAs Related to Lipid Metabolism and Non-Alcoholic Fatty Liver Disease. In Non-Coding RNAs at the Cross-Road of Cardiometabolic Diseases and Cancer; Springer: Berlin/Heidelberg, Germany, 2021; pp. 73–88. [Google Scholar]
- Yu, Y.; Du, H.; Wei, S.; Feng, L.; Li, J.; Yao, F.; Zhang, M.; Hatch, G.M.; Chen, L. Adipocyte-Derived Exosomal MiR-27a Induces Insulin Resistance in Skeletal Muscle through Repression of PPARγ. Theranostics 2018, 8, 2171. [Google Scholar] [CrossRef]
- Ando, Y.; Yamazaki, M.; Yamada, H.; Munetsuna, E.; Fujii, R.; Mizuno, G.; Ichino, N.; Osakabe, K.; Sugimoto, K.; Ishikawa, H. Association of Circulating MiR-20a, MiR-27a, and MiR-126 with Non-Alcoholic Fatty Liver Disease in General Population. Sci. Rep. 2019, 9, 18856. [Google Scholar] [CrossRef]
- Ignačáková, H. Therapeutic Targeting of MicroRNA-21 and Bile Acid-Activated Receptors in Non-Alcoholic Fatty Liver Disease. Master’s Thesis, Universidade de Lisboa, Lisboa, Portugal, 2021. [Google Scholar]
- Liu, M.; Lu, T.; Bai, Y.; Han, X.; Zhang, W.; Zhang, L.; Chen, S.; Lin, C.; Liu, C.; Yuan, C. The Critical Role of MicroRNA-21 in Non-Alcoholic Fatty Liver Disease Pathogenesis. Curr. Pharm. Des. 2023, 29, 904–913. [Google Scholar] [CrossRef]
- Holland, A.; Enrick, M.; Diaz, A.; Yin, L. Is MiR-21 A Therapeutic Target in Cardiovascular Disease? Int. J. Drug Discov. Pharmacol. 2023, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, A.; Deng, J.; Yang, Y.; Dang, L.; Ye, Y.; Li, Y.; Zhang, W. MiR-21 Attenuates Lipopolysaccharide-Induced Lipid Accumulation and Inflammatory Response: Potential Role in Cerebrovascular Disease. Lipids Health Dis. 2014, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Thavarajah, T.; Gu, W.; Cai, J.; Xu, Q. Impact of MiRNA in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e159–e170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Deng, F.; Zhang, Y.; Zhang, X.; Chen, J.; Jiang, Y. PPARγ Attenuates Hepatic Inflammation and Oxidative Stress of Non-Alcoholic Steatohepatitis via Modulating the MiR-21-5p/SFRP5 Pathway. Mol. Med. Rep. 2021, 24, 823. [Google Scholar] [CrossRef]
- Bertran, L.; Portillo-Carrasquer, M.; Barrientos-Riosalido, A.; Aguilar, C.; Riesco, D.; Martínez, S.; Culebradas, A.; Vives, M.; Sabench, F.; Castillo, D. Del Increased Secreted Frizzled-Related Protein 5 MRNA Expression in the Adipose Tissue of Women with Nonalcoholic Fatty Liver Disease Associated with Obesity. Int. J. Mol. Sci. 2022, 23, 9871. [Google Scholar] [CrossRef]
- Rodrigues, P.M.; Afonso, M.B.; Simão, A.L.; Islam, T.; Gaspar, M.M.; O’Rourke, C.J.; Lewinska, M.; Andersen, J.B.; Arretxe, E.; Alonso, C. MiR-21-5p Promotes NASH-related Hepatocarcinogenesis. Liver Int. 2023, 43, 2256–2274. [Google Scholar] [CrossRef]
- Yang, K.; Liu, C.; Shao, J.; Guo, L.; Wang, Q.; Meng, Z.; Jin, X.; Chen, X. Would Combination Be Better: Swimming Exercise and Intermittent Fasting Improve High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease in Obese Rats via the MiR-122-5p/SREBP-1c/CPT1A Pathway. Diabetes Metab. Syndr. Obes. 2024, 17, 1675–1686. [Google Scholar] [CrossRef]
- Qiang, J.; Tao, Y.F.; Bao, J.W.; Chen, D.J.; Li, H.X.; He, J.; Xu, P. High Fat Diet-Induced MiR-122 Regulates Lipid Metabolism and Fat Deposition in Genetically Improved Farmed Tilapia (GIFT, Oreochromis Niloticus) Liver. Front. Physiol. 2018, 9, 1422. [Google Scholar] [CrossRef]
- Abdou, S.M.; Abd El-Maksoud, A.M.; Ahmed, G.F.; Abd El-Aziz, H.G. MiRNA-122 as a Biomarker for Insulin Resistance and Risk of Cardiovascular Diseases in Obese Children. Gene Rep. 2024, 36, 101947. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, H.; Horie, T.; Burchett, J.M.; Restivo, J.L.; Rotllan, N.; Ramírez, C.M.; Verghese, P.B.; Ihara, M.; Hoe, H.-S. MicroRNA-33 Regulates ApoE Lipidation and Amyloid-β Metabolism in the Brain. J. Neurosci. 2015, 35, 14717–14726. [Google Scholar] [CrossRef] [PubMed]
- Jaouen, F.; Gascon, E. Understanding the Role of MiR-33 in Brain Lipid Metabolism: Implications for Alzheimer’s Disease. J. Neurosci. 2016, 36, 2558–2560. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-J.; Zhang, M.; Zhao, G.-J.; Fu, Y.; Zhang, D.-W.; Zhu, H.-B.; Tang, C.-K. MicroRNA-33 in Atherosclerosis Etiology and Pathophysiology. Atherosclerosis 2013, 227, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.; Liu, B.; Persaud, S.J. Effects of MiR-33 Deficiency on Metabolic and Cardiovascular Diseases: Implications for Therapeutic Intervention. Int. J. Mol. Sci. 2023, 24, 10777. [Google Scholar] [CrossRef]
- Nguyen, M.-A.; Hoang, H.-D.; Rasheed, A.; Duchez, A.-C.; Wyatt, H.; Cottee, M.L.; Graber, T.E.; Susser, L.; Robichaud, S.; Berber, İ. MiR-223 Exerts Translational Control of Proatherogenic Genes in Macrophages. Circ. Res. 2022, 131, 42–58. [Google Scholar] [CrossRef]
- Zhang, M.-W.; Shen, Y.-J.; Shi, J.; Yu, J.-G. MiR-223-3p in Cardiovascular Diseases: A Biomarker and Potential Therapeutic Target. Front. Cardiovasc. Med. 2021, 7, 610561. [Google Scholar] [CrossRef]
- Gu, J.; Xu, H.; Chen, Y.; Li, N.; Hou, X. MiR-223 as a Regulator and Therapeutic Target in Liver Diseases. Front. Immunol. 2022, 13, 860661. [Google Scholar] [CrossRef]
- Tabet, F.; Vickers, K.C.; Cuesta Torres, L.F.; Wiese, C.B.; Shoucri, B.M.; Lambert, G.; Catherinet, C.; Prado-Lourenco, L.; Levin, M.G.; Thacker, S. HDL-Transferred MicroRNA-223 Regulates ICAM-1 Expression in Endothelial Cells. Nat. Commun. 2014, 5, 3292. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Yang, L.-Y.; Zhao, D. MicroRNA-20a-5p Ameliorates Non-Alcoholic Fatty Liver Disease via Inhibiting the Expression of CD36. Front. Cell Dev. Biol. 2020, 8, 596329. [Google Scholar] [CrossRef]
- Gao, G.; Chen, W.; Liu, M.; Yan, X.; Yang, P. Circulating MicroRNAs as Novel Potential Biomarkers for Left Ventricular Remodeling in Postinfarction Heart Failure. Dis. Markers 2019, 2019, 5093803. [Google Scholar] [CrossRef]
- Ye, D.; Zhang, T.; Lou, G.; Xu, W.; Dong, F.; Chen, G.; Liu, Y. Plasma MiR-17, MiR-20a, MiR-20b and MiR-122 as Potential Biomarkers for Diagnosis of NAFLD in Type 2 Diabetes Mellitus Patients. Life Sci. 2018, 208, 201–207. [Google Scholar] [CrossRef]
- Wu, C.; Qin, N.; Ren, H.; Yang, M.; Liu, S.; Wang, Q. Metformin Regulating MiR-34a Pathway to Inhibit Egr1 in Rat Mesangial Cells Cultured with High Glucose. Int. J. Endocrinol. 2018, 2018, 6462793. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, M.; Wan, X.; Jin, X.; Chen, S.; Yu, C.; Li, Y. Effect of MiR-34a in Regulating Steatosis by Targeting PPARα Expression in Nonalcoholic Fatty Liver Disease. Sci. Rep. 2015, 5, 13729. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, Y.; Hu, S.; Pan, X.; Bawa, F.C.; Wang, H.H.; Wang, D.Q.-H.; Yin, L.; Zhang, Y. Hepatocyte MiR-34a Is a Key Regulator in the Development and Progression of Non-Alcoholic Fatty Liver Disease. Mol. Metab. 2021, 51, 101244. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Engler-Chiurazzi, E.B.; Cavendish, J.Z.; Povroznik, J.M.; Russell, A.E.; Quintana, D.D.; Mathers, P.H.; Simpkins, J.W. Over-Expression of MiR-34a Induces Rapid Cognitive Impairment and Alzheimer’s Disease-like Pathology. Brain Res. 2019, 1721, 146327. [Google Scholar] [CrossRef]
- Zheng, L.; Lv, G.; Sheng, J.; Yang, Y. Effect of MiRNA-10b in Regulating Cellular Steatosis Level by Targeting PPAR-α Expression, a Novel Mechanism for the Pathogenesis of NAFLD. J. Gastroenterol. Hepatol. 2010, 25, 156–163. [Google Scholar] [CrossRef]
- Kiran, S.; Mandal, M.; Rakib, A.; Bajwa, A.; Singh, U.P. MiR-10a-3p Modulates Adiposity and Suppresses Adipose Inflammation through TGF-Β1/Smad3 Signaling Pathway. Front. Immunol. 2023, 14, 1213415. [Google Scholar] [CrossRef]
- Wang, H.; Ma, M.; Li, Y.; Liu, J.; Sun, C.; Liu, S.; Ma, Y.; Yan, Y.; Tang, Z.; Shen, S. MiR-183 and MiR-96 Orchestrate Both Glucose and Fat Utilization in Skeletal Muscle. EMBO Rep. 2021, 22, e52247. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef]
- Wang, L.; Jia, X.-J.; Jiang, H.-J.; Du, Y.; Yang, F.; Si, S.-Y.; Hong, B. MicroRNAs 185, 96, and 223 Repress Selective High-Density Lipoprotein Cholesterol Uptake through Posttranscriptional Inhibition. Mol. Cell Biol. 2013, 33, 1956–1964. [Google Scholar] [CrossRef]
- Ramírez, C.M.; Rotllan, N.; Vlassov, A.V.; Dávalos, A.; Li, M.; Goedeke, L.; Aranda, J.F.; Cirera-Salinas, D.; Araldi, E.; Salerno, A. Control of Cholesterol Metabolism and Plasma High-Density Lipoprotein Levels by MicroRNA-144. Circ. Res. 2013, 112, 1592–1601. [Google Scholar] [CrossRef]
- Cheng, J.; Cheng, A.; Clifford, B.L.; Wu, X.; Hedin, U.; Maegdefessel, L.; Pamir, N.; Sallam, T.; Tarling, E.J.; de Aguiar Vallim, T.Q. MicroRNA-144 Silencing Protects against Atherosclerosis in Male, but Not Female Mice. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Yu, F.; Wang, Y.; Hui, Y.; Carnevale, K.; Fu, M.; Lu, H.; Fan, D. MicroRNA-155 Deficiency Results in Decreased Macrophage Inflammation and Attenuated Atherogenesis in Apolipoprotein E–Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, J.-J.; Deng, W.-Y.; Ren, K.; Yin, S.-H.; Yu, X.-H. CTRP12 Ameliorates Atherosclerosis by Promoting Cholesterol Efflux and Inhibiting Inflammatory Response via the MiR-155-5p/LXRα Pathway. Cell Death Dis. 2021, 12, 254. [Google Scholar] [CrossRef]
- González-López, P.; Ares-Carral, C.; López-Pastor, A.R.; Infante-Menéndez, J.; González Illaness, T.; Vega de Ceniga, M.; Esparza, L.; Beneit, N.; Martín-Ventura, J.L.; Escribano, Ó. Implication of MiR-155-5p and MiR-143-3p in the Vascular Insulin Resistance and Instability of Human and Experimental Atherosclerotic Plaque. Int. J. Mol. Sci. 2022, 23, 10253. [Google Scholar] [CrossRef]
