Abstract
Opioid use disorder remains a leading national cause of mortality. Physician opioid prescribing contributes to this crisis. In urogynecology, most of these prescriptions are aimed at addressing postoperative pain. This expert review examines the factors that contribute to postoperative pain and opioid use in urogynecologic patients. We discuss patient characteristics, physician interventions and alternative therapies that may influence postoperative pain and opioid use. By identifying patients at higher risk for postoperative pain and opioid use and utilizing evidence-based strategies to mitigate postoperative pain, physicians caring for urogynecology patients can both reduce postoperative opioid use while still providing adequate patient pain control.
1. Introduction
Opioid misuse continues to be a leading cause of morbidity and mortality in the United States (US). There were 107,941 drug overdose deaths reported in 2022 [1]. Deaths specifically involving synthetic opioids other than methadone have continued to rise. There were 73,838 overdose deaths reported in 2022 [1], with opioid-related overdose deaths increasing from 49,860 in 2019 to 81,806 in 2022 [1]. While there are other countries that are experiencing a concerning increase in opioid usage, most notably Canada, Australia and the UK, the crisis remains greatest in the US [2,3]. Opioid use in these countries, as in the US, is a combination of both prescription and illicit drugs [2,3]. In countries with much lower rates of use, the majority of opioid use is associated with heroin, as access to medications is much more limited due to cost, prescribing practices and regulatory controls [2,3]. In the US, a significant proportion of opioid abuse in young adults begins with an opioid prescription [4]. Further, overprescribing allows for opioid diversion and misuse, including by friends and family members [5], further expanding the impact of these prescriptions. Given the scope and pervasiveness of this crisis, it is imperative that physicians continue to assess the ways they can mitigate opioid use in their patients.
The often-demonstrated benefits of urogynecologic procedures include minimally invasive techniques with short postoperative recovery times and minimal postoperative pain. In the field of urogynecology, postoperative pain control is the main source of initial opioid prescribing [6,7,8]. Importantly, women undergoing vaginal reconstruction or laparoscopic sacrocolpopexy who received perioperative opioid prescriptions were significantly more likely to develop new persistent opioid usage than those that did not [8]. Multiple studies have demonstrated that prescribers generate scripts for opioids in amounts disproportionately greater than average consumption, consistently exceeding the amount used by patients [6,7]. Additionally, over 20% of the patients did not use any opioids at all [7]. As-Sanie et al. reviewed the historic prescribing patterns of gynecologists at a large academic medical center and found that prescriptions were usually twice the number of opioids that the average patient uses after hysterectomy [8]. Of the median 200 oral morphine equivalents (OME) prescribed to participants in this prospective quality initiative study, patients reported using a median of 110 morphine equivalents (interquartile range (IQR) 40–150) [8].
Additional studies have shown that these prescribing practices may not be necessary. In two different randomized controlled non-inferiority studies, Yuan et al. and Davidson et al. demonstrated that restrictive opioid prescribing was non-inferior in terms of patient satisfaction for both minor and major urogynecologic procedures [9,10]. Patient satisfaction was 92.2% in the standard protocol and 92.1% (difference −0.1; p = 0.004) in the restrictive protocol as designed by Yuan et al. [10]. Furthermore, patients in Davidson et al.’s study who received the routine opioid prescription had a median of 26 unused opioid tablets (IQR 15–28) vs. 4 (IQR 2–5) in the restricted group, despite the similarity in satisfaction [9].
In a secondary analysis of a randomized controlled trial, Ramasheshan showed that following pelvic reconstructive surgery, 34.8% of patients did not take any narcotic medications, and those that did did not use the entire prescription [11]. The use of procedure-specific, tiered opioid prescribing recommendations was associated with a significant, sustained reduction in opioid prescriptions for patients undergoing surgery for pelvic organ prolapse (POP) [12]. Following implementation, the median oral morphine equivalents decreased from 225 mg (IQR 225–300) to 71.3 (IQR 0–112.5; p < 0.0001) [12]. The clinical practice statement published by Reagan et al. states that it is safe to discharge opioid naive patients undergoing pelvic reconstructive surgery without a prescription, if patients used little to no opioids in the hospital [13].
The ability to identify urogynecology patients in the perioperative period who may have risk factors for higher postoperative pain and increased opioid usage may allow interventions to mitigate postoperative pain. Conversely, identifying those who may require less postoperative opioids can enable providers to decrease overall opioid prescribing without harming patient care or satisfaction. Such personalized prescribing practices balance the pain control needs of patients with the risks of opioid use.
2. Patient Factors Related to Postoperative Pain and Opioid Use
2.1. Age
Compared to a general gynecologic population, urogynecology patients are generally of increased age. In a population-based study within the Kaiser Permanent Healthcare Program in Southern California, Luber et al. examined the demographics of patients with pelvic floor disorder and found an age range of 30 to 89 years normally distributed around a median age of 61.5 years [14]. While this older age range may be associated with greater likelihood of medical comorbidities, in terms of opioid usage, it translates to decreased risk of postoperative pain and opioid usage. A multitude of studies have demonstrated that age is inversely correlated with postoperative pain and opioid usage [13,15,16]. DeBlanc et al. demonstrated increasing mean pain scores and oral morphine equivalents prescribed with decreasing patient age following surgery for POP [15]. The retrospective cohort study conducted by Leach et al. found a significant decrease in pain scores (3.5 to 1.9), mean oral morphine equivalents prescribed (344.2 to 254.8, p < 0.0001) and opioid refill rates (17.9 to 4.8%, p < 0.0001) with increasing patient age by decade, regardless of the surgical approach for POP [16]. In a 2019 retrospective cohort study by Willis-Gray et al., an age of less than 65 was associated with increased postoperative pain scores and total opioid usage in postoperative care unite (PACU) after surgery for POP or stress urinary incontinence (SUI) (9.0 +/− 8.3 vs. 5.1 +/− 6.0 mg, p < 0.05) [17]. In a subsequent prospective study, younger women used significantly more mean morphine equivalents (MME) during the first postoperative week compared with older women, despite no difference in average pain scores (49 +/− 79 vs. 28 +/− 40 morphine milligram equivalents) [18]. Furthermore, the odds of new and persistent opioid use following benign hysterectomy were significantly increased by younger age, with an odds ratio (OR) of 1.97 (95% confidence interval (CI), 1.69–2.30), in a retrospective cohort study by AlAshqar et al. [17]. Similarly, a prospective cohort study by Cummings et al. found that age was positively associated with greater odds of being opioid-free in the first 24 h after surgery for POP (OR 1.07, 95% CI 1.04–1.09) [19]. Among opioid users, age was inversely associated with total opioid dose [19].
The relationship between age and postoperative opioid usage can be attributed to several factors including decreased pharmacologic metabolism, which lessens the amount of medication necessary to achieve a desired effect [20,21]. It may also be due to decreased somatic pain experienced with aging secondary to the changing sensitivity of certain pain receptors. Older adults were shown to have an increased threshold for heat-related pain and peripheral small fibers, the type that process heat, have been shown to degenerate with aging [22]. Furthermore, rates of comorbid conditions that may further affect the pain processing nerves, such as diabetes, increase in older populations. The inverse relationship between age and postoperative opioid usage has been reliably shown among general, breast and orthopedic surgery patients [23], adding to the strength and validity of these findings.
