Abstract
Background and aim: ANGPTL3 is a hepatokine acting as a negative regulator of lipoprotein lipase (LPL) through its N-terminal domain. Besides this activity, the C-terminal domain of ANGPTL3 interacts with integrin αVβ3. Since integrins are involved in inflammation and in the initiation of atherosclerotic plaque, the aim of our study was to evaluate the potential direct pro-inflammatory action of ANGPTL3 through the interaction of the fibrinogen-like domain and integrin αVβ3. Methods: We utilized cultured THP-1 human-derived macrophages and evaluated their pro-inflammatory phenotype in response to treatment with human recombinant ANGPTL3 (hANGPTL3). By Western blot, RT-qPCR, biochemical analysis, and ELISA assays, we determined the expression of genes and proteins involved in lipid metabolism and inflammatory response as well as intracellular cholesterol and triglyceride levels. In addition, we evaluated the effect of hANGPTL3 on the cellular cholesterol efflux process. Results: Incubation of THP-1-derived macrophages with 100 ng/mL of hANGPTL3 increased the mRNA expression of the pro-inflammatory cytokines IL-1β, IL-6, and TNFα (respectively, 1.87 ± 0.08-fold, 1.35 ± 0.11-fold, and 2.49 ± 0.43-fold vs. control). The secretion of TNFα, determined by an ELISA assay, was also induced by hANGPTL3 (1.98 ± 0.4-fold vs. control). The pro-inflammatory effect of hANGPTL3 was partially counteracted by co-treatment with the integrin αVβ3 inhibitor RGD peptide, reducing the mRNA levels of IL-1β (3.35 ± 0.35-fold vs. 2.54 ± 0.25-fold for hANGPTL3 vs. hANGPTL3 + RGD, respectively). Moreover, hANGPTL3 reduced cholesterol efflux to apoA-I, with a parallel increase in the intracellular triglyceride and cholesterol contents by 31.2 ± 2.8% and 20.0 ± 4.1%, respectively, compared to the control. Conclusions: ANGPTL3 is an important liver-derived regulator of plasma lipoprotein metabolism, and overall, our results add a new important pro-inflammatory activity of this circulating protein. This new function of ANGPTL3 could also be related to triglyceride and cholesterol accumulation into macrophages.
1. Introduction
Dyslipidemia is a major risk factor for atherosclerosis and coronary artery disease (CAD). The first line of intervention for reducing low-density lipoprotein-cholesterol (LDL-C) is represented by the use of 3-hydroxy-3-methylglutaryl-coenzyme A inhibitors, statins, which are very effective at controlling individuals’ CAD risk. A further step forward for lipid-lowering therapies was the discovery of the Niemann-Pick C1-Like 1 (NPC1L1) inhibitor ezetimibe, utilized in combination with statins to reduce cholesterol absorption at the gastrointestinal level [1]. Finally, the ATP citrate lyase inhibitor bempedoic acid has recently been approved, showing a consistent capacity to reduce, by more than 20%, LDL-C levels and CAD risk [2]. However, the most effective therapies are represented by the inhibitors of proprotein convertase subtilisin/kexin type 9 (PCSK9), including the monoclonal antibodies evolocumab [3] and alirocumab [4] and the novel N-acetylgalactosamine (GalNAc) conjugated small-interfering ribonucleic acid (siRNA) inclisiran [5]. Nevertheless, the efficacy of all of these therapies relies on the upregulation of the LDL receptor, which is partially or totally ineffective in homozygous familial hypercholesterolemia (HoFH) patients. Within this clinical contest, the monoclonal antibody for angiopoietin-like 3 (ANGPTL3), evinacumab, has recently been approved [6]. ANGPTL3 is a member of a subfamily of angiopoietin-like proteins that are involved in the regulation of plasma lipid metabolism together with ANGPTL4 and ANGPTL8 [7,8]. ANGPTL3 is a secreted protein of 54 kDa mainly produced in the liver and can, therefore, be classified as a true hepatokine [7] (a weak expression has been observed in the kidneys, increasing upon injury [9]). The primary action of ANGPTL3 is to inhibit lipoprotein lipase (LPL), the enzyme responsible for the hydrolysis of circulating triglycerides in the capillaries of adipose and muscle tissue. LPL is expressed in a number of tissues, e.g., heart, skeletal muscle, spleen, mammary glands, lung, macrophages, as well as adipose tissue [7]. In addition, ANGPTL3 inhibits endothelial lipase (EL) and, thus, the hydrolysis of phospholipids in high-density lipoprotein (HDL), leading to reduced clearance of HDL particles and subsequently increased plasma HDL-cholesterol concentration [10]. ANGPTL3 inhibition increases the fractional catabolic rate of intermediate-density lipoprotein (IDL) and LDL. This finding supports the hypothesis that evinacumab primarily lowers LDL cholesterol by enhancing the clearance of apoB-containing lipoproteins from circulation [11].
ANGPTL3 contains an N-terminal signal peptide, an N-terminal coiled-coil domain, a linker region, and a C-terminal fibrinogen-like domain [12]. The analysis of the protein region important for regulation of lipid metabolism in ANGPTL3 has demonstrated that the N-terminal domain, and not the C-terminal fibrinogen-like domain, increased the plasma triglyceride levels in mice [13]. On the contrary, the C-terminal fibrinogen-like domain of ANGPTL3 was reported to be involved in angiogenesis via binding to integrin αVβ3 [14], a receptor also expressed in macrophages [15], with documented pro-inflammatory and pro-atherogenic activities [16]. Studies using experimental animal models demonstrated that the pharmacological inhibition of ANGPTL3 by evinacumab has a profound anti-atherosclerotic effect (−39% of atherosclerotic lesion size) in APOE*3Leiden. CETP mice [17]. Further, the role of ANGPTL3 on lipid homeostasis and CAD was observed in subjects carrying rare loss-of-function variants of ANGPTL3 [18,19]. Subjects heterozygous for ANGPTL3 loss-of-function variants had approximately 50% lower ANGPTL3 levels than noncarriers (50 ng/mL vs. 99 ng/mL). This effect was associated with a 39% lower risk of CAD [17]. From this evidence, it appears clear that ANGPTL3 is an important liver-derived regulator of lipoprotein metabolism that holds considerable promise as a target for atherosclerosis. However, little is known on the potential direct vascular effect of ANGPTL3 based on its capability to bind integrin αVβ3. In the context of the crosstalk between lipid metabolism and inflammatory responses [20], here, we explore the possible pro-inflammatory and cholesterol metabolism modulating effects of ANGPTL3 on THP-1-derived macrophages.