- Klöting, N.; Berthold, S.; Kovacs, P.; Schön, M.R.; Fasshauer, M.; Ruschke, K.; Stumvoll, M.; Blüher, M. MicroRNA Expression in Human Omental and Subcutaneous Adipose Tissue. PLoS ONE 2009, 4, e4699. [Google Scholar] [CrossRef]
- Liu, J.; Long, Y.; Xu, P.; Guo, H.; Cui, G. Pathogenesis of MiR-155 on Nonmodifiable and Modifiable Risk Factors in Alzheimer’s Disease. Alzheimers Res. Ther. 2023, 15, 122. [Google Scholar] [CrossRef]
- Massart, J.; Sjögren, R.J.O.; Lundell, L.S.; Mudry, J.M.; Franck, N.; O’Gorman, D.J.; Egan, B.; Zierath, J.R.; Krook, A. Altered MiR-29 Expression in Type 2 Diabetes Influences Glucose and Lipid Metabolism in Skeletal Muscle. Diabetes 2017, 66, 1807–1818. [Google Scholar] [CrossRef]
- Wu, P.; Wang, Q.; Jiang, C.; Chen, C.; Liu, Y.; Chen, Y.; Zeng, Y. MicroRNA-29a Is Involved Lipid Metabolism Dysfunction and Insulin Resistance in C2C12 Myotubes by Targeting PPARδ. Mol. Med. Rep. 2018, 17, 8493–8501. [Google Scholar] [CrossRef]
- Mahdy, M.M.; El-Ekiaby, N.M.; Hashish, R.M.; Salah, R.A.; Hanafi, R.S.; Azzazy, H.M.E.-S.; Abdelaziz, A.I. MiR-29a Promotes Lipid Droplet and Triglyceride Formation in HCV Infection by Inducing Expression of SREBP-1c and CaV1. J. Clin. Transl. Hepatol. 2016, 4, 293–299. [Google Scholar]
- Liu, M.; Gao, M.; Li, C.; Yu, C.; Yan, H.; Peng, C.; Li, Y.; Li, C.; Ma, Z.; Zhao, Y. Dicer1/MiR-29/HMGCR Axis Contributes to Hepatic Free Cholesterol Accumulation in Mouse Non-Alcoholic Steatohepatitis. Acta Pharmacol. Sin. 2017, 38, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-L.; Wang, P.-W.; Wang, F.-S.; Lin, H.-Y.; Huang, Y.-H. MiR-29a Modulates GSK3β/SIRT1-Linked Mitochondrial Proteostatic Stress to Ameliorate Mouse Non-Alcoholic Steatohepatitis. Int. J. Mol. Sci. 2020, 21, 6884. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hou, J.; Ye, L.; Chen, Y.; Cui, J.; Tian, W.; Li, C.; Liu, L. MicroRNA-143 Regulates Adipogenesis by Modulating the MAP2K5–ERK5 Signaling. Sci. Rep. 2014, 4, 3819. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dong, Y.; Hu, S.; Liu, T. MiR-143-3p/FNDC5 Axis: A Novel Regulator of Insulin Sensitivity. Endocrine 2024, 83, 368–377. [Google Scholar] [CrossRef]
- Blumensatt, M.; Wronkowitz, N.; Wiza, C.; Cramer, A.; Mueller, H.; Rabelink, M.J.; Hoeben, R.C.; Eckel, J.; Sell, H.; Ouwens, D.M. Adipocyte-Derived Factors Impair Insulin Signaling in Differentiated Human Vascular Smooth Muscle Cells via the Upregulation of MiR-143. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 275–283. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Xie, H.-L.; Yang, Y.-W.; Wen, J.; Liu, R.-R.; Zhao, G.-P.; Tan, X.-D.; Liu, Z.; Zheng, Y.; Zhang, J.-B. MiR-375 Upregulates Lipid Metabolism and Inhibits Cell Proliferation Involved in Chicken Fatty Liver Formation and Inheritance via Targeting Recombination Signal Binding Protein for Immunoglobulin Kappa J Region (RBPJ). Poult. Sci. 2023, 102, 102218. [Google Scholar] [CrossRef]
- García-Jacobo, R.E.; Uresti-Rivera, E.E.; Portales-Pérez, D.P.; González-Amaro, R.; Lara-Ramírez, E.E.; Enciso-Moreno, J.A.; García-Hernández, M.H. Circulating MiR-146a, MiR-34a and MiR-375 in Type 2 Diabetes Patients, Pre-diabetic and Normal-glycaemic Individuals in Relation to Β-cell Function, Insulin Resistance and Metabolic Parameters. Clin. Exp. Pharmacol. Physiol. 2019, 46, 1092–1100. [Google Scholar] [CrossRef]
- Lei, L.; Zhou, C.; Yang, X.; Li, L. Down-regulation of Micro RNA-375 Regulates Adipokines and Inhibits Inflammatory Cytokines by Targeting AdipoR2 in Non-alcoholic Fatty Liver Disease. Clin. Exp. Pharmacol. Physiol. 2018, 45, 819–831. [Google Scholar] [CrossRef]
- Zhan, J.; Lv, H.; Dai, B.; Yuan, S.; Fan, J.; Zhao, Y.; Yin, Z.; Wang, D.W.; Chen, C.; Li, H. The Nuclear and Cytoplasmic Roles of MiR-320 in Non-Alcoholic Fatty Liver Disease. Aging 2020, 12, 22019. [Google Scholar] [CrossRef]
- Du, H.; Zhao, Y.; Yin, Z.; Wang, D.W.; Chen, C. The Role of MiR-320 in Glucose and Lipid Metabolism Disorder-Associated Diseases. Int. J. Biol. Sci. 2021, 17, 402–416. [Google Scholar] [CrossRef]
- Lin, Y.; Ding, D.; Huang, Q.; Liu, Q.; Lu, H.; Lu, Y.; Chi, Y.; Sun, X.; Ye, G.; Zhu, H. Downregulation of MiR-192 Causes Hepatic Steatosis and Lipid Accumulation by Inducing SREBF1: Novel Mechanism for Bisphenol A-Triggered Non-Alcoholic Fatty Liver Disease. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2017, 1862, 869–882. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, D.; Guo, S.; Song, Y.; Zhang, X.; Gu, W.; Dong, H.; Huang, L. METTL3/MiR-192-5p/SCD1 Axis Regulates Lipid Metabolism to Affect T Cell Differentiation in Asthma. Mediat. Inflamm. 2025, 2025, 4955849. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, D.R.; Maskey, A.; Kafle, R.; Batajoo, A.; Dahal, P.; Raut, R.; Adhikari, S.; Manandhar, B.; Manandhar, K. Das Evaluation of Circulating Plasma MiR-9, MiR-29a, MiR-192, and MiR-375 as Potential Biomarkers for Predicting Prediabetes and Type 2 Diabetes in Nepali Adult Population. Noncoding RNA Res. 2024, 9, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Soronen, J.; Yki-Järvinen, H.; Zhou, Y.; Sädevirta, S.; Sarin, A.; Leivonen, M.; Sevastianova, K.; Perttilä, J.; Laurila, P.; Sigruener, A. Novel Hepatic MicroRNAs Upregulated in Human Nonalcoholic Fatty Liver Disease. Physiol. Rep. 2016, 4, e12661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Xu, J.; Kong, X.; Zou, L. Up-Regulated MiR-106b Inhibits Ox-LDL-Induced Endothelial Cell Apoptosis in Atherosclerosis. Braz. J. Med. Biol. Res. 2020, 53, e8960. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, H.; Ramírez, C.M.; Lee, S.-M.; Hoe, H.-S.; Fernández-Hernando, C.; Kim, J. MiR-106b Impairs Cholesterol Efflux and Increases Aβ Levels by Repressing ABCA1 Expression. Exp. Neurol. 2012, 235, 476–483. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, X.; Huang, Q.; Fu, Z.; Huang, Y.; Chen, Z.; Li, N.; Lin, X. The Overexpression of MiR-146a in Hippocampal Microglia via IRAK1/TRAF6/NF-ΚB Pathway Improves Cognitive Function in Diabetic Mice. Exp. Neurol. 2025, 391, 115291. [Google Scholar] [CrossRef]
- Li, K.; Ching, D.; Luk, F.S.; Raffai, R.L. MicroRNA-146a Suppression of NF-ΚB-Driven Monocyte/Macrophage Activation and Atherosclerosis Is Regulated by Cellular ApoE Expression. Arterioscler. Thromb. Vasc. Biol. 2015, 35, A155. [Google Scholar] [CrossRef]
- Xiong, J.; Yan, Z.; Shi, M.; Zhou, G.; Zhang, J.; Xu, J.; Liao, Y.; Tang, H. MiR-146a Regulates Neuroinflammation and Immune Cell Function in Neurodegenerative Diseases. Curr. Med. Sci. 2025, 45, 725–744. [Google Scholar] [CrossRef]
- Li, K.; Zhao, B.; Wei, D.; Wang, W.; Cui, Y.; Qian, L.; Liu, G. MiR-146a Improves Hepatic Lipid and Glucose Metabolism by Targeting MED1. Int. J. Mol. Med. 2020, 45, 543–555. [Google Scholar] [CrossRef]
- Zhang, Z.-C.; Liu, Y.; Xiao, L.-L.; Li, S.-F.; Jiang, J.-H.; Zhao, Y.; Qian, S.-W.; Tang, Q.-Q.; Li, X. Upregulation of MiR-125b by Estrogen Protects against Non-Alcoholic Fatty Liver in Female Mice. J. Hepatol. 2015, 63, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, K.; Cao, Y.; Luo, Y.; Liu, Y.; Zhao, C. MiR-125b Promotes the NF-ΚB-Mediated Inflammatory Response in NAFLD via Directly Targeting TNFAIP3. Life Sci. 2021, 270, 119071. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, M.; Li, E.; Yang, Y.; Li, J.; Chen, S.; Shen, W.-J.; Azhar, S.; Guo, Z.; Hu, Z. Dysregulation of MicroRNA-125a Contributes to Obesity-Associated Insulin Resistance and Dysregulates Lipid Metabolism in Mice. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158640. [Google Scholar] [CrossRef]
- Wasim, M.; Guo, J.; Wang, Z.; Parveen, R.; Chen, R.; Wang, Y.; Ma, G. MiR-137: A Therapeutic Candidate or a Key Molecular Regulator in Alzheimer’s Disease? J. Alzheimers Dis. Rep. 2025, 9, 25424823251352170. [Google Scholar] [CrossRef]
- Yu, Y.; He, C.; Tan, S.; Huang, M.; Guo, Y.; Li, M.; Zhang, Q. MicroRNA-137-3p Improves Nonalcoholic Fatty Liver Disease through Activating AMPKα. Anal. Cell. Pathol. 2021, 2021, 4853355. [Google Scholar] [CrossRef]
- Patra, D.; Roy, S.; Arora, L.; Kabeer, S.W.; Singh, S.; Dey, U.; Banerjee, D.; Sinha, A.; Dasgupta, S.; Tikoo, K. MiR-210-3p Promotes Obesity-Induced Adipose Tissue Inflammation and Insulin Resistance by Targeting SOCS1-Mediated NF-ΚB Pathway. Diabetes 2023, 72, 375–388. [Google Scholar] [CrossRef]
- Qiao, X.-R.; Wang, L.; Liu, M.; Tian, Y.; Chen, T. MiR-210-3p Attenuates Lipid Accumulation and Inflammation in Atherosclerosis by Repressing IGF2. Biosci. Biotechnol. Biochem. 2020, 84, 321–329. [Google Scholar] [CrossRef]
- Szabo, G.; Csak, T. Role of MicroRNAs in NAFLD/NASH. Dig. Dis. Sci. 2016, 61, 1314–1324. [Google Scholar] [CrossRef]
- Wang, G.; Zou, H.; Lai, C.; Huang, X.; Yao, Y.; Xiang, G. Repression of MicroRNA-124-3p Alleviates High-Fat Diet–Induced Hepatosteatosis by Targeting Pref-1. Front. Endocrinol. 2020, 11, 589994. [Google Scholar] [CrossRef]
- Shaw, T.A.; Singaravelu, R.; Powdrill, M.H.; Nhan, J.; Ahmed, N.; Özcelik, D.; Pezacki, J.P. MicroRNA-124 Regulates Fatty Acid and Triglyceride Homeostasis. iScience 2018, 10, 149–157. [Google Scholar] [CrossRef]