2.2. Medical Comorbidities
The presence of certain medical comorbidities is associated with increased postoperative opioid usage. In a single-center retrospective chart review, Dwarica et al. compared patients presenting to gynecology and urogynecology clinics and found that those presenting to the urogynecology clinic were 30% more likely to be using an opioid pain medication [24]. Urogynecology patients were noted to be older, have a higher parity, be current smokers and have a history of a previous chronic pain diagnosis [24]. In patients with a comorbid psychiatric diagnosis or those with a history of chronic pain, the rate of postoperative opioid consumption was greater compared to control subjects who underwent the same procedure across multiple studies. Palm et al. demonstrated that a diagnosis of fibromyalgia was associated with greater risk of above median use of opioids following pelvic reconstructive surgery (adjusted OR (aOR) 16.9, CI 2.24–362.9) [7]. In addition to a statistically significant increase in new persistent postoperative opioid use in patients with a preoperative fibromyalgia (aOR 1.26, 95% CI 1.07–1.47), AlAshqar et al. demonstrated that other pain diagnoses also increased the risk of new persistent postoperative opioid usage: neck pain (aOR 1.13 95% CI 1.01–1.26), back pain (aOR 1.21, 95% CI 1.04–1.42), chronic pain (aOR 1.66, 95% CI 1.36–2.04) and arthritis/joint pain (aOR 1.30, 95% CI 1.21–1.40) [25]. Further, As-Sanie et al. had patients complete the Fibromyalgia Survey prior to benign hysterectomy, a validated measure of centralized pain. Postoperatively, they found that a one-point increase in Fibromyalgia Score was associated with a 30.8 increase in mean morphine equivalents used two weeks after procedure (p < 0.001) [4]. Patients in the highest tercile of Fibromyalgia Score reported significantly higher postoperative opioid consumption each day following hysterectomy (13.9 greater oral morphine equivalents, 95% CI 3.0–24.8 p = 0.20) and postoperative pain scores (2.4 points higher, 95% CI 0.90–4.0, p = 0.004) [8]. In a systematic review, Regan et al. identified multiple studies in gynecologic surgery, not specific to urogynecology, that showed associations between endometriosis, centralized pain and chronic pain and increased postoperative opioid usage [13]. Studies specific to urogynecology showed increased postoperative opioid usage is associated with history of chronic pain and preoperative opioid use [13]. AlAshqar et al. performed a retrospective cohort study that demonstrated that the presence of some preoperative psychiatric diagnosis was associated with a statistically significant increase in new persistent opioid use postoperatively, including generalized Mood disorder (aOR 1.12, 95% CI 1.01–1.24), anxiety (aOR 1.20, 95% CI 1.09–1.33), Insomnia (aOR 1.34, 95% CI 1.18–1.52), Alcohol use (aOR 1.70, 95% CI 1.17–2.47), Substance use (aOR 1.82, 95% CI 1.21–2.75) [25]. Adjustment disorder, Attention Deficit Disorder and Schizophrenia were not significant after adjustment [25]. There was also a statistically significant difference for those using medication for a Mood disorder (aOR 1.51, 95% CI 1.40–1.64), which included Selective Serotonin Reuptake Inhibitors, Serotonin Norepinephrine reuptake inhibitors, Tricyclic Antidepressants, Monoamine oxidase inhibitors, Lithium and Anticonvulsant mood stabilizers, which were all included under the analysis [25]. In women who underwent vaginal reconstructive surgery, preoperative pelvic pain was associated with greater improvement pelvic pain scores at 3 (−3.1 +/− 2.9 vs. −0.4 +/− 1.6, p < 0.001) and 12 months (−3.4 +/− 3.0 vs. −0.6 +/− 1.6, p < 0.001); however, overall pain scores remained higher in a secondary analysis of the Outcomes Following Vaginal Prolapse Repair and Midurethral Sling trial [26]. An anxiety diagnosis preoperatively was associated with higher pain immediately, 1 to 2 days (relative odds 1.05, 95% 1.01–1.10) and two weeks after the operation (relative odds 1.53, 95% CI 1.00–2.34) in a multicenter observational study by Moss et al. [27]. This is further exacerbated in patients with Pain Catastrophizing [27], which has been described by Quartana et al. as a negative cognitive response to actual or anticipated pain [28]. Addressing patients’ medical comorbidities preoperatively may contribute to decreased opioid usage in the postoperative period. While studies such as that from Kain et al. have demonstrated that pre-procedural anxiolysis with benzodiazepines did not improve postoperative recovery [29], as discussed later in this paper, there are perioperative counseling techniques with demonstrated benefit, as well as non-medical interventions that may be especially helpful for patients with a diagnosis of anxiety.
2.3. Body Mass Index
Patient body mass index (BMI) has not clearly been associated with opioid use postoperatively among urogynecology patients. As many urogynecology procedures are elective, it is possible that procedures may be delayed in favor of non-surgical treatment options. A retrospective cohort study by Leach et al. found that preoperative opioid users tended to have a slightly higher BMI (29.2 (5.4) vs. 28.6 (10.3), p = 0.04) [30]. However, Ramaseshan et al. did not find BMI to be predictive of post-discharge opioid use in a secondary analysis of a randomized controlled trial of pelvic reconstructive surgery (p = 0.915) [11].
2.4. Race and Ethnicity
When discussing race and ethnicity in medicine, particularly in the field of gynecology, it is imperative to acknowledge the history of racism and racial exploitation that was foundational to the development of the field. Anaracha, Betsy and Lucy were enslaved Black women who were experimental subjects of Dr. Marion Sims following vaginal deliveries and sexual trauma that caused damage to their pelvic floors [31]. The procedures he subjected them to were without consent and without anesthesia [31]. There are many other instances of non-White patients being medically coerced, abused and denied appropriate care, one of the most famous being the United States Public Health Service (USPHS) or Tuskegee Syphilis study [32]. The impacts of systemic racism persist in medicine today. These manifest in many ways, including systematic undertreatment of pain. A study of medical students and residents demonstrated that about half of White trainees incorrectly believed that Black patients had physiological differences that make them feel less pain such as thicker skin or fewer nerve endings [33]. They also inaccurately rated Black patients’ pain as lower compared to White patients [33].
There is not a clear correlation between race and postoperative pain following pelvic reconstructive surgery. Studies outside of urogynecology have demonstrated that Black and Hispanic individuals have higher postoperative pain scores than non-Hispanic Whites [34]. Despite these higher pain scores, White patients were more likely to receive opioids than Black, Hispanic or Asian patients [35,36]. The previously described disparity has also been seen in postpartum patients, with Black and Hispanic women receiving less morphine milliequivalents (−3.54, 95%CI −5.88 to −1.20 and −5.03, 95%CI −6.91 to −3.15) despite reporting higher pain scores (adjusted odds ratio 2.18, 05% CI 1.63–2.91 and 1.61, 95% CI 1.26–2.06 respectively) in a retrospective cohort study by Badreldin et al. [37]. This has not been extensively studied following pelvic reconstructive surgery but may be an area for further research as our population becomes increasingly diverse given the history of inadequate pain management for non-White patients.
2.5. Language
Another area for further consideration given the increasingly diversifying United States population, is the patient’s primary language. A retrospective cohort study by Levy et al. demonstrated that non-English speaking patients received less morphine equivalents per day following gynecologic surgery (31.7 vs. 43.9 oral morphine equivalents, p < 0.01) and had their pain assessed less frequently (7.7 vs. 8.8 checks, p < 0.01) [38]. This is more likely attributable to language barrier and provider bias than physiological differences in pain perception.
2.6. Procedure Related Pain Outcomes
Shorter, more minimally invasive procedures are preferred due to their decreased association with postoperative pain [15]. For equivalent hysterectomies (uterine size, patient demographics), minimally invasive hysterectomies (vaginal, robotic or laparoscopic) were associated with decreased postoperative pain and opioid use, as measured by morphine milligram equivalents (vaginal 32.70, 95% CI 27.15–38.26), as were robotic or laparoscopic (39.91 95% CI 37.17–42.65) surgeries compared to open hysterectomy (54.97, 95% CI 48.81–61.13) [15,39]. Similarly, there was no difference between pain scores or opioid usage following robotic or laparoscopic sacrocolpopexy, and overall opioid usage and pain scores were lower for the procedure in a secondary analysis by Nilsson et al. [40]. Vaginal hysterectomy was associated with the lowest postoperative pain scores and opioid usage [15,39]. Willis-Gray et al. conducted a double-blind placebo-controlled randomized trial that demonstrated the vaginal route of surgery was associated with fewer opioid prescriptions filled [41]. Furthermore, a longer operative time was associated with greater odds of higher median opioid use in pelvic reconstructive surgery in a prospective quality study [7]. The findings supporting shorter operative times can be attributed to multiple factors including less tissue trauma, fewer incisions and less insufflation and associated abdominal distention [42]. Surgical complications and prolonged postoperative hospital stay were both independent risk factors for increased postoperative pain and opioid usage following pelvic reconstructive surgery in some studies [25] but not all [6].
3. Non-Pharmacologic Interventions for Pain
Given the significant impact of surgery on new-onset opioid use, researchers have increasingly evaluated non-medical interventions that can be utilized to improve perioperative pain management and reduce reliance on postoperative opioid prescribing. Interventions such as dedicated counseling, music therapy and massage are being introduced and evaluated. These non-medical interventions are based on the ongoing understanding of the biopsychosocial pain model. This framework conceptualizes the sensation of pain as a result of the complex interplay between psychological processes and social influences in addition to pathophysiology [43,44]. Psychological and social stressors can amplify pain perception; however, the opposite is also: true supportive environments can improve pain perception and outcomes [43,44]. While this model was designed around chronic pain management, it has demonstrated utility in the management of acute pain as well, and research regarding interventions are ongoing.
Selle et al. presented several opioid sparing strategies in a prospective cohort study including pre- and postoperative counseling regarding opioid use [45]. Patients in the intervention had significantly greater odds of being discharged without opioids (68.1% vs. 10.0%, p < 0.01) and were less likely to receive a rescue opioid prescription after discharge (1.4% vs. 9.5%, p = 0.03) [45]. Sassani et al. performed a secondary analysis of Preoperative Counseling Method and Postoperative Opioid Usage and found that despite similar pain scores, women who received preoperative phone counseling regarding opioid use prior to POP surgery had lower opioid utilization postoperatively; the median total number of 5 mg oxycodone tables was 0 (IQR 0–2, p = 0.002) [46]. Patient functional status also impacted postoperative pain and opioid usage. In a prospective cohort study by Sakai et al. examining patients undergoing pelvic reconstructive surgery, the high baseline activity (BA) group required significantly less opioid medication in the postoperative period than the low BA group (19 +/− 32 vs. 52 +/− 70 morphine milliequivalents, p = 001) [47]. Shah et al. evaluated the efficacy of therapeutic suggestion regarding opioid use in the postoperative period through a single-blinded randomized controlled trial for patients undergoing minimally invasive pelvic reconstructive procedures [48]. However, there were no significant differences in morphine milligram equivalents, postoperative pain or other subjective improvement [48].
