Abstract
Background: Topical anaesthesia of the nasal mucosa is essential for comfortable and effective nasal instrumentation. However, current methods often result in uneven anaesthesia, which can cause discomfort. This study evaluates the clinical performance of a newly developed soft mist nasal atomiser (NAA: Nasal Atomiser Adapter) for nasal topical anaesthesia. Methods: Twenty healthy adult volunteers received 1 mL of 4% lidocaine via the NAA in two doses of 0.5 mL each, administered into one nostril. Five minutes after administration, a size 7 nasopharyngeal airway was inserted into the anaesthetised nostril to assess tolerance. Comfort and anaesthetic effectiveness were rated by both participants and the attending anaesthesiologist using numeric rating scales (1–10). Results: The median total spraying time was 177.5 s (range, 152–192 s), which included the 120 s waiting period between the two 0.5 mL doses. Insertion of the nasopharyngeal airway took a median of 8.0 s (range 2–25 s). Participants rated the comfort of nasal lidocaine administration at a median of 9/10, and anaesthesia levels were rated as good to very good by both participants and clinicians. In 85% of cases, no reaction was observed during insertion of the nasopharyngeal airway; minimal reactions occurred in the remaining 15%. No adverse events were reported. Conclusions: The NAA provided effective, reliable, and safe anaesthesia of the nasal cavity, with a high level of comfort for the subject. It enabled fast and comfortable nasal instrumentation. These findings support the NAA as a promising alternative to conventional nasal anaesthetic techniques.
1. Introduction
Effective and rapid topical anaesthesia of the nasal mucosa is paramount for nasal cavity instrumentation, such as nasal fiberoptic procedures, awake nasal fiberoptic intubation, and nasogastric tube placement. Conventional topical anaesthesia of the nasal mucosa is often patchy and not consistently effective [1,2].
Although numerous methods exist to anaesthetise the nasal mucosa, achieving both effective anaesthesia and high patient comfort remains challenging. Lidocaine gel can be applied to the nostrils [3] but does not reliably reach deeper areas of the nasal mucosa. Lidocaine-soaked gauze can be inserted deeply into the nostril and left in place for several minutes [4]; however, this method is time consuming, and areas not in contact with the gauze remain unanaesthetised. The MAD (Mucosal Atomization Device) NasalTM (Teleflex Incorporated, Wayne, PA, USA) atomises drugs such as lidocaine [5] into a fine mist with particle sizes ranging from 30 to 100 µm. This allows rapid application of lidocaine mist, but the spray’s relatively high exit pressure and velocity are often perceived as uncomfortable, especially by children.
These topical anaesthetic techniques can cause various side effects, including discomfort, burning and stinging sensations, gagging, nausea, unpleasant taste, and globus sensation [6]. For patients requiring repeated nasal instrumentation, such as those dependent on feeding tubes, these side effects may lead to aversion and traumatic experiences.
Due to the protective functions of the nose—namely the filtration, heating, and humidification of inhaled air, and olfaction—the anatomy and mucosal characteristics of the nasal cavity can hinder effective topical drug delivery. Nasal hairs, and more importantly, the mucus-covered nasal mucosa, prevent foreign particles from reaching the lungs [7,8,9]. The nasal cavity contains three distinct functional zones: the vestibular, olfactory, and respiratory regions.
The vestibular region, approximately 0.6 cm2 in area, serves as the initial barrier to airborne particles. It is poorly vascularised and lined with stratified squamous and keratinised epithelial cells, along with nasal hairs. The olfactory region, covering approximately 15 cm2, is located in the most cranial part of the nasal cavity and is responsible for olfactory perception. It is richly vascularised. The respiratory region, the largest at about 130 cm2, functions primarily as the air filtration and conditioning zone through its mucus layer. Its surface area is further increased by the formation of three nasal conchae along the lateral walls, and at a microscopic level, by microvilli and cilia on the epithelial cells. The posterior section of the nasal cavity transitions into the nasopharynx.
Both nasal physiology and anatomy significantly influence the effectiveness of intranasal drug administration. While the epithelial lining provides a highly absorptive surface, the inherent permeability barrier and efficient clearance mechanisms (such as mucociliary action) can reduce drug retention and absorption.