2. Materials and Methods
2.1. Reagents and Antibodies
RPMI medium was purchased from Merck. Streptomycin, penicillin, fetal calf serum (FCS), nonessential amino acid solution, and disposable culture petri dishes and flasks were purchased from Euroclone. Molecular weight protein standards were from Thermo Fisher Scientific, Monza, Italy. BCA assay for determination of protein concentrations was purchased from Thermo Scientific. Tumor necrosis factor-α (TNFα), lipopolysaccharide (LPS), phorbol-12-myristate-13-acetate (PMA), and RGD peptides were purchased from Merck. Human recombinant ANGPTL3 was from Vinci Biochem (BPS Biosciences, San Diego, CA, USA).
2.2. Cell Culture
THP-1 human monocyte cells were cultured in RPMI medium supplemented with pen/strept, NEAA, 10% FCS, and β-mercaptoethanol 0.05 mM. THP-1 monocytes were differentiated in macrophages with phorbol 12-myristate 13-acetate (PMA; 320 nM) for 72 h.
2.3. Quantitative Real-Time PCR (qRT-PCR) Assay
Total mRNA was extracted by using iScript Sample Preparation Reagent (BIO-RAD laboratories, Milan, Italy) following the manufacturer’s instructions. iScript cDNA synthesis Kit (BIO-RAD laboratories) was used for first-strand cDNA synthesis. Thermo Sybr Green/ROX qPCR Master Mix (Carlo Erba Reagents S.r.l., Milan, Italy)), with specific primers of the genes of interest, was used for qRT-PCR analysis. The reactions were performed with the CFX96 Touch Real-Time PCR System (BIO-RAD). Data were expressed as Ct values, and the relative quantification was calculated with ΔΔCt.
2.4. Western Blot Analysis
Total protein for cell monolayers was prepared with lysis buffer containing 50 mM Tris pH 7.5, 150 mM NaCl, and 0.5% Nonidet-P40, protease and phosphatase inhibitor cocktails (Merck) for 30 min on ice. Protein samples (20 µg) and a molecular mass marker (Thermo Scientific) were separated using 4–12% gradient SDS-PAGE (BIO-RAD). The samples were then transferred to a nitrocellulose membrane using the Trans-Blot® TurboSystem (BIO-RAD laboratories, Milan, Italy). The membranes were blocked in Tris buffered saline with Tween 20 (TBS.T) containing 5% nonfat dried milk for 60 min at room temperature (RT), and blots were incubated overnight at 4 °C with the following primary antibodies: anti-ANGPTL3 (GeneTex, Milan, Italy; cod GTX104569; dilution 1:1000), anti-ANGPTL4 (Abnova, Milan, Italy; cod. H00051129-M02; dilution 1:1000), anti-CD36 (GeneTex, Milan, Italy; cod. GTX100642; rabbit polyclonal dilution 1:1000), anti-integrin αVβ3 (GeneTex, Milan, Italy; cod. GTX01084; dilution 1:500), and anti-GAPDH (GeneTex, Milan, Italy; cod. GTX100118; rabbit polyclonal, dilution 1:3000). Anti-rabbit secondary antibody was from Jackson ImmunoResearch, Milan, Italy (cod. 113-036-045, dilution 1:5000). The membranes were then washed with TBS-T and incubated for 90 min at RT to TBS-T with 5% nonfat dried milk containing the secondary antibodies (peroxidase-conjugate goat anti-rabbit and anti-mouse, Jackson Immuno Research). Immunoreactive bands were detected with Clarity Western Enhanced Chemiluminescence (ECL) chemiluminescent substrates (BIO-RAD) for 5 min, and luminescence signals were acquired with Uvitec Alliance Q9 (Uvitec, Cambridge, UK).
When used, mild stripping buffer was prepared according to Abcam’s recipe (membrane stripping for Western blot, Abcam, Milan, Italy): 15 g glycine, 1 g SDS, and 10 mL Tween 20 were dissolved in 800 mL of distilled water, and the pH was adjusted to 2.2. Upon reaching the desired pH, the volume was brought up to 1 L with distilled water. The stripping procedure was retrieved from the Abcam website as well: the membranes were incubated 2 times with an appropriate amount of stripping buffer, 10 min each; then washed 2 times with an appropriate amount of PBS, 10 min each; and finally washed 2 times with an appropriate amount of TBS-T, 5 min each. All the steps were performed at room temperature with constant agitation.
2.5. ELISA Assay for TNFα
To detect the secreted amount of TNFα, the Human TNF-alpha ELISA Kit (Quantikine R&D, Milan, Italy; cod. DTA00D) was used according to the manufacturer’s instructions. Cells were seeded in 6-well plates (200.000 cells/well) in RPMI10% FBS, and 24 h later, the treatments were performed. After 48 h, the supernatant was collected, centrifuged at 15,000 rpm for 10 min, and diluted according to the manufacturer’s instructions. Absorbance at 450 nm was obtained with a VICTOR Nivo Multimode Microplate Reader (PerkinElmer).