Connective tissue massage was associated with significantly lower pain intensity and analgesic usage (p < 0.001) following total laparoscopic and abdominal hysterectomy compared to controls in a randomized controlled trial by Dogan et al. [49]. Additionally, a randomized, placebo-controlled trial demonstrated thirty minutes of massage with lavender following gynecologic surgery improved pain scores (2.66 +/− 0.89 vs. 3.80 +/− 1.01) on the Verbal Rating Scale, providing support for aromatherapy in addition to massage [50]. Hill et al. found that altering the hospital rooms to include natural landscapes and music improved patient satisfaction with care following pelvic reconstructive surgery even though it did not change initial postoperative pain scores [51]. A systematic review by Sin et al. examined the benefits of music therapy for patients having gynecologic surgery and found a significant reduction in both pain score intensity and analgesic usage in patients who received music therapy [52]. These are only a few of the techniques that have been studied to address postoperative pain, and additional studies demonstrate benefit from both physical and psychological approaches including mediation, hypnotherapy, acupuncture and cryotherapy among many others [53].
As the vast majority of urogynecologic procedures happen on an elective basis there is ample opportunity for preoperative counseling regarding postoperative pain expectations and opioid usage as well as complementary strategies for pain management. These are low-cost alternatives that can provide both a physical and psychological benefit to patients.
There is evidence to support non-pharmacologic interventions in the peri-operative period, specifically counseling regarding expectations around opioid use and this is recommended to be included for all patients undergoing a urogynecologic procedure. Other non-pharmacologic interventions have less supporting evidence and can be implemented at low cost and risk; however, they may have insignificant benefit and are not routinely recommended.
4. Multimodal Pain Regimen
By far, one of the most impactful interventions developed to mitigate postoperative opioid use and improve surgical recovery is enhanced recovery after surgery (ERAS) protocols and, within them, multimodal pain regimens (Figure 1). ERAS protocols have been extensively studied and consistently reduce recovery time, postoperative complications and improve cost effectiveness [54,55]. ERAS relies on a multidisciplinary team to incorporate preoperative counseling and non-opioid analgesia, minimize fluid and electrolyte imbalances, maintain normothermia and prioritize minimally invasive surgical routes [54,55]. These protocols have a well-established association with lower opioid usage in urogynecologic and other surgical patients postoperatively [56]. Given the benefits seen after incorporation of ERAS protocols across other surgical subspecialties, the American Urogynecologic Society (AUGS) and International Urogynecological Associations (IUGA) released a joint clinical statement on ERAS protocols in urogynecology [57].
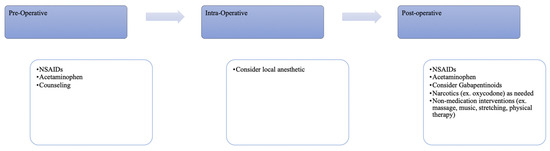
Figure 1.
Perioperative interventions to decrease postoperative opioid usage.
4.1. Preemptive Analgesia
The use of oral acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) preoperatively and postoperatively independently decreases postoperative pain following urogynecologic procedures [57]. AUGS-IUGA specifically recommends this regimen preoperatively to reduce opioid usage as a generally low-cost and low-risk intervention [53]. The primary mechanism of action of NSAIDs is via cyclooxygenase (COX) inhibition preventing the conversion of arachidonic acid to prostaglandins that mediate inflammation, pain and fever [57]. Traditional NSAIDs are nonspecific inhibitors of COX-1 and COX-2. Selective COX-2 inhibitors are sometimes preferred in ERAS protocols, as COX-2 inhibition has anti-inflammatory, analgesic and antipyretic benefits without the gastrointestinal, antiplatelet and renal effects associated with COX-1 inhibition [58]. Acetaminophen has a slightly more complex mechanism of action: it is responsible for central inhibition of COX enzymes, reducing the synthesis of prostaglandin E2 in the brain. Its lack of anti-inflammatory activity is due to weak peripheral COX inhibition [59]. Its metabolites have also been shown to act at cannabinoid receptors, modulate serotonergic and adenosine pathways and modulate neuronal excitability, also contributing to analgesic effects [60,61]. A systematic review Steinberg et al. found that the use of preemptive paracetamol, gabapentin, bupivacaine and phenothiazine resulted in less postoperative opioid usage than placebo, which lead to the development of clinical practice guidelines [62]. Similar findings were demonstrated in a study by Reagan et al., which showed patients who had a multimodal pain regimen used significantly fewer total narcotics (195.5 +/− 147.2 mg vs. 304.0 +/− 162.1, p < 0.001), despite having no difference in pain score [63]. They also were more likely to use 0 oral narcotics after discharge (34.8% vs. 10.6%, p = 0.01) [63]. However, the AUGS-IUGA does not recommend preoperative gabapentinoid use at this time due to the risk of respiratory depression and an only modest effect on postoperative pain and opioid use [57].
There is strong evidence to support preemptive analgesia in accordance with the ERAS protocol in urogynecologic surgery, and its routine use is recommended.
4.2. Local Anesthesia
The benefit of local anesthesia has been studied in urogynecology as a low-cost and generally low-risk intervention; however, its use and routes of administration are not standardized among urogynecologists. Local anesthetics reversibly block sodium channels in neuronal cell membranes, inhibiting both the initiation and propagation of nerve impulses in the sensory fibers at the site of administration [64]. Patients who received transversus abdominis plane (TAP) blocks using a standardized lateral ultrasound-guided approach in addition to conventional analgesia in the setting of robotic sacrocolpopexy had lower cumulative morphine milliequivalents postoperatively [65]. However, because of the varying routes of surgical approach and various sites of nerve blocks, these effects may not have consistent clinical significance (Table 1). Torosis et al. conducted a randomized controlled trial that demonstrated no difference in opioid use in the first 24 h following transvaginal prolapse repair with pudendal nerve block (8 (0–20) vs. 6.7 (0–15); p = 0.8) [66]. Chang-Patel et al. did find a statistically significant lower administration of morphine milliequivalents postoperatively following minimally invasive laparoscopic hysterectomy with TAP blocks (morphine milliequivalents 43.2 vs. 53.9, p = 0.002), but that difference corresponded to only approximately one 5 mg oxycodone tablet [67]. This was also true for local anesthetic injection into pelvic muscles [67]. A double-blinded placebo-controlled randomized trial found that bupivacaine injected at the puborectalis or iliococcygeus did not improve postoperative pain or opioid use following vaginal prolapse repair [68]. Following a procedure with this intervention, 24 h opiate use was 42 vs. 48 (p = 0.39), with a 48 h use of 75 vs. 37 (p = 0.09) [68]. Combining bupivacaine dexamethasone for additional anti-inflammatory benefit also had no improvement for individuals who underwent vaginal prolapse repair in a double-blind randomized controlled trial by Giugale et al. [69].
Additionally, it is not clear if this analgesic effect is secondary to tissue infiltration or local anesthetic specifically. Gluck et al. showed a lower morphine dose use in recovery and less overall postoperative analgesia use 24 h after vaginal hysterectomy in patients who had infiltration with either bupivacaine or sodium chloride compared to those with no infiltration (3.7 ± 2.3 mg vs. 5.3 ± 2.4 mg, p < 0.001) and less use of analgesia (all kinds) 24 h after surgery (54.2% vs. 79.6%, p < 0.001) [70]. Abramov et al. performed a randomized double-blinded placebo-controlled trial that showed no significant differences in postoperative pain intensity or hydromorphone consumption between bupivacaine and saline pudendal nerve blocks for transvaginal pelvic reconstruction surgery (0–3 h, 1.84 mg versus 1.77 mg; 4–7 h, 1.19 mg versus 1.20 mg; 8–18 h, 2.89 mg versus 2.35 mg) [71]. Also complicating this picture is the non-standard administration of local anesthetic. While there are standard methods for the performance of injections such as pudendal blocks, it is impossible to ensure that these are applied completely consistently across different surgeons. Additionally, the introduction of ultrasound-guided techniques may increase standardization of interventions such as TAP blocks; however, this approach is not yet universal across the urogynecologic studies, which also creates variability. Laparoscopic-guided TAP blocks have now been investigated in colorectal and bariatric surgery and have been shown to be faster and less resource-intensive, while being non-inferior to ultrasound-guided TAP blocks [72,73]. This may be a future area of investigation in urogynecology.
Furthermore, while the administration of local anesthetic is largely regarded as safe, there are risks both locally and systemically, such as central nervous system toxicity, cardiovascular toxicity, methemoglobinemia and allergic reactions [64]. At the injection site, patients can experience nerve injury, infection and hematoma, among other risks [64].
The increased use of liposomal local anesthetic in other fields such as orthopedic and dental surgery has made this agent a novel target for investigators. This involves the encapsulation of local anesthetic within a phospholipid bilayer, resulting in a sustained controlled release of local anesthetic at the administration site [74]. There has not yet been clear evidence of benefit in either pain or opioid use when liposomal anesthetic is used in urogynecologic surgery. A randomized placebo-controlled trial by Mazloomdoost et al. demonstrated that opioid use was similar between participants who received liposomal bupivacaine and those that received normal saline, and overall pain scores were low for both groups [75]. Likewise, the use of liposomal bupivacaine in posterior vaginal wall surgeries did not provide a significant decrease in postoperative pain or decrease opioid medication usage when compared to saline in a randomized control trial by Jones et al. [76]. Infiltration of lidocaine with epinephrine vs. normal saline also did not improve postoperative pain scores following sacrospinous ligament suspension in studies by both Ezzedine and Propst et al. [77,78]. Similarly, a study by Dengler et al. showed no difference in total opioid consumption through postoperative day 3 (p = 0.82) or pain scores with combination liposomal and bupivacaine injections versus plain bupivacaine injections [79]. Furthermore Jones et al. demonstrated that plain bupivacaine was not inferior to liposomal bupivacaine in regard to pain scores, morphine equivalent doses or hospital stay length [76].