In addition to patient-related factors, drug formulation, the choice of vehicle, and the delivery device are critical for successful intranasal therapy. A key challenge lies in optimizing drug deposition within the nasal cavity while minimizing pulmonary exposure due to particle escape.
Therefore a new soft mist nasal atomiser adapter (NAA; Medspray Anaesthesia, Enschede, The Netherlands) has been developed (Figure 1), utilizing the same spray-producing nozzle technology as the Trachospray (Medspray Anaesthesia, Enschede, The Netherlands) for oral topical anaesthesia. Conventional nasal spray techniques primarily deposit particles in the anterior vestibule due to inertial impaction of droplets with a median volumetric diameter greater than 50 µm, emitted at relatively high velocities. The NAA generates droplets with a diameter of 25 ± 5 µm at a lower initial velocity, resulting in a more uniform distribution throughout the nasal cavity [10].
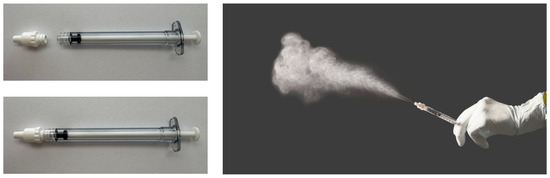
Figure 1.
Nasal Atomiser Adapter and soft mist demonstration.
Prior research has shown that the Trachospray soft mist spray device provides uniform distribution of local anaesthetic agents across the oral cavity, hypopharynx, and vocal cords [11]. It supports well-tolerated awake insertion through the vocal cords using flexible bronchoscopy [12]. This interventional study was designed to evaluate the clinical performance of the soft mist nasal atomiser device (NAA) for topical anaesthesia of the nasal cavity during nasal instrumentation.
2. Methods
The Medical Research Ethics Committee of the Radboud University Medical Centre (Radboudumc), Nijmegen, The Netherlands, reviewed and approved this interventional study. It was registered under the number NL 85958.091.23 and was conducted between December 2024 and May 2025. It was registered on ClinicalTrials.gov with the following ID: NCT06205680. All healthy volunteers provided written informed consent before participation.
A total of twenty healthy volunteers were recruited. Eligible participants were adults over 18 years of age, with a lean body weight of ≥50 kg and an American Society of Anesthesiologists (ASA) physical status classification of 1 or 2. Exclusion criteria included a history of hepatic, renal, or coagulation disorders; respiratory tract pathology; chronic rhinitis, sinusitis, or nasal obstruction; pregnancy or lactation; allergy to amide-type local anaesthetics; inability to cooperate; and lack of written informed consent. Due to the pilot design of this study, no formal sample size calculation was performed.
One 1 mL syringe containing 4% lidocaine was prepared and connected to the NAA device. The device was placed at the entrance of the most patent nostril, and the subject was instructed to breathe slowly while 0.5 mL of lidocaine was administered by the attending anaesthesiologist. After a 2 min waiting period, an additional 0.5 mL of lidocaine was administered, for a total dose of 1 mL. Five minutes later, the anaesthesiologist introduced a size 7 (28 French/Charriere) nasopharyngeal airway (Covidien llc, Mansfield, MA, USA) into the anaesthetised nostril. Subjects’ reactions, such as grimacing or retraction, were documented.
Before leaving the hospital, all participants completed a feedback form. They rated the comfort level of the nasal lidocaine administration and the degree of anaesthesia on a numeric scale from 1 to 10. The attending anaesthesiologist also rated the anaesthesia level using the same scale.
All study data and recordings were stored using an electronic data capture system, with full adherence to participant anonymity. Before analysis, data quality was assessed through three monitoring visits conducted by an independent, qualified monitor. Statistical analyses were carried out in SPSS version 29 (SPSS Inc., Chicago, IL, USA). Descriptive statistics such as mean and standard deviations (SD) for normally distributed data or alternatively median, range, and frequency distributions were used.
3. Results
A total of twenty healthy individuals meeting the study’s inclusion and exclusion criteria were enrolled. Table 1 provides an overview of their demographic characteristics.

Table 1.
Demographic characteristics of the study population.
Various time measurements were recorded throughout the procedure. The median total spraying time was 177.5 s (range, 152–192 s), which included the 120 s waiting period between the two 0.5 mL doses. Insertion of the nasopharyngeal airway took a median of 8.0 s (range 2–25 s).