- Cheng, Y.; Jung, J.; Guo, L.; Shuboni-Mulligan, D.D.; Chen, J.-F.; Hu, W.; Guo, M.-L. HIV-TAT Dysregulates Microglial Lipid Metabolism through SREBP2/MiR-124 Axis: Implication of Lipid Droplet Accumulation Microglia in NeuroHIV. Brain Behav. Immun. 2025, 123, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, L.; Xu, S.; Zhang, H.; Cheng, J.; Pan, S. Microvesicle-Shuttled MicroRNA-130b Activates the Hepatic Inflammation by Inhibiting Glucocorticoid-Receptor-Mediated Immunosuppression in High-Fat Diet-Induced Obese Mice. Vet. Sci. 2024, 11, 565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, Z.; Wang, J.; Li, S. MiR-130b Promotes Obesity Associated Adipose Tissue Inflammation and Insulin Resistance in Diabetes Mice through Alleviating M2 Macrophage Polarization via Repression of PPAR-γ. Immunol. Lett. 2016, 180, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ben-Aicha, S.; Escate, R.; Casaní, L.; Padró, T.; Peña, E.; Arderiu, G.; Mendieta, G.; Badimón, L.; Vilahur, G. High-Density Lipoprotein Remodelled in Hypercholesterolaemic Blood Induce Epigenetically Driven down-Regulation of Endothelial HIF-1α Expression in a Preclinical Animal Model. Cardiovasc. Res. 2020, 116, 1288–1299. [Google Scholar] [CrossRef]
- Goedeke, L.; Rotllan, N.; Canfrán-Duque, A.; Aranda, J.F.; Ramírez, C.M.; Araldi, E.; Lin, C.-S.; Anderson, N.N.; Wagschal, A.; De Cabo, R. MicroRNA-148a Regulates LDL Receptor and ABCA1 Expression to Control Circulating Lipoprotein Levels. Nat. Med. 2015, 21, 1280–1289. [Google Scholar] [CrossRef]
- Goedeke, L.; Rotllan, N.; Canfrán-Duque, A.; Aranda, J.F.; Ramírez, C.M.; Araldi, E.; Lin, C.-S.; Anderson, N.N.; Wagschal, A.; de Cabo, R. Identification of MiR-148a as a Novel Regulator of Cholesterol Metabolism. Nat. Med. 2015, 21, 1280–1289. [Google Scholar] [CrossRef]
- Trajkovski, M.; Hausser, J.; Soutschek, J.; Bhat, B.; Akin, A.; Zavolan, M.; Heim, M.H.; Stoffel, M. MicroRNAs 103 and 107 Regulate Insulin Sensitivity. Nature 2011, 474, 649–653. [Google Scholar] [CrossRef]
- Luo, M.; Xu, C.; Luo, Y.; Wang, G.; Wu, J.; Wan, Q. Circulating MiR-103 Family as Potential Biomarkers for Type 2 Diabetes through Targeting CAV-1 and SFRP4. Acta Diabetol. 2020, 57, 309–322. [Google Scholar] [CrossRef]
- Lorente-Cebrian, S.; Mejhert, N.; Kulyte, A.; Laurencikiene, J.; Åström, G.; Heden, P.; Ryden, M.; Arner, P. MicroRNAs Regulate Human Adipocyte Lipolysis: Effects of MiR-145 Are Linked to TNF-α. PLoS ONE 2014, 9, e86800. [Google Scholar] [CrossRef]
- Fernández-de Frutos, M.; Pardo-Marqués, V.; Torrecilla-Parra, M.; Rada, P.; Pérez-García, A.; Martín-Martín, Y.; de la Peña, G.; Gómez, A.; Toledano-Zaragoza, A.; Gómez-Coronado, D. MiR-7 Controls Cholesterol Biosynthesis through Posttranscriptional Regulation of DHCR24 Expression. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2023, 1866, 194938. [Google Scholar]
- Cuevas-Diaz Duran, R.; Wei, H.; Kim, D.H.; Wu, J.Q. Invited Review: Long Non-coding RNA s: Important Regulators in the Development, Function and Disorders of the Central Nervous System. Neuropathol. Appl. Neurobiol. 2019, 45, 538–556. [Google Scholar] [CrossRef] [PubMed]
- Maclary, E.; Hinten, M.; Harris, C.; Sethuraman, S.; Gayen, S.; Kalantry, S. PRC2 Represses Transcribed Genes on the Imprinted Inactive X Chromosome in Mice. Genome Biol. 2017, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Mongelli, A.; Mohammed, S.A.; Gorica, E.; Mengozzi, A.; Matter, C.M.; Ruschitzka, F.; Paneni, F.; Costantino, S. Long Non-Coding RNA PANDA Drives Diabetic Vascular Dysfunction by Promoting Endothelial Senescence and Oxidative Damage. Eur. Heart J. 2023, 44, ehad655-3236. [Google Scholar] [CrossRef]
- Farberov, S.; Ziv, O.; Lau, J.Y.; Ben-Tov Perry, R.; Lubelsky, Y.; Miska, E.; Kudla, G.; Ulitsky, I. Structural Features within the NORAD Long Noncoding RNA Underlie Efficient Repression of Pumilio Activity. Nat. Struct. Mol. Biol. 2025, 32, 287–299. [Google Scholar] [CrossRef]
- Farooq, U.; Notani, D. Transcriptional Regulation of INK4/ARF Locus by Cis and Trans Mechanisms. Front. Cell Dev. Biol. 2022, 10, 948351. [Google Scholar] [CrossRef]
- Li, A.; Mallik, S.; Luo, H.; Jia, P.; Lee, D.-F.; Zhao, Z. H19, a Long Non-Coding RNA, Mediates Transcription Factors and Target Genes through Interference of MicroRNAs in Pan-Cancer. Mol. Ther. Nucleic Acids 2020, 21, 180–191. [Google Scholar] [CrossRef]
- Arun, G.; Diermeier, S.D.; Spector, D.L. Therapeutic Targeting of Long Non-Coding RNAs in Cancer. Trends Mol. Med. 2018, 24, 257–277. [Google Scholar] [CrossRef]
- Singh, A.K.; Aryal, B.; Zhang, X.; Fan, Y.; Price, N.L.; Suárez, Y.; Fernández-Hernando, C. Posttranscriptional Regulation of Lipid Metabolism by Non-Coding RNAs and RNA Binding Proteins. In Proceedings of the Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 81, pp. 129–140. [Google Scholar]
- Alluri, A.; Saxena, P.; Mishra, A.; Gutti, R.K. Association of Long Non-Coding RNA in Lipid Metabolism: Implications in Leukemia. Int. J. Biochem. Cell Biol. 2025, 184, 106785. [Google Scholar] [CrossRef]
- Johnson, A.A.; Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019, 18, e13048. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, J.; Liangpunsakul, S.; Wang, L. Long non-coding RNA in liver metabolism and disease: Current status. Liver Res. 2017, 1, 163–167. [Google Scholar] [CrossRef]
- Sallam, T.; Jones, M.C.; Gilliland, T.; Zhang, L.; Wu, X.; Eskin, A.; Sandhu, J.; Casero, D.; de Aguiar Vallim, T.Q.; Hong, C. Feedback Modulation of Cholesterol Metabolism by the Lipid-Responsive Non-Coding RNA LeXis. Nature 2016, 534, 124–128. [Google Scholar] [CrossRef]
- Li, P.; Ruan, X.; Yang, L.; Kiesewetter, K.; Zhao, Y.; Luo, H.; Chen, Y.; Gucek, M.; Zhu, J.; Cao, H. A Liver-Enriched Long Non-Coding RNA, LncLSTR, Regulates Systemic Lipid Metabolism in Mice. Cell Metab. 2015, 21, 455–467. [Google Scholar] [CrossRef]
- Le, N.-A.; Walter, M.F. The Role of Hypertriglyceridemia in Atherosclerosis. Curr. Atheroscler. Rep. 2007, 9, 110–115. [Google Scholar] [CrossRef]
- Xiang, J.; Deng, Y.-Y.; Liu, H.-X.; Pu, Y. LncRNA MALAT1 Promotes PPARα/CD36-Mediated Hepatic Lipogenesis in Nonalcoholic Fatty Liver Disease by Modulating MiR-206/ARNT Axis. Front. Bioeng. Biotechnol. 2022, 10, 858558. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-X.; Zheng, F.; Xie, K.-L.; Xie, M.-R.; Jiang, L.-J.; Cai, Y. Exercise Reduces Insulin Resistance in Type 2 Diabetes Mellitus via Mediating the LncRNA MALAT1/MicroRNA-382-3p/Resistin Axis. Mol. Ther. Nucleic Acids 2019, 18, 34–44. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Guan, X.; Li, X.; Zhao, Z.; Gao, Y.; Zhang, X.; Chen, R. An Integrated Transcriptomics and Proteomics Analysis Implicates LncRNA MALAT1 in the Regulation of Lipid Metabolism. Mol. Cell. Proteom. 2021, 20, 100141. [Google Scholar] [CrossRef]
- Liu, L.; Tan, L.; Yao, J.; Yang, L. Long Non-Coding RNA MALAT1 Regulates Cholesterol Accumulation in Ox-LDL-Induced Macrophages via the MicroRNA-17-5p/ABCA1 Axis. Mol. Med. Rep. 2020, 21, 1761–1770. [Google Scholar] [CrossRef]
- Zheng, J.; Ludin, A.F.M.; Rajab, N.F.; Shaolong, L.; Jufri, N.F. The Roles of LncMALAT1 in Coronary Artery Disease Regulation and Therapeutic Perspective: A Systematic Review. iScience 2025, 28, 112945. [Google Scholar] [CrossRef]
- Wang, H.; Cao, Y.; Shu, L.; Zhu, Y.; Peng, Q.; Ran, L.; Wu, J.; Luo, Y.; Zuo, G.; Luo, J. Long Non-coding RNA (LncRNA) H19 Induces Hepatic Steatosis through Activating MLXIPL and MTORC1 Networks in Hepatocytes. J. Cell Mol. Med. 2020, 24, 1399–1412. [Google Scholar] [CrossRef]
- Lin, F.; Yi, M.; Zhou, S.; Wang, Q. LncRNA H19 Promotes Adipogenic Differentiation Disorder by Sponging MiR-130b-3p to Upregulate PPARγ in Steroid-Induced Osteonecrosis of the Femoral Head. Front. Genet. 2025, 16, 1529797. [Google Scholar] [CrossRef]
- Shi, X.; Wei, Y.-T.; Li, H.; Jiang, T.; Zheng, X.-L.; Yin, K.; Zhao, G.-J. Long Non-Coding RNA H19 in Atherosclerosis: What Role? Mol. Med. 2020, 26, 72. [Google Scholar] [CrossRef]
- Daghistani, H.; Hegazy, G.A.; Alkhalofah, M.; Alsobeihy, A.; Nasser, S.; Gad, H.; Shamrani, T.; Mufrrih, M.; Alyousfi, D. Long Noncoding RNAs in Familial Hypercholesterolemia: Biomarkers, Therapeutics, and AI in Precision Medicine. Lipids Health Dis. 2025, 24, 182. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qu, H.; Guo, M.; Zhang, Y.; Cui, Y.; Yang, Q.; Bai, R.; Shi, D. ANRIL and Atherosclerosis. J. Clin. Pharm. Ther. 2020, 45, 240–248. [Google Scholar] [CrossRef]
- Chi, J.; Li, J.; Jia, J.; Zhang, T.; Liu, X.; Yi, L. Long Non-Coding RNA ANRIL in Gene Regulation and Its Duality in Atherosclerosis. Curr. Med. Sci. 2017, 37, 816–822. [Google Scholar] [CrossRef]
- Gareev, I.; Kudriashov, V.; Sufianov, A.; Begliarzade, S.; Ilyasova, T.; Liang, Y.; Beylerli, O. The Role of Long Non-Coding RNA ANRIL in the Development of Atherosclerosis. Noncoding RNA Res. 2022, 7, 212–216. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Xiong, X.; Liu, T.; Mi, L.; Peng, X.; Rui, C.; Guo, L.; Li, S.; Li, X.; Lin, J.D. Long Noncoding RNA Licensing of Obesity-Linked Hepatic Lipogenesis and NAFLD Pathogenesis. Nat. Commun. 2018, 9, 2986. [Google Scholar] [CrossRef]
- Mi, L.; Zhao, X.-Y.; Li, S.; Yang, G.; Lin, J.D. Conserved Function of the Long Noncoding RNA Blnc1 in Brown Adipocyte Differentiation. Mol. Metab. 2017, 6, 101–110. [Google Scholar] [CrossRef]
- Tang, S.; Zhu, W.; Zheng, F.; Gui, W.; Zhang, W.; Lin, X.; Li, H. The Long Noncoding RNA Blnc1 Protects against Diet-Induced Obesity by Promoting Mitochondrial Function in White Fat. Diabetes Metab. Syndr. Obes. 2020, 13, 1189–1201. [Google Scholar] [CrossRef]
- Meng, X.; Long, M.; Yue, N.; Li, Q.; Chen, J.; Zhao, H.; Deng, W. LncRNA MEG3 Restrains Hepatic Lipogenesis via the FOXO1 Signaling Pathway in HepG2 Cells. Cell Biochem. Biophys. 2024, 82, 1253–1259. [Google Scholar] [CrossRef]
- Ghaedi, H.; Zare, A.; Omrani, M.D.; Doustimotlagh, A.H.; Meshkani, R.; Alipoor, S.; Alipoor, B. Genetic Variants in Long Noncoding RNA H19 and MEG3 Confer Risk of Type 2 Diabetes in an Iranian Population. Gene 2018, 675, 265–271. [Google Scholar] [CrossRef]
