1. Introduction
Transseptal puncture (TSP) is performed to access the left atrium (LA) from the systemic venous system by traversing the fossa ovalis (FO) within the interatrial septum (IS),
Figure 1. This approach enables the use of larger devices with improved maneuverability compared to retrograde arterial cannulation.
First introduced by Ross et al. as a diagnostic method in candidate patients for cardiac surgery [
1,
2], over the years, TSP has been employed in the electrophysiology lab for atrial fibrillation ablation, followed by procedures addressing left atrial tachycardias, left-sided accessory pathways, and left ventricular arrhythmias. TSP has also gained traction in percutaneous cardiac interventions, such as left atrial appendage closure, mitral valve repair, and transcatheter mitral annuloplasty.
Traditionally, TSP is performed using Brockenbrough needles under fluoroscopic guidance, often supplemented by transesophageal or intracardiac echocardiography (TEE/ICE). In the hands of experienced operators, the procedure is generally safe and highly successful, with an overall complication rate of approximately 1% [
3,
4]. However, the technique can be challenging, particularly for less experienced practitioners or in cases of complex anatomies, posing risks of severe and potentially life-threatening complications.
To minimize these risks, it is crucial to understand the anatomy of the fossa ovalis, recognize situations that might make the procedure more challenging, and leverage tools designed to enhance safety and ease of use. The timely identification and management of TSP-related complications are vital. This review explores the most common TSP complications, their management, and the anatomical variations that increase procedural difficulty. It also highlights the techniques and technologies available to ensure safer and more effective traversal of the FO.
2. How to Perform a Safe Transeptal Puncture
2.1. Materials and Equipment
The original needle introduced by Ross featured a curved distal end for controlled tip movement and an arrow-shaped proximal handle for orientation. Brockenbrough later modified this design, tapering the distal 1.5 cm from 18 to 21 gauge [
5,
6]. The modern Brockenbrough (BRK) needle, made of stainless steel with a stylet to reduce friction, comes in standard (19° angle) and BRK 1 (53° angle) versions, with customizable curvature and pediatric options.
Common sheaths for use with BRK needles are the Mullins and Swartz™ Braided SL (Abbott, Chicago, IL, USA). The Swartz SL offers a better pushability and torquability, suitable for targeting the superior left pulmonary vein, while the Mullins sheath is better suited for reaching the mitral valve. Today, various manufacturers produce transseptal kits with slight design differences.
2.2. Atrial Septum Anatomy and Fossa Ovalis
The IS includes the entire area between the two atria but differs from the “true” IS, which is the portion that can be safely crossed without entering the extracardiac space—only about 20% of the total septal area [
7].
The fossa ovalis, part of the true IS, is in the lower posterior IS and is typically an oval or occasionally round depression made of thin fibrous tissue. It can be divided into the following four regions: superior–anterior, inferior–anterior, superior–posterior, and inferior–posterior. Its shape varies based on atrial size, pressure, and tissue redundancy. The optimal TSP location varies according to the procedure. The fossa ovalis is surrounded by a muscular septum. The superior rim of the septum coincides with the superior vena cava, the anterior rim with the aortic valve and ascending aorta, the inferior rim with the inferior vena cava, and the posterior rim with the interatrial fold (also known as the Waterstone or Sondergaards groove). This is a portion of the atria wall in which the roofs of the left and right atrial wall fold are anteriorly separated by fat tissue. When TSP is performed in this region, there is a risk of perforating both atrial walls and the extramural epicardium with the risk of immediate or delayed tamponade.
2.3. Vascular Access Management
The first step of the TSP procedure is venous femoral puncture, typically performed on the right side for a more direct path to the right atrium (RA). However, both groins should be prepped, as the left side may be needed for invasive arterial monitoring, managing complications, or as an alternative access site in cases of unfavorable right venous anatomy.
The femoral head serves as the fluoroscopic landmark (AP projection), with the puncture positioned medial to it and below its equator. Alternatively, the femoral pulse can be palpated, and the puncture is made 4 cm below the inguinal ligament, medial to the pulse, at a 45° angle.
