Abstract
Penile cancer is a rare malignancy, with approximately 2100 cases diagnosed annually in the United States. The 5-year overall survival rate varies significantly depending on the node involvement status, at 79% in node-negative disease versus 51% for patients with inguinal metastasis. Inguinal lymph nodes are involved in micrometastatic disease in up to one out of four patients. Early inguinal lymph node dissection (ILND) has been shown to provide a survival advantage, which is why many patients undergo inguinal lymph node dissection for diagnostic and therapeutic purposes. Unfortunately, ILND is associated with high morbidity rates, which have led to potential overtreatment and decreased quality of life in the penile cancer population. Several advancements have been made to mitigate these challenges, such as dynamic sentinel node dissection, modifications to the technique or surgical templates, the introduction of minimally invasive procedures, and changes to the postoperative pathway. This manuscript examines the evolution in managing the inguinal lymph nodes in penile cancer, its associated complications, and effective strategies for their prevention and management.
1. Introduction
Penile cancer is an uncommon malignancy, accounting for 0.04 to 0.06% of all male carcinomas. However, this incidence can increase up to 10% in other areas, such as Africa or South America [1,2]. Early detection is essential but not always achievable, as patients often delay seeking clinical consultation due to embarrassment or fear [1,2,3,4].
Inguinal lymph nodes are the primary site of metastasis, and the local surgical management of these nodes is paramount for the patient’s survival. It has been identified that 25% of patients with clinically and radiographically negative disease will harbor micrometastatic disease within local inguinal lymph nodes [1,2,3,4,5,6,7,8,9,10,11,12,13]. Predicting the involvement of lymph node metastasis is challenging; effective predictive models, such as the CRiSS, are an interesting option to select patients, yet they are not broadly used nor recommended by guidelines. Therefore, the use of invasive staging methods relies heavily on characteristics within the primary lesion: tumor staging, histological findings, and grade [1,2,14]. ILND is the most accurate staging procedure, serving as a diagnostic and therapeutic tool. When performed early (within 3 months of the primary surgery), it can significantly improve overall survival [1,2,13].
Despite the benefits of ILND, it is associated with a significant complication rate that has been reported to be up to 55% [2]. It is important to emphasize that the reporting of these complications across the literature is inconsistent, leading to difficulties comparing the studies’ outcomes. These complications affect primarily the lymphatics, skin, vasculature, nerves, and the musculoskeletal system [2,13]. Since only 25% of cN0 patients harbor lymph node metastasis, 75% of patients may receive unnecessary treatment [2].
Following the initial description of the ILND technique, several efforts have been made to refine the surgical technique, improve patient selection, and mitigate complications. Additionally, alternatives like sentinel lymph node biopsy (SLNB) have been explored.
In this review, we aim to describe the historical evolution of this process, as well as the current understanding and management of the complications associated with ILND.
2. Methods
This is a comprehensive narrative literature review using Google Scholar, PubMed, and Embase databases to identify studies published between 1940 and December 2024 on inguinal lymph node dissection or sentinel lymph node biopsy for penile cancer. The search terms included “Inguinal lymph node dissection”, “ILND”, or “Inguinal lymphadenectomy”, and “Radical”, “Modified”, “VEIL”, “RAVEIL”, or “Complications”. We included level 2 to level 4 quality of evidence studies based on the Oxford Centre for Evidence-Based Medicine (CEBM) 2011 classification system. Articles that were not written in the English language were excluded. Data extracted included significant changes in surgical approaches, techniques, complication rates, complication data, and prevention strategies/management. We excluded commentaries, letters to the editor, editorial comments, and manuscripts discussing the management of metastatic disease or local management of the primary lesion. Qualitative data synthesis was performed to summarize key findings on the evolution of the surgical management of lymph nodes for penile cancer over time.
2.1. Evolution and Innovations in Lymph Node Management
In 1948, Daseler proposed a standardization of the radical inguinal lymph node dissection, emphasizing that to improve oncological outcomes, both superficial and deep nodes should be resected [15]. In 1977, Cabañas introduced the SLNB, a node or group of nodes located superomedial at the junction of the saphenous and femoral veins around the superficial epigastric vein, suggesting that it may be a reliable indicator of the presence of metastasis and, therefore, may guide more precise and less invasive therapeutic decisions [9,16]. However, biopsies directed to a specific anatomical area have a high rate of false negatives, primarily due to anatomic variations in the lymphatic drainage, making it unreliable to identify microscopic metastasis. Therefore, management strategies for clinically negative nodes shifted toward surveillance, prioritizing less invasive approaches to avoid morbidity associated with radical dissections [10,16]. This carried on until 1988, when Catalona described a modification of Daseler’s technique involving 40 patients, employing a smaller skin incision, a more limited field of dissection, preservation of the saphenous vein, avoidance of transposing the sartorius muscle to cover the exposed femoral vessels, and the use of thicker skin flaps to reduce procedure-related morbidity [16,17]. Despite achieving an approximate 25% reduction in morbidity, modified ILND is associated with significant false-negative rates of 15% to 20% [18,19].
Superficial inguinal dissection was another proposal to decrease the procedure’s morbidity. First, superficial nodes to the fascia lata are removed, followed by frozen sections of the most suspicious nodes, a procedure highly specific yet moderately sensitive for positive lymph node detection (specificity 100% and sensitivity 74%) [20]. Positive frozen sections of the superficial packet determined the need for dissection of the deep inguinal node packet, which includes nodes deep to the fascia lata within the femoral triangle. A major concern in false-negative cases lies in the difficulty of performing the re-dissection and the learning curve required for pathologists to reach proficiency in frozen sections for penile cancer, given its rarity [2,18,19,20,21,22].
In 1992, Morton et al. used colloidal blue dye to detect lymphatic pathways and first-echelon nodes to increase SLNB diagnostic accuracy [23]. A year later, Alex and Krag used radioactive 99mTc in animal models to map lymphatic drainage [24]. Building on those experiences, dynamic SLNB, a technique originally implemented in breast cancer and melanoma, was introduced for penile cancer patients in 1994 [23,24,25,26]. This technique consists of injecting peritumoral colloid particles labeled with technetium-99m, which are delivered through the lymphatic channels to the first draining nodes. These so-called sentinel nodes are detected by a gamma camera or intraoperatively with a handheld gamma probe. This offered the possibility of precise localization of the sentinel node [21,23,24,25,26,27]. Simultaneous use of blue dye with technetium-99m could further improve the technique by differentiating sentinel lymph nodes and second-echelon nodes. However, early experiences showed high false-negative rates and recurrences, which Pettaway et al. attributed to improper sampling of the lymph nodes. As a solution, a year later, an extended dynamic SLNB was proposed involving the removal of lymph nodes medial to the saphenous vein between the inguinal ligament and the superficial external pudendal vein [28]. A comparable approach known as the medial inguinal node biopsy was also suggested, focusing on the resection of the most medial inguinal node of the horizontal chain [28]. These techniques failed to reduce the false-negative rate and consequently fell out of favor [28,29].