- Zou, D.; Liu, L.; Zeng, Y.; Wang, H.; Dai, D.; Xu, M. LncRNA MEG3 Up-Regulates SIRT6 by Ubiquitinating EZH2 and Alleviates Nonalcoholic Fatty Liver Disease. Cell Death Discov. 2022, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Huang, F.; Liu, H.; Zhang, T.; Yang, M.; Sun, C. LncRNA MEG3 Functions as a CeRNA in Regulating Hepatic Lipogenesis by Competitively Binding to MiR-21 with LRP6. Metabolism 2019, 94, 1–8. [Google Scholar] [CrossRef]
- Tran, K.-V.; Nandrup-Bus, C.; DeSouza, T.; Soares, R.; Jespersen, N.Z.; Min, S.Y.; Rojas-Rodriguez, R.; Willenbrock, H.; Juhlin, T.; Severinsen, M.C.K. A Long-Non-Coding RNA, LINC00473, Confers the Human Adipose Tissue Thermogenic Phenotype through Enhanced CAMP Responsiveness. bioRxiv 2018, 339192. [Google Scholar] [CrossRef]
- Aggarwal, D.D.; Mishra, P.; Yadav, G.; Mitra, S.; Patel, Y.; Singh, M.; Sahu, R.K.; Sharma, V. Decoding the Connection between LncRNA and Obesity: Perspective from Humans and Drosophila. Heliyon 2024, 10, e35327. [Google Scholar] [CrossRef]
- Zhang, T.-N.; Wang, W.; Yang, N.; Huang, X.-M.; Liu, C.-F. Regulation of Glucose and Lipid Metabolism by Long Non-Coding RNAs: Facts and Research Progress. Front. Endocrinol. 2020, 11, 457. [Google Scholar] [CrossRef]
- Xiao, T.; Liu, L.; Li, H.; Sun, Y.; Luo, H.; Li, T.; Wang, S.; Dalton, S.; Zhao, R.C.; Chen, R. Long Noncoding RNA ADINR Regulates Adipogenesis by Transcriptionally Activating C/EBPα. Stem Cell Rep. 2015, 5, 856–865. [Google Scholar] [CrossRef]
- Petkovic, A.; Erceg, S.; Munjas, J.; Ninic, A.; Vladimirov, S.; Davidovic, A.; Vukmirovic, L.; Milanov, M.; Cvijanovic, D.; Mitic, T. LncRNAs as Regulators of Atherosclerotic Plaque Stability. Cells 2023, 12, 1832. [Google Scholar] [CrossRef]
- Chen, X.; Tan, X.-R.; Li, S.-J.; Zhang, X.-X. LncRNA NEAT1 Promotes Hepatic Lipid Accumulation via Regulating MiR-146a-5p/ROCK1 in Nonalcoholic Fatty Liver Disease. Life Sci. 2019, 235, 116829. [Google Scholar] [CrossRef]
- Liu, X.; Liang, Y.; Song, R.; Yang, G.; Han, J.; Lan, Y.; Pan, S.; Zhu, M.; Liu, Y.; Wang, Y. Long Non-Coding RNA NEAT1-Modulated Abnormal Lipolysis via ATGL Drives Hepatocellular Carcinoma Proliferation. Mol. Cancer 2018, 17, 90. [Google Scholar] [CrossRef]
- Sun, Y.; Song, Y.; Liu, C.; Geng, J. LncRNA NEAT1-MicroRNA-140 Axis Exacerbates Nonalcoholic Fatty Liver through Interrupting AMPK/SREBP-1 Signaling. Biochem. Biophys. Res. Commun. 2019, 516, 584–590. [Google Scholar] [CrossRef]
- Gernapudi, R.; Wolfson, B.; Zhang, Y.; Yao, Y.; Yang, P.; Asahara, H.; Zhou, Q. MicroRNA 140 Promotes Expression of Long Noncoding RNA NEAT1 in Adipogenesis. Mol. Cell Biol. 2016, 36, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xin, W.; Wei, W.; Tatenhorst, L.; Graf, I.; Popa-Wagner, A.; Gerner, S.T.; Huber, S.E.; Kilic, E.; Hermann, D.M. Knockdown of NEAT1 Prevents Post-Stroke Lipid Droplet Agglomeration in Microglia by Regulating Autophagy. Cell. Mol. Life Sci. 2024, 81, 30. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, E.J.; van Solingen, C.; Scacalossi, K.R.; Ouimet, M.; Afonso, M.S.; Prins, J.; Koelwyn, G.J.; Sharma, M.; Ramkhelawon, B.; Carpenter, S. The Long Noncoding RNA CHROME Regulates Cholesterol Homeostasis in Primates. Nat. Metab. 2019, 1, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Gluba-Sagr, A.; Franczyk, B.; Rysz-Górzyńska, A.; Olszewski, R.; Rysz, J. The Role of Selected LncRNAs in Lipid Metabolism and Cardiovascular Disease Risk. Int. J. Mol. Sci. 2024, 25, 9244. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, M.; Ma, Y.; Peng, Y. LncRNA TUG1 Reduces Inflammation and Enhances Insulin Sensitivity in White Adipose Tissue by Regulating MiR-204/SIRT1 Axis in Obesity Mice. Mol. Cell Biochem. 2020, 475, 171–183. [Google Scholar] [CrossRef]
- Xue, M.; Xia, F.; Wang, Y.; Zhu, L.; Li, Y.; Jia, D.; Gao, Y.; Shi, Y.; Zhang, C.; He, Y. The Role of LncRNA TUG1 in Obesity-Related Diseases. Mini Rev. Med. Chem. 2022, 22, 1305–1313. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Gu, M.; Peng, Y. LncRNA TUG1 Promotes the Brown Remodeling of White Adipose Tissue by Regulating MiR-204-targeted SIRT1 in Diabetic Mice. Int. J. Mol. Med. 2020, 46, 2225–2234. [Google Scholar] [CrossRef]
- Das, S.; Reddy, M.A.; Senapati, P.; Stapleton, K.; Lanting, L.; Wang, M.; Amaram, V.; Ganguly, R.; Zhang, L.; Devaraj, S. Diabetes Mellitus–Induced Long Noncoding RNA Dnm3os Regulates Macrophage Functions and Inflammation via Nuclear Mechanisms. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1806–1820. [Google Scholar] [CrossRef]
- Morán, I.; Akerman, I.; Van De Bunt, M.; Xie, R.; Benazra, M.; Nammo, T.; Arnes, L.; Nakić, N.; García-Hurtado, J.; Rodríguez-Seguí, S. Human β Cell Transcriptome Analysis Uncovers LncRNAs That Are Tissue-Specific, Dynamically Regulated, and Abnormally Expressed in Type 2 Diabetes. Cell Metab. 2012, 16, 435–448. [Google Scholar] [CrossRef]
- Ismail, N.; Abdullah, N.; Abdul Murad, N.A.; Jamal, R.; Sulaiman, S.A. Long Non-Coding RNAs (LncRNAs) in Cardiovascular Disease Complication of Type 2 Diabetes. Diagnostics 2021, 11, 145. [Google Scholar] [CrossRef]
- Yu, X.; Song, M.; Rong, P.; Chen, X.; Shi, L.; Wang, C.; Pang, Q. LncRNA SNHG1 Modulates Adipogenic Differentiation of BMSCs by Promoting DNMT1 Mediated Opg Hypermethylation via Interacting with PTBP1. J. Cell Mol. Med. 2022, 26, 60–74. [Google Scholar] [CrossRef]
- Cao, B.; Wang, T.; Qu, Q.; Kang, T.; Yang, Q. Long Noncoding RNA SNHG1 Promotes Neuroinflammation in Parkinson’s Disease via Regulating MiR-7/NLRP3 Pathway. Neuroscience 2018, 388, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Yi, J.; Jiang, J.; Zou, Z.; Mo, Y.; Ren, Q.; Lin, Z.; Lu, Y.; Zhang, J.; Liu, J. Identification and Validation of a Fatty Acid Metabolism-Related LncRNA Signature as a Predictor for Prognosis and Immunotherapy in Patients with Liver Cancer. BMC Cancer 2022, 22, 1037. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Wang, C.; Yang, L.; Liu, B.; Zheng, Z.; Yang, L.; Zhang, F.; Peng, J.; Huang, D.; Huang, K. The Role of Long Noncoding RNA Nron in Atherosclerosis Development and Plaque Stability. iScience 2022, 25, 103978. [Google Scholar] [CrossRef]
- Gharbi, N.; Mahmoudinasab, H.; Hooshmandi, E.; Rahimi, M.; Bayat, M.; Karimi, N.; Hojati, S.S.; Zayani, Z.; Tabrizi, R.; Borhani-Haghighi, A. Altered Expression of Long Non-Coding RNAs NRON and SNHG11 in Patients with Ischemic Stroke. Egypt. J. Med. Hum. Genet. 2024, 25, 11. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 2020, 3920196. [Google Scholar] [CrossRef]
- Liu, B.; Zhong, Y.; Huang, D.; Yang, L.; Wang, P.; Yang, L.; Zhang, F.; Li, X.; Liang, M.; Huang, K. LncRNA Nron Deficiency Protects Mice from Diet-Induced Adiposity and Hepatic Steatosis. Metabolism 2023, 148, 155609. [Google Scholar] [CrossRef]
- Cai, L.; Tu, L.I.; Li, T.; Yang, X.; Ren, Y.; Gu, R.; Zhang, Q.; Yao, H.; Qu, X.; Wang, Q. Downregulation of LncRNA UCA1 Ameliorates the Damage of Dopaminergic Neurons, Reduces Oxidative Stress and Inflammation in Parkinson’s Disease through the Inhibition of the PI3K/Akt Signaling Pathway. Int. Immunopharmacol. 2019, 75, 105734. [Google Scholar] [CrossRef]
- Xiang, H.; Tu, B.; Luo, M.; Hou, P.; Wang, J.; Zhang, R.; Wu, L. Knockdown of UCA1 Attenuated the Progression of Alcoholic Fatty Disease by Sponging MiR-214. Mamm. Genome 2022, 33, 534–542. [Google Scholar] [CrossRef]
- Huang, S.; Wu, K.; Li, B.; Liu, Y. LncRNA UCA1 Inhibits Mitochondrial Dysfunction of Skeletal Muscle in Type 2 Diabetes Mellitus by Sequestering MiR-143-3p to Release FGF21. Cell Tissue Res. 2023, 391, 561–575. [Google Scholar] [CrossRef]
- Sun, S.; Li, H.; Liu, S.; Xie, X.; Zhai, W.; Pan, J. Long Noncoding RNA UCA1 Inhibits Epirubicin-Induced Apoptosis by Activating PPARα-Mediated Lipid Metabolism. Exp. Cell Res. 2024, 442, 114271. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Du, Y.-J.; Xu, F.; Li, H.-B.; Yang, X. Potential Diagnostic Markers of Diabetic Retinopathy: Serum LncRNA MIAT, HOTTIP, SNHG16. Diabetes Metab. Syndr. Obes. 2024, 2024, 4247–4256. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Li, J.; Feng, S.; Li, Y.; Tan, L. Long Noncoding RNA Gomafu Upregulates Foxo1 Expression to Promote Hepatic Insulin Resistance by Sponging MiR-139-5p. Cell Death Dis. 2018, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Liu, S.; Jiang, Q.; Yu, T.; Huang, K. LncRNA-MIAT Increased in Patients with Coronary Atherosclerotic Heart Disease. Cardiol. Res. Pract. 2019, 2019, 6280194. [Google Scholar] [CrossRef]
- Mazidi, M.; Penson, P.; Gluba-Brzozka, A.; Rysz, J.; Banach, M. Relationship between long noncoding RNAs and physiological risk factors of cardiovascular disease. J. Cli. Lip. 2017, 11, 617–623. [Google Scholar] [CrossRef]
- Ma, M.; Duan, R.; Shen, L.; Liu, M.; Ji, Y.; Zhou, H.; Li, C.; Liang, T.; Li, X.; Guo, L. The LncRNA Gm15622 Stimulates SREBP-1c Expression and Hepatic Lipid Accumulation by Sponging the MiR-742-3p in Mice [S]. J. Lipid Res. 2020, 61, 1052–1064. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, S.; Zeng, Z.; Zheng, H.; Li, Y.; Wang, F.; Huang, Y.; Zhao, Y.; Zhuo, W.; Lv, G. Decreased LncRNA HNF4A-AS1 Facilitates Resistance to Sorafenib-Induced Ferroptosis of Hepatocellular Carcinoma by Reprogramming Lipid Metabolism. Theranostics 2024, 14, 7088. [Google Scholar] [CrossRef]
- Xiao, F.; He, Z.; Wang, S.; Li, J.; Fan, X.; Yan, T.; Yang, M.; Yang, D. Regulatory Mechanism of Circular RNAs in Neurodegenerative Diseases. CNS Neurosci. Ther. 2024, 30, e14499. [Google Scholar] [CrossRef]
- Zhao, M.; Lin, M.; Zhang, Z.; Ye, L. Research Progress of Circular RNA FOXO3 in Diseases. Glob. Med. Genet. 2025, 12, 100003. [Google Scholar] [CrossRef]