2.6. Immunocytochemistry
THP-1 cells were seeded in 24-well plates with sterile coverslips and differentiated into macrophages using 320 nM PMA for 72 h; then, after 24 h of washout, the selected treatment was performed. Each experiment included a control to define autofluorescence and a negative control for each antibody used. Cells were fixed with PFA 4% for 20 min, then washed three times with PBS, and incubated with 50 mM ammonium chloride, after which the cells were washed again three times with 1X PBS.
When needed, permeabilization was performed by adding 0.5 mL of 0.1% Triton X-100 in PBS to each well and incubating for 15 min prior to rinsing with PBS. BSA was used as the blocking agent for 1 h at room temperature. Following the blocking procedure, antibody incubation for 90 min at room temperature was performed. The primary antibodies used were anti-DDDDK (GeneTex, Milan, Italy; polyclonal, rabbit, GTX115043 1:1000 dilution) and snti-Integrin αVβ3 (GeneTex, Milan, Italy; polyclonal, rabbit, GTX01084 1:500 dilution). Goat anti-Rabbit IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (Thermo Fisher Scientific, Monza, Italy, polyclonal, goat, 1:500 dilution) and Goat anti-Rabbit IgG (H + L) Cross-Adsorbed Secondary Antibody, Pacific Blue (Thermo Fisher, polyclonal, goat, 1:500 dilution) were used as secondary antibodies and incubated for 90 min at room temperature. DAPI solution 1 µg/mL in BSA was used to stain nuclei. Coverslips were dried and mounted on a slide with a drop of mounting solution (Fluormount Aqueous Mounting, Merck Life Science S.r.l. Milan, Italy). After at least 45 min, the perimeter was sealed with a small amount of clear nail polish. The coverslips were stored in a suitable container in the dark at 4 °C. Final images were acquired using the Zeiss LSM 800 inverted laser scanning confocal microscope equipped with PMT detectors. The 488 nm laser was used for Alexa Fluor, and the 405 nm laser was used for DAPI. The software used for image acquisition was Zen 2.3 Blue Edition. Images were captured using a 63X oil immersion objective with a numerical aperture of 1.4. Intensity comparisons between images were performed using Fiji-ImageJ software (version 21.0.7).
2.7. Cholesterol Efflux
THP-1 cells were seeded in 24-well plates and differentiated in macrophages using 320 nM PMA for 72 h. Fully differentiated human THP-1 macrophages were radiolabeled with 2 μCi/mL of 1,2-3H-cholesterol (PerkinElmer, Waltham, MA, USA) in RPMI supplemented with 2.5% FCS in the presence or absence of ANGPTL3 100–300 ng/mL for 24 h. Then, the cells were treated for 18 h with RPMI + 0.2% w/v bovine serum albumin (BSA, SMerck, Darmstadt, Germany) in the presence or absence of LXR/RXR agonists, 22 (R) hydroxycholesterol (22-OH) at 5 µg/mL and 9-cis retinoic acid (9cRA) at 10 µM (both from Merck, Darmstadt, Germany), and 25 μg/mL of acetylated-LDL again in presence/absence of hANGPTL3 100–300 ng/mL. All the above treatments were performed in the presence of 2 µg/mL of acetyl-coenzyme A: cholesterol acyltransferase (ACAT) inhibitor (Sandoz 58035; Merck, Darmstadt, Germany) to maintain cholesterol in its unesterified form. Cholesterol efflux was promoted for 4 h to human apolipoprotein A-I (apoA-I) (Merck, Darmstadt, Germany) in RPMI 1640. The amount of radioactive 1,2-3H-cholesterol in each sample was evaluated using the Liquid Scintillation Counter TRI-CARB 4810TR (Perkin Elmer, Waltham, MA, USA). Cholesterol efflux was expressed as the percentage of 1,2-3H-cholesterol released in the culture medium over the total intracellular radioactivity, and data are representative of at least three independent experiments.
2.8. Triglycerides and Cholesterol Determination
Total macrophage cholesterol and triglycerides were quantified using ABX PENTRA Cholesterol CP and Triglycerides CP, respectively (Horiba, Rome, Italy; Ref. A11A01634 and A11A01640), after Folch’s lipid extraction following the manufacturer’s instructions. The total amounts were normalized by protein contents determined by the Bicinchoninic Acid (BCA) assay (Thermo Fisher Scientific, Monza, Italy).
2.9. Statistical Analysis
Statistical analysis was performed using the Prism statistical analysis package, version 5.01 (GraphPad Prism 10 Software). Data were given as mean ± SD of three independent experiments. When possible, p-values were determined by Student’s t-test. Otherwise, differences between treatment groups were evaluated by one-way ANOVA. A probability value of p < 0.05 was considered statistically significant.
3. Results
3.1. THP-1-Derived Macrophages Do Not Express but Interact with ANGPTL3
To investigate the pro-inflammatory role of ANGPTL3 on macrophages, we first performed a series of analyses in order to characterize the THP-1 cell line. We determined, by Western blot analysis, the expression of ANGPTL3, ANGPTL4, LPL, αVβ3 integrin, and CD36 protein in THP-1 monocytes and THP-1-derived macrophages in the presence or absence of LPS as inflammatory stimuli. As a positive control for the presence of ANGPTL3, a Huh7 human hepatoma cell line was also considered in this analysis. ANGPTL3 was not expressed by THP-1-derived macrophages, while a positive band at the predicted molecular weight of 54 kDa was detected from total protein extracts of THP-1 monocytes and HuH7 cells (Figure 1A). ANGPTL4 and LPL were expressed in all three cell lines, although macrophages seemed to express significantly higher levels of LPL. As predicted, the scavenger receptor CD36 is exclusively expressed by macrophages [21]. The relative expression of ANGPTL3 between the three cell types was also confirmed by real-time PCR, showing the highest levels in THP-1 cells (monocyte) and the lowest in macrophages (Figure 1B). Interestingly, LPS stimulation seems to partially reduce the transcription of ANGPTL3 (Figure 1B).