Histopathological innovations, the incorporation of single-photon emission computed tomography (SPECT/CT), and preoperative lymphoscintigraphy have greatly improved the dynamic SLNB efficacy [27,30]. Hybrid radioactive and fluorescent tracers were the latest incorporation [30,31].
One of the fluorescent tracers added to the diagnostic and surgical armamentarium was indocyanine green (ICG). ICG was first discovered by Kodak in 1955, and it rapidly became a popular diagnostic tracer measuring cardiac output and liver function [31]. Its fluorescence quality was not discovered until the 1970s, when it was found that if ICG is exposed to near-infrared light (NIR), it reflects fluorescence. This motivated its use for better visualization of vasculature, tumors, and lymphatics [31,32]. ICG has better tissue penetration and is only visible using a near-infrared fluorescent camera system, allowing for the excision of nodes in an unstained field [33]. In 2014, Brouwer et al. published the established superiority of hybrid radioactive–ICG tracers in visualizing inguinal nodes compared to blue dye during dynamic SLNB [33].
Over time, the SLNB developed into a reliable staging technique with high diagnostic accuracy and low complication rates, with a sensitivity of 92–96%, false-negative rates of 4–8%, and complication rates of 6–14% [21,23,27]. The main limitation is that the procedure should be carried out at centers with the proper expertise, equipment, and personnel, hence making its broad utilization difficult [21,23,27,34,35,36].
Moreover, in 2017, ICG-aided ILND was shown to potentially reduce lymphatic complications [37]. More recently, a randomized clinical trial involving 45 patients found that the technique was not associated with a lower complication rate, yet a significantly higher lymph node yield was retrieved with its utilization [38].
In 2003, Bishoff et al. proposed and demonstrated the feasibility of the endoscopic ILND in cadaveric models [39]. Follow-up experiences from Tobias-Machado et al. and Sotelo et al. in penile cancer patients revealed a decreased complication rate associated with the minimally invasive approach compared to the open approach [39,40]. In 2009, robotic-assisted video-endoscopic inguinal lymphadenectomy was first published. Advantages such as augmented visualization, stereoscopic cameras, and articulated mobility allowed for a theoretically more precise/controlled dissection [41]. Although high-level evidence comparing robotic versus open ILND is challenging due to the rarity of the disease, in a recent systematic review and meta-analysis, minimally invasive ILND was associated with a reduction in the complication rate at the expense of skin and infectious complications (Table 1) [34,40,41,42,43,44,45,46,47,48].

Table 1.
Complication rates of ILND by surgical approach.
Table 1.
Complication rates of ILND by surgical approach.
| Approach | Minor Complication Rate (%) | Major Complication Rate (%) | Main Complications/Comments | References |
|---|---|---|---|---|
| Open | 35–66 | 13–17 | Wound complications alone up to 77%. High rates of wound infection, dehiscence, and lymphedema. | [49,50,51,52,53,54] |
| VEIL | 12–35 | 5–15 | Lower overall complication rates compared to open, particularly cutaneous complications. | [43,49,51,52,55] |
| RA-VEIL | 9–30 | 2–17 | Similar complication rate to VEIL. There is a lack of direct comparative studies between VEIL and RA-VEIL. | [49,51,52,54,55] |
| DSLNB | 16.4–26 | 1–1.4 | Operator-dependent. Steep learning curve. Good accuracy (87%) and minimal complications. | [26,34,35,56,57] |
VEIL: Video-Endoscopic Inguinal Dissection; RA-VEIL: Robotic-Assisted Video-Endoscopic Inguinal Dissection; DSLNB: Dynamic Sentinel Lymph Node Biopsy.
Figure 1 illustrates the historical evolution of ILND management from 1948 to the present.
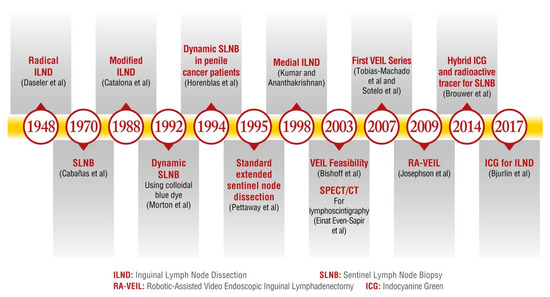
Figure 1.
The historical evolution of ILND management from 1948 to the present. ILND: Inguinal Lymph Node Dissection. SLNB: Sentinel Lymph Node Biopsy. SPECT/CT: Single-Photon Emission Computed Tomography. ICG: Indocyanine Green. VEIL: Video-Endoscopic Inguinal Lymphadenectomy. RA-VEIL: Robotic-Assisted Video-Endoscopic Inguinal Lymphadenectomy [15,16,17,23,25,27,28,30,33,37,39,40,41].
2.2. Inguinal Lymph Node Dissection Complications
ILND is associated with high morbidity, involving lymphatic, skin, vascular, nerve, and musculoskeletal complications. Several risk factors have been identified, including a high lymph node yield, high body mass index (BMI), diabetes, history of smoking, open ILND, prolonged operative time, and ASA score > 3 [45,48,49,58].
There are many reporting tools for surgical complications; Clavien–Dindo (CD) is among the most frequently utilized [59]. According to the CD classification, minor complications of ILND account for 65.7%, including seroma, lymphocele, and skin edge necrosis. On the other hand, major complications consist of about 34.3%, requiring hospital admission, operative care under general anesthesia, or life-threatening complications, including skin flap necrosis, sepsis, or thromboembolic events [45,49]. Chronologically, early complications (<30 days) include postoperative fluid collection, such as lymphocele or seroma, while leg lymphedema is the most common persistent late complication (>30 days) [45,49]. Although effective, the CD reporting tool has limitations. Mainly, the CD classification is based on the interventions required to resolve the complications rather than the patient’s reported outcomes [60]. These interventions may not correlate with the severity of the complications and may involve subjective clinical decision-making, leading to interobserver inconsistencies. Moreover, the classification does not include intraoperative complications [60]. The lack of a reliable classification has led to inconsistencies in ILND complications when defining, grading, and reporting each complication, leading to variability in the reported incidences and terminology. This lack of a clear reporting system hinders the ability to modify perioperative variables, which may help minimize the morbidity of ILND. To address this concern, the “Complications and Adverse Effects in Lymphadenectomy of the Inguinal Area (CALI)” collaboration established a standardized classification for reporting and defining ILND complications, aspiring for consistency. This classification includes intraoperative, postoperative, and sequela complications, which are further subcategorized into lymphatic, cutaneous, infectious, nerve/musculocutaneous, and vascular complications [61]. It is important to note that there are no management guidelines for many of these complications; therefore, recruiting a multidisciplinary team is essential.