- Huang, A.; Zheng, H.; Wu, Z.; Chen, M.; Huang, Y. Circular RNA-Protein Interactions: Functions, Mechanisms, and Identification. Theranostics 2020, 10, 3503–3517. [Google Scholar] [CrossRef]
- Meng, E.; Deng, J.; Jiang, R.; Wu, H. CircRNA-Encoded Peptides or Proteins as New Players in Digestive System Neoplasms. Front. Oncol. 2022, 12, 944159. [Google Scholar] [CrossRef]
- Zeng, Y.; Zheng, Z.; Liu, F.; Yi, G. Circular RNAs in Metabolism and Metabolic Disorders. Obes. Rev. 2021, 22, e13220. [Google Scholar] [CrossRef]
- Zhou, W.-Y.; Cai, Z.-R.; Liu, J.; Wang, D.-S.; Ju, H.-Q.; Xu, R.-H. Circular RNA: Metabolism, Functions and Interactions with Proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Y.-W.; Zhang, K. Interactions between CircRNA and Protein in Breast Cancer. Gene 2024, 895, 148019. [Google Scholar] [CrossRef] [PubMed]
- Rossbach, O. Artificial Circular RNA Sponges Targeting MicroRNAs as a Novel Tool in Molecular Biology. Mol. Ther. Nucleic Acids 2019, 17, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, X.; Deng, Y.; Cui, Q.; Zhu, J.; Ren, H.; Liu, Y.; Hu, X.; Zuo, J.; Peng, Y. Regulatory Roles of CircRNAs in Adipogenesis and Lipid Metabolism: Emerging Insights into Lipid-related Diseases. FEBS J. 2021, 288, 3663–3682. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Khanna, S.; Bhattacharya, A. MicroRNA Regulation of Oxidative Stress. Oxid. Med. Cell Longev. 2017, 2017, 2872156. [Google Scholar] [CrossRef]
- Abdelgwad, M.; Zakaria, R.; Marzouk, S.; Sabry, D.; Ahmed, R.; Badary, H.A.; Samir, M. The Emerging Role of Circular RNA Homeodomain Interacting Protein Kinase 3 and Circular RNA 0046367 through Wnt/Beta-Catenin Pathway on the Pathogenesis of Nonalcoholic Steatohepatitis in Egyptian Patients. Rep. Biochem. Mol. Biol. 2023, 11, 614–625. [Google Scholar] [CrossRef]
- Guo, X.-Y.; Chen, J.-N.; Sun, F.; Wang, Y.-Q.; Pan, Q.; Fan, J.-G. CircRNA_0046367 Prevents Hepatoxicity of Lipid Peroxidation: An Inhibitory Role against Hepatic Steatosis. Oxid. Med. Cell Longev. 2017, 2017, 3960197. [Google Scholar] [CrossRef]
- Wang, G.; Tong, J.; Li, Y.; Qiu, X.; Chen, A.; Chang, C.; Yu, G. Overview of CircRNAs Roles and Mechanisms in Liver Fibrosis. Biomolecules 2023, 13, 940. [Google Scholar] [CrossRef]
- Guha, S.; Sesili, S.; Mir, I.H.; Thirunavukkarasu, C. Epigenetics and Mitochondrial Dysfunction Insights into the Impact of the Progression of Non-alcoholic Fatty Liver Disease. Cell Biochem. Funct. 2023, 41, 4–19. [Google Scholar] [CrossRef]
- Arcinas, C.; Tan, W.; Fang, W.; Desai, T.P.; Teh, D.C.; Degirmenci, U.; Xu, D.; Foo, R.; Sun, L. Adipose circular RNAs exhibit dynamic regulation in obesity and functional role in adipogenesis. Nat. Metab. 2019, 1, 688–703. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Shan, K.; Liu, Y.; Zhang, Y.; Xu, L.; Xu, L. CircScd1 Promotes Fatty Liver Disease via the Janus Kinase 2/Signal Transducer and Activator of Transcription 5 Pathway. Dig. Dis. Sci. 2019, 64, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, J.; Wu, Y.; Liang, B.; Yan, M.; Sun, C.; Wang, D.; Hu, X.; Liu, L.; Hu, W. The Potential Role and Mechanism of CircRNA/MiRNA Axis in Cholesterol Synthesis. Int. J. Biol. Sci. 2023, 19, 2879. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, X.; Sun, N.; Wang, T.; Zhu, J.; Yang, S.; Song, X.; Wang, R.; Wang, X.; Zhao, Y. Differentially Expressed Circular Non-Coding RNAs in Atherosclerotic Aortic Vessels and Their Potential Functions in Endothelial Injury. Front. Cardiovasc. Med. 2021, 8, 657544. [Google Scholar] [CrossRef]
- Ning, H.; Jiang, Y.; Li, B.; Ren, J.; Wang, C.; Wei, L.; Li, L.; Ran, A.; Li, Z.; Li, J. CircABCA1 Promotes CcRCC by Reprogramming Cholesterol Metabolism and Facilitating M2 Macrophage Polarization through IGF2BP3-Mediated Stabilization of SCARB1 MRNA. Mol. Cancer 2025, 24, 199. [Google Scholar] [CrossRef]
- Holdt, L.M.; Stahringer, A.; Sass, K.; Pichler, G.; Kulak, N.A.; Wilfert, W.; Kohlmaier, A.; Herbst, A.; Northoff, B.H.; Nicolaou, A. Circular Non-Coding RNA ANRIL Modulates Ribosomal RNA Maturation and Atherosclerosis in Humans. Nat. Commun. 2016, 7, 12429. [Google Scholar] [CrossRef]
- Wouters, K.; van Gorp, P.J.; Bieghs, V.; Gijbels, M.J.; Duimel, H.; Lütjohann, D.; Kerksiek, A.; van Kruchten, R.; Maeda, N.; Staels, B.; et al. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology 2008, 48, 474–486. [Google Scholar] [CrossRef]
- Burd, C.E.; Jeck, W.R.; Liu, Y.; Sanoff, H.K.; Wang, Z.; Sharpless, N.E. Expression of Linear and Novel Circular Forms of an INK4/ARF-Associated Non-Coding RNA Correlates with Atherosclerosis Risk. PLoS Genet. 2010, 6, e1001233. [Google Scholar] [CrossRef]
- Cai, H.; Jiang, Z.; Yang, X.; Lin, J.; Cai, Q.; Li, X. Circular RNA HIPK3 Contributes to Hyperglycemia and Insulin Homeostasis by Sponging MiR-192-5p and Upregulating Transcription Factor Forkhead Box O1. Endocr. J. 2020, 67, 397–408. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Qi, Y.-F.; Xiao, Z.-X.; Chen, H.; Liu, S.-H.; Li, Z.-Z.; Zeng, Z.-F.; Wu, H.-F. CircHIPK3 Regulates Vascular Smooth Muscle Cell Calcification via the MiR-106a-5p/MFN2 Axis. J. Cardiovasc. Transl. Res. 2022, 15, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yang, Z.; Lv, B.; Xu, X. The Diagnostic and Therapeutic Role of Circular RNA HIPK3 in Human Diseases. Diagnostics 2022, 12, 2469. [Google Scholar] [CrossRef] [PubMed]
- Zaiou, M. The Emerging Role and Promise of Circular RNAs in Obesity and Related Metabolic Disorders. Cells 2020, 9, 1473. [Google Scholar] [CrossRef]
- Guo, X.-Y.; He, C.-X.; Wang, Y.-Q.; Sun, C.; Li, G.-M.; Su, Q.; Pan, Q.; Fan, J.-G. Circular RNA Profiling and Bioinformatic Modeling Identify Its Regulatory Role in Hepatic Steatosis. Biomed. Res. Int. 2017, 2017, 5936171. [Google Scholar] [CrossRef]
- Sun, C.; Fan, J.-G.; Qiao, L. Potential Epigenetic Mechanism in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2015, 16, 5161–5179. [Google Scholar] [CrossRef]
- Yu, G.; Yang, Z.; Peng, T.; Lv, Y. Circular RNAs: Rising Stars in Lipid Metabolism and Lipid Disorders. J. Cell Physiol. 2021, 236, 4797–4806. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Li, Y.; Mao, R.; Yang, H.; Zhang, Y.; Zhang, Y.; Guo, P.; Zhan, D.; Zhang, T. Circular RNA SAMD4A Controls Adipogenesis in Obesity through the MiR-138-5p/EZH2 Axis. Theranostics 2020, 10, 4705–4719. [Google Scholar] [CrossRef]
- Lu, P.; Fan, J.; Li, B.; Wang, X.; Song, M. A Novel Protein Encoded by CircLARP1B Promotes the Proliferation and Migration of Vascular Smooth Muscle Cells by Suppressing CAMP Signaling. Atherosclerosis 2024, 395, 117575. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Shi, L.; Liu, B.; Sheng, Z.; Chang, S.; Cai, X.; Shan, G. A Mammalian Conserved Circular RNA CircLARP1B Regulates Hepatocellular Carcinoma Metastasis and Lipid Metabolism. Adv. Sci. 2024, 11, 2305902. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wu, S.; Zhou, Z.; Ding, X.; Shi, R.; Thorne, R.F.; Zhang, X.D.; Hu, W.; Wu, M. CircACC1 Regulates Assembly and Activation of AMPK Complex under Metabolic Stress. Cell Metab. 2019, 30, 157–173. [Google Scholar] [CrossRef]
- Haque, S.; Harries, L.W. Circular RNAs (circRNAs) in Health and Disease. Genes 2017, 8, 353. [Google Scholar] [CrossRef]
- Jiang, R.; Li, H.; Yang, J.; Shen, X.; Song, C.; Yang, Z.; Wang, X.; Huang, Y.; Lan, X.; Lei, C. CircRNA Profiling Reveals an Abundant CircFUT10 That Promotes Adipocyte Proliferation and Inhibits Adipocyte Differentiation via Sponging Let-7. Mol. Ther. Nucleic Acids 2020, 20, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, M.; Luo, Y.; Yu, H.; Zhang, J.; Li, D.; He, Q. Maternal Obesity Alters CircRNA Expression and the Potential Role of Mmu_circRNA_0000660 via Sponging MiR_693 in Offspring Liver at Weaning Age. Gene 2020, 731, 144354. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, Z.; Feng, X.; Zang, X.; Ding, W.; Wu, F.; Zhao, Q. CircRNA/LncRNA-MiRNA-MRNA Network in Oxidized, Low-Density, Lipoprotein-Induced Foam Cells. DNA Cell Biol. 2019, 38, 1499–1511. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, T.; Feng, R.; Huang, H.; Xia, T.; Sun, C. CircARF3 Alleviates Mitophagy-Mediated Inflammation by Targeting MiR-103/TRAF3 in Mouse Adipose Tissue. Mol. Ther. Nucleic Acids 2019, 14, 192–203. [Google Scholar] [CrossRef]
- Shen, L.-P.; Zhang, W.-C.; Deng, J.-R.; Qi, Z.-H.; Lin, Z.-W.; Wang, Z.-D. Advances in the Mechanism of Small Nucleolar RNA and Its Role in DNA Damage Response. Mil. Med. Res. 2024, 11, 53. [Google Scholar] [CrossRef]
- Weng, W.; Li, H.; Goel, A. Piwi-Interacting RNAs (PiRNAs) and Cancer: Emerging Biological Concepts and Potential Clinical Implications. Biochim. Biophys. Acta (BBA) Rev. Cancer 2019, 1871, 160–169. [Google Scholar] [CrossRef]
- Russell, S.J.; LaMarre, J. Transposons and the PIWI Pathway: Genome Defense in Gametes and Embryos. Reproduction 2018, 156, R111–R124. [Google Scholar] [CrossRef]
- Majewska, K.; Wróblewska-Ankiewicz, P.; Rudzka, M.; Hyjek-Składanowska, M.; Gołębiewski, M.; Smoliński, D.J.; Kołowerzo-Lubnau, A. Different Patterns of MRNA Nuclear Retention during Meiotic Prophase in Larch Microsporocytes. Int. J. Mol. Sci. 2021, 22, 8501. [Google Scholar] [CrossRef]
- Harrison, L.J.; Bose, D. Enhancer RNAs Step Forward: New Insights into Enhancer Function. Development 2022, 149, dev200398. [Google Scholar] [CrossRef]
- Chen, Z. Progress and Prospects of Long Noncoding RNAs in Lipid Homeostasis. Mol. Metab. 2016, 5, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Ginckels, P.; Holvoet, P. Oxidative Stress and Inflammation in Cardiovascular Diseases and Cancer: Role of Non-Coding RNAs. Yale J. Biol. Med. 2022, 95, 129–152. [Google Scholar] [PubMed]