More recently, an echo-guided approach has been recommended to avoid vascular complications (inadvertent arterial puncture, lesions of the femoral artery collaterals, or artero-venous fistula). Echo-guided puncture allows for the identification of a safe area, usually cranial to the arterial bifurcation, where the vein is medial to the artery. Following venous puncture, preclosure with one or more suture-based closure device (ProGlide, ProStar, Prostyle by Abbott Vascular, Santa Clara, CA, USA, and Manta by Teleflex, Wayne, PA, USA) is recommended in the case of the insertion of large bore devices. Administering 2000 U of heparin (about 1/4 of the total dose) at this stage helps to prevent clot deposition on the TSP sheath, particularly if the procedure is prolonged, if TSP is expected to be challenging, with multiple “pull-back” maneuvers, or in the case of less experienced operators. If preclosure devices are not available or in the case of their failure, a “figure of eight” suture may serve adequately as a vascular vein closure strategy.
2.4. Transeptal Puncture Step by Step
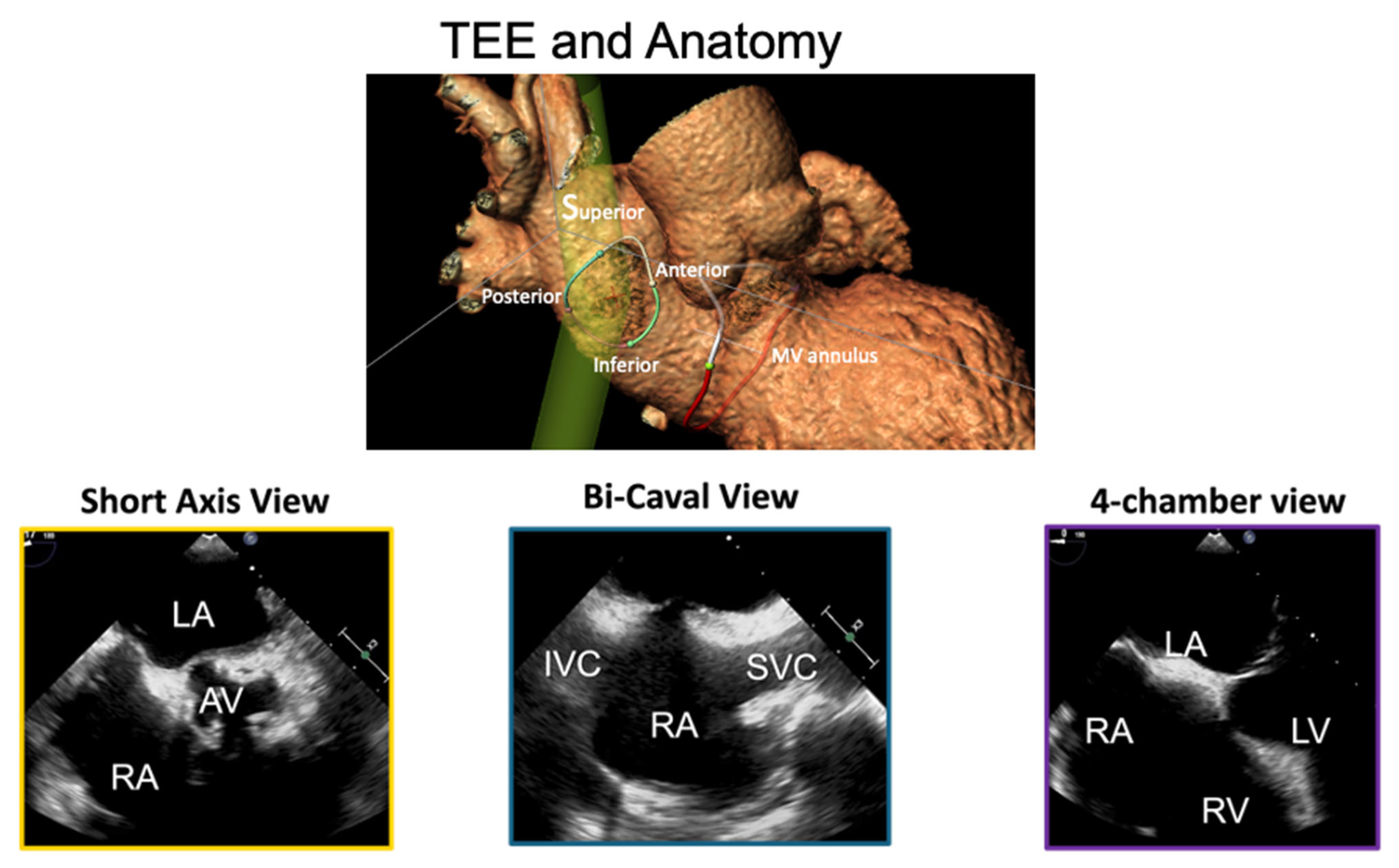
A 0.0032 guidewire is advanced in the superior vena cava. A 12F introducer sheath can be used, but in echo-guided TSP, it is not mandatory, since no tactile feedback will be needed to confirm septal engagement. The transseptal sheath and dilator are advanced over the guidewire to reach the superior vena cava (SVC) under fluoroscopic (AP projection) and TEE (bicaval view) guidance, as shown in
Figure 1. For cases involving pacemaker electrodes, to position the guidewire posterior to the leads, the advancement of the guidewire is performed in the LAO projection. When advancing the sheath, the tip is aimed leftward (toward the aorta) to avoid the cava free wall.
In cases of venous tortuosity (typical for patients with calcific peripheral arterial disease), a larger sheath and a stiff buddy wire can be used to straighten the vein.
The BRK needle is shaped according to the right atrial and fossa ovalis anatomy and the desired position of the transeptal puncture. Under fluoroscopic guidance, the BRK needle is carefully advanced until it reaches the sheath tip, avoiding overextension. The stylet remains in place to reduce friction, and the needle should rotate freely during insertion to navigate vascular tortuosity. Once the stylet is removed, the needle can be connected to a pressure line.