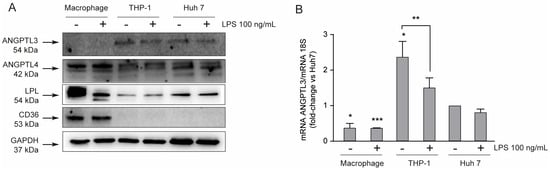
Figure 1.
THP-1-derived macrophages do not express ANGPTL3. (panel (A) THP-1 cells were seeded in 6-well trays (2.9 × 106 cells in 3 mL/well of RPMI) in the presence of 10% FCS and 320 nM PMA. After 72 h, Huh7 and THP-1 cells were seeded (5.0 × 105 Huh7 cells in 3 mL/well of MEM and 9 × 106 cells in 3 mL/well of RPMI) 10% FCS) in the presence of 10% FCS, and the medium of PMA-treated THP-1 was replaced with fresh RPMI. After 24 h, cells were treated with LPS 100 ng/mL. To determine protein levels, Western blot analysis, with indicated primary antibodies, was performed after 24 h of incubation with LPS. Antibody anti-GAPDH was utilized as the loading control. (panel (B)) To determine mRNA levels, RT-qPCR was performed after 24 h of incubation with LPS on total RNA samples. The symbols – and + indicate the absence or presence of LPS, respectively. Data are presented as mean ± SD of three different experiments. * p < 0.05, *** p < 0.001 vs. Huh7, ** p < 0.05 vs. THP-1.
We next decided to investigate the possible interaction between hANGPTL3 FLAG tag to macrophages and its potential intracellular localization. To this aim, we incubated THP-1-derived macrophages with physiological levels of ANGPTL3 (100 ng/mL) [22] and detected its localization with a specific anti-FLAG tag antibody with and without cell permeabilization. Interestingly, we found a positive signal from the cell surface of macrophages and, more importantly, at the intracellular level (Figure 2A), indicating that ANGPTL3 could interact with THP-1-derived macrophages and potentially be uptaken by the integrin αvβ3. Indeed, we observed a positive expression of integrin αvβ3 either in the presence or absence of ANGPTL3 (Figure 2B). The interaction and internalization of hANGPTL3 by THP-1-derived macrophages were confirmed by Western blot analysis (Figure 2C). Indeed, we detected an increased signal with antibody anti-ANGPTL3 of total protein extracts of THP-1 macrophages incubated for 24 h with 100–300 ng/mL of hANGPTL3. Based on these results, we decided to conduct all our experiments on THP-1-derived macrophages.
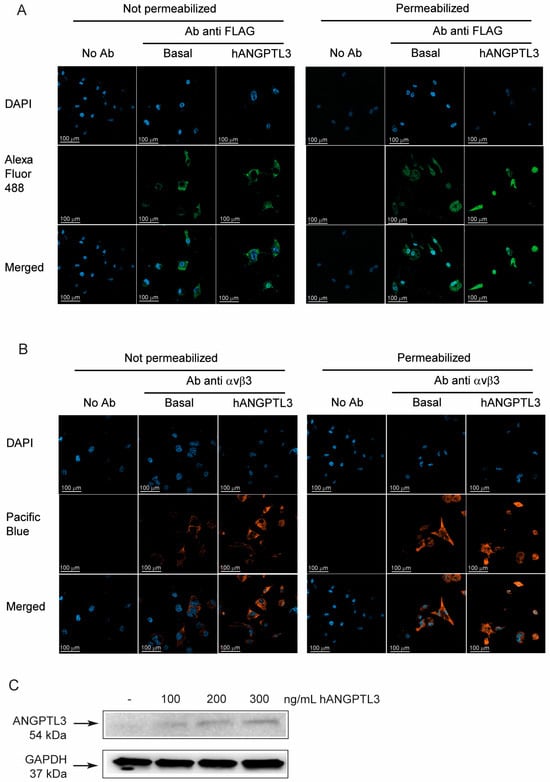
Figure 2.
Exogenous ANGPTL3 is internalized by THP-1-derived macrophages. THP-1 cells were seeded in 24-well trays (106 cells/well in 1.5 mL/well of RPMI) in the presence of 10% FCS and 320 nM PMA to differentiate into macrophages. After 72 h, the medium was replaced with fresh RPMI with 0.5% bovine serum albumin (BSA). The day after the cells were treated with hANGPTL3 100 ng/mL for 24 h, immune cytochemistry (ICC) was performed with antibody anti-FLAG recognizing the exogenous ANGPTL3 (panel (A)) and anti-αvβ3 antibody (panel (B)), with and without cell permeabilization. DAPI was used to stain cell nuclei. (panel (C)) Western blot analysis with antibody anti-ANGPTL3 of total protein extracts of THP-1-derived macrophages incubated with hANGPTL3 for 24 h at indicated concentrations. Antibody anti-GAPDH was utilized as the loading control. Blue: DAPI. Green: Alexa Fluor 488 anti-FLAG-tag ANGPTL3 antibody (anti-DDDDK antibody). Orange: Pacific Blue anti integrin αvβ3 antibody. The symbols – indicates the absence of hANGPTL3.
3.2. ANGPTL3 Shows Pro-Inflammatory Properties in THP-1-Derived Macrophages
To evaluate the potential pro-inflammatory properties of ANGPTL3, we performed a series of real-time quantitative PCR analyses on total RNA of THP-1-derived macrophages exposed for 24 h to 300 ng/mL hANGPTL3 and, as the positive control, to 10 ng/mL of TNFα. As expected, TNFα significantly induced the pro-inflammatory cytokines IL-1β and IL-6 (Figure 3A,B). Similarly, although less efficiently for IL1β, hANGPTL3 increased the mRNA expression of IL-1β, IL-6, and TNFα by 1.87 ± 0.08-fold, 1.35 ± 0.11-fold, and 2.49 ± 0.43-fold, respectively, vs. basal conditions (Figure 3A–C). To confirm these data, we determined, by an ELISA assay, the amount of secreted TNFα in the conditioned media. Incubation with 100 ng/mL of hANGPTL3 determined a 1.98 ± 0.4-fold increase in secreted TNFα vs. basal condition, confirming the pro-inflammatory properties of this hepatokine (Figure 3D).