Upon completion of the procedure, all participants completed a feedback form. The volunteers rated the method of anaesthesia as comfortable to very comfortable, with a median numeric rating scale (NRS) score of 9.0 (0 = no comfort, 10 = very comfortable). The level of anaesthesia, as reported by the participants, ranged from good to very good. The anaesthesiologist generally rated the level of anaesthesia of the nasal cavity as very good (Table 2).

Table 2.
Reports by volunteers and anaesthesiologist.
In the vast majority of volunteers (17 out of 20, i.e., 85%), insertion of the nasopharyngeal airway elicited no observable reaction. In the remaining participants (3 out of 20, i.e., 15%), only minimal reactions—such as slight frowning or forehead wrinkling—were observed.
No adverse or serious adverse events occurred during the study.
4. Discussion
This interventional study aimed to evaluate the clinical performance of a soft mist nasal atomiser device—the NAA—for topical anaesthesia of the nasal cavity during instrumentation. The NAA provided effective, reliable, and safe anaesthesia, with a high level of comfort for the subject. It enabled rapid and well-tolerated nasal instrumentation. While our pilot study was limited in scale and scope, the observed outcomes suggest that this delivery modality may offer a compelling alternative to existing intranasal anaesthetic techniques.
The anterior part of the nasal cavity is typically the most sensitive and prone to pain during nasal instrumentation. The nasal vestibule and cartilaginous anterior septum are richly innervated by branches of the trigeminal nerve, including the anterior ethmoidal and infraorbital nerves, and are covered by the highly vascular Kiesselbach’s plexus. The mucosa is thin and adheres to the underlying perichondrium and vascular plexus containing a high density of nociceptive free nerve endings, which results in increased mechanical and thermal sensitivity. At approximately 1.5 to 2 cm from the nasal vestibule, instrumentation becomes less painful due to histological and functional changes in the nasal mucosa [13].
To assess the efficacy of topical anaesthesia, a nasopharyngeal airway was inserted at least 4 cm into the nasal passage, or until resistance was encountered—typically at the posterior nasopharyngeal wall. The airway was not advanced further to avoid contact with the hypopharynx, which could trigger a gag reflex.
At a physiological level, local anaesthetics such as lidocaine exert their effect by reversibly blocking voltage-gated sodium channels in neuronal membranes, preventing the initiation and propagation of action potentials. However, effective anaesthesia depends not only on pharmacology but also on uniform and sufficient coverage of the mucosal surface, especially in highly innervated zones. Inconsistent application may lead to partial anaesthesia, leaving critical areas inadequately desensitised. Hence, the method of delivery plays a key role in both the depth and distribution of anaesthetic effect.
The effectiveness of intranasal drug delivery depends not only on the pharmacological properties of the anaesthetic but also on the characteristics of the delivery device and the administration technique [14]. Traditional methods of nasal anaesthesia, such as cotton pledgets soaked in lidocaine or viscous gels applied manually, often yield inconsistent results. These techniques can be uncomfortable for the patient and require operator skill, time, and patient cooperation. Additionally, such methods may lead to pooling of anaesthetic in the anterior cavity, with limited posterior diffusion, thus failing to effectively anaesthetise deeper structures like the nasopharynx. Conventional nasal spray devices, while more convenient, often use swirl nozzles that generate relatively large droplets, which primarily deposit in the anterior nasal cavity. Consequently, their utility might be constrained when deeper anaesthetic coverage is required. In contrast, the soft mist nozzle used in our study emitted 0.5 mL of anaesthetic solution as a fine mist, enabling broader coverage across all regions of the nasal cavity, except the nasopharynx. Effective distribution of intranasal formulations using the soft mist nozzle does not require special inhalation manoeuvres or changes in head position. Calm, regular nasal breathing during administration is sufficient while the mist is delivered into the nasal vestibule. Thereby the NAA requires minimal cooperation from the patient. This feature is particularly relevant in settings such as emergency medicine or intensive care, where patients may be obtunded, uncooperative, or cognitively impaired. Furthermore, the device’s simple mechanism and portability allow its integration into mobile care, field triage, or even telemedicine-supported home care in palliative settings. In low-resource settings, where access to sedative medications or trained personnel is limited, a reliable topical anaesthetic device may help reduce the burden on healthcare systems.