The joint statement by AUGS-IUGA highly recommends incisional injection but not tap blocks for minimally invasive gynecologic, vaginal and vulvar surgery and provided moderate evidence for the benefit of liposomal bupivacaine [57]. Given the expense of liposomal anesthetic, EXPAREL is available in 133 mg (10 mL) dose for USD 241.29 and 266 mg (20 mL) dose for USD 398.69 [80], it is not clear that it has a significant enough impact for urogynecologic patients to justify the cost.
The urogynecologic procedures referenced throughout this paper are traditionally performed under general anesthesia. However, for less intensive pelvic floor repair procedures, some physicians have performed the procedure under local anesthesia with or without sedation. A meta-analysis by Zacharakis et al. evaluated 19 studies of over 1600 urogynecologic procedures conducted with local anesthetic [81]. The comparative studies showed significantly lower mean pain scores than patients who were given general anesthesia for the first 18 h [81], i.e., at both 4–6 h and 8–18 h postoperatively (160 patients; mean difference, −1.70; 95% confidence interval: −3.12, −0.28; p = 0.02 and 160 patients; mean difference, −0.72; 95% CI: −1.17, 0.27; p = 0.002, respectively) [81]. Pain scores at >24 h did not differ among the two groups (160 patients; mean difference, −0.28; 95% CI: −0.60–0.05; p = 0.10) [81]. Outside of 24 h, there was not a difference in pain scores [81]. Athanasiou et al. compared vaginal hysterectomy and pelvic floor repair under a combined spinal epidural with local anesthetic and IV sedation [82]. They demonstrated decreased opioid need in the local anesthesia group (35% vs. 95%, p = 0.002), as well as decreased pain at rest [82]. At this time, there is not sufficient evidence to change typical urogynecologic practice. However, in patients where general anesthesia presents significant risk, it may be reasonable to perform pelvic reconstructive surgery under local anesthesia without increasing risk of postoperative pain or narcotic use.
There is evidence to support use of local anesthetic; however, it should be implemented on a case-by-case basis, as some studies have only moderate or weak evidence, as such routine use in all urogynecologic procedures is not recommended at this time. Evidence for liposomal anesthetic in urogynecologic procedures is not strong and, at this time, its use over plain anesthetic is not recommended.
4.3. Postoperative Regimens
As previously mentioned, the regimen of oral acetaminophen and NSAIDs preoperatively and postoperatively independently decreases postoperative pain following urogynecologic procedures [57]. This regimen is a cornerstone of the AUGS-IUGA postoperative medication recommendations and should be used unless contraindicated [57]. Of note, in a double-blind placebo-controlled randomized controlled trial, IV paracetamol was not superior to oral acetaminophen in terms of opioid usage (19 mg milliequivalents vs. 20 mg milliequivalents, p = 0.605) or pain scores (27.0, IQR 35.0 vs. 35.0, IQR 44.5, p = 0.465) in patients following vaginal reconstructive surgery [83]. Gabapentinoids have also been studied in urogynecology patients. These medications bind the alpha 2 delta subunit of voltage-dependent calcium channels, decreasing neuronal excitability and central sensitization; there is also evidence to support decreased microglial activation and expression of proinflammatory cytokines [84]. In pelvic reconstructive surgery, these medications have been found to have a modest effect on postoperative pain; however, more research is needed to evaluate the benefit compared to the risk of respiratory depression secondary to decreased central respiratory drive; at this time, these medications are not part of the standard AUGS-IUGA recommendations [57]. However, their use is recommended as part of the ERAS protocol following gynecologic oncologic surgery [54] and other surgical specialties [84] and recommended by the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine and the American Society of Anesthesiologists [85]. Petrikovets et al. conducted a multicenter randomized controlled trial demonstrated that an ERAS protocol that features scheduled interventions, such as those mentioned previously, Tylenol, Toradol and ice packs, was superior to the standard protocol, which included as needed interventions in decreasing postoperative pain for women after urogynecologic surgery [86]. The study group received less oral morphine equivalents during their entire hospitalization (55.7 vs. 91.2, p < 0.001) [86]. Reductions in post-pelvic reconstructive procedure pain scores and opioid usage (3.25 vs. 10.8 morphine milliequivalents, p < 0.0001) was also shown in a prospective cohort study by Trowbridge et al. [87]. Zacharakis et al. performed a systematic review evaluating ERAS protocols in urogynecologic surgery and showed that for equivalent procedures, the length of hospital stay was significantly shorter for those that had an ERAS protocol, and pain scores were non-inferior [81]. With ERAS protocols in place, same-day-of-surgery discharge for POP procedures increased to over 90% (from a baseline of 25%) in a retrospective analysis by Carter-Brooks et al. [88]. A subsequent retrospective cohort study by Carter-Brooks et al. demonstrated a similar increase in same-day discharge and decreased length of hospital stay for patients in the ERAS protocol across all age categories [89]. Furthermore, Carter-Brooks et al. demonstrated that despite no difference in PACU pain scores, the ERAS protocol was associated with decreased opioid usage, although this effect was highly dependent on patient age [89]. Younger women received 22.5 mg more than middle-aged women (p < 0.0001) and middle-aged women received 24.3 mg more than elderly women. (p < 0.0001) [89]. Given the robust data supporting ERAS protocols, in general, it is now standard practice for most urogynecologists and surgeons. While these protocols have become standardized, the implementation of all aspects should always occur on a patient-by-patient basis, balancing the risks. As previously mentioned, COX-1 inhibition can cause or exacerbate gastrointestinal injury, such as ulcers, due to loss of protective prostaglandin E2 [58]. Some of this risk can be mitigated with medications that provide gastric protection, including proton pump inhibitors, high-dose histamine 2 receptors or using selective COX-2 inhibitors; however, these come with their own dose-dependent increased cardiac risk [90,91]. The effect of the acetaminophen metabolite n-acetyl-p-benzoquinone is also dose-dependent in its relationship to hepatic injury [92]. As discussed above, gabapentinoids may be used as part of multimodal pain control; however, decreased neuronal excitability is associated with decreased central respiratory drive, which can be exacerbated by concurrent opioid usage [84] and should be carefully monitored. Additional interventions have been less successful; for example, the use of rectal diazepam for chronic pelvic pain was not effective as a post-surgical intervention for patients undergoing pelvic reconstructive surgery [93]. Studies are ongoing to investigate other ways to refine and enhance ERAS protocols for urogynecologic patients.
There is strong evidence to support postoperative analgesia following the ERAS protocol, and routine use is recommended in urogynecologic surgery.

Table 1.
Characteristics and findings of the studies regarding the use of local anesthesia.
Table 1.
Characteristics and findings of the studies regarding the use of local anesthesia.