2.3. Lymphatic Complications
The total incidence ranges from 38 to 61%, highly correlated with the total number of lymph nodes retrieved, contributing to impaired lymph flow [22,45,58]. Limb lymphedema, defined as an increased limb circumference of >10% compared to before the surgery and at postoperative day 30, has been reported in 36–62.5% of cases [58,61]. However, this number may be underestimated due to unreported and subclinical cases. Less frequent complications include lymphocele (5–24.2%), lymphorrhea (2%), scrotal lymphedema (1.3%), and lymphangitis (0.3%) [22,45,58,62,63].
Intraoperative preventive measures include cautious dissection of blood vessels and lymphatics, in addition to properly selecting the ligation method. Some retrospective studies have demonstrated a lower incidence of lymphatic leaks using clips instead of diathermy [22,50,64]. Sparing of the saphenous vein has been hypothesized to decrease short- and long-term complications [49,58,62,65,66,67,68]. Evidence from three randomized controlled trials specifically comparing saphenous vein resection versus preservation demonstrated a statistically significant reduction in lymphedema. Furthermore, each trial independently revealed an additional reduction in lower extremity phlebitis, cellulitis, sensory abnormalities, chronic pain, and cutaneous complications [49,58,62,65,66,67,68].
Placement of a closed suction drain until the drain output is <30–50 mL for 24 h may correlate with a lower incidence of lymphoceles [69,70,71]. In our institution, we use a 30 mL/24 h cutoff. When these do happen, percutaneous drainage may serve as an effective solution. Fluid analysis will reveal a clear or milky appearance with high protein compared to serum, along with a high cell count that is lymphocytic-predominant. A fluid culture is required to rule out infection. If rapid re-filling or lymphorrhea is suspected, patients may require image-guided drain placement. When persistent, lymphatic sclerotherapy to obliterate lymphatic tracts can be attempted. Sclerosing agents include ethanol, betadine, doxycycline, bleomycin, erythromycin, picibanil (OK432), or low-dose radiotherapy [69,70,71]. Another option is embolization with the instillation of a glue mixture, preferably in those small leaks where catheterization is not possible. If these strategies fail, surgical inspection for excision and ligation of lymphatics is indicated [69,70,71].
Lastly, compression stockings, sequential compression devices, and early mobilization are routinely used to decrease lymphedema occurrence [62,64,69,70]. When these arise, recommendations include compression bandages and garments in conjunction with exercise or massage therapy performed by a certified lymphedema specialist [62,64]. When selecting compression garments, awareness must be taken to avoid those that end over the surgical area, such as variceal garments, as they can injure the skin. At our institution, we prefer the use of sports underwear and post-liposuction compression garments for postoperative care. Advanced stages, based on the International Society of Lymphology Classification of lymphedema, could be managed with lymphovenous anastomosis or vascularized lymph node transfer. For more advanced lymphedemas, excisional procedures could be considered [48,51,63,71,72,73].
2.4. Cutaneous Complications
Potential causes include interruptions in the skin vasculature, weakened skin support, excessive tension on wound closure, and secondary lymphatic or infectious complications [22,45]. Cutaneous complications, also reported as “wound complications,” account for 31.1–35.4%. Seroma formation occurs in 31–48.4% of cases. Wound dehiscence, typically occurring 5–8 days following surgery, has an incidence of 14–48.4%, followed by skin flap necrosis accounting for 4.8–33%. Less reported complications include incisional skin edge necrosis (8.5–14%), hematoma (2.2–2.7%), and epidermolysis (0.09%) [22,45,49,72,74]. Risk factors for cutaneous complications include patient age, sartorius flap transposition, operative time, BMI, and concomitant pelvic lymph node dissection [34,48,58].
Surgical and perioperative key steps to help prevent cutaneous complications include the following:
- Surgical incisions:
Open surgery incisions have evolved from “S”- and “T”-shaped to adopting a horizontal incision below and parallel to the inguinal ligament following the natural skin creases or Langerhans’s lines, ensuring minimal wound tension [16,64,73,75]. Using this rationale, for the minimally invasive approach, we prefer vertical incisions rather than horizontal ones.
- 2.
- Subfascial space creation:
The subfascial space can be achieved using sharp dissection with scissors, blunt digital/endoscopic, or balloon dissection. We prefer blunt index-finger dissection to reduce the risk of flap perforation and allow for tactile feedback [75].
- 3.
- Attention to devitalized tissue:
When open ILNDs are performed, skin retraction should be performed with skin hooks or stay sutures rather than traumatic instruments such as the Allis forceps [22,39,40,41,42,43,44]. Patients who undergo open ILND and suspect a low blood supply below the flap could benefit from the use of myocutaneous flaps [22]. While creating the flap, transillumination of the skin was used to assess and maintain a proper thickness of skin flaps [76]. Distinguishing subcutaneous glistening fat from deeper thigh fat is critical to avoid thin skin flaps. A technique to recognize thin skin flaps was developed where intravenous fluorescein is injected, facilitating the observation of skin flaps under ultraviolet light [72]. Furthermore, if flaps are utilized, patient mobilization should be minimal for the first 48–72 h post-surgery to avoid increased wound tension and ensure flap health [22,62,64].
- 4.
- Wound closure/care:
We recommend using interrupted sutures when closing the port incisions to provide an outlet for potential fluid drainage in the event of accumulation. Skin closure under tension must be avoided; in these cases, local flaps such as the tensor of fascia lata should be considered [22]. We strongly discourage using any skin adhesives, such as lidocaine skin patches or tape, as they can increase the risk of epidermolysis upon removal.