However, we observed that when subjects took deep nasal breaths during lidocaine administration, some experienced transient difficulty swallowing. This suggests potential anaesthetisation of the nasohypopharyngeal region, which warrants further investigation. The soft mist may have migrated beyond the intended nasal area to the nasopharynx or oropharynx, leading to partial anaesthetisation of swallowing reflex arcs. Although this was self-limiting and not clinically concerning in our cohort, in patients with compromised airway protective reflexes—such as those with neurodegenerative disease or post-stroke dysphagia—this could theoretically increase aspiration risk. It may be advisable to investigate dose–volume relationships and mist dispersion using radiolabelled markers or imaging methods such as videoendoscopy or 3D nasal airflow modelling.
Nasal drug delivery can elicit reflex responses such as sneezing and occasionally coughing. These reactions are triggered by physical stimuli such as pressure, particulate matter, or abrupt changes in airflow. This reflexive response is protective by clearing irritants but may interfere with therapeutic efficacy or patient compliance. In our study, none of the patients exhibited a response to administration of the soft mist of lidocaine via the spray nozzle. This lack of response was reflected in the high satisfaction scores for this method of anaesthetising the nose, suggesting improved patient acceptance. Unlike pressurised spray pumps, the soft mist is not dependent on a forceful expulsion mechanism, which reduces the risk of mechanical irritation or sudden pressure changes that may trigger protective reflexes. The reduced reflex activation observed may be attributed not only to the gentleness of delivery but also to the even dispersion of the anaesthetic mist, which may mitigate localised irritation.
A particularly noteworthy observation was the lack of patient-reported pain or distress during nasopharyngeal airway (NPA) insertion following atomisation. This finding is significant given that, in daily clinical practice, NPA insertion is typically uncomfortable and often requires systemic sedation or high-dose topical anaesthesia, especially in unsedated patients. To our knowledge, no literature is currently available on the degree of pain experienced during nasopharyngeal airway (NPA) insertion in awake individuals. One study has evaluated cardiovascular responses to NPA insertion in anaesthetised patients, but did not assess subjective pain levels. Although these patients were not awake, the observed increase in blood pressure after NPA insertion suggests the procedure can elicit a significant physiological response, which could potentially be perceived as painful in awake patients [15]. Another study demonstrated that substantial doses of Remimazolam and Oxycodone were required to attenuate the response to nasopharyngeal airway insertion [16]. In awake subjects, such stimulation could translate into considerable discomfort or procedural avoidance, underscoring the potential value of effective topical anaesthesia.
Furthermore, the literature on nasogastric tube placement has consistently reported pain scores of approximately 6 on the NRS across multiple studies [17,18].
Another underexplored area is the cost-effectiveness of such devices in clinical workflows. While initial acquisition costs may be higher than traditional sprays or gauze methods, the time saved during procedures, the reduced need for systemic sedation, and the improved patient compliance may generate downstream savings. Shorter procedural times and reduced complications can translate into higher throughput and better resource allocation, particularly in high-demand environments such as outpatient clinics, ambulatory surgical centres, and emergency departments. A formal health economic analysis should be considered in future multicentre trials.
Moreover, the implementation of novel devices like the NAA should be accompanied by proper integration into clinical guidelines and training protocols. For example, anaesthesia societies and otolaryngology organisations could be engaged to evaluate whether such devices meet criteria for inclusion in recommended pre-intubation or nasendoscopy protocols.
From an ethical and patient-centred care perspective, improving procedural comfort is not merely a convenience but a quality standard. The avoidance of pain and distress in routine procedures aligns with modern frameworks such as the WHO’s “Safe Surgery Saves Lives” initiative and pain as the “fifth vital sign.” Moreover, improved tolerability may reduce procedural avoidance or refusal, thereby increasing access to essential diagnostic and therapeutic interventions.
The training implications are also significant. Traditional techniques such as pledget placement require significant manual dexterity and anatomical knowledge. In contrast, the NAA could facilitate rapid training of junior staff, paramedics, and even patients or caregivers, thus decentralizing certain forms of care. Wider adoption will depend not only on clinical evidence but also on device availability, maintenance simplicity, and ease of instruction.