| Author (Year) | Type of Study | Anesthetics | Location of Infiltration | Pain and Opioid Use Outcomes |
|---|---|---|---|---|
| Zoorob et al. [65] | Prospective Double-Blinded Randomized Controlled Trial | 0.025% Bupivacaine | TAP Block | TAP block had lower cumulative NRS pain scores at 48 h postoperatively (14.90 ± 2.2 vs. 16.60 ± 2.04, p = 0.02) and 7 days postoperatively (17.10 ± 2.63 vs. 19.75 ± 2.65, p = 0.003). Intervention group also had lower cumulative morphine milliequivalents at 7 days postoperatively (17.25 ± 10.7 vs. 29.25 ± 14.53, p = 0.005). |
| Torosis et al. [66] | Randomized Blinded Controlled Trial | 0.5% Bupivacaine, Local Anesthetic per Surgeon | Pudendal Nerve Block | Median pain scores at 24 and 48 h did not differ between groups (4 ± 2 vs. 3 ± 3; p = 0.44). No difference in opioid use in first 24 h between the groups (8 [0–20] vs. 6.7 [0–15]; p = 0.8). |
| Chang-Patel et al. [67] | Retrospective Single-Institution Cohort Study | Not listed | TAP Block | Lower mean use of opioids (MME 43.2 vs. 53.9, p = 0.002) among patients who received a TAP block (either pre or postoperatively). |
| Kaeser et al. [68] | Multicenter Double-Blinded Placebo-Controlled Randomized Trial | 0.5% Bupivacaine, 0.9% Saline | Puborectalis, Iliococcygeus | 24 h postoperative cumulative VAS pain scores for the bupivacaine and normal saline arms, 19 and 18 (p = 0.71); opiate use (24 h use was 42 vs. 48, p = 0.39; 48 h use was 75 vs. 37, p = 0.09). |
| Giugale et al. [69] | Three-Arm, Double-Blind Randomized Control Trial | 0.9% Saline, 0.25% Bupivacaine, Combination 0.25% Bupivacaine with 4 mg Dexamethasone | Trans obturator, Levator Ani, Transvaginal Pudendal Nerve | No significant difference in median pain scores on postoperative day 1 among study groups (median [interquartile range] pain score 4.0 [2.0–7.0] for placebo vs. 4.0 [2.0–5.5] for bupivacaine vs. 4.0 [1.5–5.0] for bupivacaine with dexamethasone, p = 0.92). |
| Gluck et al. [70] | Retrospective Single-Center Cohort Study | 0.9% Saline, 0.5% Bupivacaine | Local infiltration | No differences in levels of pain at all points of time. Infiltration group required a lower morphine dose in the recovery unit (3.7 ± 2.3 mg vs. 5.3 ± 2.4 mg, p < 0.001) and less use of analgesia (all kinds) 24 h after surgery (54.2% vs. 79.6%, p < 0.001). |
| Abramov et al. [71] | Randomized Double-Blinded Placebo-Controlled Trial | 0.25% Bupivacaine, 0.9% Saline | Pudendal Nerve Block | No significant differences in postoperative pain intensity or the consumption of hydromorphone (0–3 h, 1.84 mg versus 1.77 mg; 4–7 h, 1.19 mg versus 1.20 mg; 8–18 h, 2.89 mg versus 2.35 mg). |
| Mazloomdoost et al. [75] | Randomized Placebo-Controlled Trial | Liposomal Bupivacaine, 0.9% Saline | Trocar Paths, Vaginal Incision | Pain scores were lower for subjects receiving liposomal bupivacaine during the first three postoperative days. Fewer subjects in the intervention group consumed narcotic medication on postoperative day 2 (12 vs. 27; p = 0.006). |
| Propst et al. [78] | Randomized Double-Blind Trial | 0.5% Lidocaine with Epinephrine, 1.3% Bupivacaine Liposomal mixed with 0.5% Bupivacaine | Sacrospinous Ligament | Global postoperative pain differed between the arms at 36 h with median (IQR) pain score 4.0 (1.5–4.5) in the lidocaine arm and 0 (0–3) in the liposomal bupivacaine arm (p = 0.04) |
| Dengler et al. [79] | Double-Blinded Randomized Controlled Trial | 1.3% Liposomal and 0.25% plain Bupivacaine vs. 0.25% plain Bupivacaine | Pudendal Nerve Block | Median pain scores for the study and control groups, respectively, were 0 (0–2) and 2 (0–4) for postoperative day 1 (p = 0.03); 2 (1–4) and 3 (2–5) for postoperative day 2 (p = 0.05); and 2 (1–4) and 3 (2–5) for postoperative day 3 (p = 0.02). No difference between groups in total opioid consumption through postoperative day 3 (p = 0.82) |
| Zacharakis et al. [81] | Systematic Review and Meta-Analysis | Local Anesthesia, General Anesthesia | Significantly lower mean pain scores in local anesthesia group compared to general-regional anesthesia at both 4–6 h and 8–18 h postoperatively (160 patients; mean difference [MD], −1.70; 95% confidence interval [CI]: −3.12, −0.28; p = 0.02 and 160 patients; MD, −0.72; 95% CI: −1.17, 0.27; p = 0.002, respectively). Pain scores at >24 h did not differ among the two groups (160 patients; MD, −0.28; 95% CI: −0.60–0.05; p = 0.10). | |
| Athanasiou et al. [82] | Prospective Cohort Study | Combined Spinal Epidural, Local Anesthesia with IV sedation | Median pain intensity at rest was significantly lower in the local anesthesia group at 2 h, 4 h and 8 h postoperatively (median values: 0 vs. 1.9, 0 vs. 4.1 and 1 vs. 2.7, respectively) The percentage of patients needing opioids was significantly lower for the local anesthesia group (35% vs. 95%, p = 0.002) | |
| Jensen et al. [94] | Retrospective Cohort Study | Liposomal Bupivacaine, 0.25% Bupivacaine | Pudendal Nerve Block | Subjective pain was similar between groups (0 vs. 1.6 ± 2.6, p = 0.68). Difference between postoperative morphine equivalent dose for plain bupivacaine versus liposomal bupivacaine (25.3 ± 65.8 vs. 24.9 ± 31.7) |
5. Conclusions
The continued prevalence of opioid use disorder in the United States underscores the importance of not only identifying patients that are currently suffering from opioid use disorder but also identifying those at risk of increased opioid use. As a substantial percentage of patients with opioid use disorder first acquired opioids from a prescribing provider, responsible opioid prescribing is not only a medical responsibility but a necessity. Furthermore, continued research into pain management strategies that reduce the need for opioids is critical to advancing postoperative pain control for patients. Postoperative pain and opioid use can be reliably influenced by a multitude of patient factors, including age, local analgesic use comorbid conditions, procedure invasiveness, perioperative counseling and complementary pain medication/anesthesia. Clinicians can use this knowledge to identify those patients at higher risk of opioid usage in the postoperative period and consider additional pain management strategies such as those outlined in this review (Figure 2).
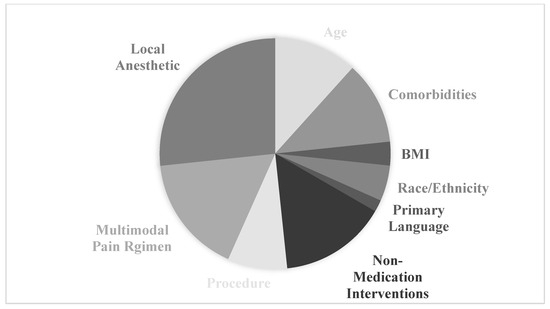
Figure 2.
Distribution of articles across factors affecting postoperative pain for patients undergoing urogynecologic procedures.
Clinical Recommendations
The current evidence supports the implementation of perioperative interventions to reduce opioid usage in urogynecology patients. Preoperative counseling regarding expectations and postoperative opioid use and pre-medication with NSAIDs and acetaminophen should be employed. Intraoperative use of local anesthesia should be considered on a case-by-case basis and should be performed with plain anesthetic. Postoperatively, scheduled use of NSAIDs and acetaminophen are recommended. As-needed use of oral narcotics is appropriate. However, we would recommend surgeons limit the quantity of opioids provided on discharge, employing a case-by-case model for the exact number prescribed through shared decision making with the patient. Finally, scheduled gabapentinoids should be considered, with the exception of patients at increased risk of respiratory compromise. Additional non-pharmacologic interventions such as opioid use and pain counseling, massage and similar techniques should be provided to patients as well. Expanding the research into non-narcotic and non-pharmacologic interventions for urogynecology patients is necessary to further develop the standard recommendations for perioperative care for these patients.
Author Contributions
J.B.L. and S.S.B. conceptualized the paper. J.B.L., S.S.B. and L.D. contributed to drafting and revising the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Thanks to Long and Boyd for their expertise and assistance throughout all aspects of writing the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- CDC Wonder: National Center for Health Statistics (NCHS), Underlying Cause of Death by Single Race 2018–2023 on CDC WONDER Online Database, Released 2025. Data Are Compiled from Data Provided by the 57 Vital Statistics Jurisdictions through the Vital Statistics Cooperative Program. Available online: http://wonder.cdc.gov/ucd-icd10-expanded.html (accessed on 5 March 2025).