Then, a “pull-back” maneuver begins with the needle arrow pointing at 5 or 6 o’clock. For posterior punctures, the arrow is maintained at 6 o’clock. It is then pulled back caudally until the needle tents the superior rim of the fossa, observed via TEE, as shown in
Figure 2. Proper tenting is typically accompanied by supraventricular extrasystolic beats. Pulling the device back a few more centimeters will result in the needle falling in the fossa. Optimal puncture locations depend on the type of procedure, device used, and anatomy.
Using TEE (SAX view) and fluoroscopy (AP projection), the needle punctures the fossa ovalis, verified by a loss of tenting on TEE and left atrial pressure confirmation. Advancing the sheath should be avoided without proper pressure tracing or if aortic pressure is detected. In experienced hands, pressure monitoring can be avoided in TEE-guided procedures, however, pressure tracing enables greater safety. Full heparinization (100 U/kg) should be administered to maintain an activated clotting time (ACT) of 250–300 s.
The sheath and needle are advanced 1–2 cm into the LA under TEE guidance. The following tips and tricks may be useful:
- -
Use fluoroscopy and TEE to confirm positioning at every step.
- -
Maintain slow, controlled movements to avoid sheath displacement.
- -
Always verify the correct puncture position using TEE, blood aspiration, pressure curves, or contrast injection before advancing the sheath.
3. Most Frequent Complications and How to Handle Them
3.1. Posterior Transeptal Puncture and Cardiac Tamponade
Cardiac tamponade is the most frequent life-threatening complication of TSP, occurring in approximately 1% of cases [
8,
9]. It is often caused by misdirected needle punctures, such as posterior wall perforation or through-and-through punctures of the left atrium due to excessive force or redundant/aneurysmal septum anatomy. The risk is higher during AF ablation due to multiple punctures, extensive catheter manipulation, and ongoing anticoagulation. Symptoms include sudden hypotension, hypoxia, sinus tachycardia, and cardiogenic shock, and diagnosis is confirmed with TTE or TEE. Tamponade may occur acutely during the procedure or present as delayed tamponade after catheter removal, often associated with atrial hematomas [
10].