Seroma formation is prevented by removing surgical drains only when the drain output has decreased to 50–30 cc/24 h [62,64,69,70]. In our practice, we remove the surgical drains once the output is consistently less than 30 cc/day. Warning patients to avoid pulling the drain accidentally during daily activities is an important consideration as well. Prophylactic incisional negative pressure wound therapy has been shown to potentially decrease seroma formation in open surgery [62,64,69,70,77,78]. Fluid analysis will differentiate seroma from lymphocele. Non-infected seroma fluid is typically clear or straw-colored and has low protein and similar glucose levels compared to serum. It generally contains few cells, particularly when diagnosed a few days after surgery [69]. Management includes needle aspiration or serial needle aspirations. A drain can be placed when various needle aspirations have been attempted. Failure of these measures will require surgical debridement, marsupialization, and excision of the seroma capsule [69]. It has been reported that when a seroma persists for over 40 days after several draining attempts, surgical management should be offered as soon as possible [69].
Regarding the management of wound dehiscence, secondary closure is recommended. The use of negative pressure wound therapy and limited and assisted mobilization has also been described [79].
Graft losses might be managed with observation when they are partial (>95%) or covering the area with wet-to-dry dressings with further re-grafting if they are total [64].
2.5. Infectious Complications
Potential causes include extensive operating time, insufficient sterilization, and fluid collections, as they may predispose bacterial growth. Infectious complications account for 16–29.8% of complications and include surgical site infection (SSI) (11.4–73%), deep incisional SSI (1.1%), sepsis (0.2%), abscess (0.1%), and infected lymphocele (0.04%) [22,58,80].
Proper sterile technique and antibiotic administration before incision are the main barriers against these complications [62,64]. Additionally, antibacterial prophylaxis until drain removal and a chlorhexidine gluconate-impregnated foam disk at the drain site can further decrease SSI and overall readmissions for infections [62,64,81].
In patients with culture-proven infections, culture-based antibiotic therapy should be administered in addition to solving the underlying cause of infection, often being a cutaneous or lymphatic complication [22,49,62,64].
2.6. Nerve/Musculoskeletal Complications
These can occur due to direct nerve injury, prolonged surgical positioning, and excessive traction. The overall incidence is 1.4–2%, primarily from inguinal paresthesia (60.6%) and chronic lower limb pain (25.8%). Chronic inguinal pain (7.7%) and difficult ambulation (5.8%) have also been reported [22,58].
Knowledge of the anatomy and nerve structure is crucial to prevent these complications during the procedure. Additionally, careful use of the retractor and the avoidance of electrocautery near nerve structures are advised. Lithotomy position can cause peripheral nerve injury, most frequently to the femoral and common peroneal nerves. Preventive measures include a recess period every 3–4 h, increased padding of vulnerable pressure points, and awareness of position errors [22,82,83].
Intraoperative direct nerve damage should be repaired with a tension-free approximation of the ends of the nerve; the assistance of a plastic surgeon can be a helpful resource. Postoperatively, electrophysiological tests can guide the assessment and management of neuropathy. Physical therapy and orthotic devices can improve symptoms, while neuropathic medications can reduce neuropathic pain. In cases of incomplete recovery, a surgical consultation for possible nerve decompression is recommended [82,83].
2.7. Vascular Complications
Vascular complications can arise from direct vessel injury, suboptimal hemostasis, or disturbance in any of Virchow’s factors. These complications are reported in 1.3–6% of ILND procedures. Deep vein thrombosis (DVT) and pulmonary embolism (PE) are responsible for 71.9% of cases, especially during the first month after surgery, followed by phlebitis (3–28%), hematoma (2.2–5%), and only a few cases from arterial thrombosis [22,45,58,64].
Based on the thromboprophylaxis guidelines, patients at moderate or high risk of thrombosis, based on Caprini or Rogers assessment tools, should be given chemical prophylaxis between 4 and 6 h postoperatively. Low-molecular-weight heparin has shown less incidence of major bleeding compared with unfractionated heparin [84,85]. Moreover, patients already receiving anticoagulant agents should discontinue the medication before surgery and restart it 4 days postoperatively since studies have shown that the highest risk for bleeding is from the time of surgery until postoperative day 4, while the risk for DVT/TE remains constant for up to 4 weeks postoperatively [86]. In our institution, we use a factor Xa inhibitor, Apixaban 2.5 mg twice a day for 30 days, and low-molecular-weight heparin during hospitalization. Use of antiembolic stockings and compression devices during the procedure, and until ambulation is resumed, is strongly encouraged. During the first postoperative hours, patients should be carefully assessed for any signs or symptoms of active bleeding to further evaluate the necessity of surgical wound re-exploration in the operating room [52,61].
3. Gaps in Knowledge and Potential Research Areas
ILND management has improved over time with the latest technologies and initiatives to enhance patient outcomes and overall cancer survival. These have led to a decrease in the ILND morbidity rate. However, there are still many gaps and inconsistencies in the literature about disease management and reporting that need to be addressed (Table 2).

Table 2.
Current literature gaps and their implications in the management of metastatic inguinal lymph nodes.
ILND selection criteria could be more accurate to minimize overtreatment and complications. Furthermore, even though an existing surgical template exists, its practical application varies among surgeons. Moreover, the reporting of ILND complications is inconsistent across studies, and guidelines for most of these complications are lacking, resulting in considerable variability throughout the literature and compromising advancements in current research. These gaps need to be addressed to improve the quality and analysis of study comparability, ultimately improving care for patients who necessitate the ILND procedure.
Additionally, liquid biopsy, including ctDNA, is a promising tool under research that can aid in the management of cancer, including penile carcinoma. It works by measuring shed circulating tumor cells and cell-free tumor DNA detection molecular analyses to assess treatment response, residual disease, and/or possible recurrences. Its use could potentially help select patients who need to undergo inguinal node surgery and avoid potential overtreatment [87,88].
4. Conclusions
ILND and SLNB techniques have evolved, reducing patients’ morbidity while maintaining appropriate oncological outcomes. Patients benefit from a holistic and multidisciplinary approach starting from the initial consultation, ensuring access to essential specialists, including psychologists, sex therapists, and lymphedema experts. Continued innovation is critical to optimizing the impact of the chosen management approach.