- Berterame, S.; Erthal, J.; Thomas, J.; Fellner, S.; Vosse, B.; Clare, P.; Hao, W.; Johnson, D.T.; Mohar, A.; Pavadia, J.; et al. Use of and barriers to access to opioid analgesics: A worldwide, regional, and national study. Lancet 2016, 387, 1644–1656. [Google Scholar] [CrossRef]
- Ju, C.; Wei, L.; Man, K.K.C.; Wang, Z.; Ma, T.-T.; Chan, A.Y.L.; Brauer, R.; Chui, C.S.L.; Chan, E.W.; Jani, Y.H.; et al. Global, regional, and national trends in opioid analgesic consumption from 2015 to 2019: A longitudinal study. Lancet Public Health 2022, 7, e335–e346. [Google Scholar] [CrossRef]
- Lankenau, S.E.; Teti, M.; Silva, K.; Bloom, J.J.; Harocopos, A.; Treese, M. Initiation into prescription opioid misuse amongst young injection drug users. Int. J. Drug Policy 2012, 23, 37–44. [Google Scholar] [CrossRef]
- Jones, C.M.; Paulozzi, L.J.; Mack, K.A. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use United States, 2008–2011. JAMA Intern. Med. 2014, 174, 802–803. [Google Scholar] [CrossRef] [PubMed]
- Ackenbom, M.F.M.; Dong, S.B.; Romanova, A.; Baranski, L.B.; Butters, M.A.; Davis, E.M.; Zyczynski, H.M. Postoperative Opioid Utilization in Older Women Undergoing Pelvic Organ Prolapse Surgery. Female Pelvic Med. Reconstr. Surg. 2021, 27, 304–309. [Google Scholar] [CrossRef]
- Palm, K.M.; Abrams, M.K.; Sears, S.B.; Wherley, S.D.; Alfahmy, A.M.; Kamumbu, S.A.; Wang, N.C.; Mahajan, S.T.; El-Nashar, S.A.; Henderson, J.W.; et al. Opioid use following pelvic reconstructive surgery: A predictive calculator. Int. Urogynecol. J. 2023, 34, 1725–1742. [Google Scholar] [CrossRef]
- As-Sanie, S.; Till, S.R.; Mowers, E.L.; Lim, C.S.; Skinner, B.D.; Fritsch, L.B.; Tsodikov, A.; Dalton, V.K.; Clauw, D.J.; Brummett, C.M. Opioid Prescribing Patterns, Patient Use, and Postoperative Pain After Hysterectomy for Benign Indications. Obstet. Gynecol. 2017, 130, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.R.W.; Paraiso, M.F.R.; Walters, M.D.; Propst, K.; Ridgeway, B.; Yao, M. A randomized controlled noninferiority trial of reduced vs routine opioid prescription after prolapse repair. Am. J. Obstet. Gynecol. 2021, 224, 619, Erratum in Am. J. Obstet. Gynecol. 2020, 223, 547.e1–547.e12. https://doi.org/10.1016/j.ajog.2020.03.017. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.S.; Propst, K.A.; Ross, J.H.; Wallace, S.L.; Paraiso, M.F.R.; Park, A.J.; Chapman, G.C.; Ferrando, C.A. Restrictive opioid prescribing after surgery for prolapse and incontinence: A randomized, noninferiority trial. Am. J. Obstet. Gynecol. 2024, 230, 340.e1–340.e13. [Google Scholar] [CrossRef]
- Ramaseshan, A.S.; Tunitsky-Bitton, E.; O’sUllivan, D.M.; Reagan, K.M.L.; Steinberg, A.C. Predictive Factors of Postdischarge Narcotic Use After Female Pelvic Reconstructive Surgery. Female Pelvic Med. Reconstr. Surg. 2019, 25, e18–e22. [Google Scholar] [CrossRef]
- Olive, E.J.; Glasgow, A.E.M.; Habermann, E.B.; Gebhart, J.B.; Occhino, J.A.; Trabuco, E.C.; Linder, B.J. Evaluating the Long-term Impact of Implementing Standardized Postoperative Opioid Prescribing Recommendations Following Pelvic Organ Prolapse Surgery. Urogynecology 2024, 30, 35–41. [Google Scholar] [CrossRef]
- Reagan, K.M.; Boyles, S.H.; Brueseke, T.J.; Linder, B.J.; Willis-Gray, M.G.; Cichowski, S.B.; Long, J.B. Postoperative Opioid Prescribing After Female Pelvic Medicine and Reconstructive Surgery. Female Pelvic Med. Reconstr. Surg. 2021, 27, 643–653. [Google Scholar] [CrossRef]
- Luber, K.M.; Boero, S.; Choe, J.Y. The demographics of pelvic floor disorders: Current observations and future projections. Am. J. Obstet. Gynecol. 2001, 184, 1496–1503. [Google Scholar] [CrossRef]
- DeBlanc, J.J.; Brummett, C.M.; Gunaseelan, V.; As-Sanie, S.; Morgan, D.M. An Analysis of Opioid Consumption and Patient Recovery after Hysterectomy by Surgical Approach. J. Women’s Health 2025, 34, 242–250. [Google Scholar] [CrossRef]
- Leach, D.A.; Habermann, E.B.; Glasgow, A.E.; Occhino, J.A. Postoperative Opioid Prescribing Following Gynecologic Surgery for Pelvic Organ Prolapse. Female Pelvic Med. Reconstr. Surg. 2020, 26, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Willis-Gray, M.G.; Husk, K.E.; Brueseke, T.J.; Wu, J.M.; Dieter, A.A. Predictors of Opioid Administration in the Acute Postoperative Period. Female Pelvic Med. Reconstr. Surg. 2019, 25, 347–350. [Google Scholar] [CrossRef]
- Willis-Gray, M.G.; Leazer, H.A.; Sun, S.; Feliciano, K.M.; Dieter, A.A.; Geller, E.J.; Connolly, A.; Chidgey, B.A.; Wu, J.M. Examining Age and Postoperative Opioid Use in the Urogynecology Population: A Prospective Study. Urogynecology 2022, 28, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.B.; Choi, U.B.; Liao, B.B.; Kohn, T.P.M.; Kohn, J.; Dumas, K.; Clifton, M. Perioperative Pain Management With Opioid Analgesics in Colpopexy Increases Risk of New Persistent Opioid Usage. Urogynecology 2023, 29, 183–190. [Google Scholar] [CrossRef]
- Chau, D.L.; Walker, V.; Pai, L.; Cho, L.M. Opiates and elderly: Use and side effects. Clin. Interv. Aging 2008, 3, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Eldesoky, E.S. Pharmacokinetic-Pharmacodynamic Crisis in the Elderly. Am. J. Ther. 2007, 14, 488–498. [Google Scholar] [CrossRef]
- Zhi, Y.; Zhang, Y.; Zhang, M.; Kong, Y. Age-associated changes in multimodal pain perception. Age Ageing 2024, 53, afae107. [Google Scholar] [CrossRef]
- van Dijk, J.F.M.; Zaslansky, R.; van Boekel, R.L.M.; Cheuk-Alam, J.M.; Baart, S.J.; Huygen, F.J.P.M.; Rijsdijk, M. Postoperative Pain and Age: A Retrospective Cohort Association Study. Anesthesiology 2021, 135, 1104–1119. [Google Scholar] [CrossRef]
- Dwarica, D.S.; Rubenstein, A.R.; Boccaccio, R.B.; Motwani, A.K.; Peck, J.D.; LeClaire, E.L.; Quiroz, L.H. Opioid Pain Medication Use in New Urogynecology Patients. Female Pelvic Med. Reconstr. Surg. 2020, 26, 622–625. [Google Scholar] [CrossRef]
- AlAshqar, A.; Ishiwata, R.; Moss, C.; Andersen, K.M.; Yanek, L.; Bicket, M.C.; Alexander, G.C.; Borahay, M.A. Predictors of new persistent opioid use after benign hysterectomy in the United States. Am. J. Obstet. Gynecol. 2022, 227, 68.e1–68.e24. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Puhl, R.M.; Cummings, D.E.; Eckel, R.H.; Ryan, D.H.; Mechanick, J.I.; Nadglowski, J.; Ramos Salas, X.; Schauer, P.R.; Twenefour, D.; et al. Joint international consensus statement for ending stigma of obesity. Nat. Med. 2020, 26, 485–497. [Google Scholar] [CrossRef]
- Moss, C.; Pandya, P.R.; Yanek, L.; Lovejoy, D.; Muñiz, K.; Chen, C.C.G.; Blomquist, J.; Jacobs, S.; Powell, A.; Handa, V.L.; et al. The impact of anxiety on postoperative pain following pelvic reconstructive surgery. Int. Urogynecol. J. 2023, 34, 1551–1557. [Google Scholar] [CrossRef]
- Quartana, P.J.; Campbell, C.M.; Edwards, R.R. Pain catastrophizing: A critical review. Expert Rev. Neurother. 2009, 9, 745–758. [Google Scholar] [CrossRef]
- Kain, Z.N.; Sevarino, F.B.; Rinder, C.; Pincus, S.; Alexander, G.M.; Ivy, M.; Heninger, G. Preoperative anxiolysis and postoperative recovery in women undergoing abdominal hysterectomy. Anesthesiology 2001, 94, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.A.; Scarlotta, L.S.; Habermann, E.B.; Glasgow, A.E.; Occhino, J.A. Characteristics of opioid users undergoing surgery for pelvic organ prolapse. Int. Urogynecol. J. 2020, 31, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Anarchalucyandbetsey. Anarcha Lucy Betsey. Available online: https://www.anarchalucybetsey.org/anarchalucyandbetsey (accessed on 10 June 2025).
- About the untreated syphilis study at Tuskegee. The U.S. Public Health Service Untreated Syphilis Study at Tuskegee. Published September 4, 2024. Available online: https://www.cdc.gov/tuskegee/about/index.html (accessed on 12 September 2025).