The prevention of wrong punctures is crucial, and the risk of tamponade is mitigated by careful TEE guidance and some other measures. In the case of risk, advancing a 0.0018” guidewire through the Brockenbrough needle can avoid inadvertent punctures while advancing the needle following the initial puncture. Another measure to reduce the risk of lesions is the use of RF-energy-supported TSP in the case of a floppy or post-cardiotomy septum, under strict echo-guidance.
Management involves reversing anticoagulation, fluid administration, and pericardiocentesis, with blood transfusion if needed. In severe cases, surgical drainage may be required for large tears or clotted blood. Treatment includes pericardial drainage and closure of the bleeding site using an atrial septum defect closure device.
For mitral procedures requiring posterior TSP, punctures near the muscular septum can lead to pericardial effusion or tamponade. Early detection is crucial; in case of doubt, at the end of the TEER procedure, a wire is readvanced through the guiding catheter and the guide is retracted in the right atrium, with the dilator ready to be able to readvance the catheter in the case of pericardial effusion. In total, 3–5 min of observation time is needed under TEE and hemodynamics monitoring. The guiding catheter will then control the bleeding and allow time to prepare an atrial septal occluder (ASD type), which will be used to definitively control the bleeding by sandwiching the walls.
3.2. Aortic Needle Puncture
Inadvertent aortic root puncture during TSP is a rare but potentially life-threatening complication, occurring in approximately 0.05% of cases [
11]. The aortic root, located anterosuperiorly to the FO, can be at risk of puncture due to anatomical variations or abnormal heart rotation, where the FO may be situated closer to the aortic root. This complication is more likely in challenging anatomies, anterior punctures, or with insufficient operator experience [
12]. TEE or ICE guidance significantly reduces this risk [
11].
Proper needle positioning can be confirmed by pressure recording or contrast injection. If an aortic puncture is identified, the dilator and sheath must not be advanced until the needle placement is verified. In cases where only the needle has entered the aorta, slow withdrawal is usually sufficient. However, if the dilator or sheath has advanced, the risk of complications such as hemopericardium increases. It is crucial to avoid pulling back the dilator if this has been already advanced in the aorta, since this will result in instantaneous tamponade and inability to manage the patient.
Management strategies vary depending on the extent of the injury [
11]. Some cases have required surgical repair, such as valve replacement due to persistent shunts or the stitching of perforations [
13]. Alternatively, occluder devices have been used successfully to close aortic perforations [
14], however, this option is not feasible when the puncture is close to the aortic valve leaflets. In borderline cases, leaving a guidewire in the aorta followed by observation allows for rapid re-advancement of the sheath and bleeding control [
15]. Monitoring hemodynamics and echocardiographic findings during a waiting period may prevent further deterioration. These measures, along with careful procedural planning, are crucial for handling this rare but critical complication.
3.3. Embolic Events
Air and thrombus embolisms are uncommon complications of TSP, but they can arise due to the improper de-airing of large devices or inadequate anticoagulation [
16]. Thrombus formation can occur in the space between the needle and dilator, particularly when the TSP system reaches the left heart. Air embolism may result from persistent air bubbles in the system, insufficient flushing, or the presence of a PFO. Also, advancement of the needle without the stylet can produce plastic debris that can embolize.
To minimize these risks, the meticulous flushing of sheaths and effective anticoagulation are essential. Heparin (1000–2000 IU) should be administered intravenously before TSP to prevent thrombus formation when a long time for TSP is anticipated.