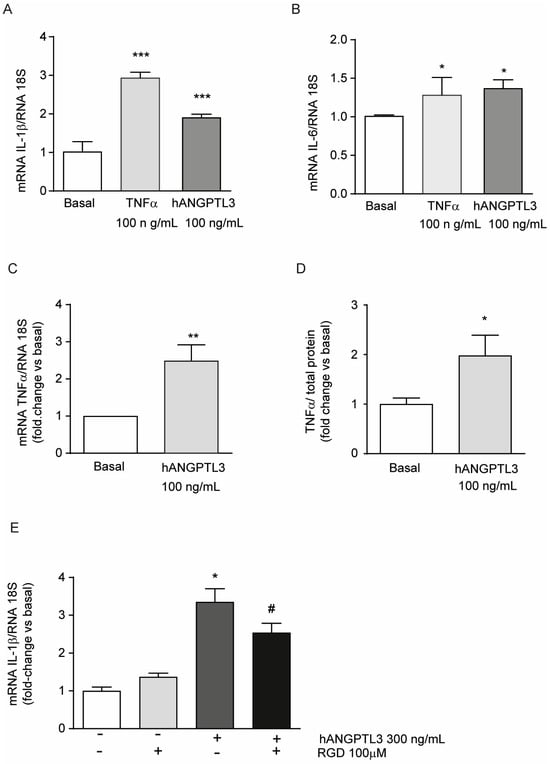
Figure 3.
ANGPTL3 produces an inflammatory response in THP-1-derived macrophages. (panels (A–E)) THP-1 cells were seeded in 48-well trays (2.5 × 105 in 300µL/well of RPMI) in the presence of 10% FCS and 0.32 µM 320 nM PMA to differentiate into macrophages. After 72 h, the medium was replaced with fresh RPMI with 0.5% BSA. The day after, the cells were stimulated with TNFα 10 ng/mL or hANGPTL3 100 ng/mL (panels (A–C)) or with hANGPTL3 100 ng/mL in the presence or absence of RGD peptide (panel (E)) for 24 h. The mRNA levels were then determined by RT-qPCR. (panel (D)) THP-1 cells were seeded in 6-well plates (2.9 × 106 cells/well in 2 mL of RPMI) in the presence of 10% FCS and 320 nM PMA to differentiate into macrophages. After 72 h, the medium was replaced with fresh RPMI with 0.5% BSA. The day after, the cells were treated with hANGPTL3 100 ng/mL for 24 h, and TNFα levels were determined by a commercially available ELISA kit. Data are presented as mean ± SD of three different experiments. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. basal, # p < 0.05 vs. hANGPTL3.
Considering the molecular structure of ANGPTL3, we hypothesized that the observed pro-inflammatory effect could be mediated by its interaction with the fibrinogen-like domain to αvβ3 integrin. RGD peptide has been shown to block this integrin in smooth muscle cells [23]. As shown in Figure 3E, the incubation of THP-1-derived macrophages with RGD peptide partially counteracted the induction of IL-1β mRNA levels by hANGPTL3 (3.35 ± 0.35-fold vs. 2.54 ± 0.25-fold for hANGPTL3 vs. hANGPTL3 + RGD, respectively). These results indicate a possible integrin-mediated mechanism for the ANGPTL3 pro-inflammatory effect.
3.3. hANGPTL3 Induces Cholesterol Accumulation and Reduces Cholesterol Efflux in THP-1-Derived Macrophages
Cholesterol accumulation in macrophages leads to the formation of the so called “foam cells” implicated in the early phases of the inflammatory response occurring in atherogenesis. We thus decided to investigate the effect of hANGPTL3 on triglycerides (TG) and cholesterol content of THP-1-derived macrophages. Cells were cultured in medium with 10% FCS containing exogenous lipoproteins for 24 h in the presence or absence of 100 ng/mL of hANGPTL3. The quantification of TG and cholesterol from total lipid extraction revealed a significant increase in TG (31.2 ± 2.8% vs. basal, Figure 4A) and cholesterol (19.6 ± 4.1% vs. basal, Figure 4B), an effect that may be implicated in the pro-inflammatory response induced by this hepatokine.
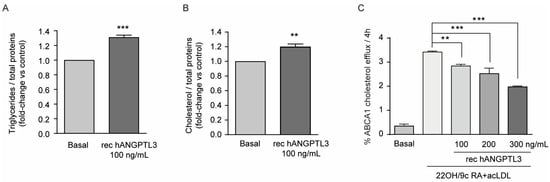
Figure 4.
ANGPTL3 induces lipid accumulation and reduces cholesterol efflux in THP-1-derived macrophages. (panel (A,B)) THP-1 cells were seeded in 6-well plates (2.9 × 106 cells/well in 2 mL of RPMI) in the presence of 10% FCS and 320 nMPMA to differentiate into macrophages. After 72 h, the medium was replaced with fresh RPMI with 0.5% BSA. The day after, the cells were treated with hANGPTL3 100 ng/mL for 24 h, and triglyceride and cholesterol contents were determined after Folch’s lipid extraction with a commercially available kit. (panel (C)) Fully differentiated human THP-1 macrophages were radiolabeled for 24 h with 2 μCi/mL of 1,2-3H-cholesterol in RPMI + 2.5% FCS in the presence or absence of hANGPTL3 100–300 ng/mL. Then, the cells were treated for 18 h with a solution of RPMI supplemented with 0.2% w/v BSA in the presence or absence of LXR/RXR agonists, 22-OH 5 µg/mL and 9cRA 10 µM, and 25 μg/mL of acLDL in the presence/absence of hANGPTL3 100–300 ng/mL. Efflux was promoted for 4 h to apoA-I and expressed as the percentage of 1,2-3H-cholesterol released in the culture medium over the total radiolabeled intracellular cholesterol. Data are presented as mean ± SD of three independent experiments: ** p < 0.01, *** p < 0.001 vs. basal.