Author Contributions
Conceptualization, L.G.M. and R.S.; methodology, L.G.M.; software, R.S.; validation, L.G.M., R.S., R.L., K.P. and M.T.M.; formal analysis, F.E.; investigation, F.E.; resources, F.E. and R.S.; data curation, F.E.; writing—original draft preparation, F.E., R.S., A.C.L.D. and L.F.; writing—review and editing, F.E., R.S.S., L.G.M.; visualization, F.E., L.G.M., R.L. and R.S.S.; supervision, R.S.; project administration, L.G.M. and F.E.; funding acquisition, R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ficarra, V.; Akduman, B.; Bouchot, O.; Palou, J.; Tobias-Machado, M. Prognostic factors in penile cancer. Urology 2010, 76 (Suppl. 2), S66–S73. [Google Scholar] [CrossRef] [PubMed]
- Pettaway, C.A.; Crook, J.M.; Pagliaro, L.C. Tumors of the Penis. In Campbell-Walsh-Wein “Urology”, 12th ed.; Partin, A.W., Dmochowski, R.R., Kavoussi, L.R., Peters, C.A., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 1742–1775. [Google Scholar]
- Ekstrom, T.; Edsmyr, F. Cancer of the penis; a clinical study of 229 cases. Acta. Chir. Scand. 1958, 115, 25–45. [Google Scholar] [PubMed]
- Beggs, J.H.; Spratt, J.S. Epidermoid Carcinoma of the penis. J. Urol. 1964, 91, 166–172. [Google Scholar] [CrossRef]
- Thomas, J.A.; Small, C.S. Carcinoma of the penis in Southern India. J. Urol. 1968, 100, 520–526. [Google Scholar] [CrossRef]
- Edwards, R.H.; Sawyers, J.L. The management of carcinoma of the penis. South. Med. J. 1968, 61, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, J.T.; Garlick, F.H.; Mammen, K.E. Results of Surgical Treatment of Carcinoma of the Penis. ANZ J. Surg. 1968, 41, 157–159. [Google Scholar] [CrossRef]
- Skinner, D.G.; Leadbetter, W.F.; Kelley, S.B. The surgical management of squamous cell carcinoma of the penis. J. Urol. 1972, 107, 273–277. [Google Scholar] [CrossRef]
- Cabanas, R.M. An approach for the treatment of penile carcinoma. Cancer 1977, 39, 456–466. [Google Scholar] [CrossRef]
- Fosså, S.D.; Hall, K.S.; Johannessen, N.B.; Urnes, T.; Kaalhus, O. Cancer of the penis. Experience at the Norwegian Radium Hospital 1974–1985. Eur. Urol. 1987, 13, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, V.; Morse, M.J.; Herr, H.W.; Sogani, P.C.; Whitmore, W.F. Penile cancer: Relation of extent of nodal metastasis to survival. J. Urol. 1987, 137, 880–882. [Google Scholar] [CrossRef] [PubMed]
- McDougal, W.S.; Kirchner, F.K.; Edwards, R.H.; Killion, L.T. Treatment of carcinoma of the penis: The case for primary lymphadenectomy. J. Urol. 1986, 136, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Teh, J.; Duncan, C.; Qu, L.; Guerra, G.; Narasimhan, V.; Pham, T.; Lawrentschuk, N. Inguinal lymph node dissection for penile cancer: A contemporary review. Transl. Androl. Urol. 2020, 9, 3210–3218. [Google Scholar] [CrossRef]
- Patel, K.N.; Bhirud, C.; Dipin, J.; Nandy, K.; Venugopal, V.; Salunke, A.; Pandya, S.J. A proposed Clino-radio-pathological Risk Scoring System (CRiSS) for prediction and management of inguinal lymph-nodes metastasis in squamous cell carcinoma of the penis. Surg. Oncol. 2021, 36, 147–152. [Google Scholar] [CrossRef]
- Daseler, E.H.; Anson, B.J.; Reimann, A.F. Radical excision of the inguinal and iliac lymph glands; a study based upon 450 anatomical dissections and upon supportive clinical observations. Surg. Gynecol. Obstet. 1948, 87, 679–694. [Google Scholar]
- Wespes, E.; Simon, J.; Schulman, C.C. Cabañas approach: Is Sentinel Node Biopsy Reliable for Staging Penile Carcinoma? Urology 1986, 28, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Catalona, W.J. Modified inguinal lymphadenectomy for carcinoma of the penis with preservation of saphenous veins: Technique and preliminary results. J. Urol. 1988, 140, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Protzel, C.; Alcaraz, A.; Horenblas, S.; Pizzocaro, G.; Zlotta, A.; Hakenberg, O.W. Lymphadenectomy in the surgical management of penile cancer. Eur. Urol. 2009, 55, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- d’Ancona, C.A.L.; de Lucena, R.G.; Querne, F.A.d.O.; Martins, M.H.T.; Denardi, F.; Netto, N.R. Long-term followup of penile carcinoma treated with penectomy and bilateral modified inguinal lymphadenectomy. J. Urol. 2004, 172, 498–501; discussion 501. [Google Scholar] [CrossRef]
- Chipollini, J.; Tang, D.H.; Manimala, N.; Gilbert, S.M.; Pow-Sang, J.M.; Sexton, W.J.; Poch, M.A.; Spiess, P.E. Evaluating the accuracy of intraoperative frozen section during inguinal lymph node dissection in penile cancer. Urol. Oncol. 2018, 36, 14.e1–14.e5. [Google Scholar] [CrossRef]
- Kroon, B.K.; Lont, A.P.; Valdés Olmos, R.A.; Nieweg, O.E.; Horenblas, S. Morbidity of dynamic sentinel node biopsy in penile carcinoma. J. Urol. 2005, 173, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Spiess, P.E.; Hernandez, M.S.; Pettaway, C.A. Contemporary inguinal lymph node dissection: Minimizing complications. World J. Urol. 2009, 27, 205–212. [Google Scholar] [CrossRef]