This pilot study has several limitations that should be acknowledged. First and foremost, the absence of a control or comparator group (e.g., a conventional atomiser, lidocaine-soaked gauze, or intranasal gel) limits the ability to draw definitive conclusions about the relative efficacy, safety, or usability of the novel atomiser. As a single-arm study, our findings are primarily descriptive and exploratory in nature. While initial outcomes are promising, future randomised controlled trials (RCTs) are needed to evaluate comparative performance, including potential superiority or non-inferiority of the device.
Second, both subject and clinician assessments, beside the registration of subjects’ reactions such as grimacing or retraction, relied on subjective measures (e.g., perceived comfort and effectiveness), which introduces the risk of bias, particularly in the absence of blinding. Participants and investigators were aware of the intervention, which may have influenced ratings through expectation effects or observer bias. Blinded or partially blinded study designs should be considered in future trials, potentially including objective endpoints such as quantitative anaesthetic efficacy or pharmacokinetic profiles.
Third, the study did not include variations in dose, route of administration, or patient characteristics (e.g., underlying nasal pathology, age variability), which limits its generalizability. Moreover, the study population consisted of healthy volunteers, and thus results may not fully extrapolate to clinical populations where mucosal conditions, anatomical variation, and comorbidities may influence atomisation performance or lidocaine absorption.
To strengthen the evidence base, future studies should include larger, diverse populations; comparative groups using established administration methods; and standardised outcome measures that allow for inferential statistical analysis. These steps will be essential in validating the utility of the novel nasal atomiser adapter in both routine clinical practice and specialised settings.
In conclusion, our study has demonstrated that effective nasal anaesthesia can be reliably achieved using a soft mist nasal atomiser device. These findings suggest that the NAA is a promising alternative to conventional nasal anaesthetic techniques. The next objective is to evaluate whether this delivery method improves patient comfort during nasal instrumentation procedures, including fiberoptic intubation, rhinolaryngeal interventions, nasogastric tube placement, and nasal administration of irritating drug formulations.
Author Contributions
Conceptualization, H.M., G.-J.v.G. and J.B.; methodology, H.M., G.-J.v.G., L.v.E. and J.B.; validation, H.M., G.-J.v.G., L.v.E. and J.B.; formal analysis, H.M., G.-J.v.G. and J.B.; investigation, H.M., G.-J.v.G. and J.B.; data curation, H.M., G.-J.v.G., L.v.E. and J.B.; writing—original draft preparation, H.M., G.-J.v.G., L.v.E. and J.B.; writing—review and editing, H.M., G.-J.v.G., L.v.E. and J.B.; visualization, H.M., G.-J.v.G., L.v.E. and J.B.; project administration, H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Medical Research Ethics Committee The study was registered under the number: NL 85958.091.23; approval date 2024-08-28; The study was registered on 12/27/2023 in ClinicalTrials.gov. by H. Markerink It was registered on ClinicalTrials.gov with ID: NCT06205680. approval date 2023-12-27.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
G.J. van Geffen and J. Bruhn are co-developers of the NAA device. Radboudumc receives royalties for the NAA.