Management strategies for thromboembolic events depend on their severity and location. For thrombus formation, a full heparin dose targeting an ACT >250 s should be administered and thrombus aspiration may be attempted, especially before crossing the septum. If the thrombus reaches the left heart, cerebral protection devices may be considered, and large thrombi might necessitate surgical intervention.
The careful de-airing of devices and systems in paramount to prevent air embolism. Silent cerebrovascular ischemia remains a concern during transcatheter left-heart procedures, emphasizing the importance of rigorous intra-procedural anticoagulation and air management to minimize embolic risks [
17].
3.4. ST-Segment Elevation
Isolated transient ST elevation in inferior leads occurs in approximately 0.6% of TSP cases and is often accompanied by vagal symptoms, including bradycardia, hypotension, and diaphoresis. This phenomenon typically resolves within 3–5 min without a rise in troponin levels. It is primarily attributed to a Bezold–Jarisch-like reflex caused by mechanical stimulation of the vagal network near the puncture site. The parasympathetic fibers in this region, which preferentially innervate the right coronary artery, may induce cholinergic vasospasm.
An alternative explanation is coronary air embolism. Gas embolism can be observed when using a diathermia on the needle to electrify the Brockenbrough needle, rather than using a dedicated RF device. Recovery can be spontaneous, but atropine or adrenaline administration is often required. If ST elevation persists, air or thrombus embolisms should be considered and managed accordingly. Proper diagnosis and prompt recognition are essential to avoid unnecessary invasive interventions.
3.5. Residual Iatrogenic Atrial Septal Defect
Residual iatrogenic ASDs are a frequent long-term complication of TSP, with sheath size being the strongest predictor [
18].
Persistent ASDs after transcatheter mitral valve interventions can have detrimental outcomes, including worsened heart failure, elevated NT-proBNP levels, and reduced six-month survival. In some cases, right-to-left or bidirectional shunts and large atrial septal defects (ASDs) (>8 mm) necessitate closure, especially after procedures that require large-bore sheaths and septal dilation. Patients with right-to-left shunts are at a higher risk of complications such as paradoxical embolism, stroke, and systemic embolization, and empiric anticoagulation may be necessary [
19].
Management strategies for residual ASDs depend on the defect’s size, shunting direction, and associated symptoms. Symptomatic patients, especially those with refractory hypoxemia, cryptogenic stroke, or worsening heart failure, should undergo percutaneous closure, typically with an Amplatzer device, which has favorable anatomical outcomes [
19]. Although prophylactic closure has been suggested for all patients undergoing mitral transcatheter edge to edge (TEER) procedures, evidence supporting this approach remains limited [
20]. TEE is essential for detecting ASDs and assessing shunt severity, offering morphological and size evaluations. However, long-term follow-up data on ASD natural history are still limited [
20].
5. Mastering Transeptal Puncture
TSP is a critical skill in structural heart interventions, requiring precision to avoid complications. Mastery begins with a thorough understanding of anatomy, fluoroscopy, and echocardiography (TEE/ICE) for real-time visualization.
Simulation-based training provides a safe, effective way to develop proficiency before performing the procedure in patients. Post-procedural echo is essential to confirm correct access and detect potential complications early. A structured learning pathway combining didactic education, hands-on simulation, and supervised clinical practice minimizes adverse events and ensures procedural success and effectiveness. Moreover, in high-volume centers, mortality and complication rates significantly decrease [
26], emphasizing the importance of experience and institutional expertise in achieving optimal outcomes.
6. Conclusions
While the technique and equipment for TSP have remained largely unchanged since their introduction in the late 1950s, the increasing use of TSP and the demand for safer and more precise punctures have led to the development of new devices and technique refinements. One of the most significant advancements has been the incorporation of intraprocedural echocardiography, which enhances precision and safety while enabling less experienced operators to perform TSP with greater confidence. Combining fluoroscopic imaging with transesophageal echocardiography can further streamline the procedure.
Despite these advancements, complications and challenges can still arise, underscoring the importance of a thorough understanding of each procedural step and potential complication.