- Morton, D.L.; Wen, D.R.; Wong, J.H.; Economou, J.S.; Cagle, L.A.; Storm, F.K.; Foshag, L.J.; Cochran, A.J. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch. Surg. 1992, 127, 392–399. [Google Scholar] [CrossRef]
- Alex, J.C.; Krag, D.N. Gamma-probe guided localization of lymph nodes. Surg. Oncol. 1993, 2, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Horenblas, S.; Jansen, L.; Meinhardt, W.; Hoefnagel, C.A.; De Jong, D.; Nieweg, O.E. Detection Of Occult Metastasis In Squamous Cell Carcinoma Of The Penis Using A Dynamic Sentinel Node Procedure. J. Urol. 2000, 163, 100–104. [Google Scholar] [CrossRef]
- Goyal, A.; Mansel, R.E. Current status of sentinel lymph node biopsy in solid malignancies. World J. Surg. Oncol. 2004, 2, 9. [Google Scholar] [CrossRef][Green Version]
- Lee, E.W.C.; Issa, A.; Oliveira, P.; Lau, M.; Sangar, V.; Parnham, A.; Fankhauser, C.D. High diagnostic accuracy of inguinal ultrasonography and fine-needle aspiration followed by dynamic sentinel lymph node biopsy in men with impalpable and palpable inguinal lymph nodes. BJU Int. 2022, 130, 331–336. [Google Scholar] [CrossRef]
- Pettaway, C.A.; Pisters, L.L.; Dinney, C.P.; Jularbal, F.; Swanson, D.A.; von Eschenbach, A.C.; Ayala, A. Sentinel lymph node dissection for penile carcinoma: The M. D. Anderson Cancer Center experience. J. Urol. 1995, 1154, 1999–2003. [Google Scholar] [CrossRef]
- Kumar, S.; Ananthakrishnan, N.; Prema, V. Predicting regional lymph node metastasis in carcinoma of the penis: A comparison between fine-needle aspiration cytology, sentinel lymph node biopsy and medial inguinal lymph node biopsy. Br. J. Urol. 1998, 81, 453–457. [Google Scholar] [CrossRef]
- Dell’Oglio, P.; de Vries, H.M.; Mazzone, E.; KleinJan, G.H.; Donswijk, M.L.; van der Poel, H.G.; Horenblas, S.; van Leeuwen, F.W.B.; Brouwer, O.R. Hybrid Indocyanine Green-99mTc-nanocolloid for Single-photon Emission Computed Tomography and Combined Radio- and Fluorescence-guided Sentinel Node Biopsy in Penile Cancer: Results of 740 Inguinal Basins Assessed at a Single Institution. Eur. Urol. 2020, 78, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A Review of Indocyanine Green Fluorescent Imaging in Surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, S.; Botero Fonnegra, C.; Reyes, A.; Hui, V.W. Developments in the Use of Indocyanine Green (ICG) Fluorescence in Colorectal Surgery. J. Clin. Med. 2024, 13, 4003. [Google Scholar] [CrossRef]
- Brouwer, O.R.; Van Den Berg, N.S.; Mathéron, H.M.; Van Der Poel, H.G.; Van Rhijn, B.W.; Bex, A.; Van Tinteren, H.; Olmos, R.A.V.; Van Leeuwen, F.W.B.; Horenblas, S. A Hybrid Radioactive and Fluorescent Tracer for Sentinel Node Biopsy in Penile Carcinoma as a Potential Replacement for Blue Dye. Eur. Urol. 2014, 65, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Wever, L.; de Vries, H.M.; Dell’Oglio, P.; van der Poel, H.G.; Donswijk, M.L.; Sikorska, K.; Leeuwen, F.W.B.; Horenblas, S.; Brouwer, O.R. Incidence and risk factor analysis of complications after sentinel node biopsy for penile cancer. BJU Int. 2022, 130, 486–495. [Google Scholar] [CrossRef]
- Akrida, I.; Michalopoulos, N.V.; Lagadinou, M.; Papadoliopoulou, M.; Maroulis, I.; Mulita, F. An updated review on the emerging role of indocyanine green (ICG) as a sentinel lymph node tracer in breast cancer. Cancers 2023, 15, 5755. [Google Scholar] [CrossRef]
- Wever, L.; de Vries, H.M.; van der Poel, H.; van Leeuwen, F.; Horenblas, S.; Brouwer, O. Minimally invasive evaluation of the clinically negative inguinal node in penile cancer: Dynamic sentinel node biopsy. Urol. Oncol. 2022, 40, 209–214. [Google Scholar] [CrossRef]
- Bjurlin, M.A.; Zhao, L.C.; Kenigsberg, A.P.; Mass, A.Y.; Taneja, S.S.; Huang, W.C. Novel Use of Fluorescence Lymphangiography During Robotic Groin Dissection for Penile Cancer. Urology 2017, 107, 267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, P.; Xie, Y.; Xu, R.; Li, Y.; Yao, K.; Liu, J.; Yan, B.; Jiang, S.; Liu, Q.; Chen, Q.; et al. Efficacy of indocyanine green fluorescence-guided inguinal lymph node dissection for penile cancer: A randomised trial. BJU Int. 2024, 133, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Bishoff, J.T.; Basler, J.W.; Teichman, J.M.; Thompson, I.M. Endoscopic subcutaneous modified inguinal lymph node dissection (ESMIL) for squamous cell carcinoma of the penis. In Journal of Urology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003; p. 78. [Google Scholar]
- Tobias-Machado, M.; Tavares, A.; Ornellas, A.A.; Molina, W.R.; Juliano, R.V.; Wroclawski, E.R. Video endoscopic inguinal lymphadenectomy: A new minimally invasive procedure for radical management of inguinal nodes in patients with penile squamous cell carcinoma. J. Urol. 2007, 177, 953–957; discussion 958. [Google Scholar] [CrossRef]
- Josephson, D.Y.; Jacobsohn, K.M.; Link, B.A.; Wilson, T.G. Robotic-assisted endoscopic inguinal lymphadenectomy. Urology 2009, 73, 167–170; discussion 170–171. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT01526486?cond=penile%20cancer&intr=Lymphadenectomy&rank=7 (accessed on 13 January 2025).