Considering the effect on intracellular lipid content, we also determined the possible influence of hANGPTL3 on cholesterol efflux. To explore the potential effect of hANGPTL3 on ABCA1-mediated cholesterol efflux, we used THP-1-derived-macrophages stimulated with the nuclear receptor LXR/RXR ligands 22-OH and 9cRA and acetylated LDL (acLDL) in order to upregulate ABCA1 expression and promote efflux to apolipoprotein A-I (apoA-I), the specific cholesterol acceptor for the ABCA1 transporter. As expected, treatment with LXR/RXR agonists resulted in an increase of about 3.2-fold of cholesterol efflux to apoA-I (Figure 4C). Co-incubation of cells with hANGPTL3 inhibited this effect in a concentration-dependent manner, reaching the maximal inhibitory effect of 55% with 300 ng/mL of hANGPTL3 (Figure 4C).
4. Discussion
In the present work, we investigated the potential biological effect of the hepatokine ANGPTL3 on macrophages utilizing human recombinant protein containing the fibrinogen-like domain and THP-1-derived macrophages. Our in vitro findings indicated that ANGPTL3 is internalized by macrophages, where it induces lipid accumulation and activates a pro-inflammatory effect by inducing the transcription of IL-1β, IL-6, and TNFα and by increasing the cell production of TNFα. Additionally, the pro-inflammatory effect is partially dampened by the αvβ3 integrin inhibitor RGD peptide, thus suggesting the involvement of the fibrinogen-like domain of ANGPTL3. Macrophages can express αvβ3 integrin in the atherosclerosis plaque [15,24], thus suggesting that ANGPTL3 could be part of the pro-inflammatory milieu promoting the development of the pathology. This hypothesis is also supported by recent experimental evidence that demonstrated the co-localization of ANGPTL3 and the macrophage marker CD68 in the atherosclerotic plaque of apoE null mice [25]. The same authors found that the absence of ANGPTL3 reduced atherosclerotic plaque development in apoE null mice of 25 weeks of age [25]. However, the protective action of the absence of ANGPTL3 was associated with reduced lipid parameters, an effect that may have had an impact on atherosclerotic plaque development. Nevertheless, the in vitro incubation of THP-1 macrophages with the ANGPTL3 fibrinogen-like domain induced a series of pro-inflammatory cytokines, including TNFα and IL-1β [25]. By using different approaches, both studies observed that αvβ3 integrin is implicated in the pro-inflammatory effect of ANGPTL3.
The role of the fibrinogen-like domain of ANGPTL3 goes potentially beyond the pro-inflammatory response in macrophages; indeed, the same portion of the protein was shown to regulate endothelial migration and neo-angiogenesis again through the activation of the αvβ3 integrin [14]. In the context of the crosstalk between lipid metabolism and inflammatory responses, pro-inflammatory signaling can deeply affect lipid metabolism, and on the other hand, an altered lipid metabolism is often associated with an abnormal immune response [20]. Macrophage cholesterol homeostasis is the result of the balance of various processes, among which are cholesterol uptake from plasma lipoproteins and cholesterol efflux to extracellular acceptors, such as HDL or apoA-I. Cholesterol uptake from native LDL is mediated by the LDL receptor (LDLr), whose expression on the cell membrane is strictly regulated by the intracellular cholesterol content. On the contrary, oxidized LDLs are internalized through the scavenger receptors, which mediate uncontrolled intracellular cholesterol uptake. Cholesterol efflux is the first step of reverse cholesterol transport, the anti-atherogenic process that removes excess cholesterol from the periphery and transports it to the liver for elimination. It occurs through aqueous diffusion and active membrane transporters and is dependent on the activity of extracellular cholesterol acceptors [26]. Our study adds a third direct cellular effect of ANGPTL3 by demonstrating that accumulation of TG and cholesterol occurs in parallel to a significant dose-dependent inhibition of ABCA1-mediated cholesterol efflux, effects that may drive cholesterol accumulation and subsequent induction of pro-inflammatory cytokines. Indeed, cholesterol accumulation in macrophages induces inflammation by stimulating the caspase-1-activating NLRP3 inflammasome, which results in cleavage and secretion of IL-1 family cytokines [18]. The ANGPTL3-induced inhibition of cholesterol efflux might be related to a possible increase in cholesterol esterification, the process by which cells protect themselves from toxic levels of free cholesterol, thus reducing the pool of free cholesterol that is available for efflux. A possible impact of ANGPTL3 on the esterifying enzyme acyl-coenzyme A cholesterol acyltransferase (ACAT) can be hypothesized, as we previously reported that silencing of ANGPTL3 by siRNA reduced cell cholesterol esterification [27]. Previous work from our group showed that proprotein convertase subtilisin/kexin type 9 (PCSK9) reduces cholesterol efflux by inhibiting ABCA1 expression [28]. We cannot exclude a direct impact of ANGPTL3 on this process; however, we have not explored this possibility but limited our attention to a functional assay, considering this phenomenon more important and with potential clinical implications. For instance, we know that, by blocking PCSK9 with monoclonal antibodies, evolocumab and alirocumab strongly reduced LDL cholesterol levels and the intraplaque inflammation and lipid core [29,30]. The effect on atherosclerotic plaque composition of the monoclonal antibody anti-ANGPTL3, evinacumab, has also been reported in patients with homozygous familial hypercholesterolemia [31,32], suggesting a potential direct vascular effect. The intracellular lipid accumulation induced by ANGPTL3 could be likely related to the inhibition of cholesteryl esters and TG hydrolysis by lipases. To date, ANGPTL3 has been shown to inhibit both LPL and EL, enzymes whose intracellular activities have never been demonstrated. On the contrary, lysosomal acid lipase is known to be involved in intracellular lipid hydrolysis, although the inhibitory effect of ANGPTL3 on this enzyme has never been explored and deserves further investigation [33].