References
- Hosseini, S.; Wei, X.; Wilkins, J.V., Jr.; Fergusson, C.P.; Mohammadi, R.; Vorona, G.; Golshahi, L. In Vitro Measurement of Regional Nasal Drug Delivery with Flonase,® Flonase® Sensimist,™ and MAD Nasal™ in Anatomically Correct Nasal Airway Replicas of Pediatric and Adult Human Subjects. J. Aerosol Med. Pulm. Drug Deliv. 2019, 32, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Moffa, A.; Costantino, A.; Rinaldi, V.; Sabatino, L.; Trecca, E.M.C.; Baptista, P.; Campisi, P.; Cassano, M.; Casale, M. Nasal Delivery Devices: A Comparative Study on Cadaver Model. Biomed Res. Int. 2019, 28, 4602651. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uri, O.; Yosefov, L.; Haim, A.; Behrbalk, E.; Halpern, P. Lidocaine gel as an anesthetic protocol for nasogastric tube insertion in the ED. Am. J. Emerg. Med. 2011, 29, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Wellenstein, D.J.; van der Wal, R.A.B.; Schutte, H.W.; Honings, J.; van den Hoogen, F.J.A.; Marres, H.A.M.; Takes, R.P.; van den Broek, G.B. Topical Anesthesia for Endoscopic Office-based Procedures of the Upper Aerodigestive Tract. J. Voice 2019, 33, 732–746. [Google Scholar] [CrossRef] [PubMed]
- Fuehner, T.; Fuge, J.; Jungen, M.; Buck, A.; Suhling, H.; Welte, T.; Gottlieb, J.; Greer, M. Topical Nasal Anesthesia in Flexible Bronchoscopy--A Cross-Over Comparison between Two Devices. PLoS ONE 2016, 11, e0150905. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chi, P.W.; Hsieh, K.Y.; Chen, K.Y.; Hsu, C.W.; Bai, C.H.; Chen, C.; Hsu, Y.P. Intranasal lidocaine for acute migraine: A meta-analysis of randomized controlled trials. PLoS ONE 2019, 14, e0224285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kashyap, K.; Shukla, R. Drug Delivery and Targeting to the Brain Through Nasal Route: Mechanisms, Applications and Challenges. Curr. Drug Deliv. 2019, 16, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Bitter, C.; Suter-Zimmermann, K.; Surber, C. Nasal drug delivery in humans. Curr. Probl. Dermatol. 2011, 40, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Cingi, C.; Bayar Muluk, N.; Mitsias, D.I.; Papadopoulos, N.G.; Klimek, L.; Laulajainen-Hongisto, A.; Hytönen, M.; Toppila-Salmi, S.K.; Scadding, G.K. The Nose as a Route for Therapy: Part 1. Pharmacotherapy. Front. Allergy 2021, 2, 638136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Angelo, D.; Kooij, S.; Verhoeven, F.; Sonvico, F.; van Rijn, C. Fluorescence-enabled evaluation of nasal tract deposition and coverage of pharmaceutical formulations in a silicone nasal cast using an innovative spray device. J. Adv. Res. 2023, 44, 227–232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Geffen, G.J.; Markerink, H.; van Barneveld, M.; Verhoeven, F.; Scheffer, G.J.; Bruhn, J. Comparative in-vitro Study of the Trachospray, a New Device for Topical Anaesthesia of the Upper Airway. Med. Devices Evid. Res. 2021, 22, 9–14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Geffen, G.J.; Markerink, H.; van Barneveld, M.; Scheffer, G.J.; Bruhn, J. Clinical evaluation of the Trachospray device for upper airway anaesthesia. Anaesthesia 2021, 76, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Peters, J.M.; Detyniecki, K.; Tatum, W.; Rabinowicz, A.L.; Carrazana, E. The nose has it: Opportunities and challenges for intranasal drug administration for neurologic conditions including seizure clusters. Epilepsy Behav. Rep. 2022, 28, 100581. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Merkus, P.; Ebbens, F.A.; Muller, B.; Fokkens, W.J. Influence of anatomy and head position on intranasal drug deposition. Eur. Arch. Oto-Rhino-Laryngol. 2006, 263, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.L.; Smith, J.E. Cardiovascular changes following insertion of oropharyngeal and nasopharyngeal airways. Br. J. Anaesth. 2004, 93, 339–342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bao, Y.; Xi, C.H.; Wang, H.J.; Chen, Y.M.; Wang, Y.; Li, K.B.; Wang, G.Y. Effective dose of remimazolam combined with oxycodone for inhibition of modified nasopharyngeal airway insertion reaction in female patients of different ages during hysteroscopic surgery. Zhonghua Yi Xue Za Zhi 2025, 105, 1283–1287. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Wolfe, T.R.; Fosnocht, D.E.; Linscott, M.S. Atomized lidocaine as topical anaesthesia for nasogastric tube placement: A randomized, double-blind, placebo-controlled trial. Ann. Emerg. Med. 2000, 35, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Cullen, L.; Taylor, D.; Taylor, S.; Chu, K. Nebulized lidocaine decreases the discomfort of nasogastric tube insertion: A randomized, double-blind trial. Ann. Emerg. Med. 2004, 44, 131–137. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).