- Sotelo, R.; Sánchez-Salas, R.; Carmona, O.; Garcia, A.; Mariano, M.; Neiva, G.; Trujillo, G.; Novoa, J.; Cornejo, F.; Finelli, A. Endoscopic lymphadenectomy for penile carcinoma. J. Endourol. 2007, 21, 364–367; discussion 367. [Google Scholar] [CrossRef]
- Greco, I.; Fernandez-Pello, S.; Sakalis, V.I.; Barreto, L.; Albersen, M.; Ayres, B.; Lopes, T.A.; Campi, R.; Crook, J.; Perdomo, H.A.G.; et al. Systematic Review and Meta-analysis of Minimally Invasive Procedures for Surgical Inguinal Nodal Staging in Penile Carcinoma. Eur. Urol. Focus 2023, 10, 567–580. [Google Scholar] [CrossRef]
- Gopman, J.M.; Djajadiningrat, R.S.; Baumgarten, A.S.; Espiritu, P.N.; Horenblas, S.; Zhu, Y.; Protzel, C.; Pow-Sang, J.M.; Kim, T.; Sexton, W.J.; et al. Predicting postoperative complications of inguinal lymph node dissection for penile cancer in an international multicentre cohort. BJU Int. 2015, 116, 196–201. [Google Scholar] [CrossRef]
- Gómez-Ferrer, A.; Collado, A.; Ramírez, M.; Domínguez, J.; Casanova, J.; Mir, C.; Wong, A.; Marenco, J.L.; Nagore, E.; Soriano, V.; et al. A single-center comparison of our initial experiences in treating penile and urethral cancer with video-endoscopic inguinal lymphadenectomy (VEIL) and later experiences in melanoma cases. Front. Surg. 2022, 9, 870857. [Google Scholar] [CrossRef]
- Muñoz Guillermo, V.; Rosino Sánchez, A.; Rivero Guerra, Á.; Barceló Bayonas, I.; Pardo Martínez, A.; Jiménez Peralta, D.; George, C.C.; Pietricica, B.N.; Morejón, E.I.; de Abia, F.I.; et al. Video endoscopic inguinal lymphadenectomy in penile cancer: Systematic review. Arch. Esp. Urol. 2019, 72, 992–999. [Google Scholar]
- Chua, K.J.; Balraj, V.; Patel, H.V.; Srivastava, A.; Doppalapudi, S.K.; Elsamra, S.E.; Jang, T.L.; Singer, E.A.; Ghodoussipour, S.B. Wound complication rates after inguinal lymph node dissection: Contemporary analysis of the NSQIP database. J. Am. Coll. Surg. 2023, 236, 18–25. [Google Scholar] [CrossRef]
- Jeanne-Julien, A.; Bouchot, O.; De Vergie, S.; Branchereau, J.; Perrouin-Verbe, M.-A.; Rigaud, J. Morbidity and risk factors for complications of inguinal lymph node dissection in penile cancer. World J. Urol. 2022, 41, 109–118. [Google Scholar] [CrossRef] [PubMed]
- La-Touche, S.; Ayres, B.; Lam, W.; Alnajjar, H.M.; Perry, M.; Watkin, N. Trial of ligation versus coagulation of lymphatics in dinamic inguinal sentinel lymph node biopsy for staging of squamous cell carcinoma of the penis. Ann. R. Coll. Surg. Engl. 2012, 94, 344–346. [Google Scholar] [CrossRef]
- Greene, A.K.; Goss, J.A. Diagnosis and staging of lymphedema. Semin. Plast. Surg. 2018, 32, 12–16. [Google Scholar] [CrossRef]
- Heyns, C.F.; Fleshner, N.; Sangar, V.; Schlenker, B.; Yuvaraja, T.B.; Van Poppel, H. Management of the Lymph Nodes in Penile Cancer. Urology 2010, 76, S43–S57. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Zheng, L.; Li, Y.; Gan, L.; Wang, Z.; Zeng, Z.; Meng, C.; Li, K.; Ma, J.; Wang, D.; et al. Comparing the safety and effectiveness of minimally invasive surgery and open inguinal lymph node dissection in penile cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2024, 50, 108553. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, Z.; Tan, Q.; Hu, X.; Liu, Y.; Wei, L.; Deng, C.; Zhou, S.; Yang, N.; Duan, G.; et al. Comparison of antegrade robotic assisted vs. laparoscopic inguinal lymphadenectomy for penile cancer. BMC Surg. 2023, 23, 55. [Google Scholar] [CrossRef]
- Singh, A.; Jaipuria, J.; Goel, A.; Shah, S.; Bhardwaj, R.; Baidya, S.; Jain, J.; Jain, C.; Rawal, S. Comparing Outcomes of Robotic and Open Inguinal Lymph Node Dissection in Patients with Carcinoma of the Penis. J. Urol. 2018, 199, 1518–1525. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Christopoulos, P.; Shilito, S.; Gall, Z.; Murby, B.; Ashworth, D.; Taylor, B.; Carrington, B.; Shanks, J.; Clarke, N.; et al. Dynamic sentinel lymph node biopsy for penile cancer: A comparison between 1- and 2-day protocols. BJU Int. 2016, 117, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Fallara, G.; Pozzi, E.; Cakir, O.O.; Tandogdu, Z.; Castiglione, F.; Salonia, A.; Alnajjar, H.M.; Muneer, A. Diagnostic Accuracy of Dynamic Sentinel Lymph Node Biopsy for Penile Cancer: A Systematic Review and Meta-analysis–ClinicalKey. Eur. Urol. Focus 2023, 9, 500–512. [Google Scholar] [CrossRef]
- Cacciamani, G.E.; Medina, L.G.; Sayegh, A.S.; La Riva, A.; Perez, L.C.; Eppler, M.B.; Gill, I.; Sotelo, R. Assessment and reporting of perioperative adverse events and complications in patients undergoing inguinal lymphadenectomy for melanoma, vulvar cancer, and penile cancer: A systematic review and meta-analysis. World J. Surg. 2023, 47, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Golder, H.; Casanova, D.; Papalois, V. Evaluation of the usefulness of the Clavien-Dindo classification of surgical complications. Cirugía Española (Engl. Ed.) 2023, 101, 637–642. [Google Scholar] [CrossRef]
- Sotelo, R.; Sayegh, A.S.; Medina, L.G.; Perez, L.C.; La Riva, A.; Eppler, M.B.; Gaona, J.; Tobias-Machado, M.; Spiess, P.E.; Pettaway, C.A.; et al. Complications and adverse events in lymphadenectomy of the inguinal area: Worldwide expert consensus. BJS Open 2024, 8, zrae056. [Google Scholar] [CrossRef] [PubMed]
- Faut, M.; Heidema, R.M.; Hoekstra, H.J.; van Ginkel, R.J.; Been, S.L.B.; Kruijff, S.; Van Leeuwen, B.L. Morbidity after inguinal lymph node dissections: It is time for a change. Ann. Surg. Oncol. 2017, 24, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Gerken, A.L.H.; Herrle, F.; Jakob, J.; Weiß, C.; Rahbari, N.N.; Nowak, K.; Karthein, C.; Hohenberger, P.; Weitz, J.; Reißfelder, C.; et al. Definition and severity grading of postoperative lymphatic leakage following inguinal lymph node dissection. Langenbecks Arch. Surg. 2020, 405, 697–704. [Google Scholar] [CrossRef]