5. Conclusions
In conclusion, in the present study, we provide in vitro evidence of the direct pro-inflammatory activity of ANGPTL3 on macrophages associated with a significant accumulation of cholesterol and TG; both effects may contribute to a pro-atherogenic effect of this hepatokine, whose pharmacological inhibition could determine a significant CV protection.
Author Contributions
Conceptualization, N.F., M.P.A., and F.Z.; methodology, I.M., I.R., G.M., and M.G.L.; formal analysis, I.M.; writing—original draft preparation, N.F.; writing—review and editing, M.P.A. and M.G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ANGPTL3 | Angiopoietin-like 3 |
| acLDL | Acetylated LDL |
| apoA-I | Apolipoprotein A-I |
| BSA | Bovine serum albumin |
| CAD | Coronary artery disease |
| EL | Endothelial lipase |
| FCS | Fetal calf serum |
| HDL | High-density lipoprotein |
| ICC | Immune cytochemistry |
| LDL | Low-density lipoprotein |
| LDLr | LDL receptor |
| LPL | Lipoprotein lipase |
| LPS | Lipopolysaccharide |
| PMA | Phorbol-12-myristate-13-acetate |
| TNFα | Tumor necrosis factor-α |
| 22-OH | Hydroxycholesterol |
| 9cRA | 9-cis retinoic acid |
References
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy After Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Nissen, S.E.; Lincoff, A.M.; Brennan, D.; Ray, K.K.; Mason, D.; Kastelein, J.J.P.; Thompson, P.D.; Libby, P.; Cho, L.; Plutzky, J.; et al. Bempedoic Acid and Cardiovascular Outcomes in Statin-Intolerant Patients. N. Engl. J. Med. 2023, 388, 1353–1364. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes After Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Ray, K.K.; Landmesser, U.; Leiter, L.A.; Kallend, D.; Dufour, R.; Karakas, M.; Hall, T.; Troquay, R.P.; Turner, T.; Visseren, F.L.; et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N. Engl. J. Med. 2017, 376, 1430–1440. [Google Scholar] [CrossRef]
- Musunuru, K.; Pirruccello, J.P.; Do, R.; Peloso, G.M.; Guiducci, C.; Sougnez, C.; Garimella, K.V.; Fisher, S.; Abreu, J.; Barry, A.J.; et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N. Engl. J. Med. 2010, 363, 2220–2227. [Google Scholar] [CrossRef]
- Lupo, M.G.; Ferri, N. Angiopoietin-Like 3 (ANGPTL3) and Atherosclerosis: Lipid and Non-Lipid Related Effects. J. Cardiovasc. Dev. Dis. 2018, 5, 39. [Google Scholar] [CrossRef]
- Mattijssen, F.; Kersten, S. Regulation of triglyceride metabolism by Angiopoietin-like proteins. Biochim. Biophys. Acta 2012, 1821, 782–789. [Google Scholar] [CrossRef]
- Tamehri Zadeh, S.S.; Toth, P.P.; Shapiro, M.D.; Surma, S.; Banach, M. ANGPTL3 vital role in different kidney diseases. Current knowledge and future perspectives. Biomed. Pharmacother. 2025, 188, 118189. [Google Scholar] [CrossRef]
- Chan, D.C.; Watts, G.F. Inhibition of the ANGPTL3/8 Complex for the Prevention and Treatment of Atherosclerotic Cardiovascular Disease. Curr. Atheroscler. Rep. 2024, 27, 6. [Google Scholar] [CrossRef]
- Reeskamp, L.F.; Millar, J.S.; Wu, L.; Jansen, H.; van Harskamp, D.; Schierbeek, H.; Gipe, D.A.; Rader, D.J.; Dallinga-Thie, G.M.; Hovingh, G.K.; et al. ANGPTL3 Inhibition with Evinacumab Results in Faster Clearance of IDL and LDL apoB in Patients with Homozygous Familial Hypercholesterolemia-Brief Report. Arter. Thromb. Vasc. Biol. 2021, 41, 1753–1759. [Google Scholar] [CrossRef]
- Fu, Z.; Yao, F.; Abou-Samra, A.B.; Zhang, R. Lipasin, thermoregulated in brown fat, is a novel but atypical member of the angiopoietin-like protein family. Biochem. Biophys. Res. Commun. 2013, 430, 1126–1131. [Google Scholar] [CrossRef]
- Ono, M.; Shimizugawa, T.; Shimamura, M.; Yoshida, K.; Noji-Sakikawa, C.; Ando, Y.; Koishi, R.; Furukawa, H. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J. Biol. Chem. 2003, 278, 41804–41809. [Google Scholar] [CrossRef]
- Camenisch, G.; Pisabarro, M.T.; Sherman, D.; Kowalski, J.; Nagel, M.; Hass, P.; Xie, M.H.; Gurney, A.; Bodary, S.; Liang, X.H.; et al. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo. J. Biol. Chem. 2002, 277, 17281–17290. [Google Scholar] [CrossRef]
- Cam, A.; de Mejia, E.G. RGD-peptide lunasin inhibits Akt-mediated NF-kappaB activation in human macrophages through interaction with the alphaVbeta3 integrin. Mol. Nutr. Food Res. 2012, 56, 1569–1581. [Google Scholar] [CrossRef]
- Chen, J.; Green, J.; Yurdagul, A., Jr.; Albert, P.; McInnis, M.C.; Orr, A.W. alphavbeta3 Integrins Mediate Flow-Induced NF-kappaB Activation, Proinflammatory Gene Expression, and Early Atherogenic Inflammation. Am. J. Pathol. 2015, 185, 2575–2589. [Google Scholar] [CrossRef]