- Hoffman, K.M.; Trawalter, S.; Axt, J.R.; Oliver, M.N. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc. Natl. Acad. Sci. USA 2016, 113, 4296–4301. [Google Scholar] [CrossRef]
- Perry, M.; Baumbauer, K.; Young, E.E.; Dorsey, S.G.; Taylor, J.Y.; Starkweather, A.R. The Influence of Race, Ethnicity and Genetic Variants on Postoperative Pain Intensity: An Integrative Literature Review. Pain Manag. Nurs. 2019, 20, 198–206. [Google Scholar] [CrossRef]
- Rosenbloom, J.M.; De Souza, E.; Perez, F.D.; Xie, J.; Suarez-Nieto, M.V.; Wang, E.; Anderson, T.A. Association of Race and Ethnicity with Pediatric Postoperative Pain Outcomes. J. Racial Ethn. Health Disparities 2023, 10, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Thurston, K.L.; Zhang, S.J.; Wilbanks, B.A.; Billings, R.; Aroke, E.N. A Systematic Review of Race, Sex, and Socioeconomic Status Differences in Postoperative Pain and Pain Management. J. PeriAnesthesia Nurs. 2023, 38, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Badreldin, N.; Grobman, W.A.; Yee, L.M. Racial Disparities in Postpartum Pain Management. Obstet. Gynecol. 2019, 134, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.A.; Kay, A.H.; Hills, N.; Chen, L.-M.; Chapman, J.S. Exploring the relationship between language, postoperative pain, and opioid use. AJOG Glob. Rep. 2024, 4, 100342. [Google Scholar] [CrossRef]
- Hessami, K.; Welch, J.; Frost, A.; AlAshqar, A.; Arian, S.E.; Gough, E.; Borahay, M.A. Perioperative opioid dispensing and persistent use after benign hysterectomy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2023, 229, 23–32.e3. [Google Scholar] [CrossRef]
- Nilsson, W.; Schmidt, M.; Turner, L.; Shepherd, J. Comparing Postoperative Pain With Laparoscopic Versus Robotic Sacrocolpopexy. J. Minim. Invasive Gynecol. 2024, 31, 200–204. [Google Scholar] [CrossRef]
- Willis-Gray, M.G.; Young, J.C.; Pate, V.; Funk, M.J.; Wu, J.M. Perioperative opioid prescriptions associated with stress incontinence and pelvic organ prolapse surgery. Am. J. Obstet. Gynecol. 2020, 223, 894.e1–894.e9. [Google Scholar] [CrossRef]
- Aarts, J.W.; E Nieboer, T.; Johnson, N.; Tavender, E.; Garry, R.; Mol, B.W.J.; Kluivers, K.B. Surgical approach to hysterectomy for benign gynecological disease. Cochrane Database Syst. Rev. 2015, 8, CD003677. [Google Scholar] [CrossRef]
- Sullivan, M.D.; Sturgeon, J.A.; Lumley, M.A.; Ballantyne, J.C. Reconsidering Fordyce’s classic article, “Pain and suffering: What is the unit?” to help make our model of chronic pain truly biopsychosocial. PAIN 2023, 164, 271–279. [Google Scholar] [CrossRef]
- Gatchel, R.J.; Peng, Y.B.; Peters, M.L.; Fuchs, P.N.; Turk, D.C. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol. Bull. 2007, 133, 581–624. [Google Scholar] [CrossRef]
- Selle, J.M.; Strozza, D.M.; Branda, M.E.; Gebhart, J.B.; Trabuco, E.C.; Occhino, J.A.; Linder, B.J.; El Nashar, S.A.; Madsen, A.M. A bundle of opioid-sparing strategies to eliminate routine opioid prescribing in a urogynecology practice. Am. J. Obstet. Gynecol. 2024, 231, 278.e1–278.e17. [Google Scholar] [CrossRef]
- Sassani, J.C.; Artsen, A.M.M.; Grosse, P.J.M.; Baranski, L.; Kunkle, L.M.; Ackenbom, M.F.M. Preoperative Counseling Method and Postoperative Opioid Usage: A Secondary Analysis of the PREOP Study. Female Pelvic Med. Reconstr. Surg. 2021, 27, 175–180. [Google Scholar] [CrossRef]
- Sakai, N.; Wu, J.M.; Willis-Gray, M. Preoperative Activity Level and Postoperative Pain After Pelvic Reconstructive Surgery. Urogynecology 2023, 29, 807–813. [Google Scholar] [CrossRef]
- Shah, N.M.; Andriani, L.A.; Mofidi, J.L.; Ingraham, C.F.; Tefera, E.A.; Iglesia, C.B. Therapeutic Suggestion in Postoperative Pain Control: A Randomized Controlled Trial. Female Pelvic Med. Reconstr. Surg. 2021, 27, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Dogan, H.; Çaltekin, M.D.; Günal, A. Short-Term Effects of Connective Tissue Massage After Hysterectomy: A Randomized Controlled Study. J. Manip. Physiol. Ther. 2022, 45, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Sahin, B.M.; Culha, I.; Gursoy, E.; Yalcin, O.T. Effect of Massage With Lavender Oil on Postoperative Pain Level of Patients Who Underwent Gynecologic Surgery: A Randomized, Placebo-Controlled Study. Holist. Nurs. Pract. 2021, 35, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.M.; Crisp, C.C.; Shatkin-Margolis, A.; Tam, T.; Yook, E.; Kleeman, S.; Pauls, R.N. The influence of postoperative environment on patient pain and satisfaction: A randomized trial. Am. J. Obstet. Gynecol. 2020, 223, 271.e1–271.e8. [Google Scholar] [CrossRef]
- Sin, W.M.; Chow, K.M. Effect of Music Therapy on Postoperative Pain Management in Gynecological Patients: A Literature Review. Pain Manag. Nurs. 2015, 16, 978–987. [Google Scholar] [CrossRef]
- Niyonkuru, E.; Iqbal, M.A.; Zhang, X.; Ma, P. Complementary Approaches to Postoperative Pain Management: A Review of Non-pharmacological Interventions. Pain Ther. 2025, 14, 121–144. [Google Scholar] [CrossRef]
- Nelson, G.; Bakkum-Gamez, J.; Kalogera, E.; Glaser, G.; Altman, A.; A Meyer, L.; Taylor, J.S.; Iniesta, M.; Lasala, J.; Mena, G.; et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations-2019 update. Int. J. Gynecol. Cancer 2019, 29, 651–668. [Google Scholar] [CrossRef]
- Kallen, A.N. ACOG Committee Opinion No. 750: Perioperative Pathways: Enhanced Recovery After Surgery. Obstet. Gynecol. 2018, 132, e120–e130. [Google Scholar] [CrossRef]
- Weston, E.; Noel, M.; Douglas, K.; Terrones, K.; Grumbine, F.; Stone, R.; Levinson, K. The impact of an enhanced recovery after minimally invasive surgery program on opioid use in gynecologic oncology patients undergoing hysterectomy. Gynecol. Oncol. 2020, 157, 469–475. [Google Scholar] [CrossRef]
- Developed by the Joint Writing Group of the International Urogynecological Association and the American Urogynecologic Society. AUGS-IUGA Joint clinical consensus statement on enhanced recovery after urogynecologic surgery. Int. Urogynecol. J. 2022, 33, 2921–2940. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Botting, R.M. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998, 104, 2S–8S. [Google Scholar] [CrossRef]
- Mirrasekhian, E.; Nilsson, J.L.Å.; Shionoya, K.; Blomgren, A.; Zygmunt, P.M.; Engblom, D.; Högestôtt, E.D.; Blomqvist, A. The antipyretic effect of paracetamol occurs independent of transient receptor potential ankyrin 1-mediated hypothermia and is associated with prostaglandin inhibition in the brain. FASEB J. 2018, 32, 5751–5759. [Google Scholar] [CrossRef] [PubMed]
- Przybyła, G.W.; Szychowski, K.A.; Gmiński, J. Paracetamol—An old drug with new mechanisms of action. Clin. Exp. Pharmacol. Physiol. 2021, 48, 3–19. [Google Scholar] [CrossRef]
- Ray, S.; Salzer, I.; Kronschläger, M.T.; Boehm, S. The paracetamol metabolite N-acetylp-benzoquinone imine reduces excitability in first- and second-order neurons of the pain pathway through actions on KV7 channels. PAIN 2019, 160, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, A.C.; Schimpf, M.O.; White, A.B.; Mathews, C.; Ellington, D.R.; Jeppson, P.; Crisp, C.; Aschkenazi, S.O.; Mamik, M.M.; Balk, E.M.; et al. Preemptive analgesia for postoperative hysterectomy pain control: Systematic review and clinical practice guidelines. Am. J. Obstet. Gynecol. 2017, 217, 303–313.e6. [Google Scholar] [CrossRef]
- Reagan, K.M.L.; O’sUllivan, D.M.; Gannon, R.; Steinberg, A.C. Decreasing postoperative narcotics in reconstructive pelvic surgery: A randomized controlled trial. Am. J. Obstet. Gynecol. 2017, 217, 325.e1–325.e10. [Google Scholar] [CrossRef]
- Ozbay, S.; Ayan, M.; Karcioglu, O. Local Anesthetics, Clinical Uses, and Toxicity: Recognition and Management. Curr. Pharm. Des. 2023, 29, 1414–1420. [Google Scholar] [CrossRef]
- Zoorob, D.M.; Tsolakian, I.; Shuffle, E.; Perring, P.; Maxwell, R.P. Addition of Transversus Abdominis Plane Block to Conventional Pain Regimens in Robotic Sacrocolpopexy Procedures—A Pilot Randomized Controlled Trial (SACROTAP). Urogynecology 2023, 29, 139–143. [Google Scholar] [CrossRef]
- Torosis, M.; Fullerton, M.; Kaefer, D.; Nitti, V.; Ackerman, A.L.; Grisales, T. Pudendal Block at the Time of Transvaginal Prolapse Repair: A Randomized Controlled Trial. Urogynecology 2024, 30, 706–713. [Google Scholar] [CrossRef]