- Zhang, X.; Price, N.L.; Fernández-Hernando, C. Non-Coding RNAs in Lipid Metabolism. Vasc. Pharmacol. 2019, 114, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Di, K.; Fan, B.; Wu, J.; Gu, X.; Sun, Y.; Khan, A.; Li, P.; Li, Z. MicroRNAs in Extracellular Vesicles: Sorting Mechanisms, Diagnostic Value, Isolation, and Detection Technology. Front. Bioeng. Biotechnol. 2022, 10, 948959. [Google Scholar] [CrossRef]
- Boon, R.A.; Vickers, K.C. Intercellular Transport of MicroRNAs. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 186–192. [Google Scholar] [CrossRef]
- Babuta, M.; Szabo, G. Extracellular Vesicles in Inflammation: Focus on the MicroRNA Cargo of EVs in Modulation of Liver Diseases. J. Leukoc. Biol. 2022, 111, 75–92. [Google Scholar] [CrossRef]
- Jopling, C. Liver-Specific MicroRNA-122: Biogenesis and Function. RNA Biol. 2012, 9, 137–142. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S. Adipose Tissue-Derived Extracellular Vesicle MicroRNAs: Diagnostic Biomarkers for the Pathophysiology Associated with Obesity. Precis. Chem. 2025, 3, 480–491. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Bezsonov, E.E.; Baig, M.S.; Popkova, T.V.; Orekhov, A.N. Mitochondrial Lipid Homeostasis at the Crossroads of Liver and Heart Diseases. Int. J. Mol. Sci. 2021, 22, 6949. [Google Scholar] [CrossRef]
- Hashemi, M.; Raesi, R.; Eslami Vaghar, M.; Hamzeh Jouneghani, S.; Hasany, S.; Bidooki, S.H.; Mirzaei, M.; Shahpasand, K.; Nabavi, N.; Farahani, N. Nanoparticles in the Stimulation of Ferroptosis in Cancer Therapy. In Autophagy, Apoptosis and Ferroptosis in Oncology: Cell Death and Cancer; World Scientific: Singapore, 2025; pp. 923–960. [Google Scholar]
- Ru, W.; Zhang, S.; Liu, J.; Liu, W.; Huang, B.; Chen, H. Non-Coding RNAs and Adipogenesis. Int. J. Mol. Sci. 2023, 24, 9978. [Google Scholar] [CrossRef]
- Vitali, C.; Wellington, C.L.; Calabresi, L. HDL and Cholesterol Handling in the Brain. Cardiovasc. Res. 2014, 103, 405–413. [Google Scholar] [CrossRef]
- Qi, L.; Xing, J.; Yuan, Y.; Lei, M. Noncoding RNAs in Atherosclerosis: Regulation and Therapeutic Potential. Mol. Cell Biochem. 2024, 479, 1279–1295. [Google Scholar] [CrossRef]
- Chen, H.; Gao, J.; Xu, Q.; Wan, D.; Zhai, W.; Deng, L.; Qie, R. MiR-145-5p Modulates Lipid Metabolism and M2 Macrophage Polarization by Targeting PAK7 and Regulating β-Catenin Signaling in Hyperlipidemia. Can. J. Physiol. Pharmacol. 2021, 99, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Kölling, M.; Kaucsar, T.; Schauerte, C.; Hübner, A.; Dettling, A.; Park, J.-K.; Busch, M.; Wulff, X.; Meier, M.; Scherf, K. Therapeutic MiR-21 Silencing Ameliorates Diabetic Kidney Disease in Mice. Mol. Ther. 2017, 25, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Duan, L.; Tian, L.; Liu, J.; Wang, S.; Gao, Y.; Yang, J. Serum MiR-21 May Be a Potential Diagnostic Biomarker for Diabetic Nephropathy. Exp. Clin. Endocrinol. Diabetes 2016, 124, 417–423. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, J.; Xiong, Y.; Mo, H.; Niu, H.; Miao, J.; Shen, W.; Zhou, S.; Wang, X.; Li, X.; Zhang, Y. MicroRNA-29b Plays a Vital Role in Podocyte Injury and Glomerular Diseases through Inducing Mitochondrial Dysfunction. Int. J. Biol. Sci. 2024, 20, 4654–4673. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, D.; Ou, L.-N.; Guo, X.-R.; Wu, B. Circulating Expression Level of LncRNA Malat1 in Diabetic Kidney Disease Patients and Its Clinical Significance. J. Diabetes Res. 2020, 2020, 4729019. [Google Scholar] [CrossRef]
- Song, P.; Chen, Y.; Liu, Z.; Liu, H.; Xiao, L.; Sun, L.; Wei, J.; He, L. LncRNA MALAT1 Aggravates Renal Tubular Injury via Activating LIN28A and the Nox4/AMPK/MTOR Signaling Axis in Diabetic Nephropathy. Front. Endocrinol. 2022, 13, 895360. [Google Scholar] [CrossRef]
- Li, B.; Leung, J.C.K.; Chan, L.Y.Y.; Li, H.-Y.; Yiu, W.-H.; Lok, S.W.Y.; Xue, R.; Zou, Y.-X.; Chen, W.; Lai, K.-N. Tubule-Specific Deletion of LincRNA-P21 Ameliorates Lipotoxic Kidney Injury. Mol. Ther. Nucleic Acids 2021, 26, 1280–1290. [Google Scholar]
- Jiang, Y.; Ma, F.; Wang, J.; Chen, X.; Xue, L.; Chen, X.; Hu, J. Up-Regulation of Long Non-Coding RNA H19 Ameliorates Renal Tubulointerstitial Fibrosis by Reducing Lipid Deposition and Inflammatory Response through Regulation of the MicroRNA-130a-3p/Long-Chain Acyl-CoA Synthetase 1 Axis. Noncoding RNA Res. 2024, 9, 1120–1132. [Google Scholar] [CrossRef]
- Zong, Y.; Wang, X.; Cui, B.; Xiong, X.; Wu, A.; Lin, C.; Zhang, Y. Decoding the Regulatory Roles of Non-Coding RNAs in Cellular Metabolism and Disease. Mol. Ther. 2023, 31, 1562–1576. [Google Scholar] [CrossRef]
- Velu, C.S.; Grimes, H.L. Utilizing AntagomiR (Antisense MicroRNA) to Knock down MicroRNA in Murine Bone Marrow Cells. In Rational Drug Design: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2012; pp. 185–195. [Google Scholar]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of MicroRNAs in Vivo with ‘Antagomirs’. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef]
- Mattes, J.; Yang, M.; Foster, P.S. Regulation of MicroRNA by Antagomirs: A New Class of Pharmacological Antagonists for the Specific Regulation of Gene Function? Am. J. Respir. Cell Mol. Biol. 2007, 36, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Xu, S.; Wang, J.; Xu, L.; Zhang, C.; Chen, K.; Lian, Z.; Zhou, J.; Xie, H.; Zheng, S. Delivery of MicroRNA-33 Antagomirs by Mesoporous Silica Nanoparticles to Ameliorate Lipid Metabolic Disorders. Front. Pharmacol. 2020, 11, 921. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Lee, H.Y.; Choi, J.Y.; Hur, J.; Kim, I.K.; Kim, Y.K.; Kang, J.Y.; Lee, S.Y. Inhibition of MicroRNA-21 by an Antagomir Ameliorates Allergic Inflammation in a Mouse Model of Asthma. Exp. Lung Res. 2017, 43, 109–119. [Google Scholar] [CrossRef] [PubMed]
- van Solingen, C.; Seghers, L.; Bijkerk, R.; Duijs, J.M.G.J.; Roeten, M.K.; van Oeveren-Rietdijk, A.M.; Baelde, H.J.; Monge, M.; Vos, J.B.; de Boer, H.C. Antagomir-mediated Silencing of Endothelial Cell Specific MicroRNA-126 Impairs Ischemia-induced Angiogenesis. J. Cell Mol. Med. 2009, 13, 1577–1585. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef]
- Ray, K.K.; Troquay, R.P.T.; Visseren, F.L.J.; Leiter, L.A.; Wright, R.S.; Vikarunnessa, S.; Talloczy, Z.; Zang, X.; Maheux, P.; Lesogor, A. Long-Term Efficacy and Safety of Inclisiran in Patients with High Cardiovascular Risk and Elevated LDL Cholesterol (ORION-3): Results from the 4-Year Open-Label Extension of the ORION-1 Trial. Lancet Diabetes Endocrinol. 2023, 11, 109–119. [Google Scholar] [CrossRef]
- Ahmad, M.; Hegele, R.A. Molecular Therapeutics in Development to Treat Hyperlipoproteinemia. Mol. Diagn. Ther. 2025, 29, 291–305. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Li, Y.; Cheng, R.; Chen, W. Research Advances in Current Drugs Targeting Hyperlipidemia. Mol. Med. Rep. 2025, 32, 1–17. [Google Scholar] [CrossRef]
- Bergmark, B.A.; Marston, N.A.; Bramson, C.R.; Curto, M.; Ramos, V.; Jevne, A.; Kuder, J.F.; Park, J.-G.; Murphy, S.A.; Verma, S. Effect of Vupanorsen on Non–High-Density Lipoprotein Cholesterol Levels in Statin-Treated Patients with Elevated Cholesterol: TRANSLATE-TIMI 70. Circulation 2022, 145, 1377–1386. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Gaudet, D.; Hegele, R.A.; Ballantyne, C.M.; Nicholls, S.J.; Lucas, K.J.; San Martin, J.; Zhou, R.; Muhsin, M.; Chang, T. Zodasiran, an RNAi Therapeutic Targeting ANGPTL3, for Mixed Hyperlipidemia. N. Engl. J. Med. 2024, 391, 913–925. [Google Scholar] [CrossRef]
- Rosenson, R.; Gaudet, D.; Hegele, R.; Ballantyne, C.; Nicholls, S.; Lucas, K.; Hellawell, J.; Chang, T.; Fu, R.; Muhsin, M. Zodasiran Silences Hepatic ANGPTL3 Leading to Deep and Durable Reductions in Atherogenic Lipids and Lipoproteins in Mixed Dyslipidemia Patients: Final Results from Arches-2, Double-Blind Period. Atherosclerosis 2024, 395, 118515. [Google Scholar] [CrossRef]
- Tang, X.L.; Hooper, A.J.; Burnett, J.R. Assessing the Clinical Potential of Plozasiran, an APOC3 SiRNA Therapy for Severe Hypertriglyceridemia. Expert Opin. Investig. Drugs 2024, 33, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.M.; Gaudet, D.; Rosenson, R.S.; Hegele, R.A.; Zhou, R.; Melquist, S.; Hellawell, J.; Leeper, N.J. Effect of Targeting Apoc-III with Plozasiran on Lipoprotein Particle Size and Number in Hypertriglyceridemia. J. Am. Coll. Cardiol. 2025, 85, 1839–1854. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.M.; Vasas, S.; Azizad, M.; Clifton, P.; Rosenson, R.S.; Chang, T.; Melquist, S.; Zhou, R.; Mushin, M.; Leeper, N.J. Plozasiran, an RNA Interference Agent Targeting APOC3, for Mixed Hyperlipidemia. N. Engl. J. Med. 2024, 391, 899–912. [Google Scholar] [CrossRef]
- Gaudet, D.; Pall, D.; Watts, G.F.; Nicholls, S.J.; Rosenson, R.S.; Modesto, K.; San Martin, J.; Hellawell, J.; Ballantyne, C.M. Plozasiran (ARO-APOC3) for Severe Hypertriglyceridemia: The SHASTA-2 Randomized Clinical Trial. JAMA Cardiol. 2024, 9, 620–630. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Rosenson, R.S.; Gencer, B.; López, J.A.G.; Lepor, N.E.; Baum, S.J.; Stout, E.; Gaudet, D.; Knusel, B.; Kuder, J.F. Small Interfering RNA to Reduce Lipoprotein (a) in Cardiovascular Disease. N. Engl. J. Med. 2022, 387, 1855–1864. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; López, J.A.G.; Knusel, B.; Gencer, B.; Wang, H.; Wu, Y.; Kassahun, H.; Sabatine, M.S. Study Design and Rationale for the Olpasiran Trials of Cardiovascular Events And LipoproteiN (a) Reduction-DOSE Finding Study (OCEAN (a)-DOSE). Am. Heart J. 2022, 251, 61–69. [Google Scholar] [CrossRef]