- Dewey, F.E.; Gusarova, V.; Dunbar, R.L.; O’Dushlaine, C.; Schurmann, C.; Gottesman, O.; McCarthy, S.; Van Hout, C.V.; Bruse, S.; Dansky, H.M.; et al. Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 211–221. [Google Scholar] [CrossRef]
- Gong, Q.; Ye, L.; Gui, H.; Liu, J.; Li, H.; Sun, Q. Association study of genetic variants of the ANGPTL3 gene and susceptibility to ischemic stroke. Neuropsychiatr. Dis. Treat. 2019, 15, 3015–3020. [Google Scholar] [CrossRef]
- Romeo, S.; Yin, W.; Kozlitina, J.; Pennacchio, L.A.; Boerwinkle, E.; Hobbs, H.H.; Cohen, J.C. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Investig. 2009, 119, 70–79. [Google Scholar] [CrossRef]
- Sima, P.; Vannucci, L.; Vetvicka, V. Atherosclerosis as autoimmune disease. Ann. Transl. Med. 2018, 6, 116. [Google Scholar] [CrossRef]
- Hayden, J.M.; Brachova, L.; Higgins, K.; Obermiller, L.; Sevanian, A.; Khandrika, S.; Reaven, P.D. Induction of monocyte differentiation and foam cell formation in vitro by 7-ketocholesterol. J. Lipid Res. 2002, 43, 26–35. [Google Scholar] [CrossRef]
- Ruscica, M.; Ferri, N.; Fogacci, F.; Rosticci, M.; Botta, M.; Marchiano, S.; Magni, P.; D’Addato, S.; Giovannini, M.; Borghi, C.; et al. Circulating Levels of Proprotein Convertase Subtilisin/Kexin Type 9 and Arterial Stiffness in a Large Population Sample: Data From the Brisighella Heart Study. J. Am. Heart Assoc. 2017, 6, e005764. [Google Scholar] [CrossRef]
- von Wnuck Lipinski, K.; Keul, P.; Ferri, N.; Lucke, S.; Heusch, G.; Fischer, J.W.; Levkau, B. Integrin-mediated transcriptional activation of inhibitor of apoptosis proteins protects smooth muscle cells against apoptosis induced by degraded collagen. Circ. Res. 2006, 98, 1490–1497. [Google Scholar] [CrossRef]
- Lin, X.; Sun, Y.; Yang, S.; Yu, M.; Pan, L.; Yang, J.; Yang, J.; Shao, Q.; Liu, J.; Liu, Y.; et al. Omentin-1 Modulates Macrophage Function via Integrin Receptors alphavbeta3 and alphavbeta5 and Reverses Plaque Vulnerability in Animal Models of Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 757926. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, C.; Dong, Y.; Zhao, J.; Yang, X.; Deng, Y.; Su, L.; Yin, J.; Zhang, Y.; Sun, F.; et al. ANGPTL3 accelerates atherosclerotic progression via direct regulation of M1 macrophage activation in plaque. J. Adv. Res. 2025, 70, 125–138. [Google Scholar] [CrossRef]
- Adorni, M.P.; Ronda, N.; Bernini, F.; Zimetti, F. High Density Lipoprotein Cholesterol Efflux Capacity and Atherosclerosis in Cardiovascular Disease: Pathophysiological Aspects and Pharmacological Perspectives. Cells 2021, 10, 574. [Google Scholar] [CrossRef]
- Rossi, I.; Marodin, G.; Lupo, M.G.; Adorni, M.P.; Papotti, B.; Dall’Acqua, S.; Ferri, N. Gene Silencing of Angiopoietin-like 3 (ANGPTL3) Induced De Novo Lipogenesis and Lipid Accumulation in Huh7 Cell Line. Int. J. Mol. Sci. 2024, 25, 3708. [Google Scholar] [CrossRef]
- Adorni, M.P.; Cipollari, E.; Favari, E.; Zanotti, I.; Zimetti, F.; Corsini, A.; Ricci, C.; Bernini, F.; Ferri, N. Inhibitory effect of PCSK9 on Abca1 protein expression and cholesterol efflux in macrophages. Atherosclerosis 2016, 256, 1–6. [Google Scholar] [CrossRef]
- Raber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Haner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; van Geuns, R.J.; Ondracek, A.S.; et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients with Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA 2022, 327, 1771–1781. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Kataoka, Y.; Nissen, S.E.; Prati, F.; Windecker, S.; Puri, R.; Hucko, T.; Aradi, D.; Herrman, J.R.; Hermanides, R.S.; et al. Effect of Evolocumab on Coronary Plaque Phenotype and Burden in Statin-Treated Patients Following Myocardial Infarction. JACC Cardiovasc. Imaging 2022, 15, 1308–1321. [Google Scholar] [CrossRef]
- Schonck, W.A.M.; Reijman, M.D.; Wiegman, A.; Ibrahim, S.; Corpeleijn, W.E.; Planken, R.N.; Hovingh, G.K.; Stroes, E.S.G.; Nurmohamed, N.S.; Reeskamp, L.F. Decreased LDL-Cholesterol Exposure Following ANGPTL3 Inhibition Reduces Coronary Plaque Development in Homozygous Familial Hypercholesterolemia. JACC Cardiovasc. Imaging 2024, 17, 1258–1260. [Google Scholar] [CrossRef]
- Reeskamp, L.F.; Nurmohamed, N.S.; Bom, M.J.; Planken, R.N.; Driessen, R.S.; van Diemen, P.A.; Luirink, I.K.; Groothoff, J.W.; Kuipers, I.M.; Knaapen, P.; et al. Marked plaque regression in homozygous familial hypercholesterolemia. Atherosclerosis 2021, 327, 13–17. [Google Scholar] [CrossRef]
- Pritchard, A.B.; Strong, A.; Ficicioglu, C. Persistent dyslipidemia in treatment of lysosomal acid lipase deficiency. Orphanet J. Rare Dis. 2020, 15, 58. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).