- Chang-Patel, E.J.; Wong, J.M.; Gould, C.H.; Demirel, S. The Effect of Transversus Abdominis Plane Block Timing on Milliequivalents of Opioid Use and Immediate Postoperative Pain Scores in Patients Undergoing Minimally Invasive Hysterectomy: A Retrospective Cohort Study. J. Minim. Invasive Gynecol. 2024, 31, 237–242. [Google Scholar] [CrossRef]
- Kaeser, C.T.; Rothenberger, R.; Zoorob, D.; Whiteside, J.L.M. Bupivacaine Use After Posterior Colporrhaphy to Reduce Postoperative Pain: A Multicenter, Double-Blinded, Placebo-Controlled, Randomized Clinical Trial. Female Pelvic Med. Reconstr. Surg. 2022, 28, 72–76. [Google Scholar] [CrossRef]
- Giugale, L.E.; Baranski, L.A.B.; Meyn, L.A.; Schott, N.J.; Emerick, T.D.M.; Moalli, P.A. Preoperative Pelvic Floor Injections With Bupivacaine and Dexamethasone for Pain Control After Vaginal Prolapse Repair: A Randomized Controlled Trial. Obstet. Gynecol. 2021, 137, 21–31. [Google Scholar] [CrossRef]
- Gluck, O.; Amram, S.; Feldstein, O.; Barber, E.; Tamayev, L.; Weiner, E.; Oren, B.; Ginath, S. The Effect of Preemptive Local Infiltration on Postoperative Pain After Vaginal Hysterectomy: A Retrospective Study. J. Minim. Invasive Gynecol. 2023, 30, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Abramov, Y.; Sand, P.K.; Gandhi, S.; Botros, S.M.; Miller, J.-J.R.; Koh, E.-K.; Goldberg, R.P. The effect of preemptive pudendal nerve blockade on pain after transvaginal pelvic reconstructive surgery. Obstet. Gynecol. 2005, 106, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Meena, S.; Anand, G. Randomized single blind trial to compare the short term post-operative outcome and cost analysis of laparoscopic versus ultrasound guided transversus abdominis plane block in patients undergoing bariatric surgery. Surg. Endosc. 2023, 37, 7136–7143. [Google Scholar] [CrossRef] [PubMed]
- Iaquinandi, F.; Mongelli, F.; Christoforidis, D.; Cianfarani, A.; Pini, R.; Saporito, A.; Popeskou, S.G.; La Regina, D. Laparoscopic vs. ultrasound-guided transversus abdominis plane (TAP) block in colorectal surgery: A systematic review and meta-analysis of randomized trials. Surg. Endosc. 2024, 38, 1119–1130. [Google Scholar] [CrossRef]
- Prabhakar, A.; Ward, C.T.; Watson, M.; Sanford, J.; Fiza, B.; Moll, V.; Kaye, R.J.; Hall, O.M.; Cornett, E.M.; Urman, R.D.; et al. Liposomal bupivacaine and novel local anesthetic formulations. Best Pract. Res. Clin. Anaesthesiol. 2021, 35, E1–E2, Erratum in Best Pract. Res. Clin. Anaesthesiol. 2019, 33, 425–432. https://doi.org/10.1016/j.bpa.2019.07.012. [Google Scholar] [CrossRef]
- Mazloomdoost, D.; Pauls, R.N.; Hennen, E.N.; Yeung, J.Y.; Smith, B.C.; Kleeman, S.D.; Crisp, C.C. Liposomal bupivacaine decreases pain following retropubic sling placement: A randomized placebo-controlled trial. Am. J. Obstet. Gynecol. 2017, 217, 598.e1–598.e11. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.L.; Gruber, D.D.; Fischer, J.R.; Leonard, K.; Hernandez, S.L. Liposomal bupivacaine efficacy for postoperative pain following posterior vaginal surgery: A randomized, double-blind, placebo-controlled trial. Am. J. Obstet. Gynecol. 2018, 219, 500.e1–500.e8. [Google Scholar] [CrossRef] [PubMed]
- Ezzedine, D.; Dhariwal, L.; Wasenda, E.M.; Salamon, C.; Caraballo, R. Pudendal Nerve Block With Liposomal Bupivacaine for Sacrospinous Ligament Suspension. Urogynecology 2024, 30, 98–106. [Google Scholar] [CrossRef]
- Propst, K.; O’sUllivan, D.M.; Steinberg, A.C. Randomized double-blind trial of short- versus long-acting analgesia at the sacrospinous ligament. Int. Urogynecol. J. 2019, 30, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Dengler, K.L.; Craig, E.R.; DiCarlo-Meacham, A.M.; Welch, E.K.; Brooks, D.I.; Vaccaro, C.M.; Gruber, D.D. Preoperative pudendal block with liposomal and plain bupivacaine reduces pain associated with posterior colporrhaphy: A double-blinded, randomized controlled trial. Am. J. Obstet. Gynecol. 2021, 225, 556.e1–556.e10. [Google Scholar] [CrossRef]
- EXPAREL Cost 2024. The Price of Long-Lasting Non-Opioid EXPAREL for Pain After Surgery. Exparelpro.com. 2024. Available online: https://www.exparelpro.com/value/total-hip-arthroplasty (accessed on 25 May 2025).
- Zacharakis, D.; Diakosavvas, M.; Prodromidou, A.; Kathopoulis, N.; Angelou, K.; Kalantzis, C.; Ntounis, T.; Athanasiou, S.; Grigoriadis, T. Enhanced Recovery Protocols in Urogynecologic and Pelvic Floor Reconstructive Surgery: A Systematic Review and Meta-Analysis. Urogynecology 2023, 29, 21–32. [Google Scholar] [CrossRef]
- Athanasiou, S.; Zacharakis, D.; Grigoriadis, T.; Papalios, T.; Pitsouni, E.; Valsamidis, D.; Hadzillia, S. Vaginal hysterectomy with anterior and posterior repair for pelvic organ prolapse under local anesthesia: Results of a pilot study. Int. Urogynecol. J. 2020, 31, 2109–2116. [Google Scholar] [CrossRef]
- Crisp, C.C.; Khan, M.; Lambers, D.L.; Westermann, L.B.; Mazloomdoost, D.M.; Yeung, J.J.; Kleeman, S.D.; Pauls, R.N. The Effect of Intravenous Acetaminophen on Postoperative Pain and Narcotic Consumption After Vaginal Reconstructive Surgery: A Double-Blind Randomized Placebo-Controlled Trial. Female Pelvic Med. Reconstr. Surg. 2017, 23, 80–85. [Google Scholar] [CrossRef]
- Verret, M.; Lauzier, F.; Zarychanski, R.; Perron, C.; Savard, X.; Pinard, A.-M.; Leblanc, G.; Cossi, M.-J.; Neveu, X.; Turgeon, A.; et al. Perioperative Use of Gabapentinoids for the Management of Postoperative Acute Pain: A Systematic Review and Meta-analysis. Anesthesiology 2020, 133, 1159, Erratum in Anesthesiology 2020, 133, 265–279. https://doi.org/10.1097/ALN.0000000000003428. [Google Scholar] [CrossRef]
- Chou, R.; Gordon, D.B.; de Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, E.H.; Degenhardt, E.; et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J. Pain 2016, 17, 508–510, Erratum in J. Pain 2016, 17, 131–157. https://doi.org/10.1016/j.jpain.2015.12.008. [Google Scholar] [CrossRef]
- Petrikovets, A.; Sheyn, D.; Sun, H.H.; Chapman, G.C.; Mahajan, S.T.; Pollard, R.R.; El-Nashar, S.A.; Hijaz, A.K.; Mangel, J. Multimodal opioid-sparing postoperative pain regimen compared with the standard postoperative pain regimen in vaginal pelvic reconstructive surgery: A multicenter randomized controlled trial. Am. J. Obstet. Gynecol. 2019, 221, 511.e1–511.e10. [Google Scholar] [CrossRef]
- Trowbridge, E.R.; Vollum, K.G.; Sarosiek, B.M.M.; Chang, E.S.; Hullfish, K.L. Enhanced Recovery Program for Outpatient Female Pelvic Reconstructive Surgery. Female Pelvic Med. Reconstr. Surg. 2021, 27, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Carter-Brooks, C.M.; Du, A.L.; Ruppert, K.M.; Romanova, A.L.; Zyczynski, H.M. Implementation of a urogynecology-specific enhanced recovery after surgery (ERAS) pathway. Am. J. Obstet. Gynecol. 2018, 219, 495.e1–495.e10. [Google Scholar] [CrossRef] [PubMed]
- Carter-Brooks, C.M.M.; Romanova, A.L.; DeRenzo, J.S.; Shepherd, J.P.M.; Zyczynski, H.M. Age and Perioperative Outcomes After Implementation of an Enhanced Recovery After Surgery Pathway in Women Undergoing Major Prolapse Repair Surgery. Female Pelvic Med. Reconstr. Surg. 2021, 27, e392–e398. [Google Scholar] [CrossRef]
- Yang, M.; He, M.; Zhao, M.; Zou, B.; Liu, J.; Luo, L.-M.; Li, Q.-L.; He, J.-H.; Lei, P.-G. Proton pump inhibitors for preventing non-steroidal anti-inflammatory drug induced gastrointestinal toxicity: A systematic review. Curr. Med. Res. Opin. 2017, 33, 973–980. [Google Scholar] [CrossRef]
- Stiller, C.O.; Hjemdahl, P. Lessons from 20 years with COX-2 inhibitors: Importance of dose-response considerations and fair play in comparative trials. J. Intern. Med. 2022, 292, 557–574. [Google Scholar] [CrossRef]
- Shingina, A.; Mukhtar, N.; Wakim-Fleming, J.; Alqahtani, S.; Wong, R.J.; Limketkai, B.N.; Larson, A.M.; Grant, L. Acute Liver Failure Guidelines. Am. J. Gastroenterol. 2023, 118, 1128–1153. [Google Scholar] [CrossRef]
- Aldrich, E.R.; Tam, T.Y.; Saylor, L.M.; Crisp, C.C.; Yeung, J.; Pauls, R.N. Intrarectal diazepam following pelvic reconstructive surgery: A double-blind, randomized placebo-controlled trial. Am. J. Obstet. Gynecol. 2022, 227, 302.e1–302.e9. [Google Scholar] [CrossRef]
- Jensen, S.; Amasyali, A.S.; Keheila, M.; Feldkamp, A.; Maldonado, J.; Wagner, H.J.; Baldwin, D.D.; Staack, A. Liposomal versus plain bupivacaine for pain control following vaginal reconstruction. Can. J. Urol. 2023, 30, 11703–11707. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).