- Warden, B.A.; Duell, P.B. Antisense Oligonucleotide Targeting Apolipoprotein (a) Treatment of Lipoprotein Disorders Treatment of Cardiovascular Diseases. Drugs Future 2022, 47, 11–25. [Google Scholar] [CrossRef]
- Ogieuhi, I.J.; Callender, K.; Odukudu, G.O.; Obi, E.S.; Muzofa, K.; Babalola, A.E.; Ugiomoh, O.M.-A.; Umenzeakor, K.H.; Akingbola, A.; Ayoson, C.O. Antisense Oligonucleotides in Dyslipidemia Management: A Review of Clinical Trials. High. Blood Press. Cardiovasc. Prev. 2025, 32, 33–47. [Google Scholar] [PubMed]
- Wang, Z. The Guideline of the Design and Validation of MiRNA Mimics. In MicroRNA and Cancer: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2010; pp. 211–223. [Google Scholar]
- Hassan, M.; Elzallat, M.; Aboushousha, T.; Elhusseny, Y.; El-Ahwany, E. MicroRNA-122 Mimic/MicroRNA-221 Inhibitor Combination as a Novel Therapeutic Tool against Hepatocellular Carcinoma. Noncoding RNA Res. 2023, 8, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, H.; Ren, J.; Geng, Q.; Song, J.; Lee, C.; Cao, C.; Zhang, J.; Xu, N. MicroRNA-223 Inhibits Tissue Factor Expression in Vascular Endothelial Cells. Atherosclerosis 2014, 237, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Sallam, T.; Jones, M.; Gilliland, T.; Li, Z.; Wu, X.; Eskin, A.; Sandhu, J.; Casero, D.; Vallim, T.; Cynthia, H. Feedback Regulation of Cholesterol Metabolism by Lexis, a Lipid-Responsive Non-Coding RNA. Circulation 2015, 132, A13674. [Google Scholar] [CrossRef]
- Rayner, K.J.; Suárez, Y.; Dávalos, A.; Parathath, S.; Fitzgerald, M.L.; Tamehiro, N.; Fisher, E.A.; Moore, K.J.; Fernández-Hernando, C. MiR-33 Contributes to the Regulation of Cholesterol Homeostasis. Science 2010, 328, 1570–1573. [Google Scholar] [CrossRef]
- Huang, L.; Luo, J.; Gao, W.; Song, N.; Tian, H.; Zhu, L.; Jiang, Q.; Loor, J.J. Crispr/Cas9-Induced Knockout of MiR-24 Reduces Cholesterol and Monounsaturated Fatty Acid Content in Primary Goat Mammary Epithelial Cells. Foods 2022, 11, 2012. [Google Scholar] [CrossRef]
- Ijee, S.; Chambayil, K.; Chaudhury, A.D.; Bagchi, A.; Modak, K.; Das, S.; Benjamin, E.S.B.; Rani, S.; Paul, D.Z.; Nath, A. Efficient Deletion of MicroRNAs Using CRISPR/Cas9 with Dual Guide RNAs. Front. Mol. Biosci. 2024, 10, 1295507. [Google Scholar] [CrossRef]
- Laterza, O.F.; Scott, M.G.; Garrett-Engele, P.W.; Korenblat, K.M.; Lockwood, C.M. Circulating MiR-122 as a Potential Biomarker of Liver Disease. Biomark. Med. 2013, 7, 205–210. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Y.; Zheng, R.; Guo, Y.; Wang, Y.; Guo, H.; Fei, M.; Sun, S. Plasma MicroRNA-122 as a Biomarker for Viral-, Alcohol-, and Chemical-Related Hepatic Diseases. Clin. Chem. 2010, 56, 1830–1838. [Google Scholar] [CrossRef]
- Tobaruela-Resola, A.L.; Milagro, F.I.; Elorz, M.; Benito-Boillos, A.; Herrero, J.I.; Mogna-Peláez, P.; Tur, J.A.; Martínez, J.A.; Abete, I.; Zulet, M.Á. Circulating MiR-122-5p, MiR-151a-3p, MiR-126-5p and MiR-21-5p as Potential Predictive Biomarkers for Metabolic Dysfunction-Associated Steatotic Liver Disease Assessment. J. Physiol. Biochem. 2024, 1–14. [Google Scholar] [CrossRef]
- Tobaruela-Resola, A.L.; Milagro, F.I.; Mogna-Pelaez, P.; Moreno-Aliaga, M.J.; Abete, I.; Zulet, M.Á. The Use of Circulating MiRNAs for the Diagnosis, Prognosis, and Personalized Treatment of MASLD. J. Physiol. Biochem. 2025, 81, 589–609. [Google Scholar] [CrossRef]
- Cermelli, S.; Ruggieri, A.; Marrero, J.A.; Ioannou, G.N.; Beretta, L. Circulating MicroRNAs in Patients with Chronic Hepatitis C and Non-Alcoholic Fatty Liver Disease. PLoS ONE 2011, 6, e23937. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-L.; Pan, Q.; Zhang, R.-N.; Shen, F.; Yan, S.-Y.; Sun, C.; Xu, Z.-J.; Chen, Y.-W.; Fan, J.-G. Disease-Specific MiR-34a as Diagnostic Marker of Non-Alcoholic Steatohepatitis in a Chinese Population. World J. Gastroenterol. 2016, 22, 9844. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yan, X.; Xu, G.; Pang, Z.; Weng, J.; Yin, J.; Li, M.; Yu, L.; Chen, Q.; Sun, K. A Novel Plasma LncRNA ENST00000416361 Is Upregulated in Coronary Artery Disease and Is Related to Inflammation and Lipid Metabolism. Mol. Med. Rep. 2020, 21, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, Y.; Wu, G.; Chen, X.; Liu, Y.; Wang, X.; Yu, J.; Li, C.; Chen, X.; Jose, P.A. Plasma Long Non-Coding RNA, CoroMarker, a Novel Biomarker for Diagnosis of Coronary Artery Disease. Clin. Sci. 2015, 129, 675–685. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, Y.; Chen, X.; Wu, G.; Zhang, X.; Liu, Y.; Yu, J.; Wang, X.; Fu, J.; Li, C. Circulating ‘LncRNA OTTHUMT00000387022’from Monocytes as a Novel Biomarker for Coronary Artery Disease. Cardiovasc. Res. 2016, 112, 714–724. [Google Scholar] [CrossRef]
- Hsu, S.; Yu, B.; Wang, X.; Lu, Y.; Schmidt, C.R.; Lee, R.J.; Lee, L.J.; Jacob, S.T.; Ghoshal, K. Cationic Lipid Nanoparticles for Therapeutic Delivery of SiRNA and MiRNA to Murine Liver Tumor. Nanomedicine 2013, 9, 1169–1180. [Google Scholar] [CrossRef]
- Schachner-Nedherer, A.-L.; Fuchs, J.; Vidakovic, I.; Höller, O.; Schratter, G.; Almer, G.; Fröhlich, E.; Zimmer, A.; Wabitsch, M.; Kornmueller, K. Lipid Nanoparticles as a Shuttle for Anti-Adipogenic MiRNAs to Human Adipocytes. Pharmaceutics 2023, 15, 1983. [Google Scholar] [CrossRef]
- Hashemi, M.; Ghadyani, F.; Hasani, S.; Olyaee, Y.; Raei, B.; Khodadadi, M.; Ziyarani, M.F.; Basti, F.A.; Tavakolpournegari, A.; Matinahmadi, A. Nanoliposomes for Doxorubicin Delivery: Reversing Drug Resistance, Stimuli-Responsive Carriers and Clinical Translation. J. Drug Deliv. Sci. Technol. 2023, 80, 104112. [Google Scholar] [CrossRef]
- Albakr, L.; Alqahtani, F.Y.; Aleanizy, F.S.; Alomrani, A.; Badran, M.; Alhindas, H.; Al-Mohanna, F. Improved Delivery of MiR-1296 Loaded Cationic Nanoliposomes for Effective Suppression of Triple Negative Breast Cancer. Saudi Pharm. J. 2021, 29, 446–455. [Google Scholar] [CrossRef]
- Qin, Z.-X.; Zuo, L.; Zeng, Z.; Ma, R.; Xie, W.; Zhu, X.; Zhou, X. GalNac-SiRNA Conjugate Delivery Technology Promotes the Treatment of Typical Chronic Liver Diseases. Expert. Opin. Drug Deliv. 2025, 22, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qin, G.; Shi, L.; Li, R.; Shen, L.; Cao, W.; Tao, Y. Comparative Metabolism of an N-Acetylgalactosamine–Conjugated Small Interfering RNA, Inclisiran, among Various in Vitro Systems and Correlations with in Vivo Metabolism in Rats. Drug Metab. Dispos. 2025, 53, 100089. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, W.; Zheng, S.; Yuan, Y.; Wen, W.; Cui, W.; Xue, L.; Sun, X.; Shang, H.; Zhang, H. Targeting ANGPTL3 by GalNAc-Conjugated SiRNA ANGsiR10 Lowers Blood Lipids with Long-Lasting and Potent Efficacy in Mice and Monkeys. Mol. Ther. Nucleic Acids 2023, 31, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Sutaria, D.S.; Jiang, J.; Elgamal, O.A.; Pomeroy, S.M.; Badawi, M.; Zhu, X.; Pavlovicz, R.; Azevedo-Pouly, A.C.P.; Chalmers, J.; Li, C. Low Active Loading of Cargo into Engineered Extracellular Vesicles Results in Inefficient MiRNA Mimic Delivery. J. Extracell. Vesicles 2017, 6, 1333882. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Bala, S.; Bukong, T.; Szabo, G. Exosome-Mediated Delivery of Functionally Active MiRNA-155 Inhibitor to Macrophages. Nanomedicine 2014, 10, 1517–1527. [Google Scholar] [CrossRef]
- Jin, Y.; Lee, J.S.; Min, S.; Park, H.; Kang, T.J.; Cho, S. Bioengineered Extracellular Membranous Nanovesicles for Efficient Small-Interfering RNA Delivery: Versatile Platforms for Stem Cell Engineering and In Vivo Delivery. Adv. Funct. Mater. 2016, 26, 5804–5817. [Google Scholar] [CrossRef]
- Tai, Z.; Ma, J.; Ding, J.; Pan, H.; Chai, R.; Zhu, C.; Cui, Z.; Chen, Z.; Zhu, Q. Aptamer-Functionalized Dendrimer Delivery of Plasmid-Encoding LncRNA MEG3 Enhances Gene Therapy in Castration-Resistant Prostate Cancer. Int. J. Nanomed. 2020, 15, 10305–10320. [Google Scholar] [CrossRef]
- Ning, H.; Jiang, Y.; Li, B.; Ren, J.; Ran, A.; Li, W.; Xiao, B. Targeted Delivery of CircPDHK1 SiRNA via Aptamer Functionalized Lipid Nanoparticles Inhibits CcRCC Growth and Migration. Int. J. Pharm. 2025, 677, 125666. [Google Scholar] [CrossRef]
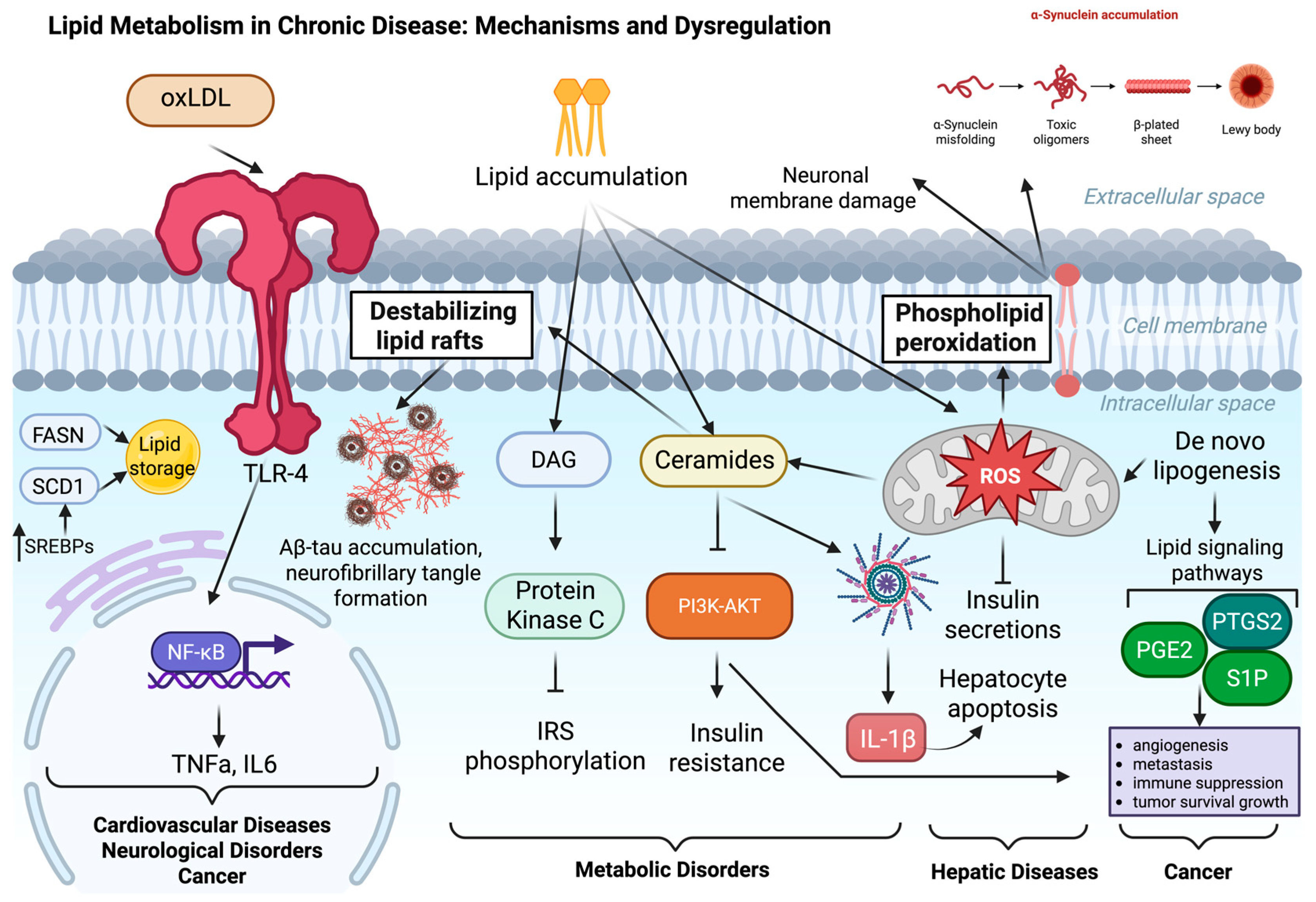

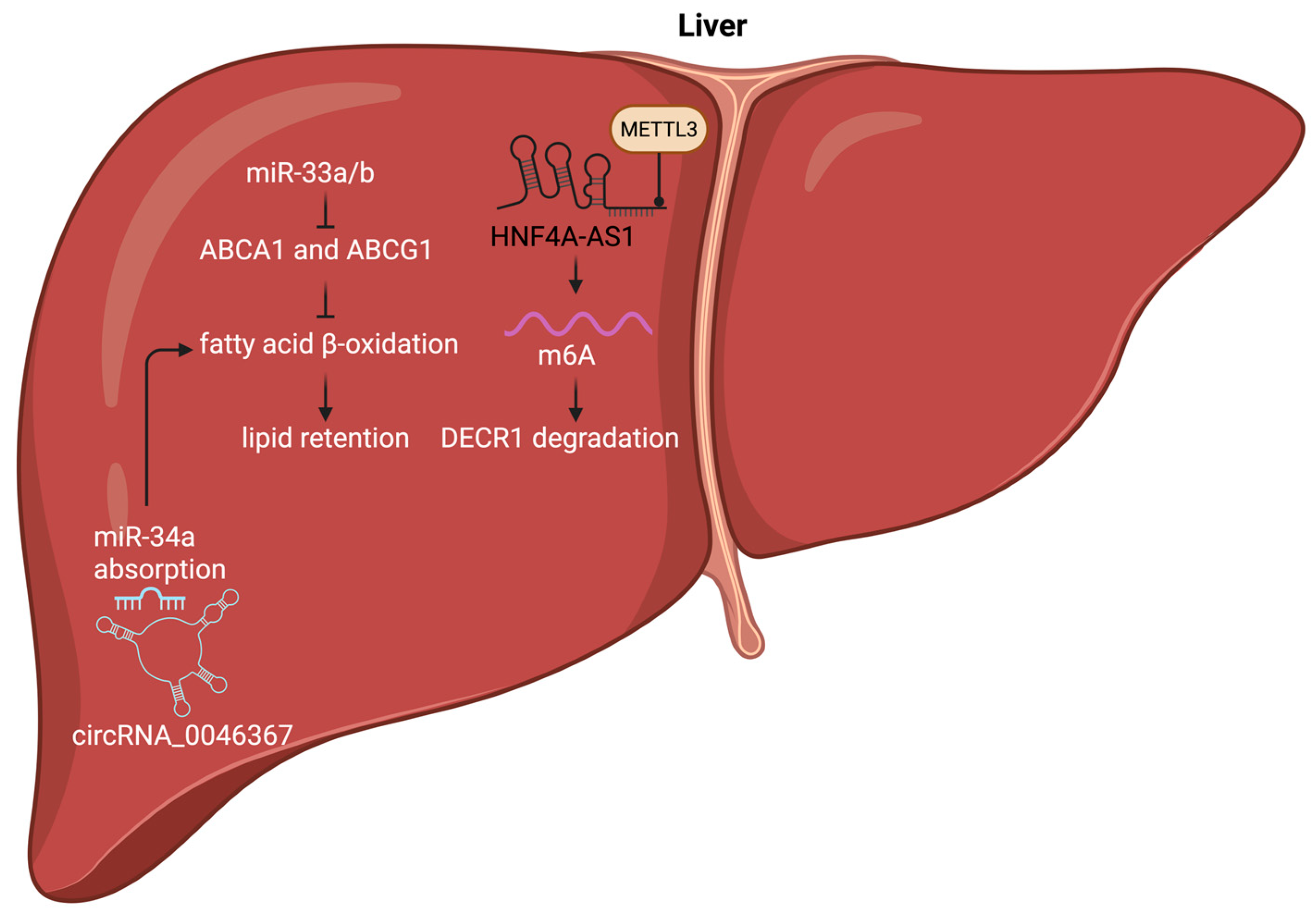
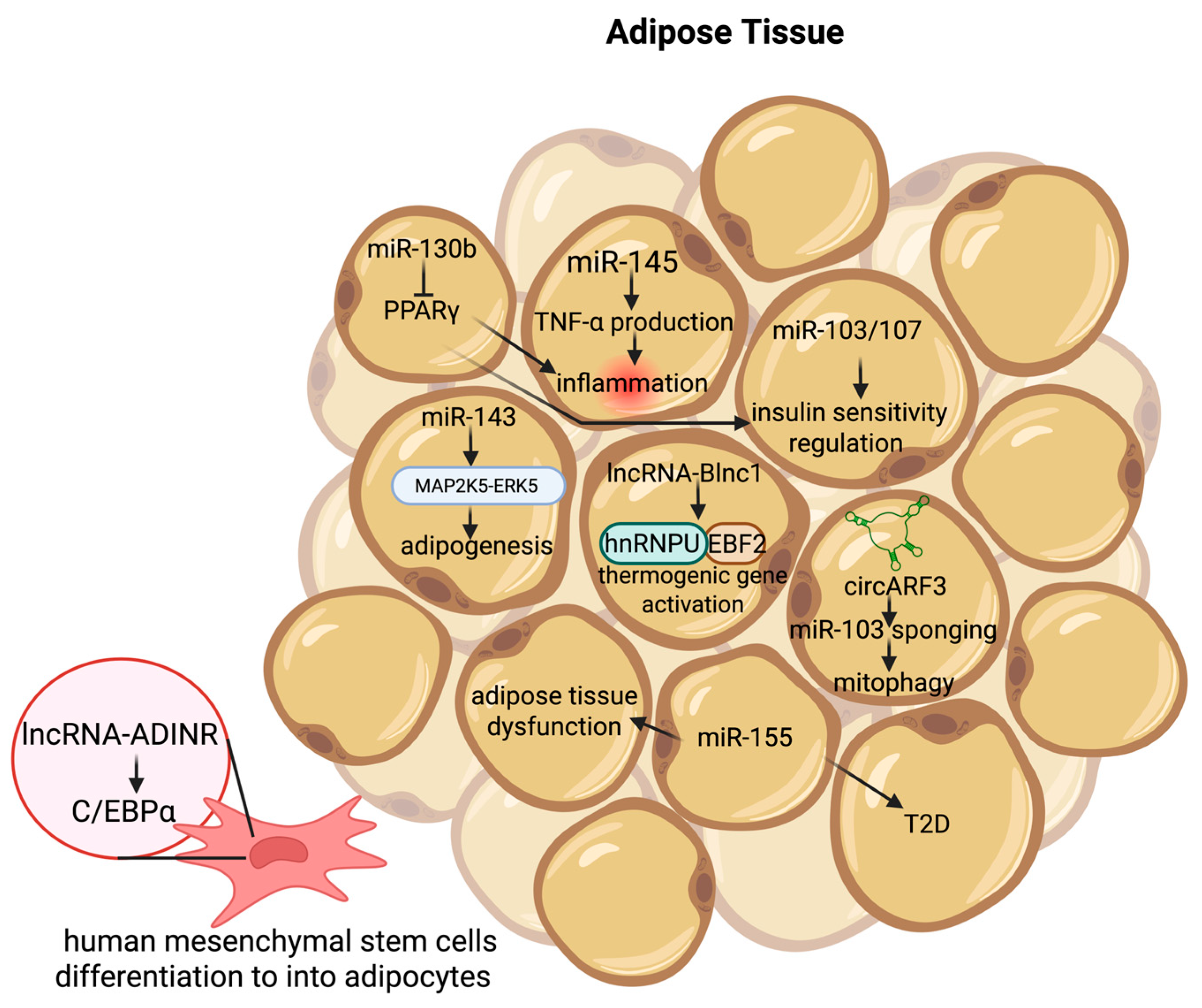
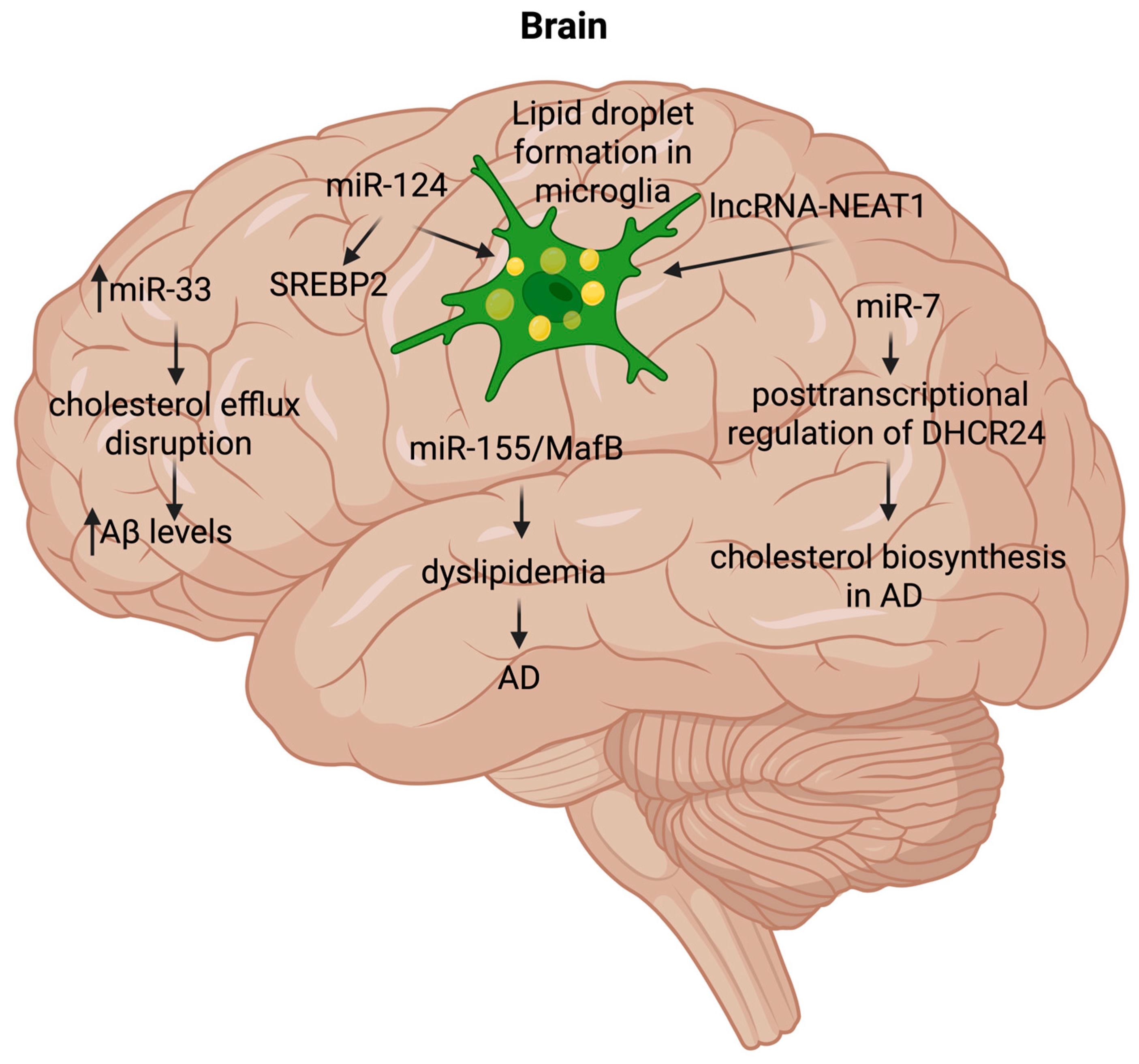
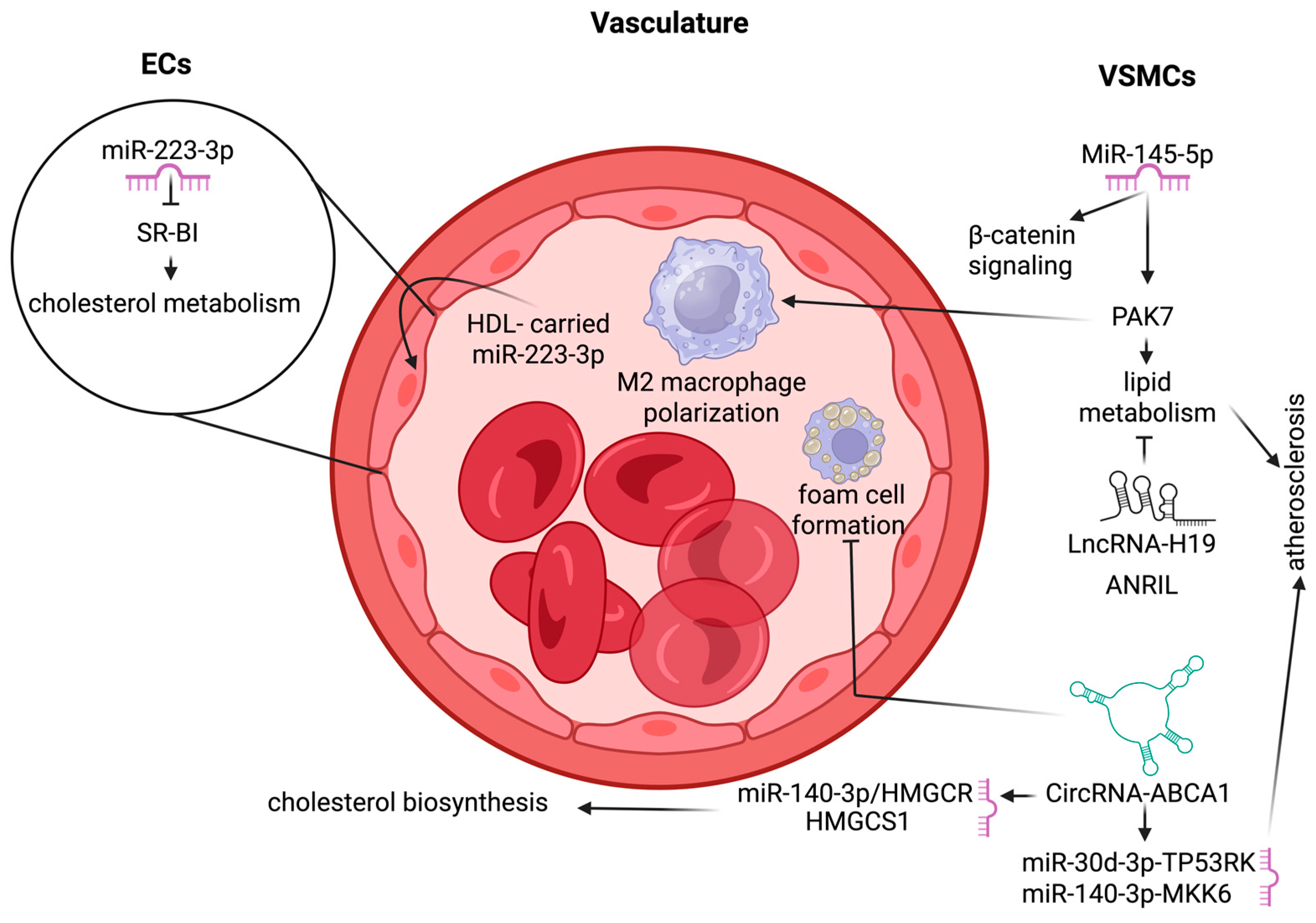
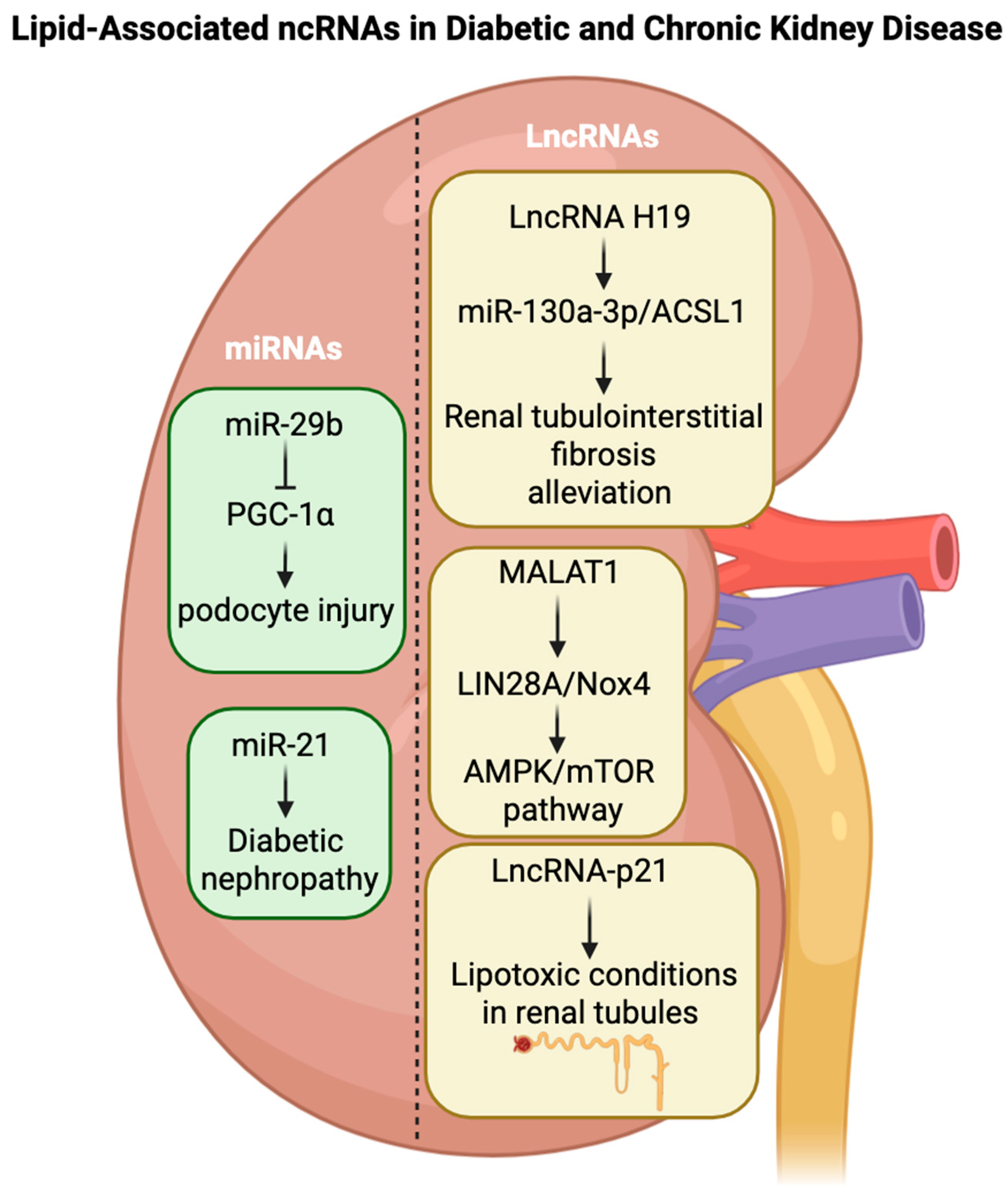
| miRNA | Target | Effect | Disease | References |
|---|---|---|---|---|
| miR-27b | HMGCR | Cholesterol synthesis | MASLD | [109,110] |
| miR-27a | Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) | Triglyceride accumulation, Adipogenesis | T2D, MASLD | [111,112] |
| miR-21 | Secreted Frizzled-Related Protein 5 (SFRP5), vascular cell adhesion molecule 1 (VCAM-1), Peroxisome Proliferator-Activated Receptor Alpha (PPARα), PTEN/AKT signaling, TNFα, IL-6 | Liver lipid-induced inflammation and fibrosis, decrease fatty acid oxidation, and increase lipid accumulation and Lipogenesis | MASLD/NASH, Atherosclerosis, T2D, DKD | [113,114,115,116,117,118,119,120] |
| miR-122 | SREBP-1c, Carnitine palmitoyltransferase 1A (CPT1A), PPARα | Promotes DNL by targeting suppressors of lipogenesis, increases VLDL production, and causes hypertriglyceridemia | MASLD/NASH, T2D | [108,121,122,123] |
| miR-33a/b | CPT1A, ATP-binding cassette transporter A1 and G1 (ABCA1, ABCG1) | Represses fatty acid oxidation, reduces cholesterol, and Aβ efflux | Atherosclerosis, AD | [124,125,126,127] |
| miR-223 | Scavenger Receptor Class B Type 1 (SCARB1 or SR-B1), ABCA1 | Dysregulation of HDL cholesterol uptake and inflammation | Atherosclerosis, CVD, MASLD | [128,129,130,131] |
| miR-20a-5p | CD36 | Lipid uptake and storage | MASLD, T2D, CVD | [132,133,134] |