- Sood, A.; Rudzinski, J.K.; Spiess, P.E.; Pettaway, C.A. The acute complications after surgery for penile carcinoma and strategies for their management: A systematic review of the literature. Semin. Oncol. Nurs. 2022, 38, 151285. [Google Scholar] [CrossRef]
- Gupta, M.K.; Patel, A.P.; Master, V.A. Technical considerations to minimize complications of inguinal lymph node dissection. Transl. Androl. Urol. 2017, 6, 820–825. [Google Scholar] [CrossRef]
- Zhang, X.; Sheng, X.; Niu, J.; Li, H.; Li, D.; Tang, L.; Li, Q.; Li, Q. Sparing of saphenous vein during inguinal lymphadenectomy for vulval malignancies. Gynecol. Oncol. 2007, 105, 722–726. [Google Scholar] [CrossRef]
- Zhang, S.H.; Sood, A.K.; Sorosky, J.I.; Anderson, B.; Buller, R.E. Preservation of the saphenous vein during inguinal lymphadenectomy decreases morbidity in patients with carcinoma of the vulva. Cancer 2000, 89, 1520–1525. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, H.; Liu, L.; Chen, Z.; Chen, J.; Qi, L.; Zu, X. Saphenous vein sparing during laparoscopic bilateral inguinal lymphadenectomy for penile carcinoma patients. Int. Urol. Nephrol. 2016, 48, 363–366. [Google Scholar] [CrossRef]
- Papanikolaou, A.; Minger, E.; Pais, M.-A.; Constantinescu, M.; Olariu, R.; Grobbelaar, A.; Lese, I. Management of postoperative seroma: Recommendations based on a 12-year retrospective study. J. Clin. Med. 2022, 11, 5062. [Google Scholar] [CrossRef]
- Pouwer, A.W.; Hinten, F.; van der Velden, J.; Smolders, R.G.V.; Slangen, B.F.M.; Zijlmans, H.J.M.A.A.; IntHout, J.; van der Zee, A.G.J.; Boll, D.; Gaarenstroom, K.N.; et al. Volume-controlled versus short drainage after inguinofemoral lymphadenectomy in vulvar cancer patients: A Dutch nationwide prospective study. Gynecol. Oncol. 2017, 146, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.; Kim, J.; Ratnam, L.; Itkin, M. Lymphatic intervention, the frontline of modern lymphatic medicine: Part II. Classification and treatment of the lymphatic disorders. Korean J. Radiol. 2023, 24, 109–132. [Google Scholar] [CrossRef]
- Ciudad, P.; Sabbagh, M.D.; Agko, M.; Huang, T.C.T.; Manrique, O.J.; Carmen Román, L.; Reynaga, C.; Delgado, R.; Maruccia, M.; Chen, H.-C. Surgical management of lower extremity lymphedema: A comprehensive review. Indian J. Plast. Surg. 2019, 52, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Hu, X.; Ren, S.; Liao, D.; Yang, Z.; Liu, Y.; Lia, T.; Wu, K.; Xiong, S.; Yang, W.; et al. Comparison of different surgical methods and strategies for inguinal lymph node dissection in patients with penile cancer. Sci. Rep. 2022, 12, 2560. [Google Scholar] [CrossRef] [PubMed]
- Wevers, K.P.; Poos, H.P.A.M.; Van Ginkel, R.J.; Van Etten, B.; Hoekstra, H.J. Early mobilization after ilio-inguinal lymph node dissection for melanoma does not increase the wound complication rate. Eur. J. Surg. Oncol. 2013, 39, 185–190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sudhir, R.; Krishnappa, R.S.; Khanna, S.; Sekon, R.; Koul, R. Video endoscopic inguinal lymphadenectomy (VEIL): Minimally invasive radical inguinal lymphadenectomy technique. Indian J. Surg. Oncol. 2012, 3, 257–261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kandasamy, S.G.; Chandran, K.R.; Pooleri, G.K. Minimal invasive approaches in lymph node management of carcinoma of penis: A review. Indian J. Urol. 2022, 38, 15–21. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Toyserkani, N.M.; Thomsen, J.B.; Sørensen, J.A. Prophylactic incisional negative pressure wound therapy shows promising results in prevention of wound complications following inguinal lymph node dissection for Melanoma: A retrospective case-control series. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 1178–1183. [Google Scholar] [CrossRef]
- Asciutto, K.C.; Acosta, S.; Borgfeldt, C. Negative pressure wound therapy (NPWT) in groin wounds after lymphadenectomy in vulvar cancer patients. Vivo 2020, 34, 3511–3517. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.D.; Manna, B. Wound dehiscence. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Petroze, R.T.; Holton, L. Complications of inguinal lymphadenectomy. Surg. Technol. 2020, 52, 270–279. [Google Scholar]
- Dargan, D.; Hindocha, S.; Hadlett, M.; Wright, R.; Beck, D.; McConville, S.; Hartley-Large, D.; Mortimer, K.; Brackley, P. Groin dissections in skin cancer: Effect of a change in prophylactic antibiotic protocol. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Bychkov, Y.; Mutua, D.; Bhullar, R. A Review of Lithotomy Position-Related Intraoperative Peripheral Nerve Injury and Preventative Measures. ASA Monit. 2022, 86, 39–40. [Google Scholar] [CrossRef]
- Yıkılmaz, T.N.; Öztürk, E.; Hamidi, N.; Başar, H.; Yaman, Ö. Management of obturator nevre injury during pelvic lymph node dissection. Turk. J. Urol. 2019, 45, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, L.; Tempelhoff, G.-F.V.; Kirkpatrick, C.; Schneider, D.M.; Hommel, G.; Pollow, K. Comparison of unfractionated versus low molecular weight heparin for deep vein thrombosis prophylaxis during breast and pelvic cancer surgery: Efficacy, safety, and follow-up. Clin. Appl. Thromb. Hemost. 1998, 4, 268–273. [Google Scholar] [CrossRef]
- Saluja, M.; Gilling, P. Venous thromboembolism prophylaxis in urology: A review. Int. J. Urol. 2017, 24, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Uroweb–European Association of Urology. Uroweb.org. Available online: http://uroweb.org/guidelines/compilations-of-all-guidelines/ (accessed on 21 October 2024).
- Gerke, M.B.; Jansen, C.S.; Bilen, M.A. Circulating Tumor DNA in Genitourinary Cancers: Detection, Prognostics, and Therapeutic Implications. Cancers 2024, 16, 2280. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Moon, S.C.; Shumaker, L.A.; Patel, S.; Galgano, S.J.; Ferguson, J.E.; Basu, A.; Peyton, C.C. Utility of Tumor-Informed Circulating Tumor DNA for Monitoring Treatment Response in Nonmetastatic, Locally Advanced Penile and Primary Urethral Cancers. JCO Precis. Oncol. 2025, 9, e2500045. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).