| miR-34a | Sterol Regulatory Element Binding Transcription Factor 1 (SREBF1), PPARα | Increases lipogenesis, less mitochondrial and peroxisomal fatty acid oxidation | MASLD/NASH, T2D, AD | [135,136,137,138] |
| miR-10b | PPARα, transforming growth factor beta 1 (TGF-β1) | Fatty acid oxidation and lipid accumulation | MASLD | [139,140] |
| miR-96 | Adipose Triglyceride Lipase (ATGL), ABCA1, SREBP2 | Represses intramuscular lipolysis, fatty acid oxidation, and uptake of HDL-associated cholesteryl esters | Atherosclerosis | [141,142,143] |
| miR-144 | ABCA1 | Decrease cholesterol efflux and HDL levels | Atherosclerosis | [144,145] |
| miR-155 | ABCA1, Liver X Receptor α (LXRα) | Decrease cholesterol efflux and HDL formation | Atherosclerosis, T2D, AD | [146,147,148,149,150] |
| miR-29 | HMGCR, SREBP-1c, Insulin Receptor Substrate 1 (IRS1), AKT3, PPARδ, GSK3β/SIRT1 | Impairs insulin signaling, decreases fatty acid synthesis, and cholesterol production | MASLD/NASH, T2D, DKD | [151,152,153,154,155] |
| miR-143 | oxysterol-binding protein-related protein 8 (ORP8), IRS1, MAP2K5–ERK5 | Impairs insulin signaling, Adipogenesis | Atherosclerosis, T2D | [156,157,158] |
| miR-375 | Adiponectin Receptor 2 (AdipoR2) | Decreases lipid oxidation | MASLD, T2D | [159,160,161] |
| miR-320 | IRS1, CD36 | Increase lipid accumulation | MASLD, T2D | [162,163] |
| miR-192 | SREBF1, SCD1, PPARα | Increase lipid accumulation | MASLD/NASH, T2D | [164,165,166] |
| miR-106b | ABCA1, PTEN | Impairs cholesterol efflux | Atherosclerosis, AD, MASLD | [167,168,169] |
| miR-146a | Mediator complex subunit 1 (MED1), Tumor necrosis factor receptor-associated factor 6 (TRAF6) | Increase lipid accumulation | MASLD, AD, Atherosclerosis | [170,171,172,173] |
| miR-125 | ELOVL fatty acid elongase 6 (Elovl6), NF-κB signaling, Sphingosine Kinase 1 (SphK1) | Increase lipid accumulation and inflammation | T2D, MASLD, AD | [174,175,176] |
| miR-137 | AMP-activated protein kinase (AMPKα), Calcium channel subunit (CACNA1C), PTGS2 | Decrease lipid accumulation, decrease inflammation, | MASLD, AD | [177,178] |
| miR-210 | Insulin-like Growth Factor 2 (IGF2), Suppressor of Cytokine Signaling 1 (SOCS1) | Decrease lipid accumulation and inflammation, | Atherosclerosis, T2D | [179,180] |
| miR-124 | Preadipocyte factor-1 (Pref1), SREBP2 | Regulates triglyceride contents in hepatocytes, Lipid droplet formation | MASLD, Atherosclerosis | [181,182,183,184] |
| miR-130b | PPARγ, TNFα | Inflammation in adipose tissue and insulin sensitivity | Obesity, T2D | [185,186] |
| miR-126 | SCARB1, hypoxia-inducible factor 1α (HIF1α) | Suppressing vascular inflammation | CVD | [187] |
| miR-148a | LDL receptors (LDLR), ABCA1 | Regulates LDL-cholesterol uptake | Atherosclerosis | [188,189] |
| miR-103 | Caveolin-1 (CAV1), SFRP4 | Decreases adipocyte size and enhances insulin-stimulated glucose uptake | MASLD/NASH, T2D | [190,191] |
| miR-107 | CAV1 | Decreases adipocyte size and enhances insulin-stimulated glucose uptake | MASLD/NASH, T2D | [190] |
| miR-145 | TNFα, NF-κB | Inflammation in adipose tissue | Obesity, Atherosclerosis | [192] |
| miR-7 | LXR, ABCA1, 24-dehydrocholesterol reductase (DHCR24) | Regulates cholesterol efflux | AD, T2D | [193] |
| lncRNA | Target | Effect | Disease | References |
|---|---|---|---|---|
| lnc-LeXis | RNA-binding protein RALY | Increases the hepatic cholesterol | MASLD/NASH | [204,205] |
| lnc-LSTR | TDP-43, Cyp8b1 | Regulates bile acid composition, which influences lipid absorption and metabolism | Atherosclerosis | [206,207] |
| lnc-MALAT1 | ABCA1, miRNA-17-5p, PPARα/CD36, miR-206, miR-382-3p | Inhibits glucose uptake and lipogenesis, increases cholesterol accumulation | Atherosclerosis, MASLD, T2D, DKD | [208,209,210,211,212] |
| lnc-H19 | MLX-interacting protein-like (MLXIPL), mTORC1, PPARγ, miR-130b-3p | Increases lipid synthesis and accumulation, adipogenesis | MASLD, Atherosclerosis, DKD | [213,214,215] |
| lnc-ANRIL | Cyclin-dependent kinase inhibitor (CDKN2A/B), PRC1/2, NF-κB, ADIPOR1, VAMP3, C11ORF10 | Impair cholesterol efflux, glycolipid metabolism | Atherosclerosis | [216,217,218,219] |
| lnc-BLNC1 | Early B-cell factor 2 (EBF2), uncoupling protein 1 (UCP1), SREBP1c | Exacerbate insulin resistance, lipid accumulation, and adipogenesis | MASLD, T2D | [220,221,222] |
| lnc-MEG3 | mir-21, Low-density lipoprotein receptor-related protein 6 (LRP6), Sirtuin 6 (SIRT6), FOXO1, Acetyl-CoA Carboxylase 1 (ACC1) | FFA-induced lipid accumulation | MASLD, T2D | [223,224,225,226] |
| lnc-LINC00473 | UCP1, Cyclic adenosine monophosphate (cAMP), Perilipin 1 (PLIN1) | Lipolysis, respiration process, and mitochondrial oxidative metabolism | T2D | [227,228] |
| lnc-ADINR | c/EBPα, histone methyltransferase complex (MLL3/4), PA1 | Adipogenesis | Obesity | [229,230] |
| lnc-NEAT1 | ATGL, CCAAT/enhancer-binding protein α (CEBPα), PPARγ, miR-140, AMPK/SREBP-1 signaling, miR-146a-5p/ROCK1 | Adipogenesis, lipolysis | MASLD, Atherosclerosis, AD | [231,232,233,234,235,236] |
| lnc-CHROME | miR-27b, miR-33a/b, miR-128, ABCA1 | Cholesterol secretion and HDL synthesis, | Atherosclerosis | [237,238] |
| lnc-TUG1 | miR-204/SIRT1, PPARα | Fatty acid β-oxidation | Obesity, T2D | [239,240,241] |
| lnc-Dnm3os | NF-κB, Nucleolin | Glucose uptake and increasing free fatty acid, inflammation-induced lipid accumulation | T2D | [242] |
| lnc-HI-LNC45 | Unknown | β-cell dysfunction | T2D | [243,244] |
| lnc-SNHG1 | Glutathione peroxidase 4 (GPX4), Nuclear factor erythroid 2-related factor 2 (NRF2), Nuclear Receptor Coactivator 4 (NCOA4), CD98, Polypyrimidine tract-binding protein 1 (PTBP1), miR-7/NLRP3, | Fatty acid β-oxidation, ferroptosis, adipogenic differentiation | PD | [245,246,247] |
| lnc-NRON | PER2/Rev-Erbα/FGF21, AMPK | Hepatic lipid homeostasis enhances adipose function via triacylglycerol hydrolysis | MASLD, T2D, Atherosclerosis | [248,249,250,251] |
| lnc-UCA1 | PPARα, miR-30a-3p, retinoid X receptor α (RXRα), miR-143-3p, miR-214 | Promotes lipid accumulation, mitochondrial dysfunction | T2D, MASLD, PD | [252,253,254,255] |
| lnc-MIAT (GOMAFU) | IL-1β, IL-6, and Tumor necrosis factor alpha (TNFα), HIF1α, FOXO1, miR-139-5p | Increases lipid content and reduces collagen content in the plaques, microvascular dysfunction, and promotes hepatic insulin resistance | Atherosclerosis, CVD, T2D | [256,257,258,259] |
| lnc-Gm15622 | SREBP-1c, miR-742-3p | Lipid accumulation in hepatocytes | MASLD | [260] |
| lnc-HNF4A-AS1 | Methyltransferase-like 3 (METTL3) | Regulates intracellular polyunsaturated fatty acids (PUFA) | MASLD | [261] |
| circRNA | Target | Effect | Disease | References |
|---|---|---|---|---|
| circRNA-0046367 circRNA-0046366 | miR-34a/PPARα | β-oxidation of fatty acids | MASLD/NASH | [271,272,273] |
| circRNA-0001452 circRNA-0001453 circRNA-0001454 | miR-466i-3p, miR-669c-3p, AMPK | Promotes the transcription and translation of lipogenic genes | MASLD | [274] |
| circRNA-0071410 | miR-9-5p | Fibrosis development | MASLD/NASH | [272,275] |
| circRNA-SCD1 | JAK2/STAT5 | Hepatic lipid droplet formation | MASLD | [276,277] |
| circRNA-ABCA1 | miR-140-3p/MAP2K6 | Promotes cholesterol efflux to apolipoprotein A-I (apoA-I), β-oxidation of fatty acids | CVD, Atherosclerosis | [278,279,280] |
| circRNA-ANRIL | INK4/ARF, pescadillo homologue 1 (PES1) | Glycolipid metabolism, plaque growth | Atherosclerosis | [219,281,282,283] |
| circRNA-HIPK3 | miR-192-5p, FOXO1, miR-190b, miR-106a-5p/MFN2 | Effect of oleate on adipose deposition, Insulin resistance | Atherosclerosis, T2D, MASLD | [272,284,285,286,287] |
| circRNA-021412 | miR-1972, Lipin 1 (LPN1) | Promotes liver steatosis and inflammation | MASLD | [288,289,290] |
| circRNA-SAMD4A | miR-138-5p/EZH2 | Dysregulated adipogenesis and insulin signaling | Obesity | [291] |
| circRNA-LARP1B | cAMP/PKA signaling, Phosphodiesterase 4C (PDE4C) | Promotes lipid accumulation | Atherosclerosis | [292,293] |
| circRNA-ACC1 | miR-338-3p, AMPK-ABCA1/ABCG1 | β-oxidation of fatty acids | MASLD | [294,295] |
| circRNA-FUT10 | let-7c/let-e, PPAR1β | Inhibit the differentiation of adipocytes | Obesity, T2D | [296] |
| circRNA-0000660 | miR-693, Insulin-like Growth Factor Binding Protein 1 (IGFbp1) | Liver lipid accumulation | Obesity | [297] |
| circRNA-0003546 | miR-326, PDE3B | Increases Lipid efflux | Atherosclerosis | [298] |
| circRNA-0092317 | miR-543, miR-326, PDE3B | Increases Lipid efflux | Atherosclerosis | [298] |
| circRNA-ARF3 | miR-103, TNF receptor-associated factor 3 (TRAF3) | Inflammation in adipose tissue | Obesity | [299] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayani, Z.; Matinahmadi, A.; Tavakolpournegari, A.; Moosavi, S.S.; Bidooki, S.H. The Crosstalk Between Non-Coding RNAs and Lipid Metabolism in Chronic Disease Progression. Lipidology 2025, 2, 19. https://doi.org/10.3390/lipidology2040019
Zayani Z, Matinahmadi A, Tavakolpournegari A, Moosavi SS, Bidooki SH. The Crosstalk Between Non-Coding RNAs and Lipid Metabolism in Chronic Disease Progression. Lipidology. 2025; 2(4):19. https://doi.org/10.3390/lipidology2040019
Chicago/Turabian StyleZayani, Zoofa, Arash Matinahmadi, Alireza Tavakolpournegari, Seyedeh Safoora Moosavi, and Seyed Hesamoddin Bidooki. 2025. "The Crosstalk Between Non-Coding RNAs and Lipid Metabolism in Chronic Disease Progression" Lipidology 2, no. 4: 19. https://doi.org/10.3390/lipidology2040019
APA StyleZayani, Z., Matinahmadi, A., Tavakolpournegari, A., Moosavi, S. S., & Bidooki, S. H. (2025). The Crosstalk Between Non-Coding RNAs and Lipid Metabolism in Chronic Disease Progression. Lipidology, 2(4), 19. https://doi.org/10.3390/lipidology2040019






